SUMMARY
Many neurodegenerative diseases are associated with pathological aggregation of proteins in neurons.
Autophagy is a natural self-cannibalization process that can act as a powerful mechanism to remove aged and damaged organelles as well as protein aggregates. It has been shown that promoting autophagy can attenuate or delay neurodegeneration by removing protein aggregates.
In this paper, we will review the role of autophagy in Alzheimer’s disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease (HD) and discuss opportunities and challenges of targeting autophagy as a potential therapeutic avenue for treatment of these common neurodegenerative diseases.
Keywords: autophagy, neurodegenerative disease, Alzheimer’s disease (AD), Parkinson’s Disease (PD), Huntington’s Disease (HD)
Introduction: An overview of autophagy machinery
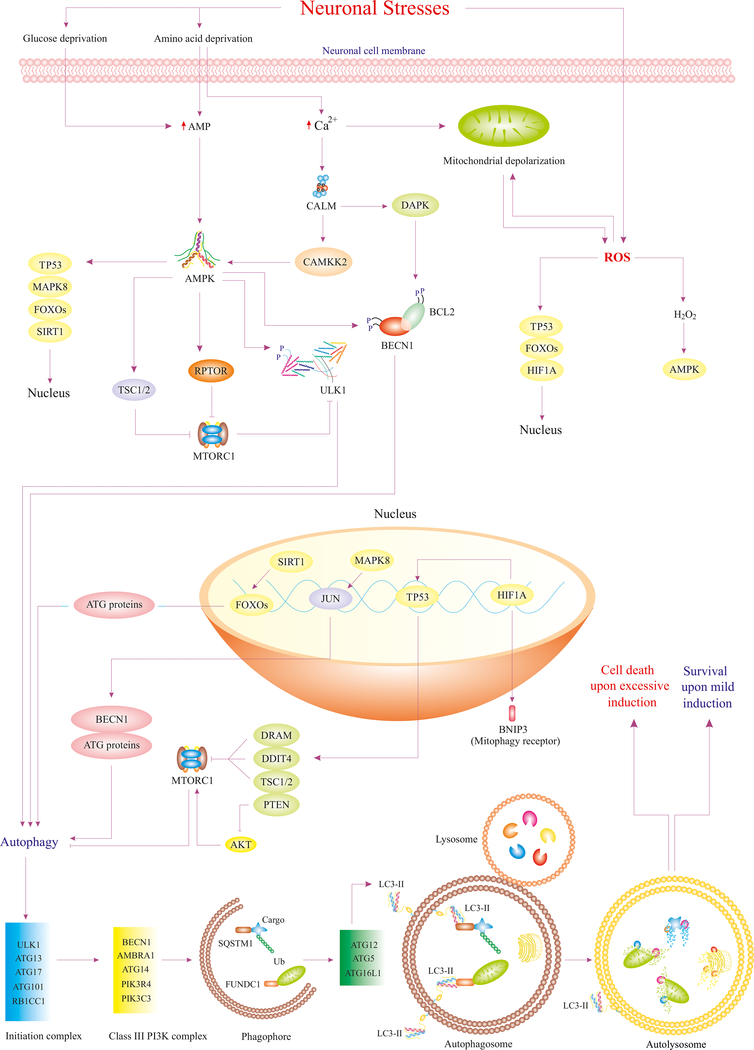
Macroautophagy (hereafter autophagy) is a eukaryotic intracellular process that includes long-lived and damaged organelles, aggregated and misfolded proteins, as well as superfluous cellular components by forming a transitory double-membrane structure; namely, the phagophore.The phagophore hen matures into a double-membrane autophagosome. Mature autophagosomes then fuse with lysosomes to degrade and recycle autophagy products into their building blocks, thus providing energy and substrates for cellular biosynthesis.1–5 Triggers for neuronal autophagy include several possible causative factors including metabolic stress, ischemia and reperfusion (I/R) injury, nutrient deprivation, inflammation, physical trauma, neurotoxins, and neurodegeneration.6–9 Autophagy maintains cellular homeostasis, removes aggregated/misfolded proteins, mitigates endoplasmic reticulum (ER) stress, and restores energy via recycling of glucose, fatty acids, and amino acids.10–12 Therefore, autophagy initially acts as a cytoprotective process in neurons which is referred to as adaptive autophagy (a.k.a. basal or mild autophagy). However, persistent or excessive induction of autophagy may contribute to neuronal cell death, which is referred to as maladaptive autophagy (also called excessive or unchecked autophagy).13–15 Proteins encoded by the family of autophagy-targeted genes (ATG) are hierarchically implicated in initiation, nucleation, and maturation steps from formation of autophagosomes to autolysosomes (Fig. 1).4 Although the ATG protein family is a major constituent of autophagy processes, various upstream signaling pathways and molecules are also present to regulate autophagy.5 For instance, protein kinase AMP-activated catalytic subunit alpha 2 (PRKAA2, also known as AMPK) promotes autophagy by inhibiting mechanistic target of rapamycin kinase complex 1 (MTORC1), resulting in dephosphorylation and activation of the autophagy initiation complex, so-called unc-51 like autophagy activating kinase 1 (ULK1) (Fig. 1).1
Figure 1.
Various neuronal stresses such as nutrient deprivation and ROS trigger diverse pathways that regulate autophagy. Deprivation of amino acids and glucose substantially accelerate cytosolic level of AMP and Ca2+ in neurons. Enhanced AMP level triggers activation of AMPK (master regulator of autophagy), which upregulates ATG genes, blocks MTORC1, and activates ULK1, ultimately leading to autophagy initiation. Increased cytosolic level of Ca2+ also mediates AMPK activation and mitochondrial depolarization, which accounts for ROS production. ROS, in turn, activates certain transcriptional factors and AMPK thus, leading to autophagy. Eventually, ATG proteins are recruited to various steps of phagophore and autophagosome formation, resulting in clearance of protein aggregates and impaired organelles via fusion with lysosome. Although basal autophagy alleviates neurodegeneration, its excessive induction may lead to various styles of cell death 1,8,110,111.
Abbreviations: DAPK (death associated protein kinase), CAMKK2 (calcium/calmodulin dependent protein kinase kinase 2), JUN/C-Jun (Jun proto-oncogene, AP-1 transcription factor subunit), FUNDC1 (FUN14 domain containing 1), Ub (ubiquitin), AMP (adenosine monophosphate), HIF1A/HIF1α (hypoxia inducible factor 1 subunit alpha), RPTOR/raptor (regulatory associated protein of MTOR complex 1), MAPK8/JNK1 (mitogen-activated protein kinase 8), DDIT4 (DNA damage inducible transcript 4), PIK3R4/VPS15 (phosphoinositide-3-kinase regulatory subunit 4), DRAM (DNA damage regulated autophagy modulator), RB1CC1/FIP200 (RB1 inducible coiled-coil 1), BNIP3 (BCL2 interacting protein 3).
Recent, evidence indicated a pivotal role of autophagy in the pathogenesis of cardiovascular, infectious, and neurodegenerative diseases.5,16–19 Therapeutic induction of basal autophagy has been reported to mitigate onset and progression of disease in mouse models of AD, PD, and HD.20,21 This review aims to update the role of autophagy in neurodegenerative diseases and summarize possible novel therapeutic avenues for the treatment of these diseases.
Dementia
Autophagy has been shown to promote neuronal survival by eliminating pathological protein aggregates and maintaining energy balance In addition, it supports neuronal plasticity, which has the potential to prevent the onset and development of neurodegenerative diseases.22 Conversely, dysfunctional autophagy has been shown to result in the accumulation of defective protein aggregates and autophagosomes leading to neurotoxicity, cell death, and neurodegeneration.6 Moreover, long-term induction of autophagy could serve as a pro-death mechanism leading to the exacerbation of neurodegenerative diseases including AD, PD, and HD.13,22
Dementia is considered the greatest global challenge for health and social care in the 21st century.23,24 In contrast to historical views, it is now possible to improve the trajectory of symptoms even if the underlying disease may not be curable. This provides an opportunity for prevention and intervention that transforms future dementia care. The strongest risk factor for dementia including AD is advancing age.25–27 In addition, vascular contributions to cognitive impairment and dementia (VCID) are increasingly recognized as a major risk factor for dementia;28 However the underlying mechanisms and specific links between vascular pathology and dementia are complex and only partially understood.
Adaptive autophagy in AD
AD is the most prevalent neurodegenerative disease characterized by extracellular deposits of β-amyloid (Aβ) peptides and hyper-phosphorylated MAPT/tau protein as neurofibrillary tangles.22,29,30 Yet, microscopic examination of post-mortem AD brains reveals extensive cerebral small vessel disease (CSVD) pathology, including tortuous microvessels with poorly-stained endothelium in affected cortical tissue. The consistent epidemiological association of multiple vascular risk factors with the incidence of AD suggests that CSVD is a plausible causal factor to the pathogenesis and development of AD. CSVD is highly prevalent in the general population with up to 95% of subjects affected at age 65 years and older, particularly if they have vascular risk factors,31. CVSD is also recognized as a major risk factor for dementia.32–35 Together, these observations highlight the role for CSVD as an important pathophysiological link to vascular dementia and AD. Accordingly, mitigating the consequences of cerebrovascular disease is a promising strategy to reduce dementia risk.
In this respect, increasing evidence indicates that autophagy dysfunction such as accumulation of autophagosomes and downregulation of autophagy proteins occurs in AD pathophysiology.36 In AD brains, autophagosomes, and multivesicular and multilamellar bodies, were abundantly found, particularly in synaptic terminals under neuritis, suggesting a role for defective autophagy in the neurodegenerative process of AD.37 In brains from AD patients, beclin 1 (BECN1) ), a core autophagy component, was attenuated.38 In this study, BECN1 knockout in a murine model of AD enhanced intracellular Aβ accumulation, and its extracellular deposition, indicating that BECN1 may possess therapeutic potential to revive autophagy and alleviate AD.38 Genomic studies identified phosphatidylinositol binding clathrin assembly protein (PICALM) involvement in AD pathology through modulation of autophagy and related MAPT/tau removal.39 In line with this finding, zebrafish PICALM transgenic models failed to execute autophagy and MAPT removal.39 Thus, it was concluded that adaptive autophagy dysregulation is a contributing factor to the onset and progression of AD pathology. Furthermore, circulating levels of autophagy marker “ATG5” and mitophagy marker parkin RBR E3 ubiquitin protein ligase (“PRKN”) were downregulated in AD patients compared to healthy controls.40,41 This suggests that autophagy and mitophagy processes are impaired upon AD insult. Recently, microtubule end binding motif of activity dependent neuroprotector homeobox (ADNP) was found to induce autophagy. To this end, ADNP was defined as a novel bridge between autophagy and microtubules, which could be utilized for reinitiating defective autophagy in AD and other brain diseases.42 Taken together, adaptive autophagy is often impaired in AD and designing new strategies to combat defective autophagy is imperative in AD management.
Chaperone-mediated autophagy (CMA) is a type of autophagy that degrades cellular proteins without formation of autophagosomes.43 In CMA, cargo is directly transported into the lysosomes, which is mediated by heat shock protein family A [Hsp70] member 8) (HSC70 ) and lysosomal associated membrane protein 2 (LAMP2) 43 Exposing murine primary hippocampal neurons under treatment of oligomeric Aβ to trehalose and lactulose reduced neuroinflammation and improved autophagy and CMA. This result suggests that trehalose and lactulose could be developed and optimized for the treatment or prevention of AD.44 MAPT fragments, after being degraded by autophagy, can be a substrate of CMA by binding to HSC70 and entering the lysosome.45 However, CMA dysfunction may lead to inefficient translocation of MAPT fragments, which potentiates MAPT aggregation, lysosomal dysfunction, and ultimately AD pathology.45
The tryptophan-aspartic acid (WD) domain of ATG16L1 is of paramount importance for noncanonical autophagy pathways; i.e., alternative autophagy mechanisms that do not involve the full complement of ATG proteins typically needed for autophagosome formation.46 For example, a murine study showed that dysfunction in noncanonical autophagy due to the absence of the WD domain resulted in the Aβ buildup, MAPT/tau hyperphosphorylation, and neurodegeneration associated with an AD-like phenotype. Absence of the WD domain leads to failure of Aβ receptor recycling in microglial cells. Given that inhibition of neuroinflammation ameliorates memory loss and neurodegeneration,47,48 it was concluded that the impairment of noncanonical autophagy due to absence of the WD domain might trigger severe neuroinflammation. This study implies that autophagy is a pro-survival mechanism that could attenuate the onset or progression of AD.49
Similarly, it was reported that branched chain amino acid aminotransferase (BCAT) is upregulated leading to the accumulation of Aβ due to the suppression of autophagy via BCAT-mediated hyperactivation of MTORC1, suggesting that autophagy activation ameliorates AD via eliminating Aβ aggregates.50 Also, berberine has been reported to attenuate the accumulation of MAPT/tau via autophagy activation in a PIK3C3/VPS34-BECN1-dependent manner.51 Similarly, repetitive transcranial magnetic stimulation (rTMS) alleviates AD via promoting autophagy and reducing apolipoprotein E (APOE).52 Furthermore, an in vitro model of AD revealed that β-asarone (derived from Acorus tatarinowii Schott) effectively counters those proteins implicated in AD pathogenesis including amyloid beta precursor protein (APP) ), presenilin-1 (PSEN1/PS-1), beta-secretase 1 (BACE1), and Aβ via potentiating autophagy, which is demonstrated by LC3-II and BECN1 upregulation.53 Moreover, in the SAMP8 mouse AD model, treatment with Yishen Huazhuo decoction enhanced Aβ removal via upregulation of BECN1 and downregulation of MTORC1 and promoted autophagosome formation, resulting in enhanced autophagy.54 In sum, adaptive (mild) autophagy is a cytoprotective mechanism that mitigates AD, whereas dysfunctional autophagy promotes neuronal injury and AD.
Maladaptive autophagy in AD
A growing body of evidence shows that autophagy could serve as a pro-degenerative mechanism in AD. It has been shown that aberrant autophagosomes contain Aβ, which ultimately results in extracellular accumulation of Aβ and ultimately AD, suggesting that abnormal autophagy processes could be an underlying cause of this disease.55 It was postulated that autophagy is altered to facilitate Aβ plaque formation during AD.56 In line with this notion, it was found that AD is associated with increased accumulation of Aβ and derivatives, aberrant autophagosomes and compromised Aβ clearance.57 Conversely, persistent Aβ accumulation results in excessive autophagy induction, which leads to apoptotic or autophagic cell death and ultimately exacerbation of AD.58 Jiang et al, revealed that Aβ aggregates induce the overexpression of NADPH oxidase 2 (NOX4) which enhances ROS generation and results in the hyperactivation of autophagy and neuronal death.58 Estrogen has been reported to alleviate cognitive damage in an animal model of vascular dementia via downregulation of BECN1 and LC3-II (two core autophagy proteins) and upregulating WNT-CTNNB1/β-catenin signaling cascade elements, which abolishes maladaptive autophagy.59 In summary, aberrant autophagy and excessive autophagy induction (maladaptive autophagy) are two major mechanisms in the pathophysiology of AD pathogenesis.
Adaptive autophagy in HD
HD is a neurodegenerative disorder characterized by the ubiquitous presence of mutated huntingtin (mHTT) in the brain. Similar to AD, autophagy activation plays a cardinal role in degrading protein aggregates and alleviating HD symptoms. As a result, autophagy deregulation or dysfunction can contribute to the onset of HD.60 In this regard, ATG7 polymorphism was correlated with the early onset of HD.61 Walter, et al. demonstrated that inducing AMPK activation was a promising approach for HD alleviation due to the reinvigoration of adaptive autophagy in an mTORC1-independent manner.62 In a murine model of HD, BECN1 overexpression in the early disease progression rewired defective autophagy, reduced mHTT aggregates, and alleviated neuronal pathology.63 Moreover, in a mouse model of HD, defective autophagic flux was found to be an essential and early event following mHTT aggregation in neurons.64 Furthermore, Synder and colleagues reported that RASD family member 2 (RASD2) binds mHTT and exacerbates its cytotoxicity.65 These investigators went on to reveal that RASD2 knockdown dampened autophagy, although overexpression of RASD2 enhanced autophagy in PC12 cells.66 The underlying mechanism appears to be that RASD2 binds BECN1 and reduces its interaction with BCL2 apoptosis regulator (BCL2), leading to BECN1-mediated autophagy.66 However, mHTT and its binding with RASD2, compromised RASD2-mediated autophagy and triggered autophagy defects.66 Genomic screening showed that homeodomain interacting protein kinase 3 (HIPK3)() is a negative regulator of autophagy and positive modulator of mHTT both in vitro and in vivo models of HD.67 mHTT also enhanced HIPK3 levels, and thereby, attenuated adaptive autophagy. To this end, inactivation of HIPK3 could be a therapeutic strategy to revitalize defective autophagy upon HD.67
Cuervo et al showed that LAMP2 and HSC70 (two markers of CMA) were significantly upregulated in both cellular and murine model of HD.68 They concluded that due to the adaptive autophagy defect in the early stage of HD, CMA increases as a compensatory mechanism. However, this compensatory CMA declines with age.68 Furthermore, some studies have shown that mHTT aggregates can be degraded by CMA; thus, modulating CMA pathways and components could be a therapeutic approach for the treatment of HD.69
It was found that overexpression of transcription factor EB (TFEB), a regulator of autophagy genes, did not significantly influence removal of mHTT aggregates. However, Beclin-1 overexpression in the early stage of the disease provoked autophagic removal of mHTT aggregates in a murine model of HD. Based on this study, activating Beclin-1-mediated autophagy in the early stage of the disease imparts neuroprotection within HD.63 Also, protective effects of rutin flavonoid found in apples, tea, and buckwheat against HD was attributed to mild autophagy induction, attenuation of oxidative stress, and the induction of insulin/insulin-like growth factor 1 (IGF1) signaling in a Caenorhabditis elegans HD model. This implies that maintaining a basic level of autophagy in neurons along with antioxidants could be implicated in the management of HD.70 Moreover, it was revealed that upregulation of glutamine synthetase 1 (GS1) inhibited activation and phosphorylation of mTORC1 and ribosomal protein S6 kinase B1 (S6K), respectively, thereby mitigating neuronal motility defects caused by mHTT aggregates.71 WD repeat and FYVE domain containing 3 (Wdfy3) is an autophagy adaptor protein (interacts with LC3II) playing a role in the brain clearance of protein aggregates. Depletion of Wdfy3 was reported to exacerbate HD pathogenesis and accelerated mHTT aggregates, indicating the cytoprotective role of autophagy and its adaptors in the alleviation of HD.72 Overexpression of cytochrome P450 family 46 subfamily a member 1 (CYP46A1), a cardinal enzyme in brain cholesterol metabolism, attenuated the size and quantity of mHTT aggregates in a neuroblastoma cell HD model which was linked to mild autophagy induction.73 Huntingtin (HTT) plays various roles in mitophagy, selective autophagy of mitochondria, such as promoting mitophagy initiation and the recruitment of mitophagy receptors. Franco-Iborrait et al reported that mHTT impaired the formation of mitophagy protein complexes leading to mitophagy defects, oxidative stress, and neurodegeneration in HD. This suggests that HD impairs selective autophagy which results in mitochondrial dysfunction and disease progression. Thus, restoring selective autophagy mechanisms such as mitophagy might alleviate HD.74
Maladaptive autophagy in HD
Similar to AD, excessive autophagy induction may lead to neuronal cell death. Sharma et al reported that upon HD onset and progression, mHTT induced DNA damage which upregulated a DNA sensor, namely, cGMP-AMP synthase (cGAS) leading to the inflammation and exacerbation of HD. Furthermore, they found that cGAS mediated excessive autophagy induction via promoting cGAS/stimulator of interferon response CGAMP interactor 1 (STING) pathway and enhancing the formation of LC3II and autophagosomes which contributed to the progression of HD.75 Overall, autophagy induction at the basic level is a cytoprotective mechanism, which prevents the onset or progression of HD; however, unrestrained autophagy induction might adversely affect HD progression and recovery.
Adaptive autophagy in PD
PD is a neurodegenerative disorder caused by dopaminergic neurons loss in the brain due to the aggregation of aberrant α-synuclein. Mutated α-synuclein triggers autophagy dysfunction, which relates to PD development and progression.76 Microglial ATG5 knockout in mice rendered PD-like phenotypes such as impaired cognitive recognition or motor coordination.77 The mechanism was that autophagy inhibition activated NLR family pyrin domain containing 3 (NLRP3) () through phosphodiesterase 10A (PDE10A) )-cAMP axis, which ultimately led to pro-inflammatory cytokines such as macrophage migration inhibitory factor (MIF) ().77 Therefore, activating defective autophagy could be a clinical therapy for the management of PD patients. PTEN induced kinase 1 (PINK1) (PTEN induced kinase 1) is a serine-threonine kinase with role in mitophagy.78 Upon PINK1 deficiency, excessive ROS production, deregulation of calcium homeostasis and electron transport chain can induce alterations in mitochondrial quality and quantity. Therefore, modulation of PINK1 may confer protection against different forms of PD via re-initiation of defective mitophagy.78 Overall, defective mitophagy and autophagy exacerbates PD pathogenesis and progression.
CMA activation contributes to the clearance of α-synuclein. Whereas, its depletion contributes to aggresome and oligomer formation in the pathology of PD.79 Kabuta and associates found that ubiquitin C-terminal hydrolase L1 (UCHL1) () abnormally interacted with LAMP2, heat shock protein family A [Hsp70] member 8) (HSPA8) (heat shock protein 90 alpha family class B member 1 (HSP90AB1) (), and thereby, impaired CMA.80 Therefore aberrant interactions between UCHL1 and CMA components can contribute to PD pathogenesis. Thus, targeting the inhibition of UCHL1 may alleviate defective CMA in PD.80
Ample evidence indicates that strategies to boost neuronal autophagy flux (i.e., lysosomal degradation capacity of autophagolysosomes) may attenuate PD-associated pathogenesis. For example, in a murine PD model, autophagy was activated via mucolipin 1/Ca2+/calcineurin/TFEB signaling cascade leading to the alleviation of PD and reduced cell death. TFEB overexpression imparted similar effects, suggesting that TFEB-mediated autophagy acts as a pro-survival mechanism in PD.81 Melibiose and lactulose are trehalose analogs, which improved motor deficits and upregulated LC3II and anti-oxidant genes including nuclear factor erythroid 2-related factor 2 (Nrf-2), superoxide dismutase 2 (SOD2), and NAD(P)H dehydrogenase (NQO1) in a murine PD model, indicating that anti-PD effects of melibiose and lactulose are attributed to autophagy activation.82 Similarly, the neuroendocrine peptide, apelin-36, had neuroprotective effects and eliminated α-synuclein aggregates via upregulation of Beclin-1, LC3II, and anti-oxidant genes such as glutathione (GSH) and SOD and the suppression of apoptosis signal-regulating kinase 1 (ASK1)/ C-Jun N-terminal kinase 1 (JNK1)/caspase-3 signaling cascade in a murine PD model.This further indicates the pivotal role of autophagy in the degradation of α-synuclein and alleviation of PD.83 Also, the anti-PD function of extracts of Eucommia ulmoides Oliver leaves was shown to evoke autophagy induction, which rescued the loss of neural vasculature and dopaminergic neurons, blocked apoptosis, and mediated α-synuclein elimination in zebrafish PD model.84 Moreover, exosomes secreted from human umbilical cord mesenchymal stem cells (hucMSCs) averted apoptotic cell death and dopaminergic neuron loss by enhancing autophagy in the SH-SY5Y cells PD model.85
Maladaptive autophagy in PD
It was revealed that brain-derived neurotrophic factor anti-sense (BDNF-AS), a long non-coding RNA, was upregulated in the murine model of PD leading to the suppression of excessive autophagy induction and apoptosis via targeting microRNA (miR)-125b-5p.86 Therefore, it can be interpreted that unnecessary autophagy induction might adversely affect PD progression. Overall, mild autophagy induction is a potential strategy to reverse PD progression, while over-activation of autophagy might pose destructive effects in neurons upon PD.
Targeting autophagy for the management of neurodegenerative diseases
A growing body of evidence suggests that pathology of neurodegenerative diseases may be attributed to abnormal protein aggregates which also serve as substrates of autophagy. Therefore, therapeutic activators of autophagy may alleviate these diseases in both in vivo and in vitro models.87 However, when autophagy is defective, therapeutic inducers of autophagy may not enhance the removal of protein aggregates and in some cases may lead to autophagosome accumulation, which would exacerbate disease pathology. Understanding pathways downstream and upstream from autophagy might help identify novel therapeutic targets. Rapamycin and temsirolimus (a soluble analogue of rapamycin) are the leading autophagy inducers in neurodegenerative diseases and function by inhibiting MTORC1.88–90 Inhibition of MTORC1 may cause adverse effects in patients such as impaired wound healing and immunosuppression due to non-autophagic roles of MTORC1.91,92 However, until recently several autophagy inducers independent of MTORC1 were screened for possible clinical utility. For instance, lithium induces autophagy by inhibition of inositol monophosphatase 1 (IMPA1) and depletion of inositol-3-phosphate [Ins(1,4,5)P3], representing a possible candidate for HD treatment.93 Similarly, carbamazepine mediates autophagy by reducing Ins(1,4,5)P3 through inhibition of inositol synthesis thus, exhibiting anti-AD effects in mouse models.94 Although psychotropics such as lithium and carbamazepine are used in the clinical management of the psychiatric aspects of HD, proof of a neuroprotective effect in human HD is lacking. Trehalose and disaccharide trehalose also act independent of MTORC1 and induce autophagy via un-identified mechanisms, and exert neuroprotection in murine amyotrophic lateral sclerosis (ALS) and tauopathy.95–97
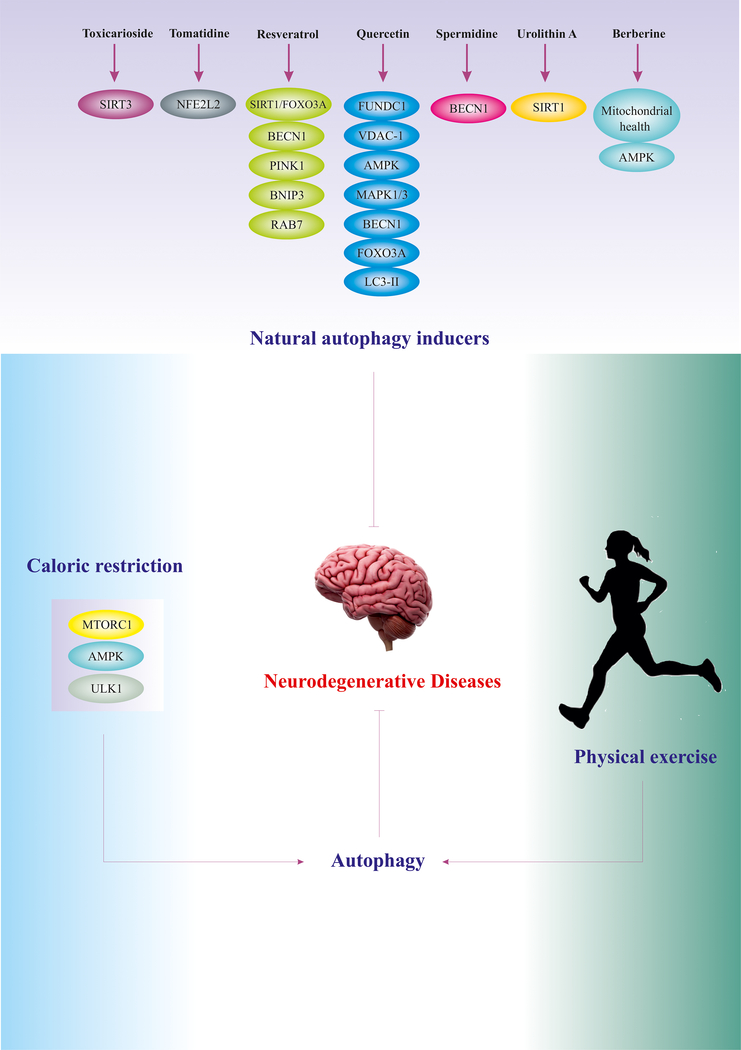
Besides, futures studies could be supported by the use of nanomedicine and drug delivery systems for specific and optimized targeting of autophagy in neurodegenerative diseases.98,99 Table 1 lists both natural and pharmaceutical modulators of autophagy in the context of neurodegenerative diseases. Figure 2 summarizes holistic strategies including physical exercise and caloric restriction, as well as natural therapeutics for the management of neurodegenerative diseases via autophagy regulation.
Table 1.
Therapeutic modulators of autophagy.
| Modulators | Name/Source | Function | Ref |
|---|---|---|---|
| A769662 | Direct AMPK activator | Induces AMPK-mediated autophagy and clearance of α-synuclein in neurons | 100 |
| AUTEN-67 | Autophagy enhancer-67 | Induces autophagy by modulating MTORC1 in AD | 101 |
| CysC | Cystatin C | Induces autophagy by manipulating AMPK-MTORC1 axis and inhibits aggregation of Aβ | 102 |
| Digoxin | Natural compound | Induces autophagy in a TFEB-dependent fashion | 103 |
| GSK621 | Specific AMPK agonist | Induces AMPK-mediated autophagy and clearance of α-synuclein in neurons | 100 |
| Latrepirdine | Pharmaceutical compound | Induces autophagy in MTORC1-dependent manner, ameliorates yeast model of AD, and upregulates ATG5 | 104,105 |
| Metformin | N,N-dimethylbiguanide | Exerts neuroprotection and reduces cognitive decline via AMPK-mediated autophagy | 106 |
| MSL | Synthetic autophagy upregulator | Activates calcineurin, leading to dephosphorylation of TFEB that translocates to nucleus and upregulate ATG genes, and blocks neurodegeneration inflammation | 107 |
| Resveratrol | Found in peanuts, red grapes, and blueberries | Enhances degradation of α-synuclein in in vivo models of PD via inducing AMPK-SIRT1-mediated autophagy | 108 |
| RSVA314 and RSVA405 | Synthetic molecules | Activate CAMKK2-AMPK axis, leading to autophagy induction and Aβ clearance | 109 |
Abbreviations: TFEB (transcription factor EB), SIRT1 (sirtuin 1)
Figure 2.
Holistic strategies for targeting autophagy in the management of neurodegenerative diseases. Natural autophagy inducers manipulate autophagy regulators via various mechanisms, leading to basal autophagy induction (likely, in a dose dependent manner). Numerous studies have substantiated the vital role of physical exercise and caloric restriction as non-invasive strategies for autophagy induction 2,112–115.
Abbreviations: NFE2L2/NRF2 (nuclear factor, erythroid 2 like 2), FOXO3 (forkhead box O3), SIRT3 (sirtuin 3), RAB7 (RAB7, member RAS oncogene family), VDAC1 (voltage dependent anion channel 1), MAPK1/3 (mitogen-activated protein kinase 1/3), MAP1LC3B/LC3B (microtubule associated protein 1 light chain 3 beta)
Concluding remarks
Evidence to date suggests that autophagy defects play a pivotal role in the pathogenesis of neurodegenerative diseases. A better understanding of autophagy dysregulation in the context of neurodegenerative diseases promises to provide novel therapeutic targets. Specifically, balancing basal autophagy induction while avoiding excessive induction of autophagy is important to improve neuronal survival.
Footnotes
Conflict of interest: None of the authors had any conflict of interest to declare.
Data sharing and data accessibility: No original data were used for this work that would need data repository, although the corresponding authors will respond to any reasonable request for this publication.
References
- 1.Ajoolabady A, Aghanejad A, Bi Y, et al. Enzyme-based autophagy in anti-neoplastic management: From molecular mechanisms to clinical therapeutics. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2020:188366. [DOI] [PubMed] [Google Scholar]
- 2.Ajoolabady A, Aslkhodapasandhokmabad H, Aghanejad A, Zhang Y, Ren J. Mitophagy Receptors and Mediators: Therapeutic Targets in the Management of Cardiovascular Ageing. Ageing Research Reviews. 2020:101129. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Ren J. Role of autophagy and regulatory mechanisms in alcoholic cardiomyopathy. Biochim Biophys Acta Mol Basis Dis 2018;1864(6 Pt A):2003–2009. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Sowers JR, Ren J. Targeting autophagy in obesity: from pathophysiology to management. Nature Reviews Endocrinology. 2018;14(6):356–376. [DOI] [PubMed] [Google Scholar]
- 5.Ren J, Zhang Y. Targeting autophagy in aging and aging-related cardiovascular diseases. Trends in pharmacological sciences. 2018;39(12):1064–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corti O, Blomgren K, Poletti A, Beart PM. Autophagy in neurodegeneration: New insights underpinning therapy for neurological diseases. Journal of Neurochemistry. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Giorgi C, Bouhamida E, Danese A, Previati M, Pinton P, Patergnani S. Relevance of Autophagy and Mitophagy Dynamics and Markers in Neurodegenerative Diseases. Biomedicines. 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghislat G, Knecht E. Regulation of Autophagy by Amino Acid Starvation Involving Ca2+. In: Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Elsevier; 2015:69–79. [Google Scholar]
- 9.Ren J, Sowers JR, Zhang Y. Metabolic stress, autophagy, and cardiovascular aging: from pathophysiology to therapeutics. Trends in Endocrinology & Metabolism. 2018;29(10):699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peker N, Gozuacik D. Autophagy as a cellular stress response mechanism in the nervous system. Journal of molecular biology. 2020;432(8):2560–2588. [DOI] [PubMed] [Google Scholar]
- 11.Ajoolabady A, Wang S, Kroemer G, et al. ER stress in Cardiometabolic Diseases: from Molecular Mechanisms to Therapeutics. Endocrine Reviews. 2021. [DOI] [PubMed] [Google Scholar]
- 12.Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nature Reviews Cardiology. 2021:1–23. [DOI] [PubMed] [Google Scholar]
- 13.Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS neuroscience & therapeutics. 2012;18(3):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galluzzi L, Vitale I, Abrams J, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death & Differentiation. 2012;19(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Whaley-Connell AT, Sowers JR, Ren J. Autophagy as an emerging target in cardiorenal metabolic disease: From pathophysiology to management. Pharmacology & therapeutics. 2018;191:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J-G, Chu J, Praticò D. Downregulation of autophagy by 12/15Lipoxygenase worsens the phenotype of an Alzheimer’s disease mouse model with plaques, tangles, and memory impairments. Molecular Psychiatry. 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 17.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nature Reviews Immunology. 2013;13(10):722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu NN, Tian H, Chen P, Wang D, Ren J, Zhang Y. Physical exercise and selective autophagy: benefit and risk on cardiovascular health. Cells. 2019;8(11):1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic research in cardiology. 2011;106(6):1173–1191. [DOI] [PubMed] [Google Scholar]
- 20.Nixon RA. The role of autophagy in neurodegenerative disease. Nature medicine. 2013;19(8):983–997. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Wang L, Qin X, et al. ALDH2 contributes to melatonin-induced protection against APP/PS1 mutation-prompted cardiac anomalies through cGAS-STING-TBK1-mediated regulation of mitophagy. Signal Transduct Target Ther 2020;5(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Yosef T, Damri O, Agam G. Dual role of autophagy in diseases of the central nervous system. Frontiers in cellular neuroscience. 2019;13:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Li C, Zhang Y, Ren J. Interrelationship between Alzheimer’s disease and cardiac dysfunction: the brain-heart continuum? Acta Biochim Biophys Sin (Shanghai). 2020;52(1):1–8. [DOI] [PubMed] [Google Scholar]
- 25.Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67(8):1340–1352. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimers Dement 2008;4(5):316–323. [DOI] [PubMed] [Google Scholar]
- 27.Seshadri S, Drachman DA, Lippa CF. Apolipoprotein E epsilon 4 allele and the lifetime risk of Alzheimer’s disease. What physicians know, and what they should know. Arch Neurol 1995;52(11):1074–1079. [DOI] [PubMed] [Google Scholar]
- 28.Corraini P, Henderson VW, Ording AG, Pedersen L, Horváth-Puhó E, Sørensen HT. Long-term risk of dementia among survivors of ischemic or hemorrhagic stroke. Stroke. 2017;48(1):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebadi M, Brown-Borg H, Ren J, et al. Therapeutic efficacy of selegiline in neurodegenerative disorders and neurological diseases. Curr Drug Targets. 2006;7(11):1513–1529. [DOI] [PubMed] [Google Scholar]
- 30.Dhanasekaran M, Ren J. The emerging role of coenzyme Q-10 in aging, neurodegeneration, cardiovascular disease, cancer and diabetes mellitus. Curr Neurovasc Res. 2005;2(5):447–459. [DOI] [PubMed] [Google Scholar]
- 31.Longstreth WT Jr., Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. [DOI] [PubMed] [Google Scholar]
- 32.Corriveau RA, Bosetti F, Emr M, et al. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol 2016;36(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Flier WM, van Straaten EC, Barkhof F, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36(10):2116–2120. [DOI] [PubMed] [Google Scholar]
- 34.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–1252. [DOI] [PubMed] [Google Scholar]
- 36.Di Meco A, Curtis ME, Lauretti E, Praticò D. Autophagy dysfunction in Alzheimer’s disease: mechanistic insights and new therapeutic opportunities. Biological psychiatry. 2020;87(9):797–807. [DOI] [PubMed] [Google Scholar]
- 37.Nixon RA, Wegiel J, Kumar A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. Journal of Neuropathology & Experimental Neurology. 2005;64(2):113–122. [DOI] [PubMed] [Google Scholar]
- 38.Pickford F, Masliah E, Britschgi M, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. The Journal of clinical investigation. 2008;118(6):2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau K, Fleming A, Imarisio S, et al. PICALM modulates autophagy activity and tau accumulation. Nature communications. 2014;5(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellazzi M, Patergnani S, Donadio M, et al. Autophagy and mitophagy biomarkers are reduced in sera of patients with Alzheimer’s disease and mild cognitive impairment. Scientific reports. 2019;9(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren J, Sun M, Zhou H, et al. FUNDC1 interacts with FBXL2 to govern mitochondrial integrity and cardiac function through an IP3R3-dependent manner in obesity. Science advances. 2020;6(38):eabc8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sragovich S, Merenlender‐Wagner A, Gozes I. ADNP plays a key role in autophagy: from autism to schizophrenia and Alzheimer’s disease. Bioessays. 2017;39(11):1700054. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Navarro S, Hurt E. Linking gene regulation to mRNA production and export. Current opinion in cell biology. 2011;23(3):302–309. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y-S, Lai D-M, Huang H-J, et al. Prebiotic Lactulose Ameliorates the Cognitive Deficit in Alzheimer’s Disease Mouse Model through Macroautophagy and Chaperone-Mediated Autophagy Pathways. Journal of Agricultural and Food Chemistry. 2021. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Martinez-Vicente M, Krüger U, et al. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Human molecular genetics. 2009;18(21):4153–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nature reviews Molecular cell biology. 2012;13(1):7–12. [DOI] [PubMed] [Google Scholar]
- 47.Jha NK, Sharma A, Jha SK, et al. Alzheimer’s disease-like perturbations in HIV-mediated neuronal dysfunctions: understanding mechanisms and developing therapeutic strategies. Open Biol 2020;10(12):200286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das BC, Dasgupta S, Ray SK. Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer’s disease. Neural Regen Res 2019;14(11):1880–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heckmann BL, Teubner BJ, Boada-Romero E, et al. Noncanonical function of an autophagy protein prevents spontaneous Alzheimer’s disease. Science advances. 2020;6(33):eabb9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris M, El Hindy M, Usmari-Moraes M, et al. BCAT-induced autophagy regulates Aβ load through an interdependence of redox state and PKC phosphorylation-implications in Alzheimer’s disease. Free Radical Biology and Medicine. 2020. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Chen Y, Liang Y, Chen H, Ji X, Huang M. Berberine mitigates cognitive decline in an alzheimer’s disease mouse model by targeting both tau hyperphosphorylation and autophagic clearance. Biomedicine & Pharmacotherapy. 2020;121:109670. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Dong GY, Wang LX. High‐frequency transcranial magnetic stimulation protects APP/PS1 mice against Alzheimer’s disease progress by reducing APOE and enhancing autophagy. Brain and Behavior 2020:e01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang N, Wang H, Li L, Li Y, Zhang R. β-Asarone Inhibits Amyloid-β by Promoting Autophagy in a Cell Model of Alzheimer’s Disease. Frontiers in pharmacology. 2020;10:1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K, Sun W, Xu J, et al. Yishen Huazhuo Decoction induces autophagy to promote the clearance of Aβ1–42 in SAMP8 mice: mechanism research of a traditional Chinese formula against Alzheimer’s disease. CNS & Neurological Disorders Drug Targets. 2020. [DOI] [PubMed] [Google Scholar]
- 55.Ling D, Magallanes M, Salvaterra PM. Accumulation of amyloid-like Aβ1–42 in AEL (autophagy–endosomal–lysosomal) vesicles: potential implications for plaque biogenesis. ASN neuro 2014;6(2):AN20130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geng P, Zhang J, Dai W, et al. Autophagic degradation deficit involved in sevoflurane-induced amyloid pathology and spatial learning impairment in APP/PS1 transgenic mice. Frontiers in Cellular Neuroscience. 2018;12:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen J, Fang F, Guo S-H, et al. Amyloid β-derived diffusible ligands (ADDLs) induce abnormal autophagy associated with Aβ aggregation degree. Journal of Molecular Neuroscience. 2018;64(2):162–174. [DOI] [PubMed] [Google Scholar]
- 58.Jiang S, Zhao Y, Zhang T, et al. Galantamine inhibits β‐amyloid‐induced cytostatic autophagy in PC 12 cells through decreasing ROS production. Cell Proliferation. 2018;51(3):e12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Zhao L, Li N, et al. Estrogen Exerts Neuroprotective Effects in Vascular Dementia Rats by Suppressing Autophagy and Activating the Wnt/β-Catenin Signaling Pathway. Neurochemical Research. 2020;45(9):2100–2112. [DOI] [PubMed] [Google Scholar]
- 60.Tan C-C, Yu J-T, Tan M-S, Jiang T, Zhu X-C, Tan L. Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiology of aging. 2014;35(5):941–957. [DOI] [PubMed] [Google Scholar]
- 61.Metzger S, Saukko M, Van Che H, et al. Age at onset in Huntington’s disease is modified by the autophagy pathway: implication of the V471A polymorphism in Atg7. Human genetics. 2010;128(4):453–459. [DOI] [PubMed] [Google Scholar]
- 62.Walter C, Clemens LE, Müller AJ, et al. Activation of AMPK-induced autophagy ameliorates Huntington disease pathology in vitro. Neuropharmacology. 2016;108:24–38. [DOI] [PubMed] [Google Scholar]
- 63.Brattås PL, Hersbach BA, Madsen S, Petri R, Jakobsson J, Pircs K. Impact of differential and time-dependent autophagy activation on therapeutic efficacy in a model of Huntington disease. Autophagy. 2020:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heng MY, Detloff PJ, Paulson HL, Albin RL. Early alterations of autophagy in Huntington disease-like mice. Autophagy. 2010;6(8):1206–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324(5932):1327–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mealer RG, Murray AJ, Shahani N, Subramaniam S, Snyder SH. Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. Journal of Biological Chemistry. 2014;289(6):3547–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu Y, Sun X, Lu B. HIPK3 modulates autophagy and HTT protein levels in neuronal and mouse models of Huntington disease. Autophagy. 2018;14(1):169–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koga H, Martinez-Vicente M, Arias E, Kaushik S, Sulzer D, Cuervo AM. Constitutive upregulation of chaperone-mediated autophagy in Huntington’s disease. Journal of Neuroscience. 2011;31(50):18492–18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bauer PO, Goswami A, Wong HK, et al. Harnessing chaperone-mediated autophagy for the selective degradation of mutant huntingtin protein. Nature biotechnology. 2010;28(3):256–263. [DOI] [PubMed] [Google Scholar]
- 70.Cordeiro LM, Machado ML, da Silva AF, et al. Rutin protects Huntington’s disease through the insulin/IGF1 (IIS) signaling pathway and autophagy activity: Study in Caenorhabditis elegans model. Food and Chemical Toxicology. 2020:111323. [DOI] [PubMed] [Google Scholar]
- 71.Vernizzi L, Paiardi C, Licata G, et al. Glutamine Synthetase 1 Increases Autophagy Lysosomal Degradation of Mutant Huntingtin Aggregates in Neurons, Ameliorating Motility in a Drosophila Model for Huntington’s Disease. Cells. 2020;9(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fox LM, Kim K, Johnson CW, et al. Huntington’s disease pathogenesis is modified in vivo by Alfy/Wdfy3 and selective macroautophagy. Neuron. 2020;105(5):813–821. e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nóbrega C, Conceição A, Costa RG, et al. The cholesterol 24-hydroxylase activates autophagy and decreases mutant huntingtin build-up in a neuroblastoma culture model of Huntington’s disease. BMC Research Notes. 2020;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franco-Iborra S, Plaza-Zabala A, Montpeyo M, Sebastian D, Vila M, Martinez-Vicente M. Mutant HTT (huntingtin) impairs mitophagy in a cellular model of Huntington disease. Autophagy. 2020:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma M, Rajendrarao S, Shahani N, Ramírez-Jarquín UN, Subramaniam S. Cyclic GMP-AMP synthase promotes the inflammatory and autophagy responses in Huntington disease. Proceedings of the National Academy of Sciences. 2020;117(27):15989–15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Senkevich K, Gan-Or Z. Autophagy lysosomal pathway dysfunction in Parkinson’s disease; evidence from human genetics. Parkinsonism & related disorders. 2020;73:60–71. [DOI] [PubMed] [Google Scholar]
- 77.Cheng J, Liao Y, Dong Y, et al. Microglial autophagy defect causes parkinson disease-like symptoms by accelerating inflammasome activation in mice. Autophagy. 2020;16(12):2193–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chu CT. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Human molecular genetics. 2010;19(R1):R28–R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Archives of neurology. 2010;67(12):1464–1472. [DOI] [PubMed] [Google Scholar]
- 80.Kabuta T, Furuta A, Aoki S, Furuta K, Wada K. Aberrant interaction between Parkinson disease-associated mutant UCH-L1 and the lysosomal receptor for chaperone-mediated autophagy. Journal of Biological Chemistry. 2008;283(35):23731–23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhuang X-X, Wang S-F, Tan Y, et al. Pharmacological enhancement of TFEB-mediated autophagy alleviated neuronal death in oxidative stress-induced Parkinson’s disease models. Cell death & disease. 2020;11(2):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin C-H, Wei P-C, Chen C-M, et al. Lactulose and Melibiose Attenuate MPTP-Induced Parkinson’s Disease in Mice by Inhibition of Oxidative Stress, Reduction of Neuroinflammation and Up-Regulation of Autophagy. Frontiers in Aging Neuroscience. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu J, Gao W, Shan X, et al. Apelin-36 mediates neuroprotective effects by regulating oxidative stress, autophagy and apoptosis in MPTP-induced Parkinson’s disease model mice. Brain research. 2020;1726:146493. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S, Yu Z, Xia J, et al. Anti-Parkinson’s disease activity of phenolic acids from Eucommia ulmoides Oliver leaf extracts and their autophagy activation mechanism. Food & Function. 2020;11(2):1425–1440. [DOI] [PubMed] [Google Scholar]
- 85.Chen H-X, Liang F-C, Gu P, et al. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death & Disease. 2020;11(4):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fan Y, Zhao X, Lu K, Cheng G. LncRNA BDNF-AS promotes autophagy and apoptosis in MPTP-induced Parkinson’s disease via ablating microRNA-125b-5p. Brain Research Bulletin. 2020;157:119–127. [DOI] [PubMed] [Google Scholar]
- 87.Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nature reviews neurology. 2012;8(2):108–117. [DOI] [PubMed] [Google Scholar]
- 88.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PloS one. 2010;5(4):e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang T, Yu J-T, Zhu X-C, et al. Temsirolimus promotes autophagic clearance of amyloid-β and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacological research. 2014;81:54–63. [DOI] [PubMed] [Google Scholar]
- 90.Hashemi V, Farhadi S, Chaleshtari MG, et al. Nanomedicine for improvement of dendritic cell-based cancer immunotherapy. International immunopharmacology. 2020;83:106446. [DOI] [PubMed] [Google Scholar]
- 91.Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nature Reviews Neuroscience. 2015;16(6):345–357. [DOI] [PubMed] [Google Scholar]
- 92.Rostami N, Nikkhoo A, Ajjoolabady A, et al. S1PR1 as a novel promising therapeutic target in cancer therapy. Molecular diagnosis & therapy. 2019;23(4):467–487. [DOI] [PubMed] [Google Scholar]
- 93.Sarkar S, Floto RA, Berger Z, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. The Journal of cell biology. 2005;170(7):1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li L, Zhang S, Zhang X, et al. Autophagy enhancer carbamazepine alleviates memory deficits and cerebral amyloid-β pathology in a mouse model of Alzheimer’s disease. Current Alzheimer Research. 2013;10(4):433–441. [DOI] [PubMed] [Google Scholar]
- 95.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. Journal of Biological Chemistry. 2007;282(8):5641–5652. [DOI] [PubMed] [Google Scholar]
- 96.Rodríguez-Navarro JA, Rodríguez L, Casarejos MJ, et al. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiology of disease. 2010;39(3):423–438. [DOI] [PubMed] [Google Scholar]
- 97.Castillo K, Nassif M, Valenzuela V, et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2013;9(9):1308–1320. [DOI] [PubMed] [Google Scholar]
- 98.Alizadeh L, Zarebkohan A, Salehi R, Ajjoolabady A, Rahmati-Yamchi M. Chitosan-based nanotherapeutics for ovarian cancer treatment. Journal of drug targeting. 2019;27(8):839–852. [DOI] [PubMed] [Google Scholar]
- 99.Khiavi MA, Safary A, Barar J, Ajoolabady A, Somi MH, Omidi Y. Multifunctional nanomedicines for targeting epidermal growth factor receptor in colorectal cancer. Cellular and Molecular Life Sciences. 2020;77(6):997–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao J, Perera G, Bhadbhade M, Halliday GM, Dzamko N. Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells. Journal of Biological Chemistry. 2019;294(39):14241–14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang C-C, Wang C, Peng X, Gan B, Guan J-L. Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. Journal of Biological Chemistry. 2010;285(5):3499–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mathews PM, Levy E. Cystatin C in aging and in Alzheimer’s disease. Ageing research reviews. 2016;32:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang C, Niederstrasser H, Douglas PM, et al. Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nature communications. 2017;8(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bharadwaj PR, Verdile G, Barr RK, et al. Latrepirdine (Dimebon™) Enhances Autophagy and Reduces Intracellular GFP-Aβ 42 Levels in Yeast. Journal of Alzheimer’s Disease. 2012;32(4):949–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bharadwaj P, Bates K, Porter T, et al. Latrepirdine: molecular mechanisms underlying potential therapeutic roles in Alzheimer’s and other neurodegenerative diseases. Translational psychiatry. 2013;3(12):e332–e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang T, Yu JT, Zhu XC, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre‐activation of AMPK‐dependent autophagy. British journal of pharmacology. 2014;171(13):3146–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lim H, Lim Y-M, Kim KH, et al. A novel autophagy enhancer as a therapeutic agent against metabolic syndrome and diabetes. Nature communications. 2018;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Y, Li X, Zhu JX, et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19(3):163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambsud P. Novel synthetic small‐molecule activators of AMPK as enhancers of autophagy and amyloid‐β peptide degradation. The FASEB Journal. 2011;25(1):219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simon H-U, Friis R, Tait SW, Ryan KM. Retrograde signaling from autophagy modulates stress responses. Science signaling. 2017;10(468). [DOI] [PubMed] [Google Scholar]
- 111.Mrakovcic M, Fröhlich LF. p53-mediated molecular control of autophagy in tumor cells. Biomolecules. 2018;8(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferreira-Marques M, Aveleira CA, Carmo-Silva S, Botelho M, de Almeida LP, Cavadas C. Caloric restriction stimulates autophagy in rat cortical neurons through neuropeptide Y and ghrelin receptors activation. Aging (Albany NY). 2016;8(7):1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh S, Singh AK, Garg G, Rizvi SI. Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life sciences. 2018;193:171–179. [DOI] [PubMed] [Google Scholar]
- 114.Jeong JH, Yu KS, Bak DH, et al. Intermittent fasting is neuroprotective in focal cerebral ischemia by minimizing autophagic flux disturbance and inhibiting apoptosis. Experimental and therapeutic medicine. 2016;12(5):3021–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Almeida MF, Silva CM, Chaves RS, et al. Effects of mild running on substantia nigra during early neurodegeneration. Journal of sports sciences. 2018;36(12):1363–1370. [DOI] [PubMed] [Google Scholar]