Abstract
Background and Purpose
The purpose of this study was to investigate the frequency of symptomatic in-stent restenosis (ISR) and its contribution to non-procedural symptomatic infarction in the Stenting and Aggressive Medical Management for the Prevention of Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial.
Methods
Patients without a peri-procedural primary endpoint were followed to determine the occurrence of any of the following events: ischemic stroke, cerebral infarct with temporary signs (CITS) or TIA in the territory of the stented artery. Vascular imaging performed after these events were reviewed for ISR. Annual rates for symptomatic ISR were calculated using Kaplan Meier estimates.
Results
Of 183 patients in the stenting group without a peri-procedural primary endpoint, 27 (14.8%) had a symptomatic infarction (stroke or CITS) and 16 (8.7%) had TIA alone in the territory during a median follow-up of 35.0 months. Of the 27 patients with infarctions, 17 (9.3%) had an ischemic stroke and 10 (5.5%) had a CITS alone. Adequate vascular imaging to evaluate ISR was available in 24 patients with infarctions (showing ISR in 16 (66.7%)) and in 10 patients with TIA alone (showing ISR in 8 (80%)). The 1,2 and 3-year rates (with 95% confidence limits) for symptomatic ISR in the SAMMPRIS stent cohort were 9.6% (6.1% – 14.9%), 11.3% (7.5% – 17.0%), 14.0% (9.6% – 20.2%), respectively.
Conclusions
Symptomatic ISR occurred in at least one of seven patients in SAMMPRIS by 3 years of follow-up and was likely responsible for the majority of non-procedural cerebral infarctions.
Clinical Trial Registration
https://clinicaltrials.gov. Unique identifier NCT00576693
Keywords: Intracranial stenosis, angioplasty and stenting, clinical trial, restenosis
Introduction
Enrollment in the Stenting and Aggressive Medical Management for the Prevention of Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial was stopped early owing to a higher rate of 30-day stroke and death in the stenting arm relative to aggressive medical management.1 Aggressive medical management and follow-up continued and long term follow-up (mean 32.4 months) showed no late gain for the percutaneous transluminal angioplasty and stent (PTAS) arm: stroke rates after 30-day procedural events were similar between medical and interventional arms2. Beyond 30 days, 21 (10%) of 210 patients in the medical group and 19 (10%) of 191 patients in the PTAS group had a primary endpoint.2
One factor that may have been responsible for many of the non-procedural strokes after intracranial PTAS for intracranial atherosclerotic disease (ICAD) is in-stent restenosis (ISR).3–15 Although previous studies have described the incidence of ISR after PTAS for ICAD, with or without the Wingspan stent, most of these studies include both symptomatic and asymptomatic ISR.3–15 We undertook this analysis to estimate the rate of symptomatic ISR in SAMMPRIS.
Methods
SAMMPRIS was a randomized, multi-center clinical trial.1, 2, 16 The study design has been published.1, 16 Eligibility criteria included either transient ischemic attack (TIA) or non-disabling stroke within 30 days prior to enrollment attributable to angiographically-verified 70% to 99% stenosis of a major intracranial artery. Enrollment in the trial began in November 2008 and was stopped in April 2011. Medical treatment and follow up of enrolled patients was completed in April 2013. The SAMMPRIS study was approved by local institutional review boards at each site.
The present study is a post-hoc analysis of the data collected in the trial. Patients assigned to the PTAS arm that had successful (no peri-procedural complications) PTAS or angioplasty alone and any of the following events more than 30 days after enrollment were identified: (1) an ischemic stroke in the territory of the target artery, or (2) cerebral infarction with transient signs (CITS) in the territory, or (3) TIA in the territory. Ischemic stroke in the territory of the qualifying artery was a primary endpoint and was defined as a new focal neurological deficit of sudden onset, lasting at least 24 hours that was not associated with a hemorrhage on brain imaging. A CITS, which was considered a subtype of TIA and not a primary endpoint in SAMMPRIS, was defined as a new focal neurological deficit of sudden onset that lasted for less than 24 hours but was associated with a new infarct on brain imaging. TIA was defined as a new focal neurological deficit of sudden onset that lasted for less than 24 hours and was not associated with a new infarct on brain imaging if performed. Subjects were not followed for recurrent events after a primary endpoint but were for CITS and TIA. Details regarding any repeat revascularization procedures of the target artery performed prior to a primary endpoint were collected. Study records, including case report forms, procedure and progress notes, discharge or death summaries, electronically archived imaging, and records of central end-point adjudication on all patients with any of the events described above were reviewed.
PTAS Procedure
The Gateway PTA Balloon Catheter and Wingspan Stent System (Boston Scientific Corporation, Stryker Neurovascular, Fremont CA) were used for PTAS in the trial. Specific aspects of the study protocol for PTAS procedure, post-procedure care, and aggressive medical management have been published.1, 17 A 600 mg loading dose between 6 – 24 hours before PTAS was required if the patient was not on daily clopidogrel (75 mg) for five days prior to PTAS.
Central Adjudication of Non-Procedural Primary Endpoints in PTAS Group
Detailed analyses of the 30-day outcomes of the stented patients in SAMMPRIS have been reported previously.18–20 Beyond 30 days, clinical evaluations of treated patients were required at 4 month intervals to a common termination date 2 years after enrollment of the last patient. Patients with a suspected stroke were examined by a study neurologist and any brain imaging was reviewed. Vascular imaging was obtained at the discretion of the investigators or treating physicians. All ischemic strokes (i.e., neurological signs lasting more than 24 hours) and TIAs lasting > 1 hour were reported by sites and centrally adjudicated. TIAs associated with an infarct were classified as CITS. For the present investigation, all reported TIAs were reviewed blinded to treatment assignment by two study investigators to determine if they met the definition of a CITS or TIA in the territory.
ISR
All available vascular imaging (Computed Tomographic Angiography (CTA), Magnetic Resonance Angiography (MRA), and Digital Subtraction Angiography (DSA)) for all patients who had a primary endpoint, CITS or TIA in the territory beyond 30 days of enrollment was reviewed by two investigators. If patients had a CTA or MRA followed by DSA, the DSA study was used to determine ISR. ISR was defined as greater than 50% stenosis within or immediately adjacent (within 5 mm) of the implanted stent and >20% absolute luminal loss.3 This was scored as definite, probable, absent (50% or less stenosis), or indeterminate (unable to evaluate owing to the quality of the study or vascular imaging unavailable). Disagreements were resolved by consensus. Symptomatic ISR was defined as probable or definite ISR associated with ischemic symptoms in the territory.
Data Analysis
The analysis included patients that underwent angioplasty alone or PTAS who did not have a primary endpoint during the first 30 days after enrollment in the study. The Kaplan-Meier method was used to estimate the cumulative probability of symptomatic ISR versus time after enrollment. A patient having an adjudicated ischemic event in the territory associated with ISR was deemed to have had an endpoint at the time of the first such event. A patient not having an adjudicated ischemic event with ISR was censored at the time of a primary endpoint or the last study visit. In addition, patients having one or more adjudicated ischemic events whose ISR status could not be evaluated were censored at the first such event. Patients that were lost or who withdrew were censored at the last study visit completed. Analyses were done using SAS 9.3.
Results
Study Patients and Follow-Up
Of 224 patients in the stent arm, 4 patients declined the procedure after randomization and in 7 patients the procedure was aborted before the lesion was accessed (3 of whom had a peri-procedural stroke). An additional 30 patients had a primary endpoint within 30 days after enrollment. The remaining 183 patients underwent a procedure (4 of whom had angioplasty only) and form the basis for the present analysis. Median follow up of the 183 patients was 35.0 months (inter-quartile range 27.8 – 42.3 months). Nine patients were either lost to follow-up or withdrew consent beyond 30 days after enrollment (3 within the first year and 6 after 2 years of follow-up).
Ischemic Events Beyond Peri-procedural Period
Of the 183 patients in this study, 17 (9.3%) had an ischemic stroke in the territory during follow-up. The age, gender, symptomatic artery, timing of symptom onset, disability and imaging findings for these 17 patients are listed in Table 1. Two of the 17 patients had TIAs or CITS preceding a stroke (patients 6 and 15 in Table 1). The 15 remaining ischemic stroke patients did not have a CITS or TIA in the territory prior to the ischemic stroke.
Table 1.
Ischemic Stroke in the Territory of the Stented Artery after 30 days *
| Number | Age at Enrollment (yrs) | Gender | Symtomatic Artery | Months after Enrollment Stroke Occurred | ISR Status | Modality | Disabling Stroke1 |
|---|---|---|---|---|---|---|---|
| 1 | 72.8 | Male | Intracranial carotid | 21.0 | ISR | DSA | Yes |
| 2 | 49.6 | Male | Vertebral | 25.4 | No ISR | CTA | No |
| 3 | 56.0 | Male | Basilar | 7.1 | ISR | DSA | No |
| 4 | 79.2 | Male | Intracranial carotid | 4.8 | ISR | CTA | No |
| 5 | 60.6 | Male | Vertebral | 7.0 | No ISR | DSA | Yes |
| 6** | 73.7 | Female | Intracranial carotid | 5.8 | ISR | DSA | Yes |
| 7 | 55.7 | Male | Intracranial carotid | 3.5 | No ISR | DSA | No |
| 8 | 53.9 | Female | Intracranial carotid | 7.7 | ISR | DSA | No |
| 9 | 45.4 | Male | Basilar | 4.0 | ISR | DSA | No |
| 10 | 63.4 | Male | MCA stem (M1) | 14.2 | ISR | CTA | No |
| 11 | 62.9 | Male | Intracranial carotid | 8.1 | No Imaging | No | |
| 12 | 51.2 | Male | MCA stem (M1) | 3.0 | ISR | CTA | No |
| 13 | 64.2 | Male | MCA stem (M1) | 26.2 | No ISR | DSA | No |
| 14 | 56.8 | Male | MCA stem (M1) | 24.2 | No ISR | MRA | No |
| 15*** | 71.4 | Male | Basilar | 28.2 | ISR | DSA | No |
| 16 | 70.1 | Female | Basilar | 26.0 | ISR | DSA | No |
| 17 | 61.7 | Female | MCA stem (M1) | 2.2 | ISR | DSA | No |
Two other patients in SAMMPRIS had primary endpoints beyond 30 days after enrollment. One did not have angioplasty or stenting (procedure was aborted) and had a subsequent ischemic stroke and another had a symptomatic brain hemorrhage after repeat angioplasties for asymptomatic ISR.
Patient 6 had several TIAs in the territory associated with ISR (the first one at 1.6 months after enrollment) leading to dual antiplatelet therapy and repeat angioplasties. The ischemic stroke occurred three days after an attempted repeat angioplasty that was aborted.
Patient 15 had a CITS in the territory 3.4 months after enrollment unrelated to ISR and was placed on dual antiplatelet therapy.
For patients not having an ischemic stroke in the territory, the first occurring ischemic event in the territory after the 30-day peri-procedural period was CITS in 10 patients (5.5% of the 183 patients) and TIA in 16 patients (8.7% of the 183 patients). The age, gender, symptomatic artery, timing of symptom onset, and ISR status/imaging modality for these 26 patients are listed in Table 2. In total, of the 183 patients 27 patients (14.8%) had a symptomatic infarction (17 ischemic strokes and 10 CITS alone) in the territory after the 30-day peri-procedural period.
Table 2.
TIAs and CITS in the territory of the stented after 30 days
| Patient Number | Age at Enrollment (yrs) | Gender | Symptomatic Artery | Adjudicated Event | Months After Enrollment | ISR Status (Modality) | Anti- Platelet Rx Post Event | Months of Follow-up After CITS/TIA | Subsequent Events (Time after Initial Adjudicated Event) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65.0 | Female | MCA stem (M1) | CITS | 4.0 | ISR (DSA) | DAPT | 34.7 | TIA (8.5 months) |
| 2 | 53.2 | Male | Basilar | CITS | 11.2 | No ISR (DSA) | 15.7 | . | |
| 3 | 70.7 | Male | MCA stem (M1) | CITS | 4.9 | ISR (DSA) | DAPT | 26.6 | . |
| 4 | 62.9 | Female | MCA stem (M1) | CITS | 4.8 | ISR (DSA) | DAPT | 23.1 | CITS (1.0 month) |
| 5 | 65.2 | Male | Vertebral | CITS | 27.5 | No ISR (DSA) | 19.6 | . | |
| 6 | 48.8 | Female | MCA stem (M1) | CITS | 3.6 | ISR (MRA) | Aspirin | 5.5 | . |
| 7 | 56.2 | Male | MCA stem (M1) | CITS | 9.9 | Indeterminate (CTA) | 20.9 | . | |
| 8 | 61.0 | Male | Vertebral | CITS | 1.4 | ISR (CTA) | DAPT | 45.5 | . |
| 9 | 48.7 | Female | Basilar | CITS | 3.5 | No Imaging | 27.4 | TIA (4.2 months) | |
| 10 | 69.8 | Female | MCA stem (M1) | CITS | 3.4 | No ISR (DSA) | 38.8 | . | |
| 11 | 59.3 | Male | Intracranial carotid | TIA | 4.8 | ISR (DSA) | Aspirin | 24 | TIA (12.3 months) |
| 12 | 53.6 | Female | Intracranial carotid | TIA | 3.5 | No Imaging | 24.9 | . | |
| 13 | 32.9 | Female | MCA stem (M1) | TIA | 7.3 | ISR (DSA) | Aspirin | 36.4 | TIA (8 days) |
| 14 | 53.4 | Male | MCA stem (M1) | TIA | 24.3 | ISR (DSA) | Aspirin | 17.8 | . |
| 15 | 68.1 | Male | Intracranial carotid | TIA | 25.8 | ISR (DSA) | DAPT | 18.3 | CITS (2.3 months) |
| 16 | 45.5 | Male | MCA stem (M1) | TIA | 35.9 | No Imaging | 12.1 | . | |
| 17 | 44.4 | Male | Basilar | TIA | 3.0 | ISR (CTA) | Aspirin | 45.3 | TIA × 3 (2 days, 4.6 months, 13.1 months) |
| 18 | 72.7 | Male | Basilar | TIA | 12.5 | No ISR (CTA) | 24.4 | . | |
| 19 | 56.9 | Male | Vertebral | TIA | 9.6 | No Imaging | 29.2 | . | |
| 20 | 72.7 | Female | MCA stem (M1) | TIA | 6.0 | No Imaging | 19.7 | . | |
| 21 | 51.1 | Male | Vertebral | TIA | 6.1 | No Imaging | 39.4 | . | |
| 22 | 60.0 | Female | Intracranial carotid | TIA | 4.6 | No Imaging | 23.2 | . | |
| 23 | 35.8 | Male | MCA stem (M1) | TIA | 3.5 | No ISR (MRA) | 28.5 | . | |
| 24 | 49.8 | Female | MCA stem (M1) | TIA | 9.8 | ISR (DSA) | DAPT | 17 | . |
| 25 | 59.8 | Female | Basilar | TIA | 18.0 | ISR (DSA) | DAPT | 27.3 | Angioplasty at 18 months |
| 26 | 59.4 | Male | Basilar | TIA | 11.7 | ISR (DSA) | DAPT | 22.7 | . |
DAPT = Dual antiplatelet
Frequency and Location of Symptomatic In-Stent Restenosis
Of the 27 patients with a cerebral infarct in the territory beyond 30 days, vascular imaging to assess for ISR was adequate in 24 patients, indeterminate in 1, and missing in 2. In the 24 patients with adequate imaging, definite or probable ISR was present in 16 (66.7%) and no ISR was seen in 8 (33.3%) (Tables 1 and 2). Two ischemic stroke patients assessed as having ISR presented with stent occlusion (Patients 1 and 15 in Table 1). In the first, the proximal internal carotid was occluded, with flow through a narrowed channel in the distal aspect of the stent from an ophthalmic artery collateral. In the second, the patient presented with acute basilar thrombosis and severe ISR was identified during a thrombectomy procedure. Of the 17 patients with ischemic stroke after 30 days (Table 1), 11 underwent DSA, four had CTA only, one had MRA only and one had no imaging. Of the 10 patients with CITS after 30 days (Table 2) 6 underwent DSA, two had CTA only, one had MRA only and one had no imaging.
Of the 16 patients with a TIA alone in the territory beyond 30 days, vascular imaging to assess for ISR was adequate in 10 patients (7 with DSA, 2 with CTA, 1with MRA) and missing in 6. In the 10 patients with adequate imaging, definite or probable ISR was present in eight (80%) and no ISR was seen in two (20%) (Table 2). Excluding the 9 patients with indeterminate or missing vascular studies yielded an overall symptomatic ISR frequency of 24 of 174 (13.8%) patients in this study. The median time to symptomatic ISR among these 24 patients was 6.0 months (interquartile range = 3.8 – 16.1 months, range = 1.4 – 28.2 months). The 1,2 and 3 year Kaplan Meier rates (with 95% confidence limits) for symptomatic ISR among all 183 patients followed beyond 30 days in the stenting arm were 9.6% (6.1% – 14.9%), 11.3% (7.5% – 17.0%), 14.0% (9.6% – 20.2%), respectively (Figure 1).
Figure 1.
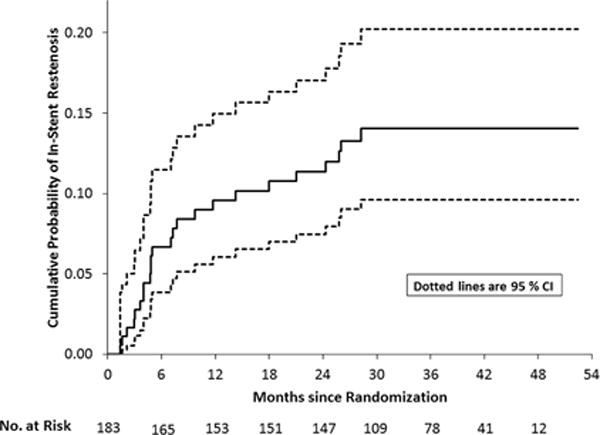
Kaplan Meier ISR curves
Of the 174 patients who either had no ischemic events reported during follow-up (n=140) or had ischemic events for which an adequate vascular imaging study was performed (n=34), the frequencies of symptomatic ISR according to the treated artery were 10 (13.0%) of 77 for the middle cerebral artery, 6 (19.4%) of 31 for the internal carotid artery, 7 (18.4%) of 38 for the basilar artery, and 1 (3.6%) of 28 for the vertebral artery.
Subsequent Events in Patients with TIA and CITS Associated with ISR
The mean follow up after the first ischemic event for the 28 patients with CITS or TIA (2 in Table 1 who had TIA or CITS preceding a stroke and 26 in Table 2) was 24.9 months (standard deviation = 10.2 months, range = 4.2 – 45.5 months). Of these 28 patients, 14 had ISR, 6 did not have ISR, and ISR was indeterminate in 8 at the time of their first ischemic event. Treatment after the first ischemic event in 14 patients with symptomatic ISR included dual antiplatelet therapy in 9 patients (patient 6 in Table 1 and 8 in Table 2) and aspirin alone in 5 patients (Table 2). Of the 14 patients with symptomatic ISR, 2 underwent angioplasty (patient 6, Table 1 and patient 25, Table 2). One of the two underwent multiple repeat angioplasties and suffered an ischemic stroke (a primary endpoint) three days after an aborted attempt at repeat angioplasty. The second underwent repeat angioplasty for restenosis of a mid-basilar lesion 18 months after enrollment and remained asymptomatic for the duration of subsequent follow up (27 months). Of the remaining 12 patients with ISR none had a subsequent ischemic stroke in the territory. Two had a CITS in the territory (patients 4 and 15 in Table 2). Four had recurrent TIAs in the territory (three had single events and one had three – patients 1, 11, 13 and 17 in Table 2).
Of the 14 patients with TIA or CITS who did not have ISR or in whom ISR was indeterminate, all were treated medically and one had a subsequent ischemic stroke in the territory (a primary endpoint). This was patient 15 in table 2, who had a CITS and no evidence of ISR 3.4 months after enrollment and then ischemic stroke at 28 months associated with ISR. One additional patient with a CITS at 3.5 months (patient 9, table 2) had a recurrent TIA at 4 months and then remained asymptomatic for the remaining 27 months of follow up. The remaining 12 patients had no further ischemic events in the territory for the duration of follow up.
Discussion
SAMMPRIS provides unique data on the risk of symptomatic ISR after use of the Wingspan stent system. Symptomatic ISR was most frequent in the first year after PTAS and was associated with a majority of the cerebral infarcts and TIAs in the territory of the stented artery during follow-up. ISR is an important cause of non-procedural ischemic events after intracranial stenting.
Most of the existing literature on the incidence of ISR includes both symptomatic and asymptomatic lesions. In the US Wingspan registry, Levy et al reported a 29.7% overall rate of ISR (25/84 lesions in 68 patients) on follow-up vascular imaging obtained between 1.5 and 15.5 months after stenting.3 Eight of the 68 patients (12%) were symptomatic, 4 of whom had ISR and 4 had acute thrombosis. Fifteen of the 25 patients with ISR underwent repeat angioplasty. The symptomatic ISR rate of 12% is similar to the 13.8% symptomatic ISR in SAMMPRIS but the length of follow-up was longer in SAMMPRIS. The definition of ISR employed in the US Wingspan registry was identical to the present study.3
We cannot provide data on asymptomatic ISR rates in SAMMPRIS because follow up vascular imaging was only allowed after the development of ischemic symptoms. This decision was made because the risk and benefit of angioplasty for asymptomatic ISR is unknown.
Asian studies have reported much lower rates of symptomatic ISR. Yu and colleagues reported ISR in 11 of 66 patients on routine angiographic follow up at one year, none of which were symptomatic .4 Shin reported ISR in 17 of 69 lesions (24.6%) at a median of 12 months, of which only 3 were symptomatic.5 The different rates of symptomatic ISR in SAMMPRIS and the Asian studies may be related to differences in study design. The Asian case series included routine surveillance for ISR with follow up vascular imaging. Some patients with asymptomatic ISR underwent repeat angioplasty, with or without stenting. Others may have had changes in their anti-platelet regimen or other medications based on the results of the vascular imaging. Either of these interventions may have had an impact on reducing the rate of symptomatic ISR.
One pattern of ISR that was described in the US Wingspan Registry was progressive stenosis of the supraclinoid carotid.15 ISR in this location was common especially in younger patients and was often recurrent. Turk, et al., reported on a subset of 93 treated lesions that met study criteria for imaging surveillance, out of a total of 144 patients with 155 treated lesions. They dichotomized the cohort by age older than 55 years and age 55 years and younger and investigated subgroups by lesion location. ISR was more common in the younger patients (45% 14/31 versus 24% (15/62), odds ratio 2.6, 95% confidence interval 1.0 – 6.5). Within the younger group, ISR was more common in anterior circulation (13 of 26 treated) than posterior circulation (1 of 5 treated). Five of the 14 younger patients with ISR were symptomatic. Eight of nine lesions involving the supraclinoid segment developed ISR, with ischemic symptoms in 4 of the 8. The possibility of a non-atherosclerotic pathology underlying these lesions has been raised.15
In the present analysis of SAMMPRIS data, 6 of 31 (19.4%) patients with intracranial carotid stenosis who were stented developed symptomatic ISR and only one of these underwent multiple repeat angioplasties for recurrent distal internal carotid artery/M1 segment ISR (Patient 6 in Table 1). The low incidence of distal internal carotid artery/M1 segment symptomatic ISR observed in SAMMPRIS, relative to the Wingspan registry described above, may be related to the inclusion criteria in the SAMMPRIS trial that required patients less than 50 years of age to have at least two atherosclerotic risk factors to be enrolled.
The optimal management of patients with symptomatic ISR is unclear. This study provides unique data on the outcome of symptomatic ISR treated medically or with angioplasty. All 5 patients with CITS and ISR were treated medically only (four with dual antiplatelet therapy) and all 5 patients remained stroke free during a median follow-up of 26.6 months, although one had a TIA and another had a CITS (see CITS patients in Table 2). Two of the nine patients with TIAs and ISR underwent angioplasty. The remaining 7 patients with TIA and ISR were treated medically (three with dual antiplatelet therapy and four with aspirin). None had an ischemic stroke, although one had a CITS and three had recurrent TIAs during follow up (see Table 2). While ISR accounted for a large number of ischemic events, including delayed stroke, in this cohort, the risk of a subsequent event on medical therapy appears to be low. These data suggest that the risk of subsequent ischemic events in most patients with symptomatic ISR may be time-limited.
This post hoc study has some important limitations. ISR was diagnosed by DSA in the large majority of cases, but one was based on MRA which may overestimate the degree of stenosis. The rate of symptomatic ISR may be underestimated. It is possible that not all TIAs were reported by the sites. Additionally, the 95% confidence intervals around the rates of symptomatic ISR are quite wide owing to the sample size. Nevertheless, this is the largest prospective study of symptomatic ISR in patients undergoing stenting with the Wingspan stent in the USA. This study does not provide any data on the rate or clinical significance of asymptomatic ISR as routine vascular imaging was not part of the SAMMPRIS protocol. Additionally, we have no data on the long-term outcome of patients after stroke associated with ISR because once patients were evaluated 90 days after the stroke, they were no longer followed in the trial.
In conclusion, symptomatic ISR occurred in at least one in seven patients during a median follow-up of 35 months in SAMMPRIS, and was associated with the majority of symptomatic infarcts in the territory of the stented artery beyond the peri-procedural period. Taken together with the peri-procedural outcomes in SAMMPRIS, these data show it will be necessary to substantially lower both the rate of peri-procedural stroke as well as the rate of symptomatic ISR for stenting to have a role in the treatment of intracranial stenosis.
Acknowledgments
Sources of Funding: NIH U01 NS058728.
Footnotes
Disclosures: DF received research/salary support from Siemens, Microvention, Penumbra and Sequent. Medtronic, Sequent, Codman and Shurtleff, Penumbra, Microvention consulting fees. Codman& Shurtleff (REVIVE) royalties. Vascular Simulations LLC. TT received personal fees from Gore and Boehringer Ingelheim for participating as a stroke adjudicator in clinical trials unrelated to this work. MC received other support from Astra Zeneca and Stryker Neurovascular (formerly Boston Scientific Neurovascular) related to the SAMMPRIS trial.
References
- 1.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (sammpris): The final results of a randomised trial. Lancet. 2014;383:333–341. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Pride L, et al. Wingspan in-stent restenosis and thrombosis: Incidence, clinical presentation, and management. Neurosurgery. 2007;61:644–650. doi: 10.1227/01.NEU.0000290914.24976.83. discussion 650–641. [DOI] [PubMed] [Google Scholar]
- 4.Yu SC, Leung TW, Lee KT, Wong LK. Angioplasty and stenting of intracranial atherosclerosis with the wingspan system: 1-year clinical and radiological outcome in a single asian center. J Neurointerv Surg. 2014;6:96–102. doi: 10.1136/neurintsurg-2012-010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin YS, Kim BM, Suh SH, Jeon P, Kim DJ, Kim DI, et al. Wingspan stenting for intracranial atherosclerotic stenosis: Clinical outcomes and risk factors for in-stent restenosis. Neurosurgery. 2013;72:596–604. doi: 10.1227/NEU.0b013e3182846e09. discussion 604. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Huang Q, Zhang Y, Deng B, Liu J, Hong B, et al. A single-center study of wingspan stents for symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci. 2013;20:362–366. doi: 10.1016/j.jocn.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Huang Q, Zhang Y, Liu J, Hong B, Xu Y, et al. Wingspan stents for the treatment of symptomatic atherosclerotic stenosis in small intracranial vessels: Safety and efficacy evaluation. AJNR Am J Neuroradiol. 2012;33:343–347. doi: 10.3174/ajnr.A2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma MM, Yin Q, Xu GL, Zhang RL, Zhu SG, Fan XY, et al. [predictors of wingspan in-stent restenosis for the treatment of symptomatic intracranial arterial stenosis] Zhonghua Yi Xue Za Zhi. 2011;91:1303–1307. [PubMed] [Google Scholar]
- 9.Costalat V, Maldonado IL, Vendrell JF, Riquelme C, Machi P, Arteaga C, et al. Endovascular treatment of symptomatic intracranial stenosis with the wingspan stent system and gateway pta balloon: A multicenter series of 60 patients with acute and midterm results. J Neurosurg. 2011;115:686–693. doi: 10.3171/2011.5.JNS101583. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Wang L, Deng JP, Gao L, Zhang T, Zhao ZW, et al. Treatment of symptomatic intracranial atherosclerotic stenosis with a normal-sized gateway() balloon and wingspan() stent. The Journal of international medical research. 2010;38:1968–1974. doi: 10.1177/147323001003800610. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Huang QH, Zhang YW, Liu JM, Hong B, Xu Y, et al. [intracranial angioplasty with wingspan stents for symptomatic atherosclerotic stenosis in small vessels:A study of long-term follow-up] Zhonghua Yi Xue Za Zhi. 2010;90:3323–3326. [PubMed] [Google Scholar]
- 12.Moskowitz SI, Kelly ME, Haynes J, Fiorella D. Dynact evaluation of in-stent restenosis following wingspan stenting of intracranial stenosis. J Neurointerv Surg. 2010;2:2–5. doi: 10.1136/jnis.2009.000505. [DOI] [PubMed] [Google Scholar]
- 13.Fiorella DJ, Levy EI, Turk AS, Albuquerque FC, Pride GL, Jr, Woo HH, et al. Target lesion revascularization after wingspan: Assessment of safety and durability. Stroke. 2009;40:106–110. doi: 10.1161/STROKEAHA.108.525774. [DOI] [PubMed] [Google Scholar]
- 14.Albuquerque FC, Levy EI, Turk AS, Niemann DB, Aagaard-Kienitz B, Pride GL, Jr, et al. Angiographic patterns of wingspan in-stent restenosis. Neurosurgery. 2008;63:23–27. doi: 10.1227/01.NEU.0000335067.53190.A2. discussion 27–28. [DOI] [PubMed] [Google Scholar]
- 15.Turk AS, Levy EI, Albuquerque FC, Pride GL, Jr, Woo H, Welch BG, et al. Influence of patient age and stenosis location on wingspan in-stent restenosis. AJNR Am J Neuroradiol. 2008;29:23–27. doi: 10.3174/ajnr.A0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chimowitz MI, Lynn MJ, Turan TN, Fiorella D, Lane BF, Janis S, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis. 2011;20:357–368. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turan TN, Lynn MJ, Nizam A, Lane B, Egan BM, Le NA, et al. Rationale, design, and implementation of aggressive risk factor management in the stenting and aggressive medical management for prevention of recurrent stroke in intracranial stenosis (sammpris) trial. Circulation. Cardiovascular quality and outcomes. 2012;5:e51–e60. doi: 10.1161/CIRCOUTCOMES.112.966911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derdeyn CP, Fiorella D, Lynn MJ, Barnwell SL, Zaidat OO, Meyers PM, et al. Impact of operator and site experience on outcomes after angioplasty and stenting in the sammpris trial. J Neurointerv Surg. 2013;5:528–533. doi: 10.1136/neurintsurg-2012-010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derdeyn CP, Fiorella D, Lynn MJ, Rumboldt Z, Cloft HJ, Gibson D, et al. Mechanisms of stroke after intracranial angioplasty and stenting in the sammpris trial. Neurosurgery. 2013;72:777–795. doi: 10.1227/NEU.0b013e318286fdc8. discussion 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorella D, Derdeyn CP, Lynn MJ, Barnwell SL, Hoh BL, Levy EI, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (sammpris) Stroke. 2012;43:2682–2688. doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]


