Abstract
Introduction
Preliminary studies showed that coronavirus disease 2019 (COVID-19) disrupts body immune system, including dysregulation of cytokine interleukin-6 (IL-6). IL-6 inhibitors agents have been used as treatment options for COVID-19, yet their benefit as therapeutic agents remains unclear.
Objective
We performed a systematic review and meta-analysis to synthesize the available evidence on the potential therapeutic effect of IL-6 inhibitor agents for the treatment of COVID-19.
Methods
Two authors initially screened and reviewed the relevant studies from available databases. The data extracted will be tabulated and analyzed for the outcomes. The primary outcome was mortality. Secondary outcomes included discharge from the hospital, length of stay, and requirement for mechanical ventilation. The quality of each study was assessed using OCEBM ratings.
Results
We reviewed 18 studies with a total of 3303 subjects. Tocilizumab was the most commonly used in the studies (15 studies). Meta-analysis of included studies revealed significant reduction in mortality with tocilizumab and sarilumab (RR = 0.61, 95% CI 0.49–0.76). Other outcomes including hospital discharge (RR = 1.04, 95% CI 0.86–1.24), length of stay (mean difference –1.96 days, 95% CI –4.24 to 0.33) or requirement for mechanical ventilation (RR = 0.68, 95% CI 0.32–1.45) revealed no differences of IL-6 inhibitor agents compared to controls.
Conclusions
Available evidence suggests that IL-6 inhibitor agents reduce the risk of mortality in COVID-19, especially in severe conditions. Further well-designed trials are needed for assessing its efficacy and safety for COVID-19.
Keywords: COVID-19, SARS-CoV-2, Interleukin-6, IL-6
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has set major healthcare issues and economic burden worldwide. Since its outbreak in December 2019, researchers and physicians around the globe are working continuously through clinical trials and ongoing research to find the cure and vaccine for COVID-19. Currently, treatments of COVID-19 are mainly repurposing drugs or symptomatic with no definitive treatment directed against the virus [1].
In the absence of specific treatment or antiviral drugs been proven against SARS-CoV-2, researchers have proposed many therapeutics agents used as adjunctive treatments for COVID-19 patients apart from supplemental oxygen therapy or mechanical ventilation. Cytokine storm is one of the main mechanisms of the disease and is believed to trigger an exaggerated immune response in the host and has been observed more frequently in severe COVID-19 patients. The dysregulation of inflammatory markers including IL-6 has been associated with COVID-19 complications, such as acute respiratory distress syndrome (ARDS) and other organ injuries, including neurological and cardiovascular diseases [[2], [3], [4]].
Monoclonal antibody against IL-6 receptors or IL-6 inhibitors has shown to be an effective agent in COVID-19 patients with severe illness [3 4]. These drugs targeting IL-6 as inflammatory mediators will decrease inflammatory response in cytokine storm, minimizing the incidence of jeopardizing complications, such as ARDS, and improving clinical outcome and decrease in mortality rate [3,4]. Tocilizumab and other IL-6 inhibitors have been approved by the Food and Drug Administration (FDA) for the management of severely ill patients with COVID-19 [4,5]. This review summarizes current evidence regarding interleukin-6 (IL-6) inhibitors drugs for the treatment of COVID-19.
Methods
This study was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [6].
Search strategy
A systematic literature search of the available studies was conducted on PubMed and medRxiv for articles published until November 2020. Additionally, we examined the bibliography of the selected articles for further potential studies. The search terms used were “interleukin 6,” OR “IL-6,” OR “tocilizumab,” OR "sarilumab," OR "siltuximab." AND “COVID-19,” OR “SARS-CoV-2” and their derivates.
Inclusion andexclusion criteria
We included only studies that compared the effectiveness of the IL-6 inhibitors with placebo or other agents for treatment of COVID-19. The following inclusion criteria were utilized for article selection: [1] involve human patients with COVID-19 [2]; be either a randomized controlled trial, prospective trial, retrospective analysis [3]; reported at least one clinical outcome, including hospital discharge, length of stay, mortality, and requirement for mechanical ventilation
The literatures were restricted to English language articles only. The exclusion criteria were as follows: (i) non-original studies, such as meta-analyses, conference papers, comments, or consensus documents; (ii) case reports or case series; (iii) single-arm trials; (iv) studies that did not report outcomes for IL-6 inhibitor drugs in COVID-19, and studies that did not compare the outcomes with IL-6 inhibitor drugs compared to the placebo or control.
The primary outcome was mortality. Secondary outcomes included discharge from hospital, length of stay, and requirement for mechanical ventilation.
Study selection and data extraction
Two authors (VOW and RBB) independently screened and examined the titles and abstract, followed by full-text review, using pre-defined criteria. In the event of disagreement between the two authors, consensus discussion would help to resolve the issue and make a final decision. Studies that entirely fulfilled our inclusion criteria were retrieved and additional articles were added based on the bibliography of the articles retrieved through the outlined search strategy. If the reviewers could not reach an agreement, the first author’s will be consulted for the final decision.
We extracted and tabulated the following data: author(s), year of publication, study design, country, baseline characteristics, details of the regimen of IL-6 inhibitors and comparative agents, and clinical outcomes.
Data analysis
Statistical analysis was conducted using the software Review Manager v.5.3 (Cochrane Collaboration). The degree of heterogeneity was evaluated with the Q statistic generated from the χ2 test. Heterogeneity was assessed using the I2 measure. Heterogeneity was defined as significant when the P-value was <0.05 or the I2 > 50%. A random-effects model was applied when data were considered heterogeneous. The pooled risk ratio (RR) and 95% confidence interval (CI) were calculated for the outcomes of mortality, hospital discharge, and requirement for mechanical ventilation. In case of continuous variables, the mean difference for each study was calculated and plotted. P- value of <0.05 was considered statistically significant [7,8].
Study quality assessment
We assessed the quality of evidence using The Oxford Center for Evidence-Based Medicine Quality ratings and classified the evidence ratings ranged from 1 to 5, with 1 representing high quality studies such as randomized controlled trial (RCT) and 5 representing case reports [9].
Results
Study characteristics
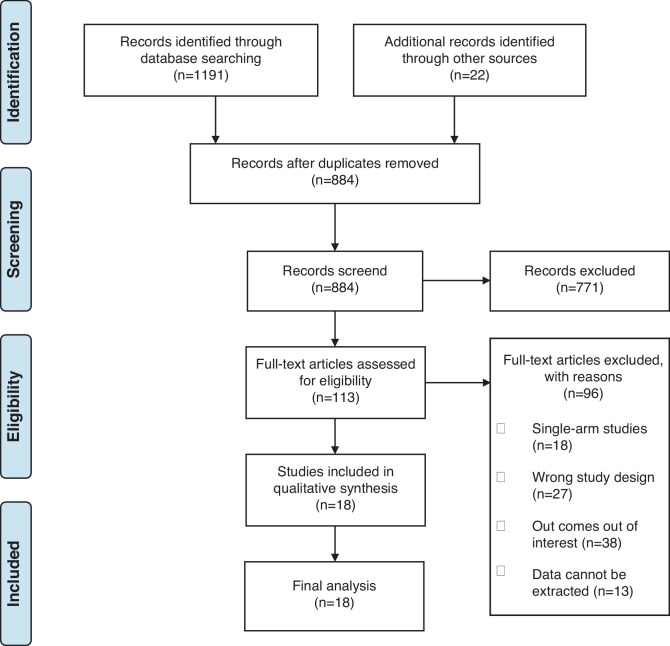
The search strategy initially generated 1213 articles. After removal of duplicates and abstract screening, 113 full-text articles were subsequently assessed for its eligibility. Finally, 18 articles were included in the final review including 1 RCT, 14 cohorts and 3 case control. Fig. 1 shows the PRISMA flow chart of study selection. Table 1 summarizes the baseline characteristics of all the 18 studies, including the study quality ratings.
Fig. 1.
Flow diagram of included studies.
Table 1.
Baseline characteristics of patients in the included studies.
| Authors | Study Type | Country Location | No. of participants, n (%) | Severity, n (%) | Age, Median (IQR, y) or Mean ± SD | Study Quality Level |
|---|---|---|---|---|---|---|
| Guaraldi et al. 2020 [10] | Retrospective Cohort | Modena, Italy | 544 COVID-19 patients | Severe, 544 (100%) | 67 (56−77) | 2 |
| Campochiaro et al. 2020 [11] | Retrospective Cohort | Milan, Italy | 65 COVID-19 patients | Severe, 65 (100%) | Treatment = 64 (53−75), Control = 60 (55−75.5) | 2 |
| Klopfenstein et al. 2020 [12] | Case Control | France | 45 COVID-19 patients | Critical, 20 (44%) | Treatment = 76.8 (52−93), Control = 70.7 (33−96) | 3 |
| Somers et al. 2020 [13] | Retrospective Cohort | United States | 154 COVID-19 patients | Severe, 154 (100%) | 58 ± 14.9 | 2 |
| Quartuccio et al. 2020 [14] | Retrospective Cohort | Udine, Italy | 111 COVID-19 patients | NR | Treatment = 62.4 ± 11.8, Control = 56.2 ± 14.2 | 2 |
| Rojas-Marte et al. 2020 [15] | Case Control | United States | 193 COVID-19 patients | Critical, 121 (62.7%); Very Severe, 59 (30.6%) | 60.4 ± 13.8 | 3 |
| Kewan et al. 2020 [16] | Retrospective Cohort | United States | 51 COVID-19 patients | Severe, 51 (100%) | 65 (53−74) | 2 |
| Klopfenstein et al. 2020 [17] | Case Control | France | 206 COVID-19 patients | Critical, 30 (14.6%) | Treatment = 75.6 ± 11.3, Control = 74.3 ± 11 | 3 |
| De Rossi et al. 2020 [18] | Retrospective Cohort | Brescia, Italy | 158 COVID-19 patients | NR | Treatment = 62.9 ± 12.5, Control = 71 ± 14.6 | 2 |
| Eimer et al. 2020 [19] | Retrospective Cohort | Sweden | 87 COVID-19 patients | Moderate, 11 (13%) | Treatment = 58 (49−63), Control = 55 (52−64.8) | 2 |
| Severe, 76 (87%) | ||||||
| Canziani et al. 2020 [20] | Retrospective Cohort | Italy | 128 COVID-19 patients | NR | 63 ± 10 | 2 |
| Patel et al. 2020 [21] | Retrospective Cohort | United States | 83 COVID-19 patients | Severe, 42 (51%) | Treatment = 68 (25−96), Control = 67 (20−91) | 2 |
| Severe, 41 (49%) | ||||||
| Gokhale et al. 2020 [22] | Retrospective Cohort | India | 161 COVID-19 patients | Severe, 161 (100%) | Treatment = 52 (44−57), Control = 55 (48−65) | 2 |
| Biran et al. 2020 [23] | Retrospective Cohort | United States | 630 COVID-19 patients | Severe, 630 (100%) | Treatment = 62 (53−71), Control = 65 (56−74) | 2 |
| Garcia et al. 2020 [24] | Retrospective Cohort | Spain | 171 COVID-19 patients | NR | Treatment = 61.5 ± 12.4, Control = 61.4 ± 16 | 2 |
| Della-Torre et al. 2020 [25] | Prospective Cohort | Milan, Italy | 56 COVID-19 patients | Severe, 56 (100%) | Treatment = 56 (49−60), Control = 57 (52−60) | 2 |
| Gritti et al. 2020 [26] | Prospective Cohort | Italy | 60 COVID-19 patients | Severe, 30 (100%) | Treatment = 64 (57−66), Control = 65.5 (56−70) | 2 |
| Stone et al. 2020 [27] | Randomized Controlled Trial | United States | 242 COVID-19 patients | NR | Treatment = 61.6 (46.4−69.7), Control = 56.5 (44.7−67.8) | 1 |
Of the 18 non-randomized articles reported the use of IL-6 inhibitor and its comparator for COVID-19, 16 studies using tocilizumab [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24],27], 1 using sarilumab [25], and 1 using siltuximab [26]. The therapeutic interventions, drug dosing, IL-6 markers, and clinical outcomes are presented in Table 2 . Most of the included studies were from Italy, with 7 studies, followed by the United States (US) with 6 studies, France with 2 studies, Spain with 1 study, Sweden with 1 study, and India with 1 study, respectively. Overall, this review included a total of 3303 patients, including 1265 in the tocilizumab group, 28 in the sarilumab group, 30 in the siltuximab group and 1980 in the control group. All the subjects in intervention group also received standard treatment in addition with IL-6 inhibitor drugs.
Table 2.
Patients group and clinical outcomes of COVID-19 patients in the included studies.
| Authors | Treatment (no. of patients) | Control (no. of patients) | Baseline IL-6 level, pg/mL (Median [range] or Mean ± SD) | Discharge, n (%) | Length of Stay, days (Median [range] or Mean ± SD) |
Death, n (%) | Mechanical Ventilation, n (%) | Other outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | ||||||||
| Guaraldi et al. 2020 [10] | Tocilizumab 8 mg/kg i.v. repeated after 12 h (n = 88), 324 mg s.c. once (n = 91) + standard treatment | Standard treatment (hydroxychloroquine/ azithromycin/antiretrovirals/low molecular weight heparin), (n = 365) | 178.6 (67.6−402) | NR | 12 (6−17) | 8 (4−14) | 86 (16%) | 90 (17%) | |
| Campochiaro et al. 2020 [11] | Tocilizumab 400 mg i.v. once, repeated after 24 h in case of worsening + standard treatment (n = 32) | Standard treatment (hydroxychloroquine 400 mg daily, lopinavir/ritonavir 400/100 mg twice daily, ceftriaxone 2 g for 6 days, azithromycin 500 mg daily, enoxaparin 4000 UI once a day) (n = 33) | NR | 36 (55%) | 13.5 (10−16.7) | 14 (12−15.5) | 16 (25 %) | 1 (3%) | Patients with age <75 years had a higher survival (HR 1.46, 1.03–2.08, p = 0.03) |
| Klopfenstein et al. 2020 [12] | Tocilizumab 1 or 2 doses + standard treatment (n = 20) | Standard treatment (hydroxychloroquine/lopinavir-ritonavir and antibiotics,) (n = 25) | NR | 22 (49%) | 13 ± 7 | 17 ± 12 | 16 (36%) | 8 (18%) | |
| Somers et al. 2020 [13] | Tocilizumab 8 mg/kg i.v. once + standard treatment (n = 78) | Standard treatment (n = 76) | NR | 74 (48%) | 20.4 (13.8–35.8) | 22.9 (16.3–28.5) | 41 (27%) | 154 (100%)a | Rate of Superinfection, Treatment = 42 (54%); Control = 20 (26%) |
| Quartuccio et al. 2020 [14] | Tocilizumab 8 mg/kg i.v. once + standard treatment (n = 42) | Standard treatment (antivirals/antimalarials/glucocorticoids/antibiotics/anticoagulant) (n = 69) | Treatment = 63.5 (37−136), Control = 18.5 (10−33) | 79 (72%) | NR | NR | 4 (4%) | 26 (23%) | Recovered or Improvement, Treatment = 30 (71%), Control = 69 (100%) |
| Rojas-Marte et al. 2020 [15] | Tocilizumab one dose + standard treatment (n = 96) | Standard treatment (hydroxychloroquine/remdesivir/corticosteroids/anticoagulants and azithromycin) (n = 97) | NR | NR | 14.5 ± 8.8 | 16.5 ± 10.8 | 90 (50.8%) | 121 (62.7%) a | Bacteremia was more commonly in the control group compared to tocilizumab group (23.7% vs. 12.5%, P = 0.04), whereas fungemia was similar in both groups (4% vs. 3% P = 0.7). |
| Kewan et al. 2020 [16] | Tocilizumab 8 mg/kg i.v. up to 400 mg once + standard treatment (n = 28) | Standard treatment (hydroxychloroquine/corticosteroids and azithromycin) (n = 23) | Treatment = 14 (8−59), Control = 35 (16−55) | 24 (47%) | 11 (6−22.25) | 7(5−13.5) | 5 (10%) | 32 (63%) a | Among patients aged >65 years and required invasive ventilation, tocilizumab group had higher rate of clinical improvement [40% vs. 13%, p = 0.20], shorter median time to clinical improvement [8 days (5–14.5) vs. 12.5 (7.75–17.5), p = 0.53], and shorter median duration of vasopressor support [2.5 days (1.75–3.25) vs. 6.5 days (4.25–9.5), p = 0.011], compared to control |
| Klopfenstein et al. 2020 [17] | Tocilizumab 8 mg/kg i.v. one or two doses + standard treatment (n = 30) | Standard treatment (hydroxychloroquine/lopinavir-ritonavir/corticosteroids and antibiotics) (n = 176) | Treatment = 549 (3−4156), Control = 179 (66−399) | 98 (48%) | 17 ± 10.1 | 15.2 ± 12 | 74 (36%) | 39 (22%) | |
| De Rossi et al. 2020 [18] | Tocilizumab 400 mg i.v (n = 43) or 324 mg s.c (n = 47) + standard treatment | Standard treatment (hydroxychloroquine 400 mg daily, lopinavir 800 mg plus ritonavir 200 mg per day) (n = 68) | NR | NR | NR | NR | 41 (26%) | 19 (12%) | Twelve patients in treatment group (13.3%) had pulmonary embolism, three reported died. |
| Eimer et al. 2020 [19] | Tocilizumab 8 mg/kg i.v. once + routine care (n = 29) | Routine care (n = 58) | Treatment = 351 (154−1193), Control = 180.5 (105.8−335.2) | 31 (36%) | 20.5 [16.5−30] | 30 [21.5−30], | 24 (28%) | 77 (89%) | Treatment group had shorter length of stay (days) in ICU (12 [6.8−17.2] vs 20 [9.8−30], p = 0.04) and hospital (20.5 [16.5−30] vs 30 [21.5−30], p = 0.04), compared to control group Blood stream infection (17.2% vs. 24.1%, P = 0.65), ventilator-associated pneumonia(20.7% vs. 32.8%, P = 0.36), and pulmonary embolism(20.7% vs. 22.4%, P = 1.00) were more commonly in the control group compared to treatment group |
| Canziani et al. 2020 [20] | Tocilizumab 8 mg/kg i.v. repeated after 24 h + standard treatment (n = 64) | standard treatment (hydroxy- chloroquine/direct antivirals/antibiotics [ceftriaxone, azithromycin, piperacillin and ta- zobactam]/glucocorticoids [IV methylprednisolone 1–2 mg/kg/day]/prophylactic enoxaparin) (n = 64) | 179 ± 193 | NR | NR | NR | 41 (32%) | 38/102 (34%) | The use of tocilizumab was not associated with the risk of thrombotic vascular events, bleeding, or infection (p > 0.05). |
| Patel et al. 2020 [21] | Tocilizumab 8 mg/kg i.v. repeated after 24 h + standard treatment (n = 42) | standard treatment (hydroxy- chloroquine/ antivirals/vitamin C) (n = 41) | Treatment; Severe = 61 ± 107, Critical = 342 ± 783 | 33 (40%) | NR | NR | 22 (27%) | 2 (2%) | At the time of last follow up (day 7), treatment group had lower rate of hospitalization (19% vs 48.8%) compared to control group |
| Gokhale et at. 2020 [22] | Tocilizumab 400 mg i.v + standard treatment once (n = 70) | standard treatment (antibiotics, hydroxychloroquine 400 mg once daily, ivermectin 12 mg once daily, oseltamivir 75 mg twice daily, low molecular weight heparin 1 mg/ kg s.c once daily, methylprednisolone 125500 mg i.v once daily) (n = 91) | NR | 56 (35%) | 14 (9-25.5) | 6 (3–14) | 94 (58%) | 10 (6%) | |
| Biran et al. 2020 [23] | Tocilizumab 4 mg/kg i.v one or two doses + standard treatment once (n = 210) | Standard treatment (n = 420) | Treatment = 29 (9−96), Control = 18.5 (7−49.75) | 416 (66%) | NR | NR | 358 (57%) | 587 (93%) | Among 286 patients with C-reactive protein levels of 15 mg/dL or higher, tocilizumab exposure was associated with decreased hospitalrelated mortality (HR 0·48, 95% CI 0·30–0·77; p = 0·0025) |
| Garcia et al. 2020 [24] | Tocilizumab 4−6 mg/kg/12 h i.v up to three doses + standard treatment (n = 94) | Standard treatment (lopinavir/ritonavir 400/100 mg BID for 7−14 days + hydroxychloroquine 200−400 mg/12 h for 5 days + azithromycin 250−500 mg/24 h for 5 days) (n = 77) | NR | 136 (79.5%) | 11.2 ± 6.2 | 14.7 ± 10.6 | 25 (15%) | 13 (8%) | Comorbidities, oxygen requirement upon admission, C-reactive protein level >16 mg/dl, and complications were significantly associated with mortality and ICU admission |
| Della-Torre et al. 2020 [25] | Sarilumab 400 mg i.v once + standard treatment (n = 28) | Standard treatment (lopinavir/ritonavir, hydroxychloroquine and azithromycin) (n = 28) | Treatment = 67.5 (37.5-127), Control = 46 (34–117) | 34 (61%) | 12 (8–20) | 13 (10–20) | 7 (12.5%) | 13 (23%) | Median time to death was significantly longer in the sarilumab group (19 days, IQR 13–26 vs 4 days, IQR 3–4; p = 0.006) compared to control. At 28-day follow-up, CRP returned to normal value in 86% patients treated with sarilumab and in 61% patients in the control group (p = 0.06) Adverse events were reported more frequently in the sarilumab group compared to the control group (43 vs 36%) Serum IL-6 level, PaO2/FiO2 ratio, the percentage of lung consolidation and total volume of consoli- dated lung were associated with clinical improvement in patients treated with sarilumab |
| Gritti et al. 2020 [26] | Siltuximab 11 mg/kg i.v one or two doses + standard treatment (n = 30) | Standard treatment (antivirals, hydroxychloroquine, and low molecular weight heparin) (n = 30) | Treatment = 129.86 (74.56-237.88) | 16/30 (53%)a | 33 (7–58) | 22.9 (2–45) | 10/30 (33%)a | 5/30 (17%)a | Adverse events in Siltuximab group: cerebrovascular events, 1/30 (3%), infection 13/30 (43%) The 30-day mortality rate was significantly lower in the siltuximab-treated than the matched-control cohort patients (HR 0·462, 95% CI 0·221– 0·965); p = 0·0399). |
| Stone et al. 2020 [27] | Tocilizumab 8 mg/kg i.v. single dose + standard treatment (n = 161) | Standard treatment (antivirals, hydroxychloroquine, and glucocorticoids) (n = 81) | Treatment = 23.6 (14-49.9), Control = 25.4 (14.6-40.3) | 219 (90%) | 6.0 (4.0-7.0) | 6.0 (5.0-6.0) | 12 (0.5%) | 19 (0.8%) | Patients in tocilizumab treatment had fewer serious infections than patients who received standard care. |
Study by Gritti et al [26] was not included in meta-analysis because its only reported the outcomes in treatment group.
A single dose of 400 mg or 8 mg/kg intravenous was the most commonly reported regimen of Tocilizumab. In 8 out of 16 studies [[10], [11], [12],17,[20], [21], [22], [23], [24]] suggested that the second dose of Tocilizumab may be administered based on physician judgment. In one study, Della-Torre et al. [25] used intravenous injection of sarilumab of 400 mg. Gritti et al. [26] also used 11 mg/kg of intravenous injection of siltuximab. The use of hydroxychloroquine, antivirals, azithromycin, or anticoagulants were the most commonly reported regimen included in standard treatment.
Clinical outcome
Hospital discharge and length of stay
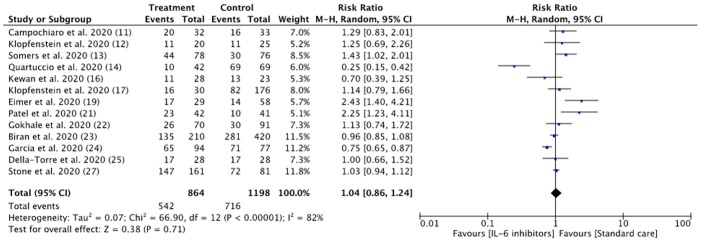
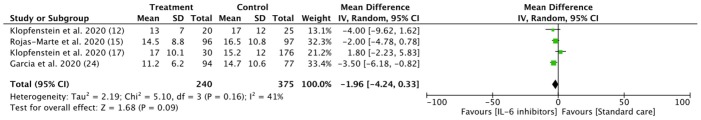
Analysis of the thirteen included studies revealed that patients with COVID-19 had a similar risk to be discharged from hospital during study follow-up in the IL-6 treatment group compared with the control group (62.7% vs 59.8%; RR = 1.04, 95% CI 0.86–1.24; I2 = 82%) ( Fig. 2 ). The patient’s group and clinical outcomes are shown in Table 2. Evidence from 3 case control and 1 cohort studies in patients treated with tocilizumab suggested potential but uncertain effects on decreasing length of hospital stay (weighted MD –1.96, 95% CI –4.24 to 0.33) (Fig. 3 ). A prospective cohort study reported longer duration of follow up in the siltuximab treatment group compared (33 days [7–58]) to standard care (22.9 days [2–45]). [26].
Fig. 2.
Risk of hospital discharge between IL-6 inhibitors and control groups. (Note = All of the studies were using Tocilizumab, except for Della-Torre et al. [25] with Sarilumab).
Fig. 3.
IL-6 inhibitors in reducing length of hospital stay. Weights are from random-effects analysis. (Note = All of the studies included were using Tocilizumab, Only studies reported the data in mean ± standard deviation [SD] were included in the analysis).
Mortality
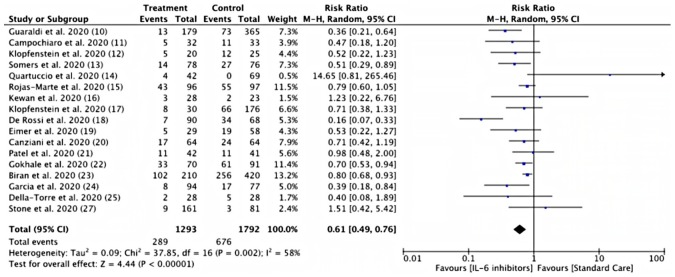
Pooled analysis from thirteen studies showed a lower risk of mortality in the IL-6 inhibitor treatment group compared to the control group, the difference also reach statistical significance (22.35 % vs 37.72 %; RR = 0.61, 95% CI 0.49–0,76; I2 = 58% p < 0,00001) ( Fig. 4 ). Two cohort studies reported [23,26] the IL-6 inhibitor treatment group's decreased mortality rate. Prospective studies in Italy [25] reported that the sarilumab treatment group had a longer median time to death than the control group (19 days, IQR 13–26 vs four days, IQR 3–4; p = 0.006). A prospective study of siltuximab treatment in COVID-19 reported a lower 30-day mortality rate than the control group (HR 0.462, 95% [CI] 0·221–0·965; p = 0·0399) [26].
Fig. 4.
Risk of mortality between IL-6 inhibitors and control groups. (Note = All of the studies were using Tocilizumab, except for Della-Torre et al. [25] with Sarilumab).
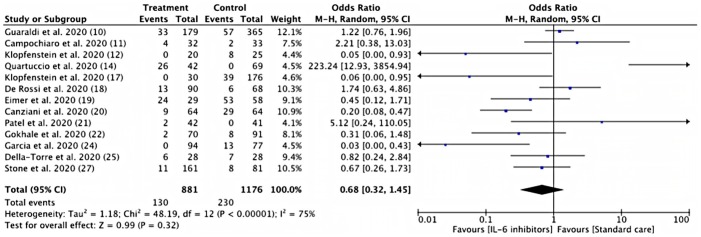
Risk of mechanical ventilation
Pooled estimates from thirteen studies showed a similar risk of mechanical ventilation between IL-6 Inhibitor treatment group and control group (14.75% vs 19.55%; RR = 0.68, 95% CI 0.32–1.45; I2 = 75%) (Fig. 5 ). A siltuximab treatment group in the prospective study showed the potential to reduced mechanical ventilation risk, although the results are not significant (HR 0.615; 95% CI 0.362–1.044) [26].
Fig. 5.
Risk of requirement for mechanical ventilation between IL-6 inhibitors and control groups. (Note = All of the studies were using Tocilizumab, except for Della-Torre et al. [25] with Sarilumab).
Discussion
COVID-19 primarily infects pneumocyte type II and cells expressing angiotensin-converting enzyme (ACE-2), which also serve as receptor and entry point for the virus [30]. COVID-19 replication causing pyroptosis (apoptosis induced by inflammation) effects on its target cell, thus activating innate immunity and leading to the synthesis of pro-inflammatory cytokine by myeloid cells. COVID-19 also inhibits the synthesis of type I Interferon, which attenuates the body's immune response to the virus and provides a suitable environment for the virus to replicate rapidly. The Rapid increase of viral load and viral cytopathic effects induced a rapid progression of the inflammatory process, which lead to cytokine storm syndrome [30]. CSS, marked by the uncontrolled release of the pro-inflammatory cytokine, may cause an increase of gas exchange, reducing pulmonary tissue oxygenation [32]. Among those cytokines, several studies [[33], [34], [35]] suggest that IL-6 plays a crucial role in CSS's pathogenesis in COVID-19.
Monocytes and macrophages produce IL-6 after stimulated by Toll-like receptors and work through two different signal pathways. The first pathways are the classic pathway, in which IL-6 bind to transmembrane IL-6 (mIL-6R) and IL-6 soluble receptor (sIL-6R). The complex then binds to gp130 and triggered gene expression. The trans pathways are the second pathways, in which the complex of IL-6 and its receptors bind to signal transducer glycoprotein (gp130) and initiated intracellular signal transduction, thus initiated activation of other pathways promoting cellular proliferation, differentiation, immune regulation, and oxidative stress [31].
The primary consideration of IL-6 inhibitors in the treatment of COVID-19 may be based on the ability to inhibit IL-6, which plays a central role in acute inflammation and cytokine release syndrome [28]. Anti-IL-6 monoclonal antibodies (siltuximab) prevent IL-6 to binding to its receptors (both membranes bound and soluble receptors) and inhibit the formation between gp130 and hexameric signalling complex on the cell surface, thus hinder the activation of signal transducer and transcription signalling pathway [36]. Anti-IL-6 receptor monoclonal antibodies (sarilumab and tocilizumab) bind to both transmembrane (mIL-6R) and soluble (sIL-6R) IL-6 receptor and inhibits both the classical and trans pathway of IL-6 signal transduction [37].
This study investigates the therapeutic effect of IL-6 Inhibitors based on four clinical outcomes; hospital discharge, length of stay, mortality rate, and mechanical ventilation risk. Based on an analysis of twelve studies, it was found that IL-6 inhibitors do not provide a beneficial effect on hospital discharge and length of stay. However, one retrospective study reported treatment of tocilizumab in critically ill COVID-19 patients might reduce the length of stay in ICU and hospital [19]. A retrospective study in the USA also suggested an early administration of IL-6 Inhibitors (Sarilumab and tocilizumab) may help reduce the length of stay and needed mechanical ventilation [28].
The result of this review suggested the benefit of IL-6 inhibitors in reducing mortality rate based on an analysis of thirteen studies. This result is in line with several recent systematic reviews that reported a lower mortality rate in the tocilizumab group than the control group [29,[38], [39], [40], [41]]. COVID-19 replication causing pyroptosis of the target cell, thus inducing synthesis of pro-inflammatory cytokine [28]. The rapid increase of pro-inflammatory cytokine may lead to a cytokine storm, causing septic shock and multiple organ failure [30]. IL-6 inhibitors may inhibit the pro-inflammatory cytokine, thus lower the disease's mortality. An observational study found that patient with rapid progressing COVID-19 respiratory failure may benefit from siltuximab treatment to reduce hyper inflammation driven by cytokine and mortality rate [26]. A multicenter study in COVID-19 patient requiring ICU admission reported the favorable results of tocilizumab treatment to reduce hospital-related mortality, especially in patients required mechanical ventilator support and those younger than 65 years [23].
The analysis results of thirteen studies in mechanical ventilation risk suggested that IL-6 inhibitors do not provide benefits to reducing mechanical ventilation risk in COVID-19 patients. The recent RCT reported the Tocilizumab was not effective in preventing intubation in moderately ill COVID-19 patients, with a hazard ratio of 0.83 compared to the control group (95% confidence interval [CI], 0.38 to 1.81; P = 0.64), this study suggested the Tocilizumab failure to affect clinical outcome possibly because elevated Interleukins level represent host responses to infection rather than a self-amplifying inflammatory loop that would benefit from IL-6 inhibitors. [27] Unlike our study, a previous systematic review reported the benefit of tocilizumab to lower mechanical ventilation risk [39]. A prospective study in Germany reported IL-6 as a strong predictor of mechanical ventilation [31], these results showed the potential of IL-6 inhibitors to lower the mechanical ventilation risk. Tocilizumab administered to non-critically ill COVID-19 patients in the early stage of inflammatory flare may reduce mechanical ventilation use [24]. Two retrospective studies [20,21] reported a lower risk of mechanical ventilation initiation in patients receiving tocilizumab than those who do not receive anti-cytokine therapy.
Additionally, previous study shows that COVID-19-associated neurological diseases were linked with elevated levels of IL‐6, and other inflammatory markers in the cerebrospinal fluid [42]. Conversely, increased serum levels of IL‐6 was associated with an incidence of encephalopathy in a COVID-19 patient. Furthermore, the treatment with tocilizumab resolved the neuropsychiatric manifestations of the patient [42,43]. These findings may be indicative of IL-6 role in blood brain barrier disruption and the potential role of IL-6 inhibitor agents in treating COVID-19-related neurological diseases.
There are some limitations regarding this review. First, the majority of included studies were using tocilizumab as treatment option, therefore lacking of another type of IL-6 drugs to be included in meta-analysis. In addition, most of the subjects were not homogenous in terms of baseline characteristics and most of the treatment group had more severe disease at baseline compared with controls. Most of the studies included were retrospective studies with small sample size, which may result in low quality of evidence. Future well-designed randomized trials are needed for testing its efficacy to provide high-quality evidence to support the findings of this study.
Conclusion
IL-6 inhibitors agents have shown potential benefit in reducing risk of mortality in COVID-19 patients especially in severe disease. However, the appropriate dosage and drug administration remains unclear. Clinicians should consider the use of IL-6 inhibitor agents in well-established clinical trials to evaluating the benefit and risk of the drugs.
Funding
No funding Sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X., Han M., Li T., Sun W., Wan D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syn- drome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40:511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins J.P.T., Green S., editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration; Oxford (UK): 2011. 7.7.3.5. Medians and interquartile ranges.https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges.htm Available: [Google Scholar]
- 8.Higgins J.P.T., Altman D.G., Gotzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.OCEBM Levels of Evidence Working Group . Oxford Centre for Evidence-Based Medicine; Oxford: 2011. The oxford levels of evidence 2. [Google Scholar]
- 10.Guaraldi G., Meschiari M., Cozzi-Lepri A. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campochiaro C., Della-Torre E., Cavalli G. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klopfenstein T., Zayet S., Lohse A. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020;50(5):397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somers E.C., Eschenauer G.A., Troost J.P. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Preprint. medRxiv. 2020 doi: 10.1101/2020.05.29.20117358. 2020.05.29.20117358. Published 2020 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quartuccio L., Sonaglia A., McGonagle D. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas-Marte G., Khalid M., Mukhtar O. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM. 2020;113(8):546–550. doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kewan T., Covut F., Al-Jaghbeer M.J., Rose L., Gopalakrishna K.V., Akbik B. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100418. Published 2020 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klopfenstein T., Zayet S., Lohse A. Impact of Tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int J Infect Dis. 2020;99:491–495. doi: 10.1016/j.ijid.2020.08.024. S1201-9712(20)30653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rossi N., Scarpazza C., Filippini C. Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eimer J., Vesterbacka J., Svensson A.K. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J Intern Med. 2021;289(3):434–436. doi: 10.1111/joim.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canziani L.M., Trovati S., Brunetta E. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients [published online ahead of print, 2020 Jul 8] J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel K., Gooley T.A., Bailey N. Use of the IL-6R Antagonist Tocilizumab in Hospitalized COVID-19 Patients. J Intern Med. 2020;289(3):430–433. doi: 10.1111/joim.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gokhale Y., Mehta R., Karnik N., Kulkarni U., Gokhale S. Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100467. Published 2020 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biran N., Ip A., Ahn J. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia E.M., Caballero V.R., Albiach L. Tocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. Preprint. medRxiv. 2020 doi: 10.1101/2020.06.05.20113738. 2020.06.05.20113738. [DOI] [Google Scholar]
- 25.Della-Torre E., Campochiaro C., Cavalli G. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gritti G., Raimondi F., Ripamonti D. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. Preprint. medRxiv. 2020 doi: 10.1101/2020.04.01.20048561. 2020.04.01.20048561. [DOI] [Google Scholar]
- 27.Stone J.H., Frigault M.J., Serling-Boyd N.J. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Early administration of interleukin-6 inhibitors for patients with severe COVID-19 disease is associated with decreased intubation, reduced mortality, and increased disch. [DOI] [PMC free article] [PubMed]
- 29.Kaye A., Siegel R. The efficacy of IL-6 inhibitor Tocilizumab in reducing severe COVID-19 mortality: a systematic review. PeerJ. 2020;8 doi: 10.7717/peerj.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herold T., Jurinovic V., Arnreich C., Lipworth B., Hellmuth J., von Bergwelt-Baildon M. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1) doi: 10.1016/j.jaci.2020.05.008. 128–136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atal S., Fatima Z. IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut Med. 2020;34(4):223–231. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science. [DOI] [PubMed] [Google Scholar]
- 34.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palanques-Pastor T., López-Briz E., Poveda Andrés J. Involvement of interleukin 6 in SARS-CoV-2 infection: siltuximab as a therapeutic option against COVID-19. Eur J Hosp Pharm. 2020;27(5):297–298. doi: 10.1136/ejhpharm-2020-002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40(6):511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarfraz A. Tocilizumab and COVID-19: a meta-analysis of 2120 patients with severe disease and implications for clinical trial methodologies. Turk J Med Sci. 2020;(November) doi: 10.3906/sag-2010-131. Epub ahead of print. PMID: 33244947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotak S. Use of tocilizumab in COVID-19: a systematic review and meta-analysis of current evidence. Cureus. 2020;12(October (10)) doi: 10.7759/cureus.10869. PMID: 33178522; PMCID: PMC7652362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berardicurti O. Mortality in tocilizumab-treated patients with COVID-19: a systematic review and meta-analysis. Clin Exp Rheumatol. 2020;38(November–December (6)):1247–1254. Epub 2020 Dec 3. PMID: 33275094. [PubMed] [Google Scholar]
- 41.Hariyanto T.I. Efficacy and safety of tocilizumab for coronavirus disease 2019 (Covid-19) patients: a systematic review and meta-analysis. Drug Res (Stuttg) 2021;(January) doi: 10.1055/a-1336-2371. Epub ahead of print. PMID: 33401328. [DOI] [PubMed] [Google Scholar]
- 42.Muccioli L., Pensato U., Cani I., Guerra L., Provini F., Bordin G. COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J Neuroimmunol. 2020;349(December) doi: 10.1016/j.jneuroim.2020.577400. Epub 2020 Sep 24. PMID: 33032013; PMCID: PMC7513756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espíndola O.M., Gomes Y.C.P., Brandão C.O., Torres R.C., Siqueira M., Soares C.N. Inflammatory cytokine patterns associated with neurological diseases in coronavirus disease 2019. Ann Neurol. 2021;89(May (5)):1041–1045. doi: 10.1002/ana.26041. Epub 2021 Feb 24. PMID: 33547819; PMCID: PMC8014707. [DOI] [PMC free article] [PubMed] [Google Scholar]