Abstract
Aim
To quantify recruitment, retention and differential retention rates and associated trial, participant and intervention characteristics in randomised controlled trials (RCTs) evaluating the effect of exercise therapy in people with multimorbidity.
Data sources
MEDLINE, EMBASE, CINAHL and CENTRAL from 1990 to April 20, 2020.
Study selection
RCTs including people with multimorbidity comparing exercise therapy with a non-exposed comparator group reporting at least one of the following outcomes: physical function, health-related quality of life, depression symptoms, or anxiety symptoms.
Data extraction and synthesis
Recruitment rates (proportion of people randomised/proportion of people eligible), retention rates (proportion of people providing the outcomes of interest/proportion randomised) and differential retention rates (difference in proportion of people providing the outcomes in the intervention group and comparator group) were calculated. Meta-analysis using a random-effects model was used to estimate pooled proportions. Methodological quality was assessed using Cochrane ´Risk of Bias tool 2.0´ for individual studies, and the GRADE approach was used to assess the overall quality of the evidence.
Results
Twenty-three RCTs with 3363 people were included. The pooled prevalence for recruitment rate was 75% (95%CI 66 to 84%). The pooled prevalence for retention rate was 90% (95%CI 86 to 94%) at the end of the intervention (12 weeks; interquartile range (IQR) (12 to 12)). Meta-regression analyses showed that increasing age and including a higher proportion of people with hypertension was associated with lower retention rates. Retention rates did not differ between the intervention and comparator groups. The overall quality of the evidence was deemed very low.
Conclusion
Three in four eligible people with multimorbidity were randomised to RCTs using exercise therapy, of which nine out of 10 provided end of treatment outcomes with no difference seen between the intervention and comparison groups. However, the results must be interpreted with caution due to large differences between the included studies.
Trial registration
ClinicalTrials.govCRD42020161329. Registered on 28 April 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-021-05346-x.
Keywords: Multimorbidity, Recruitment, Retention, Exercise therapy, Randomised controlled trial, Systematic review
Background
Multimorbidity, defined as the coexistence of two or more chronic conditions, is a major priority in health care and research [1, 2]. A possible explanation is that multimorbidity is becoming a rapidly escalating problem in most healthcare systems because of its increasing prevalence with age and association with increased mortality, worse functional status and reduced health-related quality of life (HRQoL) [2–5]. This increasing burden combined with the complexity of multimorbidity challenges the current perspectives of standard care, which focus on single disease-oriented management programs rather than specific patient-oriented care [6, 7].
Chronic conditions such as osteoarthritis, hypertension, type 2 diabetes mellitus, depression, heart failure, ischemic heart disease, and chronic obstructive pulmonary disease are among the leading causes of global disability, affecting hundreds of millions of people worldwide [8]. These conditions often coexist and are linked by a common risk factor (physical inactivity) and pathogenesis (systemic low-grade inflammation) which potentially causes a cascade of reactions resulting in the development of a ‘vicious cycle’ of chronic diseases and poor outcomes [9, 10].
Randomised controlled trials (RCTs) are the gold standard of experimental study designs [11]. However, RCTs with poor recruitment and retention rates are considered a threat to the validity of the results [12] and it is widely agreed that research that identifies strategies for improving recruitment and retention is a priority [13]. Prior systematic reviews within the medical field have reported wide ranges of recruitment and retention rates [14–17], and individual studies have identified that recruiting and retaining patients in multimorbidity in clinical trials is challenging [18, 19]. Possible sources of poor recruitment and retention include lack of good communication between the patient and recruitment staff and negative attitude of research staff [20].
Exercise therapy appears to be a safe and effective treatment for people with multimorbidity [21]; however, a comprehensive summary of recruitment and retention rates in people with multimorbidity participating in RCTs of exercise therapy is lacking. Evaluating recruitment and retention rates, identifying strategies to improve recruitment and retention, and determining if retention between exercise and control groups are different in exercise therapy RCTs would help in the design and conduct of future RCTs for people with multimorbidity by providing a realistic perspective on crucial parts of the RCT beneficial for both clinical and research practise. Therefore, we investigated the recruitment, retention and differential retention rates of people with multimorbidity participating in RCTs evaluating the effect of exercise therapy. We also examined trial, participant and intervention characteristics associated with improved recruitment, retention and differential retention.
Methods
Protocol and registration
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA Checklist: Additional file 1) [22] and was based on a protocol with pre-specified study selection, eligibility criteria, data extraction and strategy for data synthesis [23] in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [24]. The protocol was registered at PROSPERO (CRD42020161329) and was also made publicly available via the Open Science Framework website [25, 26] before completion of the title/abstract screening phase.
Information sources
We used the same search strategy developed from our previous systematic review which investigated the effect of exercise therapy in people with multimorbidity [23]. Information was retrieved from the following sources:
Searching MEDLINE via PubMed, EMBASE via Ovid, CINAHL (including preCINAHL) via EBSCO and the Cochrane Central Register of Controlled Trials (CENTRAL) up to October 12, 2019, with no restriction on language. Only RCTs published since 1990 were included as the reporting and treatment of multimorbidity have changed considerably in recent years. Searches were repeated for the period from October 2019 to April 20, 2020, in the same databases to identify additional studies published before manuscript submission.
Screening the reference lists of the latest Cochrane reviews investigating the effect of therapeutic exercise on the following conditions: osteoarthritis, hypertension, diabetes type 2, depression, heart disease or heart failure, and chronic obstructive pulmonary disease.
Screening the reference lists of included RCTs.
Screening The World Health Organization’s International Clinical Trials Registry Platform (ICTRP) http://apps.who.int/trialsearch/ which comprise the 16 primary registries of the WHO registry network and ClinicalTrials.gov.
Web of Science (WoS) was used for citation tracking by searching studies citing the RCTs included in this systematic review.
The following constructs were used for the literature search in MEDLINE via PubMed: osteoarthritis, co-existing health problem, diabetes mellitus, depression, hypertension, pulmonary disease, chronic obstructive, myocardial ischemia, exercise and randomised controlled trial. They were combined with the Boolean operators OR/AND, searched as Title/Abstract (i.e., TIAB), and as keywords Medical Subject Headings (i.e., MeSH). The detailed search strategy in MEDLINE (https://osf.io/84vzn/) was made publicly available at Open Science Framework [26] and was adjusted to fit the other databases.
Eligibility criteria
Study design
English language RCTs published in peer-reviewed journals or unpublished RCTs from registries with available and relevant data.
Type of participants
Studies including at least 80% of the people with at least two of the following conditions: osteoarthritis of the hip or knee, heart failure, ischemic heart disease, hypertension (systolic blood pressure >90 and diastolic blood pressure >140), type 2 diabetes mellitus, chronic obstructive pulmonary disease and depression as defined by the studies or calculated based on baseline participants characteristics. This pragmatic approach was pre-specified and adopted to capture all the studies which included people with multimorbidity, given the expected inconsistency of reporting of the conditions across trials. Studies including children and adolescents (i.e., mean age <18 years) were excluded.
Types of intervention
Studies which included exercise therapy interventions with or without additional pharmacotherapy or other adjuvant interventions (e.g. weight loss) were eligible for inclusion. Exercise therapy is defined as ‘a regimen or plan of physical activities designed and prescribed for specific therapeutic goals with the purpose of restoring normal physical function or to reduce symptoms caused by diseases or injuries’ [27]. Intervention arms delivering unstructured exercise programs (e.g. providing a pedometer or booklet to the people without a specific plan for physical activity) were excluded.
Type of outcomes of the individual studies
Studies assessing at least one of the following outcomes were eligible for inclusion:
Physical outcome: Objectively measured and self-reported physical function (e.g. 6-min walking test, 36-item Short-Form Health Survey (SF-36))
Psychosocial outcome: HRQoL (e.g. EQ-5D questionnaire), depression symptoms or anxiety symptoms (e.g. Hospital Anxiety and Depression Scale)
The rationale for including these outcomes is based on a consensus study that identified outcomes for multimorbidity intervention studies [28] and the fact that they are generic and widely used across the conditions of interest. Additionally, to avoid multiplicity, we used a hierarchy of selection rules for the outcomes described elsewhere [23].
The primary outcome measures of this systematic review were as follows:
Recruitment rates: Proportion of eligible people recruited (proportion of people randomised/proportion of people eligible). The proportion of people eligible included those saying no to being included.
Retention rates: Proportion of randomised people (proportion of people providing the outcomes of interest/proportion randomised) providing physical (i.e. physical function) and/or psychosocial outcomes (i.e. HRQoL, depression symptoms and anxiety symptoms) at the end of the intervention and the follow-up closest to 12 months.
Differential retention rates: Difference in proportion of people providing physical (i.e. physical function) and/or psychosocial outcomes (i.e. HRQoL, depression symptoms and anxiety symptoms) in the intervention and comparator group, at the end of the intervention and the follow-up closest to 12 months.
Study selection
The identified studies from the literature search were uploaded to EndNote X9. Two reviewers (LKH and AB) independently screened titles and abstracts, and all studies deemed eligible by at least one of the reviewers were checked independently in full text. Disagreement between the reviewers in inclusion was discussed until consensus was reached. If consensus could not be reached, a third author’s opinion (CBJ) was sought to achieve consensus. We checked whether multiple reports from the same study were published by juxtaposing author names, treatment comparisons, sample sizes or outcomes. If multiple reports of the same studies provided different study characteristics (e.g. number of people and presence of comorbidities), we contacted the authors for clarifications.
Risk of bias and overall quality assessment of the evidence
Two reviewers (LKH and AB) independently assessed the methodological quality of the included studies using the Cochrane ‘Risk of Bias Tool 2.0’ [24]. The Risk of Bias Tool was applied because all the included studies were effect estimation studies. Poor methodology in the studies therefore influence recruitment and retention rates. Bias was assessed in five distinct domains: bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome (blinding) and bias in the selection of the reported result. Within each domain, the two reviewers answered one or more signalling questions (e.g. Was the allocation sequence random? Were people aware of their assigned intervention during the trial?) which led to judgements of ‘low risk of bias’, ‘some concerns’, or ‘high risk of bias’. The judgements within each domain led to an overall risk-of-bias judgement for the result being assessed.
The overall quality of evidence for the estimates was evaluated by two reviewers (LKH and AB) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [29]. GRADE is a systematic approach to rate the quality of evidence across studies for specific outcomes. It is based on five domains that involve the methodological flaws of the studies (i.e. risk of bias), the heterogeneity of results across studies (i.e. inconsistency), the generalizability of the findings to the target population (i.e. indirectness), the precision of the estimates (i.e. imprecision) and the risk of publication bias.
Data collection process
Our data extraction sheet was developed based on the Cochrane Collaboration data collection form for intervention reviews: RCTs only [30] and are available at open science framework [26]. Thereafter, pilot testing was performed using three of the included RCTs randomly chosen to refine the data extraction sheet before extracting data from all the included studies. Two reviewers (LKH and AB) performed data extraction for all included studies.
Data extraction
All data were extracted at a study level (e.g. we evaluated whether age was associated with increased/reduced recruitment rates across studies). To calculate recruitment rates, we extracted the number of people randomised and the number of people eligible. Similarly, to calculate retention and differential retention rates, we extracted the number of people providing outcomes in the intervention and comparator groups, at end of the intervention and closest to 12-month follow-up. Additionally, we extracted the following data to investigate the impact of the study, intervention, comparator and outcomes characteristics on the outcomes of interest.
Trial characteristics
Trial design (e.g. factorial, open design), country and clinical location (in case of multilocation studies, primary investigator affiliation applied), recruitment strategy used (e.g. one-to-one, news advertisement, online) and retention strategy used (e.g. financial incentives, phone reminders), recruitment strategy length (in months), the total number of people assessed for eligibility, location of the recruitment (e.g. hospital, community of GP practice), patient public involvement (people involved in the intervention development), eligibility assessment strategy (e.g. via registry, database, in person, via phone call pre-screening) and reasons for people to dropout.
Participant characteristics
Age, % female, body mass index (BMI), socioeconomic status (labelled as `low SES` when the majority of people are described as having low education levels, low income, being unemployed or sample otherwise labelled as `low SES`), baseline severity of the conditions and number, and type and severity of other conditions.
Intervention and comparator characteristics
Components of intervention (i.e. therapeutic exercise, exercise + diet), type of exercise/comparator intervention (i.e. aerobic, neuromuscular, strengthening or a combination), frequency of the sessions (times per week), intensity of the session (% of maximum pulse, or % of 1 repetition maximum), volume of the sessions, mode of delivery (i.e. one-to-one, group or self-help) setting (i.e. home-based, clinic-based or a combination), duration of the interventions (in weeks), supervision (i.e. yes, no or a combination), tailoring (i.e. intervention developed according to guidelines and individual people’s needs), and adherence to intervention (i.e. the total number of sessions attended out of the total number of sessions available).
Outcome characteristics
Time points assessed and the magnitude of objectively and subjectively measured changes (e.g. change in HRQoL). As previously mentioned, a hierarchy of selection rules for the outcomes was applied. We prioritised data extraction of outcome measures important for the participants [28] and generic over disease-specific measures [23]. For objectively measured physical function, we prioritised (1) the 6-min walking test, (2) incremental shuttle walking test and (3) any other outcome measure related to daily function (e.g. chair stand test). For self-reported physical function, we prioritised outcomes in the following order: (1) the SF-36 physical function subscale, (2) the SF-36 role function subscale and (3) any other self-reported measure of physical function. For HRQoL outcomes, we prioritised (1) the EQ-5D questionnaire and (2) any other HRQoL questionnaires (e.g. The Minnesota living with heart failure questionnaire). For depression symptoms, we prioritised (1) The Beck Depression Inventory (BDI) and (2) any other depression questionnaires (e.g. the Hospital Anxiety and Depression Scale (HADS depression). For anxiety symptoms, we prioritised (1) State Trait Anxiety Inventory questionnaire and (2) any other anxiety questionnaires (e.g. HADS anxiety).
If we were unable to extract the abovementioned data from the included RCTs, we emailed the corresponding author of each study with a checklist of the data we aimed to obtain. If the corresponding author did not reply, we contacted a second author as well for obtaining the information and so forth. After 3 days, we sent a reminder including the last author of the study. After 7 days, a reminder was re-sent to the corresponding and last author. Another reminder followed 10 days later. Finally, we considered the data as missing if no communication from the authors was received 15 days after sending the first email.
Summary measures and synthesis of results
Recruitment, retention, and differential retention rates of people with multimorbidity were the outcome measures being calculated. Estimates of these rates were pooled using random-effects proportion meta-analyses (Stata V.16.1 metaprop command) [31]. Binomial proportion 95% CIs for individual studies were calculated around study-specific and pooled prevalence based on the score-test statistic. Heterogeneity was examined as between-study variance and calculated as the I-squared statistic measuring the proportion of variation in the combined estimates due to between-study variance [24]. An I-squared value of 0% indicated that no inconsistency existed between the results of individual trials, where an I-squared value of 100% indicated maximal inconsistency.
Additional analyses
We pre-specified subgroup and meta-regression analyses to explore heterogeneity. Relevant study-level covariates, able to decrease inconsistencies measured as the I-squared statistic (and thus the between-study variance Tau-square), were investigated to explore possible association between study, participants, intervention and comparator group characteristics on recruitment, retention and differential retention rates. In accordance with the Cochrane Handbook, we performed meta-regression analyses when at least 10 studies reported data for the relevant covariates [24].
Results
Study selection
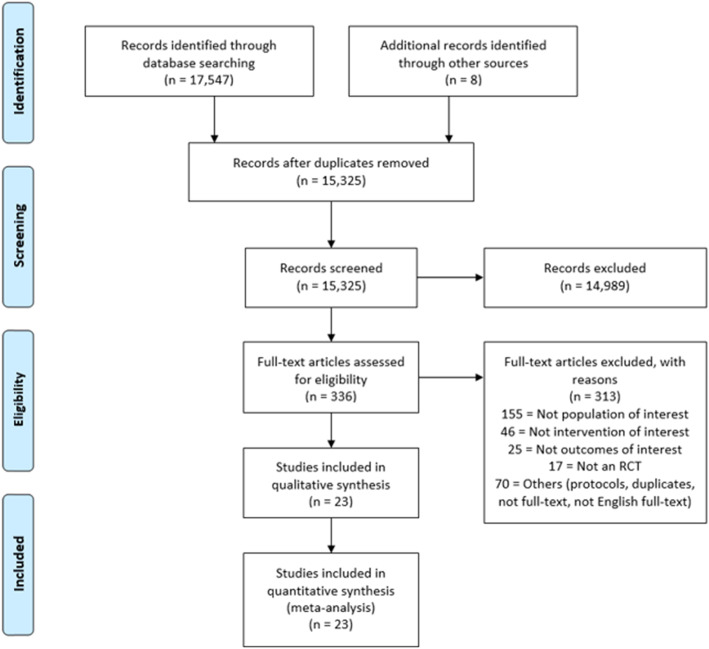
A total of 17,547 studies were identified by the search strategy. After removing duplicates, assessing title and abstracts, and full-text assessment of the remaining studies, we included 23 RCTs published in 24 papers (Fig. 1).
Fig. 1.
Flow diagram of the included RCTs. RCT = randomised controlled trial
Study characteristics
Table 1 summarises the characteristics of the included RCTs. The studies were conducted across 18 countries, including Europe [33, 34, 36, 40, 42, 43, 45, 47, 48, 52, 55], USA [32, 35, 37–39, 46, 50, 54], Australia [41], and Asia [44, 49, 51, 53]. A total of 18 studies reported the type of recruitment strategy used with 50% using a mix of both direct (i.e. potential people approached individually) and indirect (i.e. potential people approached, e.g. via news advertisement or flyers) strategies. The recruitment setting was classified as outpatient (k=13) and at hospitals (k=7) with recruitment length varying widely from 2 to 53 months.
Table 1.
Characteristics of the included studies
| Author, publication year | Country, recruitment setting | Recruitment length, strategy used | Intervention length, outcome measures | Proportion of people for each multimorbidity condition, population characteristics |
|---|---|---|---|---|
| Gary et al. [32] |
USA University clinic |
18 months Direct and indirect approacha,b |
12 weeks PF, HRQoL, DEP |
KOA 68%, HYP 88%, T2DM 31%, DEP 44%, HF 100%, COPD 34% 32 people, mean age 68 years, 100% female, mean BMI 33.5 |
| Koukouvou et al. [33] |
Greece Hospital |
2 months Direct and indirect approacha,b |
26 weeks HRQoL, DEP, ANX |
HYP 12%, DEP 100%, HF 100% 26 people, mean age 52 years, 0% female, mean BMI 28.1 |
| Kulcu et al. [34] |
Turkey University school |
n/a |
8 weeks HRQoL, DEP, ANX |
DEP 100%, HF 100% 53 people, mean age 59 years, 27% female, n/a |
| Gary et al. [35] |
USA Outpatient clinic |
14 months Direct and indirect approacha,b |
12 weeks PF, HRQoL, DEP |
HYP 88%, T2DM 32%, DEP 100%, HF 100% 74 people, mean age 65.8 years, 57% female, n/a |
| Asa et al. [36] |
Sweden n/a |
n/a |
8 weeks PF, HRQoL, DEP, ANX |
T2DM 100%, HF 100% 20 people, mean age 67.4 years, 20% female, mean BMI 29 |
| Blumenthal et al. [37] (UPBEAT) |
USA Outpatient clinics |
53 months Direct and indirect approacha,b |
16 weeks DEP |
HYP 19%, DEP 100%, HF 100%, 101 people, mean age 63.9 years, 32% female, mean BMI 31 |
| Blumenthal et al. [38] (HF-ACTION) |
USA, Canada, France 82 medical centres |
47 months Direct approacha |
12 weeks DEP |
HYP 61%, DM* 10%, DEP 100%, HF 100% 653 people, mean age 56 years, 92% female, mean BMI 31.5 |
| Gary et al. [39] |
USA Outpatient clinic |
6 months Indirect approachb |
12 weeks PF, HRQoL, DEP |
HYP 50%, T2DM 50%, DEP 70%, HF 100% 24 people, mean age 60 years, 50% female, mean BMI 34 |
| Oerkild et al. [40] |
Denmark Rehabilitation unit |
19 months Direct and indirect approacha,b |
12 weeks PF, HRQoL, DEP, ANX |
HYP 73%, T2DM 23%, DEP 18%, HF 100%, COPD 28% 40 people, mean age 76.9 years, 43% female, mean BMI 27 |
| Leung et al. [41] |
Australia Hospital |
41 months Direct approacha |
12 weeks PF, HRQoL, DEP, ANX |
KOA 60%, HYP 55%, T2DM 19% IHD 33%, COPD 100% 42 people, mean age 73 years, 36% female, mean BMI 27.4 |
| Nolte et al. [42] and Edelmann et al. [43] (Ex-DHF-P) |
Germany 3 University hospitals |
7 months n/a |
12 weeks PF, HRQoL, DEP |
HYP 82%, T2DM 14%, DEP 64%, HF 100% 67 people, mean age 65 years, 56% female, mean BMI 31 |
| Keihani et al. [44] |
Iran n/a |
n/a |
8 weeks PF, DEP, ANX |
DEP 100%, HF 100% 65 people, mean age 61.2 years, 40% female, mean BMI 26.1 |
| Pibernik-Okanovic et al. [45] |
Croatia Hospital |
19 months Indirect approachb |
6 weeks HRQoL, DEP |
T2DM 100%, DEP 100% 209 people, mean age 58.1 years, 54% female, mean BMI 30 |
|
Schneider et al. [46] |
USA University school |
21 months Direct and indirect approacha,b |
12 weeks DEP |
T2DM 100%, DEP 100% 29 people, mean age 53.4, 100% female, mean BMI 34.6 |
| Hinrichs et al. [47] (Homefit) |
Germany General practices |
14 months Direct and indirect approacha,b |
12 weeks PF, HRQoL |
KOA 60%, HOA 46%, HYP 90%, T2DM 40%, HF 33%, IHD 29%, COPD 22% 209 people, mean age 79.8 years, 74% female, mean BMI 30.7 |
| Bernocchi et al. [48] |
Italy Hospital |
15 months Direct approacha |
16 weeks PF, HRQoL |
HF 100%, COPD 100% 112 people, mean age 70.5 years, 18% female, mean BMI 28.1 |
| Abdelbasset et al. [49] |
Saudi Arabia University hospital |
4 months Direct approacha |
6 weeks DEP |
HYP 20%, DEP 100%, HF 100% 69 people, mean age 52.7 years, 28% female, mean BMI 30 |
| de Groot et al. [50] (ACTIVE II) |
USA Medical practices |
48 months Direct and indirect approacha,b |
12 weeks PF, HRQoL, DEP |
T2DM 100%, DEP 100% 140 people, n/a |
| Leung et al. [51] |
Hong Kong Outpatient clinic |
3 months Direct approacha |
12 weeks PF, HRQoL |
HYP 100%, T2DM 96% 54 people, mean age 64 years, 48% female, mean BMI 27.3 |
| Rodriguez-Manas et al. [52] (MID-Frail) |
Europe (7 countries) 74 study sites |
15 months Direct approacha |
18 weeks PF, HRQoL |
HYP 87%, T2DM 100%, HF 9% 964 people, mean age 78 years, 49% female, mean BMI 29.6 |
| Soliman et al. [53] |
Saudi Arabia n/a |
5 months n/a |
12 weeks DEP |
DEP 100%, COPD 100% 34 people, mean age 69.7 years, 44% female, mean BMI 26.8 |
| Gretebeck et al. [54] |
USA Community centres |
n/a Direct and indirect approacha,b |
10 weeks PF |
A* 36%, HYP 83%, T2DM 100% 111 people, mean age 70.5 years, 61% female, mean BMI 32.7 |
| Campo et al. [55] |
Italy Hospital |
15 months Direct approacha |
24 weeks PF, HRQoL, DEP, ANX |
HYP 86%, T2DM 30%, HF 100% 235 people, mean age 76.5 years, 23% female, mean BMI 27 |
aDirect approach (potential people approached individually), bindirect approach (potential people approached e.g. via news advertisement or flyers), DM* diabetes mellitus, not specified if type 1 or 2; A* arthritis, not specified which form (e.g. osteoarthritis); DEP depression symptoms, HF heart failure, T2DM type 2 diabetes mellitus, COPD chronic obstructive pulmonary disease, KOA knee osteoarthritis, HOA hip osteoarthritis, IHD ischemic heart disease, BMI body mass index (kg/m2), PF physical function, HRQoL health-related quality of life, ANX anxiety symptoms, n/a not applicable
Participants characteristics
A total population of 3363 people with multimorbidity participated in the 23 RCTs. The most common diseases reported were heart failure (k=16), depression (k=15), type 2 diabetes mellitus (k=15), hypertension (k=14), chronic obstructive pulmonary disease (k=6), osteoarthritis of the knee (k=4) or hip (k=4). The number of conditions reported varied from two to seven with the most common combination being heart failure and depression [33–35, 37, 38, 44, 49] (Table 1). The mean age of the people was 65.5 (SD 8.4) years with 46% being females and the average BMI was 29.8 (SD 2.6).
Intervention and comparator groups
The most commonly applied type of exercise therapy was aerobic exercise only (k=11) [32, 34–38, 40, 44, 49, 50, 53], followed by exercise programs combining aerobic, strengthening, balance and flexibility exercises (k=8) [33, 39, 42, 43, 45–48, 55], and Tai Chi (k=2) [41, 51] or resistance training only (k=2) [52, 54]. The duration of the exercise therapy varied from 1 to 26 weeks with a median (interquartile range (IQR)) of 12 weeks (12 to 16). Sessions per week varied from 2 to 14 with a median of 3 session IQR (3 to 3). Comparator groups varied widely and included usual care, medication, cognitive behavioural therapy, health condition education, general practitioner consultations and stretching and flexibility exercises.
Synthesis of results
Recruitment rates
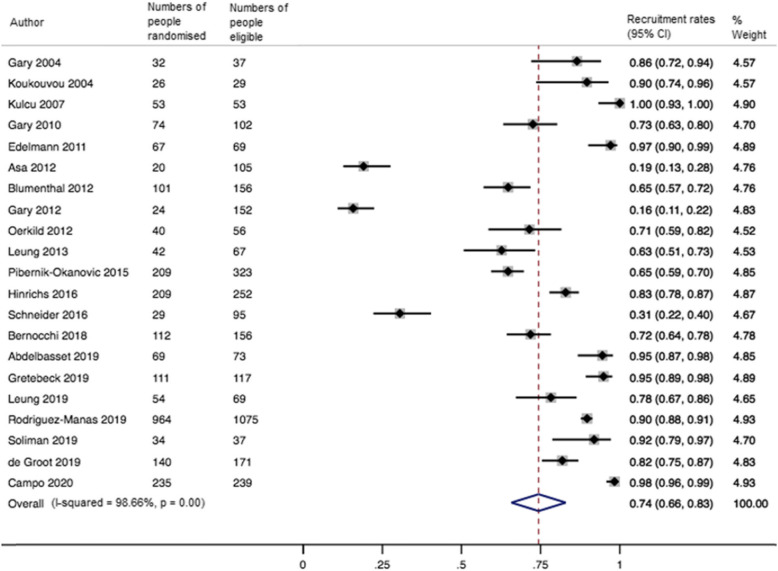
The pooled recruitment rate (k=21) was 0.74 (95% CI 0.66 to 0.83; I2=99%) (Fig. 2). Meta-regression analyses showed no impact of recruitment strategy and trial, intervention and participant characteristics on recruitment rates (Supplementary Table 1).
Fig. 2.
Forest plot for the recruitment rates in RCTs of exercise therapy in people with multimorbidity. 95% CI = confidence interval
Retention rates
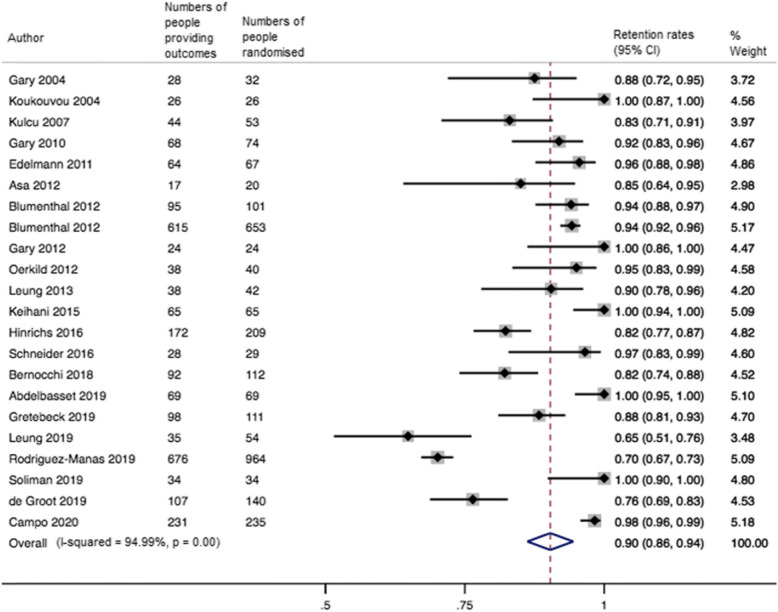
The pooled retention rate (k=22) was 0.90 (95% CI 0.86 to 0.94; I2=95%) at the end of the intervention (median 12 weeks IQR 12 to 12) (Fig. 3). Meta-regression analyses showed that there was no difference in retention rates between physical and psychosocial outcomes and that increasing age (slope −0.01; 95% CI −0.01 to 0.01; Tau2=.006) and including a higher proportion of people with hypertension (slope −0.01; 95% CI −0.01 to −0.01; Tau2=.006) were associated with lower retention rates (Supplementary Table 1 and Supplementary Figures 2a and b). This suggests that for every year the age increase, the retention rates were reduced with 1%. Similarly, for each additional percentage of people with hypertension included in the study, the retention rates were reduced by 1%.
Fig. 3.
Forest plot for the retention rates in RCTs of exercise therapy in people with multimorbidity at the end of the intervention (median 12 weeks IQR 12 to 12). 95% CI = 95% confidence interval
Ten studies were included in the meta-analysis at the follow-up time closest to 12 months. The pooled retention rate was 0.80 (95% CI 0.68 to 0.92; I2=98%).
Differential retention rates
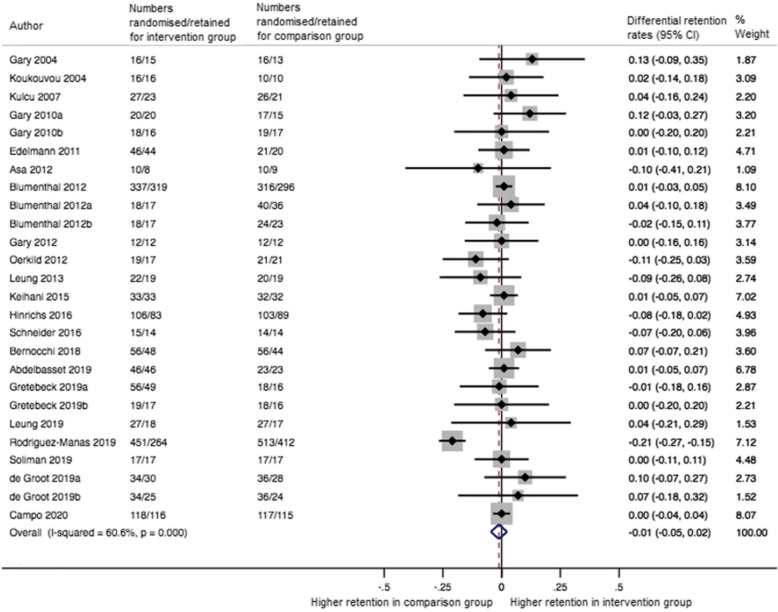
The pooled differential retention rate (k=22) was −0.01 (95% CI −0.05 to 0.02; I2=61%) at the end of the intervention (median 12 weeks IQR 12 to 12) (Fig. 4). Meta-regression analyses showed no impact of outcome domain (physical vs. psychosocial outcomes) and intervention and comparator characteristics on differential retention rates (Supplementary Table 2).
Fig. 4.
Forest plot for the differential retention rates in RCTs of exercise therapy in people with multimorbidity at the end of the intervention (median 12 weeks IQR 12 to 12). 95% CI = 95% confidence interval
Ten studies were included in the meta-analysis at the follow-up time closest to 12 months. The pooled differential retention was 0.01 (95% CI −0.06 to 0.08; I2=73%).
Risk of bias within studies
Overall, the risk of bias assessment showed that none of the studies was judged as having ‘low risk of bias’, 91% as having ‘some concerns’, and 9% as having ‘high risk of bias’, respectively (Supplementary Figure 1). A randomisation process with a low risk of bias was seen in 78% of the studies, and only 9% had bias due to deviations from intended interventions. The domain causing the greatest risk of bias was the measurement of the outcome as the outcome assessors in all the studies were participants filling out self-completed questionnaires, which made blinding particularly difficult.
Quality of the evidence
The overall quality of the evidence, including the reasons for grading the evidence, was summarised in Table 2. Overall, the quality of evidence was deemed as very low. We started the GRADE assessment from low as recommended for observational studies since although this systematic review included RCTs they did not test the effect of recruitment and retention strategies on effect estimates. Inconsistency and indirectness were the two reasons for downgrading the quality of the evidence due to the inclusion of people with depression and heart failure in most of the studies and the inability to explain the inconsistency of the estimates with meta-regression analyses.
Table 2.
Summary of findings
| People with multimorbidity participating in exercise therapy RCTs | ||||
|
Patient or population: People with two or more chronic conditions Intervention: Exercise therapy Comparison: Usual care, education, or other non-exercise groups | ||||
| Outcomes follow-up | Proportion (95% CI) | I-squared | Numbers of people (studies) | Quality of the evidence (GRADE) |
| Recruitment rates | 0.74 (0.66, 0.83) | 99% | 2645 (21 RCTs) | ⊕ ⊖ ⊖ ⊖a,b (Very low) |
|
Retention rates Timepoint: end of treatment |
0.90 (0.86, 0.94) | 95% | 3154 (22 RCTs) | ⊕ ⊖ ⊖ ⊖a,b (Very low) |
|
Differential retention rates* Timepoint: end of treatment |
−0.01 (−0.05, 0.02) | 61% | 3154 (22 RCTs) | ⊕ ⊖ ⊖ ⊖a,b (Very low) |
*The basis of the differential retention rate is calculated by subtracting numbers providing intervention outcomes from numbers providing comparison outcomes
RCTs (randomised controlled trials), CI (95% Confidence Interval), GRADE (Grading of Recommendations Assessment, Development and Evaluation)
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
aDowngraded one due to considerable heterogeneity without a plausible explanation
bDowngraded one due to indirectness of people
Discussion
This systematic review investigating recruitment and retention rates included 23 exercise therapy RCTs with the participation of more than 3300 people with multimorbidity performed in 18 countries. On average, 74% of the eligible people were randomised to RCTs. Of these, 90% provided outcomes at the end of the intervention assessment. Recruiting people with increasing age and/or with chronic hypertension plus a coexisting condition showed lower retention rates. Retention rates did not vary between the intervention groups and comparison groups.
Recruitment rates in people with multimorbidity
Three out of four eligible people were randomised to RCTs of exercise therapy. This recruitment rate was higher (74% vs 64%) than in previous RCTs including people with multimorbidity patient-centred interventions, without an exercise component [18, 19]. However, the population in our systematic review was younger and the combination of diseases was different. Similarly, in exercise RCTs, recruitment rates were higher when including people with multimorbidity rather than when including people with single chronic conditions such as chronic obstructive pulmonary disease (54%), heart failure (41%) or depression (65%) [56–58]. Comparing with these previous findings, this suggests that for exercise therapy RCTs, eligible people with multimorbidity might be easier to recruit compared to people with a single condition. However, the indirect comparisons of the recruitment rates need to be interpreted with caution due to differences in participant characteristics and setting of interventions.
Retention and differential rates in people with multimorbidity
Overall, nine in 10 people with multimorbidity provided end of treatment outcomes, with no difference between retaining people in the intervention and comparison groups. The overall retention rates were slightly higher (90% versus 86%) than previously reported retention rates of multimorbidity RCTs in general [18, 19]. Additionally, the rates found were similar to RCTs including people with either chronic obstructive pulmonary disease (82%), heart failure (97%) or depression (100%) [56–58], suggesting that people with multimorbidity are just as likely to provide end of treatment outcomes as people with a single disease. However, for longer-term follow-up assessment, the retention rates found diminished from 90 to 80%. This is somewhat expected and highlights the need of putting extra effort into retaining participants for long-term outcome assessments [12]. Additionally, our findings suggest that including people with increasing age and/or people with hypertension plus another coexisting condition is potentially less likely to provide end of treatment outcomes than younger people and/or people with multimorbidity consisting of other chronic conditions. Increasing age has previously been found to negatively affect attrition rates in RCTs [59]. However, while we are unsure why hypertension in this study is associated with lower retention rates, it appears that retaining people with hypertension in exercise trials is particularly challenging [60]. Finally, our findings also suggest that retention rates do not vary between the intervention and comparison groups as previously hypothesised.
Strengths and limitations
This systematic review with meta-analyses followed a pre-specified protocol made publicly available prior to completion of the title/abstract screening and followed recommended guidelines for conducting and reporting systematic reviews [22, 24]. Additionally, authors of the included RCTs were emailed to obtain missing data, which enabled us to perform meta-regression analyses with more complete information on the trial, participant and intervention characteristics associated with recruitment, retention and differential retention.
This systematic review has some limitations. The number of RCTs included is small and from many different countries. However, this was expected since multimorbidity is a relatively new concept and to improve chances of identifying all studies available, a comprehensive search strategy developed for a previously published systematic review was used [23]. Additionally, the prevalence of people with osteoarthritis, hypertension, type 2 diabetes mellitus, depression, heart failure, ischemic heart disease and chronic obstructive pulmonary disease varied considerably across all the included RCTs. Most people randomised had either depression, heart failure or a combination of both diseases, which limits the generalizability of our findings to the whole population with multimorbidity. Furthermore, as expected by the multimorbidity definition, we found considerable heterogeneity when pooling the results, and only a few of the trial, participant and intervention characteristics were able to explain some of the heterogeneous results for the meta-analyses. Finally, reporting of both recruitment and retention strategies was inconsistent. For example, none of the included RCTs reported any form of reminders for non-responders and only one reported the use of financial incentives [46].
Implication for future research
The optimal strategy for recruiting and retaining people with multimorbidity in exercise RCTs remains unclear, partially due to the inconsistent reporting of recruitment and retention strategies in existing trials. Therefore, it is important that future exercise RCTs including people with multimorbidity should describe the strategies used in a detailed and transparent way and, if feasible and available, use existing strategies proven to be effective. For example, using patient and public involvement may increase recruitment and retention rates as it helps to ensure that the research focuses on issues relevant to patients and the public [61]. Additionally, the use of an open trial design and telephone reminders for non-responders to postal interventions to improve recruitment [62] and the use of monetary incentives to increase retention [63] has proven to be effective. Finally, in order to enhance the evidence base for strategies that are useful to improve recruitment and retention rates, future exercise RCTs should consider testing the effect of different recruitment and retention strategies by performing Studies Within A Trial [64], which are self-contained studies embedded within a trial aimed at evaluating or exploring different ways of delivering or organising a specific trial process.
Conclusions
Three in four eligible people with multimorbidity were randomised to exercise therapy RCTs, of which nine out of 10 provided end of treatment outcomes, with no difference seen between the intervention and comparison groups. Enrolling people with increasing age and/or with chronic hypertension plus a coexisting condition could appear to lead to lower retention rates. However, the results must be interpreted with caution due to large differences between the included studies.
Supplementary Information
Additional file 1. PRISMA 2009 Checklist.
Additional file 2: Supplementary Figure 1. ‘Risk of bias’ summary shown as percentage of 23 individual ‘Risk of bias’ items for each included study. Supplementary Table 1. Impact of covariates on recruitment and retention rates. Supplementary Table 2. Impact of covariates on differential retention rates. Supplementary Figure 2. Bubble plot for the impact of age (a) and proportion of included participants with hypertension (b) on retention rates.
Acknowledgements
We would like to thank Gregers Aagaard and Margit Dybkjær, two people living with multiple chronic conditions, and Tue Dybkjær, a partner of a person with multimorbidity, for their feedback on the choice of the outcome measures for this systematic review and feedback on the presentation of the findings of this systematic review, respectively. We would also like to thank the MOBILIZE scientific advisory board consisting of Prof. Sallie Lamb, Prof. Alan Silman, Prof. Bente Klarlund Pedersen, Prof. Ewa M. Roos and Prof. Rod Taylor. Additionally, we would also like to thank the authors of the included studies which provided us the data requested via email: Dr. Timo Hinrichs, Prof. Evangelia Kouidi, Dr. Olga Laosa Zafra, Dr. Regina Leung, Prof. Rebecca Gary, Prof. Yeşim Kurtaiş Aytür, Dr. Frank Edelmann and Dr. Bodil Oerkild.
Abbreviations
- RCTs
Randomised controlled trials
- HRQoL
Health-related quality of life
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- MEDLINE
Medical Literature Analysis and Retrieval System Online
- CENTRAL
Cochrane Central Register of Controlled Trials
- ICTRP
International Clinical Trials Registry Platform
- WoS
Web of Science
- TIAB
Title/Abstract
- MeSH
Medical Subject Heading
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- SD
Standard deviation
- SE
Standard error
- 95% CI
95% confidence interval
- BMI
Body mass index
- SES
Socio economic status
- IQR
Interquartile range
Authors’ contributions
STS procured the funding for this systematic review. AB and CBJ designed the search strategy. LKH and AB independently screened title/abstracts, full texts, extracted the data from the included studies, assessed the risk of bias, performed the GRADE assessment and contacted the study authors to retrieve additional information. LKH did the data analysis, and both AB and CBJ provided statistical expertise. LKH wrote the first draft of the manuscript and AB provided feedback. LKH is the guarantor and drafted the manuscript. The authors read, provided feedback and approved the study design, methods, protocol and manuscript drafts as well as approved the final manuscript.
Funding
European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (MOBILIZE, grant agreement No 801790), Næstved, Slagelse and Ringsted Hospitals’ Research Fund and The Association of Danish Physiotherapists Research Fund. The funding sources were not involved in any aspect of this systematic review.
Availability of data and materials
The dataset and statistical script necessary to reproduce the analyses presented in this systematic review has been made available online at the Open Science Framework page of the MOBILIZE project [26].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lasse K. Harris, Email: lasse.kindler.harris@regionh.dk
Søren T. Skou, Email: stskou@health.sdu.dk
Carsten B. Juhl, Email: cjuhl@health.sdu.dk
Madalina Jäger, Email: msaracutu@health.sdu.dk.
Alessio Bricca, Email: abricca@health.sdu.dk.
References
- 1.Lancet T. Making more of multimorbidity: an emerging priority. Lancet (London, England) 2018;391:1637. doi: 10.1016/S0140-6736(18)30941-3. [DOI] [PubMed] [Google Scholar]
- 2.Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Rev. 2011;33(2):451–474. doi: 10.1007/BF03391611. [DOI] [Google Scholar]
- 3.Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Fam Med. 2003;1(1):15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortin M, Hudon C, Dubois MF, Almirall J, Lapointe L, Soubhi H. Comparative assessment of three different indices of multimorbidity for studies on health-related quality of life. Health Qual Life Outcomes. 2005;3:1–7. doi: 10.1186/1477-7525-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey GE, Ramsay EN, Vitry AI, Gilbert AL, Luszcz MA, Ryan P, Roughead EE. Comorbid chronic diseases, discordant impact on mortality in older people: a 14-year longitudinal population study. J Epidemiol Community Health. 2010;64(12):1036–1042. doi: 10.1136/jech.2009.088260. [DOI] [PubMed] [Google Scholar]
- 6.Sinnott C, Mc Hugh S, Browne J, Bradley C. GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open. 2013;3(9):e003610. doi: 10.1136/bmjopen-2013-003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pefoyo AJK, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15(1):415. doi: 10.1186/s12889-015-1733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D, Duan L, Erskine H, Feigin VL, Ferrari AJ, Fitzmaurice C, Fleming T, Graetz N, Guinovart C, Haagsma J, Hansen GM, Hanson SW, Heuton KR, Higashi H, Kassebaum N, Kyu H, Laurie E, Liang X, Lofgren K, Lozano R, MacIntyre MF, Moradi-Lakeh M, Naghavi M, Nguyen G, Odell S, Ortblad K, Roberts DA, Roth GA, Sandar L, Serina PT, Stanaway JD, Steiner C, Thomas B, Vollset SE, Whiteford H, Wolock TM, Ye P, Zhou M, Ãvila MA, Aasvang GM, Abbafati C, Ozgoren AA, Abd-Allah F, Aziz MIA, Abera SF, Aboyans V, Abraham JP, Abraham B, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NME, Aburto TC, Achoki T, Ackerman IN, Adelekan A, Ademi Z, Adou AK, Adsuar JC, Arnlov J, Agardh EE, al Khabouri MJ, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Allen PJ, AlMazroa MAA, Alsharif U, Alvarez E, Alvis-Guzman N, Ameli O, Amini H, Ammar W, Anderson BO, Anderson HR, Antonio CAT, Anwari P, Apfel H, Arsenijevic VSA, Artaman A, Asghar RJ, Assadi R, Atkins LS, Atkinson C, Badawi A, Bahit MC, Bakfalouni T, Balakrishnan K, Balalla S, Banerjee A, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Basu S, Basu A, Baxter A, Beardsley J, Bedi N, Beghi E, Bekele T, Bell ML, Benjet C, Bennett DA, Bensenor IM, Benzian H, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta Z, Bienhoff K, Bikbov B, Abdulhak AB, Blore JD, Blyth FM, Bohensky MA, Basara BB, Borges G, Bornstein NM, Bose D, Boufous S, Bourne RR, Boyers LN, Brainin M, Brauer M, Brayne CEG, Brazinova A, Breitborde NJK, Brenner H, Briggs ADM, Brooks PM, Brown J, Brugha TS, Buchbinder R, Buckle GC, Bukhman G, Bulloch AG, Burch M, Burnett R, Cardenas R, Cabral NL, Nonato IRC, Campuzano JC, Carapetis JR, Carpenter DO, Caso V, Castaneda-Orjuela CA, Catala-Lopez F, Chadha VK, Chang JC, Chen H, Chen W, Chiang PP, Chimed-Ochir O, Chowdhury R, Christensen H, Christophi CA, Chugh SS, Cirillo M, Coggeshall M, Cohen A, Colistro V, Colquhoun SM, Contreras AG, Cooper LT, Cooper C, Cooperrider K, Coresh J, Cortinovis M, Criqui MH, Crump JA, Cuevas-Nasu L, Dandona R, Dandona L, Dansereau E, Dantes HG, Dargan PI, Davey G, Davitoiu DV, Dayama A, de la Cruz-Gongora V, de la Vega SF, de Leo D, del Pozo-Cruz B, Dellavalle RP, Deribe K, Derrett S, Des Jarlais DC, Dessalegn M, deVeber GA, Dharmaratne SD, Diaz-Torne C, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duber H, Durrani AM, Edmond KM, Ellenbogen RG, Endres M, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Fahimi S, Farzadfar F, Fay DFJ, Felson DT, Fereshtehnejad SM, Fernandes JG, Ferri CP, Flaxman A, Foigt N, Foreman KJ, Fowkes FGR, Franklin RC, Furst T, Futran ND, Gabbe BJ, Gankpe FG, Garcia-Guerra FA, Geleijnse JM, Gessner BD, Gibney KB, Gillum RF, Ginawi IA, Giroud M, Giussani G, Goenka S, Goginashvili K, Gona P, de Cosio TG, Gosselin RA, Gotay CC, Goto A, Gouda HN, Guerrant R, Gugnani HC, Gunnell D, Gupta R, Gupta R, Gutierrez RA, Hafezi-Nejad N, Hagan H, Halasa Y, Hamadeh RR, Hamavid H, Hammami M, Hankey GJ, Hao Y, Harb HL, Haro JM, Havmoeller R, Hay RJ, Hay S, Hedayati MT, Pi IBH, Heydarpour P, Hijar M, Hoek HW, Hoffman HJ, Hornberger JC, Hosgood HD, Hossain M, Hotez PJ, Hoy DG, Hsairi M, Hu H, Hu G, Huang JJ, Huang C, Huiart L, Husseini A, Iannarone M, Iburg KM, Innos K, Inoue M, Jacobsen KH, Jassal SK, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jonas JB, Joseph J, Juel K, Kan H, Karch A, Karimkhani C, Karthikeyan G, Katz R, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Khader YS, Khalifa SEAH, Khan EA, Khan G, Khang YH, Khonelidze I, Kieling C, Kim D, Kim S, Kimokoti RW, Kinfu Y, Kinge JM, Kissela BM, Kivipelto M, Knibbs L, Knudsen AK, Kokubo Y, Kosen S, Kramer A, Kravchenko M, Krishnamurthi RV, Krishnaswami S, Defo BK, Bicer BK, Kuipers EJ, Kulkarni VS, Kumar K, Kumar GA, Kwan GF, Lai T, Lalloo R, Lam H, Lan Q, Lansingh VC, Larson H, Larsson A, Lawrynowicz AEB, Leasher JL, Lee JT, Leigh J, Leung R, Levi M, Li B, Li Y, Li Y, liang J, Lim S, Lin HH, Lind M, Lindsay MP, Lipshultz SE, Liu S, Lloyd BK, Ohno SL, Logroscino G, Looker KJ, Lopez AD, Lopez-Olmedo N, Lortet-Tieulent J, Lotufo PA, Low N, Lucas RM, Lunevicius R, Lyons RA, Ma J, Ma S, Mackay MT, Majdan M, Malekzadeh R, Mapoma CC, Marcenes W, March LM, Margono C, Marks GB, Marzan MB, Masci JR, Mason-Jones AJ, Matzopoulos RG, Mayosi BM, Mazorodze TT, McGill NW, McGrath JJ, McKee M, McLain A, McMahon BJ, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mensah G, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Mitchell PB, Mock CN, Moffitt TE, Ibrahim NM, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Montine TJ, Moore AR, Moran AE, Morawska L, Mori R, Moschandreas J, Moturi WN, Moyer M, Mozaffarian D, Mueller UO, Mukaigawara M, Murdoch ME, Murray J, Murthy KS, Naghavi P, Nahas Z, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KMV, Nash D, Nejjari C, Neupane SP, Newman LM, Newton CR, Ng M, Ngalesoni FN, Nhung NT, Nisar MI, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh IH, Ohkubo T, Omer SB, Opio JN, Ortiz A, Pandian JD, Panelo CIA, Papachristou C, Park EK, Parry CD, Caicedo AJP, Patten SB, Paul VK, Pavlin BI, Pearce N, Pedraza LS, Pellegrini CA, Pereira DM, Perez-Ruiz FP, Perico N, Pervaiz A, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Phillips D, Phillips B, Piel FB, Plass D, Poenaru D, Polanczyk GV, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman S, Raju M, Rakovac I, Rana SM, Razavi H, Refaat A, Rehm J, Remuzzi G, Resnikoff S, Ribeiro AL, Riccio PM, Richardson L, Richardus JH, Riederer AM, Robinson M, Roca A, Rodriguez A, Rojas-Rueda D, Ronfani L, Rothenbacher D, Roy N, Ruhago GM, Sabin N, Sacco RL, Ksoreide K, Saha S, Sahathevan R, Sahraian MA, Sampson U, Sanabria JR, Sanchez-Riera L, Santos IS, Satpathy M, Saunders JE, Sawhney M, Saylan MI, Scarborough P, Schoettker B, Schneider IJC, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Serdar B, Servan-Mori EE, Shackelford K, Shaheen A, Shahraz S, Levy TS, Shangguan S, She J, Sheikhbahaei S, Shepard DS, Shi P, Shibuya K, Shinohara Y, Shiri R, Shishani K, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Simard EP, Sindi S, Singh JA, Singh L, Skirbekk V, Sliwa K, Soljak M, Soneji S, Soshnikov SS, Speyer P, Sposato LA, Sreeramareddy CT, Stoeckl H, Stathopoulou VK, Steckling N, Stein MB, Stein DJ, Steiner TJ, Stewart A, Stork E, Stovner LJ, Stroumpoulis K, Sturua L, Sunguya BF, Swaroop M, Sykes BL, Tabb KM, Takahashi K, Tan F, Tandon N, Tanne D, Tanner M, Tavakkoli M, Taylor HR, te Ao BJ, Temesgen AM, Have MT, Tenkorang EY, Terkawi AS, Theadom AM, Thomas E, Thorne-Lyman AL, Thrift AG, Tleyjeh IM, Tonelli M, Topouzis F, Towbin JA, Toyoshima H, Traebert J, Tran BX, Trasande L, Trillini M, Truelsen T, Trujillo U, Tsilimbaris M, Tuzcu EM, Ukwaja KN, Undurraga EA, Uzun SB, van Brakel WH, van de Vijver S, Dingenen RV, van Gool CH, Varakin YY, Vasankari TJ, Vavilala MS, Veerman LJ, Velasquez-Melendez G, Venketasubramanian N, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Waller S, Wallin MT, Wan X, Wang L, Wang JL, Wang Y, Warouw TS, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Wessells KRR, Westerman R, Wilkinson JD, Williams HC, Williams TN, Woldeyohannes SM, Wolfe CDA, Wong JQ, Wong H, Woolf AD, Wright JL, Wurtz B, Xu G, Yang G, Yano Y, Yenesew MA, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis M, Yu C, Kim KY, Zaki MES, Zhang Y, Zhao Z, Zhao Y, Zhu J, Zonies D, Zunt JR, Salomon JA, Murray CJL. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleeson M, Bishop N, Stensel D, Lindley M, Mastana S, Nimmo M. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 10.Reeuwijk KG, de Rooij M, van Dijk GM, Veenhof C, Steultjens MP, Dekker J. Osteoarthritis of the hip or knee: which coexisting disorders are disabling? Clin Rheumatol. 2010;29(7):739–747. doi: 10.1007/s10067-010-1392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.1996.03540080059030. [DOI] [PubMed] [Google Scholar]
- 12.Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, Koletzko B, Lucas A. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child. 2008;93(6):458–461. doi: 10.1136/adc.2007.127316. [DOI] [PubMed] [Google Scholar]
- 13.Tudur Smith C, Hickey H, Clarke M, Blazeby J, Williamson P. The trials methodological research agenda: results from a priority setting exercise. Trials. 2014;15:32. 10.1186/1745-6215-15-32. [DOI] [PMC free article] [PubMed]
- 14.Robinson KA, Dennison CR, Wayman DM, Pronovost PJ, Needham DM. Systematic review identifies number of strategies important for retaining study participants. J Clin Epidemiol. 2007;60:757–765. doi: 10.1016/j.jclinepi.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toerien M, Brookes ST, Metcalfe C, de Salis I, Tomlin Z, Peters TJ, et al. A review of reporting of participant recruitment and retention in RCTs in six major journals. Trials. 2009;10:1–12. doi: 10.1186/1745-6215-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters SJ, Dos Anjos Henriques-Cadby IB, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7(3):1–10. doi: 10.1136/bmjopen-2016-015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcdonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;8(7):1–8. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salisbury C, Man M-S, Bower P, Guthrie B, Chaplin K, Gaunt DM, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet (London, England) 2018;392(10141):41–50. doi: 10.1016/S0140-6736(18)31308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ Br Med J. 2012;345(sep03 1):e5205. doi: 10.1136/bmj.e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhatre S, Jefferson A, Cook R, Meeker CR, Kim JH, Hartz KM, Wong YN, Caruso A, Newman DK, Morales KH, Jayadevappa R. Patient-centered recruitment and retention for a randomized controlled study. Trials. 2018;19(1):205. doi: 10.1186/s13063-018-2578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bricca A, Harris LK, Jäger M, Smith SM, Juhl CB, Skou ST. Benefits and harms of exercise therapy in people with multimorbidity: a systematic review and meta-analysis of randomised controlled trials. Ageing Res Rev. 2020;63:101166. doi: 10.1016/j.arr.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bricca A, Harris LK, Saracutu M, Smith SM, Juhl CB, Skou ST. The benefits and harms of therapeutic exercise on physical and psychosocial outcomes in people with multimorbidity: Protocol for a systematic review. J comorbidity. 2020;10:2235042X20920458. doi: 10.1177/2235042X20920458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJWV, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster ED, Deardorff A. Open Science Framework (OSF) J Med Libr Assoc. 2017;105(2):203–206. [Google Scholar]
- 26.OSF | MOBILIZE [Internet]. Available from: https://osf.io/84vzn/.
- 27.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SM, Wallace E, Salisbury C, Sasseville M, Bayliss E, Fortin M. A Core Outcome Set for Multimorbidity Research (COSmm) Ann Fam Med. 2018;16(2):132–138. doi: 10.1370/afm.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schünemann HJ, JPT H, Vist GE, Glasziou P, Akl EA, Skoetz NGG. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: JPT H, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6,0 (updated July 2019) Cochrane. 2019. p. 2019. [Google Scholar]
- 30.The Cochrane Collaboration. Data Collections Forms for intervention reviews [Internet]. Available from: https://dplp.cochrane.org/data-extraction-forms.
- 31.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gary RA, Sueta CA, Dougherty M, Rosenberg B, Cheek D, Preisser J, Neelon V, McMurray R. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Hear Lung J Acute Crit Care. 2004;33(4):210–218. doi: 10.1016/j.hrtlng.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Koukouvou G, Kouidi E, Iacovides A, Konstantinidou E, Kaprinis G, Deligiannis A. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J Rehabil Med. 2004;36(1):36–41. doi: 10.1080/11026480310015549. [DOI] [PubMed] [Google Scholar]
- 34.Kulcu DG, Kurtais Y, Tur BS, Gulec S, Seckin B. The effect of cardiac rehabilitation on quality of life, anxiety and depression in patients with congestive heart failure. A randomized controlled trial, short-term results. Eura Medicophys. 2007;43(4):489–497. [PubMed] [Google Scholar]
- 35.Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119–131. doi: 10.1016/j.jpsychores.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Åsa C, Maria S, Katharina SS, Bert A. Aquatic exercise is effective in improving exercise performance in patients with heart failure and type 2 diabetes mellitus. Evid Based Complement Altern Med. 2012;2012:1–8. doi: 10.1155/2012/349209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, O'Hayer CVF, Mabe S, Johnson J, Doraiswamy PM, Jiang W, Schocken DD, Hinderliter AL. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (understanding the prognostic benefits of exercise and antidepressant therapy) study. J Am Coll Cardiol. 2012;60(12):1053–1063. doi: 10.1016/j.jacc.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumenthal JA, Babyak MA, O’Connor C, Keteyian S, Landzberg J, Howlett J, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized tria. J Am Med Assoc. 2012;308(5):465–474. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gary RA, Cress ME, Higgins MK, Smith AL, Dunbar SB. A combined aerobic and resistance exercise program improves physical functional performance in patients with heart failure: a pilot study. J Cardiovasc Nurs. 2012;27(5):418–430. doi: 10.1097/JCN.0b013e31822ad3c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oerkild B, Frederiksen M, Hansen JF, Prescott E. Home-based cardiac rehabilitation is an attractive alternative to no cardiac rehabilitation for elderly patients with coronary heart disease: results from a randomised clinical trial. BMJ Open. 2012;2(6):1–9. doi: 10.1136/bmjopen-2012-001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung RWM, McKeough ZJ, Peters MJ, Alison JA. Short-form Sun-style t’ai chi as an exercise training modality in people with COPD. Eur Respir J. 2013;41(5):1051–1057. doi: 10.1183/09031936.00036912. [DOI] [PubMed] [Google Scholar]
- 42.Nolte K, Herrmann-Lingen C, Wachter R, Gelbrich G, Düngen HD, Duvinage A, Hoischen N, von Oehsen K, Schwarz S, Hasenfuss G, Halle M, Pieske B, Edelmann F. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol. 2015;22(5):582–593. doi: 10.1177/2047487314526071. [DOI] [PubMed] [Google Scholar]
- 43.Edelmann F, Gelbrich G, Dngen HD, Fröhling S, Wachter R, Stahrenberg R, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol. 2011;58(17):1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 44.Keihani D, Kargarfard M, Mokhtari M. Cardiovascular effects of exercise rehabilitation on quality of life, anxiety and depression in patients with heart failure. J Ment Heal Princ. 2014;1(17):13–19. [Google Scholar]
- 45.Pibernik-Okanović M, Hermanns N, Ajduković D, Kos J, Prašek M, Šekerija M, et al. Does treatment of subsyndromal depression improve depression-related and diabetes-related outcomes? A randomised controlled comparison of psychoeducation, physical exercise and enhanced treatment as usual. Trials. 2015;16(1):1–13. doi: 10.1186/s13063-015-0833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider KL, Panza E, Handschin B, Ma Y, Busch AM, Waring ME, Appelhans BM, Whited MC, Keeney J, Kern D, Blendea M, Ockene I, Pagoto SL. Feasibility of pairing behavioral activation with exercise for women with type 2 diabetes and depression: the get it study pilot randomized controlled trial. Behav Ther. 2016;47(2):198–212. doi: 10.1016/j.beth.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinrichs T, Bücker B, Klaaßen-Mielke R, Brach M, Wilm S, Platen P, Mai A. Home-based exercise supported by general practitioner practices: ineffective in a sample of chronically ill, mobility-limited older adults (the HOMEfit Randomized Controlled Trial) J Am Geriatr Soc. 2016;64(11):2270–2279. doi: 10.1111/jgs.14392. [DOI] [PubMed] [Google Scholar]
- 48.Bernocchi P, Vitacca M, La Rovere MT, Volterrani M, Galli T, Baratti D, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: A randomised controlled trial. Age Ageing. 2018;47(1):82–88. doi: 10.1093/ageing/afx146. [DOI] [PubMed] [Google Scholar]
- 49.Abdelbasset WK, Alqahtani BA, Alrawaili SM, Ahmed AS, Elnegamy TE, Ibrahim AA, Soliman GS. Similar effects of low to moderate-intensity exercise program vs moderate-intensity continuous exercise program on depressive disorder in heart failure patients: a 12-week randomized controlled trial. Med (Baltimore). 2019;98(32):e16820. doi: 10.1097/MD.0000000000016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Groot M, Shubrook JH, Hornsby WG, Pillay Y, Mather KJ, Fitzpatrick K, et al. Program ACTIVE II: outcomes from a randomized, multistate community-based depression treatment for rural and urban adults with type 2 diabetes. Diabetes Care. 2019;42(7):1185–1193. doi: 10.2337/dc18-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung LY. ling, Chan AW kiu, Sit JW hung, Liu T, Taylor-Piliae RE. Tai Chi in Chinese adults with metabolic syndrome: a pilot randomized controlled trial. Complement Ther Med. 2019;46(March):54–61. doi: 10.1016/j.ctim.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Mañas L, Laosa O, Vellas B, Paolisso G, Topinkova E, Oliva-Moreno J, Bourdel-Marchasson I, Izquierdo M, Hood K, Zeyfang A, Gambassi G, Petrovic M, Hardman TC, Kelson MJ, Bautmans I, Abellan G, Barbieri M, Peña-Longobardo LM, Regueme SC, Calvani R, de Buyser S, Sinclair AJ, on behalf of the European MID‐Frail Consortium Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle. 2019;10(4):721–733. doi: 10.1002/jcsm.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soliman GS, Abdelbasset WK. Efficacy of aerobic training on pulmonary functions and depression in elderly COPD patients. J Adv Pharm Educ Res. 2019;9(4):39–45.
- 54.Gretebeck KA, Blaum CS, Moore T, Brown R, Galecki A, Strasburg D, Chen S, Alexander NB. Functional exercise improves mobility performance in older adults with type 2 diabetes: a randomized controlled trial. J Phys Act Heal. 2019;16(6):461–469. doi: 10.1123/jpah.2018-0240. [DOI] [PubMed] [Google Scholar]
- 55.Campo G, Tonet E, Chiaranda G, Sella G, Maietti E, Bugani G, et al. Exercise intervention improves quality of life in older adults after myocardial infarction: randomised clinical trial. Heart. 2020;106(21):1658–64. 10.1136/heartjnl-2019-316349. [DOI] [PubMed]
- 56.Chen Y, Funk M, Wen J, Tang X, He G, Liu H. Effectiveness of a multidisciplinary disease management program on outcomes in patients with heart failure in China: a randomized controlled single center study. Heart Lung. 2018;47(1):24–31. doi: 10.1016/j.hrtlng.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Fleck MP. Exercise and severe depression: preliminary results of an add-on study. J Affect Disord. 2011;133(3):615–618. doi: 10.1016/j.jad.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 58.Greening NJ, Williams JEA, Hussain SF, Harvey-Dunstan TC, Bankart MJ, Chaplin EJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ. 2014;349(jul08 5). 10.1136/bmj.g4315. [DOI] [PMC free article] [PubMed]
- 59.Peterson JC, Pirraglia PA, Wells MT, Charlson ME. Attrition in longitudinal randomized controlled trials: home visits make a difference. BMC Med Res Methodol. 2012;12(1):178. doi: 10.1186/1471-2288-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naci H, Salcher-Konrad M, Dias S, Blum MR, Sahoo SA, Nunan D, Ioannidis JPA. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53(14):859–869. doi: 10.1136/bjsports-2018-099921. [DOI] [PubMed] [Google Scholar]
- 61.Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem. 2017;3(1):1–11. doi: 10.1186/s40900-017-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2(2):MR000013. 10.1002/14651858.MR000013.pub6. [DOI] [PMC free article] [PubMed]
- 63.Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open. 2014;4(2):e003821. 10.1136/bmjopen-2013-003821. [DOI] [PMC free article] [PubMed]
- 64.Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, Collett C, Cotton S, Devane D, el Feky A, Flemyng E, Galvin S, Gardner H, Gillies K, Jansen J, Littleford R, Parker A, Ramsay C, Restrup L, Sullivan F, Torgerson D, Tremain L, Westmore M, Williamson PR. Trial Forge Guidance 1: what is a Study Within A Trial (SWAT)? Trials. 2018;19(1):139. doi: 10.1186/s13063-018-2535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PRISMA 2009 Checklist.
Additional file 2: Supplementary Figure 1. ‘Risk of bias’ summary shown as percentage of 23 individual ‘Risk of bias’ items for each included study. Supplementary Table 1. Impact of covariates on recruitment and retention rates. Supplementary Table 2. Impact of covariates on differential retention rates. Supplementary Figure 2. Bubble plot for the impact of age (a) and proportion of included participants with hypertension (b) on retention rates.
Data Availability Statement
The dataset and statistical script necessary to reproduce the analyses presented in this systematic review has been made available online at the Open Science Framework page of the MOBILIZE project [26].