Abstract
Although core decompression (CD) is often performed in the early stage of osteonecrosis of the femoral head (ONFH), the procedure does not always prevent subsequent deterioration and the effects of CD are not fully clarified. The aim of this study is to evaluate the efficacy of CD for steroid-associated ONFH in rabbits. Twelve male and 12 female New Zealand rabbits were injected intramuscularly 20 mg/kg of methylprednisolone once and were divided into the disease control and CD groups. In the disease control group, rabbits had no treatment and were euthanized at 12 weeks postinjection. In the CD group, rabbits underwent left femoral CD at 4 weeks postinjection and were euthanized 8 weeks postoperatively. The left femurs were collected to perform morphological, biomechanical, and histological analysis. Bone mineral density and bone volume fraction in the femoral head in the CD group were significantly higher than in the disease control group. However, no difference in the mechanical strength was observed between the two groups. Histological analysis showed that alkaline phosphatase and CD31 positive cells significantly increased in the males after CD treatment. The number of empty lacunae in the surrounding trabecular bone was significantly higher in the CD group. The current study indicated that CD improved the morphological properties, but did not improve the mechanical strength in the femoral head at early-stage ONFH. These data suggest the need for additional biological, mechanical strategies, and therapeutic windows to improve the outcome of early-stage steroid-associated ONFH.
Keywords: core decompression, gender difference, mechanical testing, micro-CT, osteonecrosis of the femoral head, rabbits
1 |. INTRODUCTION
Osteonecrosis of the femoral head (ONFH) is a disease that commonly affects young adults, 30–40 years of age, and has the potential for collapse of the femoral head and subsequent osteoarthritis of the hip. Although the pathogenesis of ONFH has not been completely clarified, it was well established that corticosteroids, alcohol, and trauma are associated with ONFH.1 In the United States, 10,000–20,000 new patients with ONFH are diagnosed every year2; 5%–18% of total hip arthroplasties in the USA each year are performed for ONFH.3
Core decompression (CD) is commonly performed as a joint-preserving surgery for treating small to medium-sized lesions in the early precollapse stage of ONFH.1,4 However, CD does not prevent the collapse of the necrotic femoral head in all cases. Thus, additional therapeutic measures are being investigated to improve the outcome, such as the use of cells and scaffolds.5 However, to evaluate the effects of these ancillary treatments, the biological and mechanical consequences of CD alone must be clearly delineated.
In Asia, alcohol is the most common etiology for ONFH in male patients, whereas corticosteroid use is the most common in female patients.6–8 However, several clinical studies reported that male patients undergoing steroid administration due to systemic lupus erythematosus or acute respiratory disease syndrome developed ONFH more often than in female patients.9–11 In addition, Ikemura et al.12,13 found that the incidence of steroid-associated osteonecrosis in male rabbits was significantly higher than in female rabbits, which may relate to cytochrome P450A activity. However, whether there are gender differences in the efficacy of the CD for ONFH is unknown.
The primary aim of this study is to evaluate the efficacy of CD in steroid-associated ONFH in rabbits. The secondary aim is to investigate whether gender differences affected the efficacy of CD. We hypothesized that CD would produce a predictable, injury-triggered, increased response in bone formation, despite the ongoing impairment in bone healing in the femoral head.
2 |. MATERIALS AND METHODS
2.1 |. Animal experiments
The experimental design was approved and performed following our Institution’s Animal Care and Use Committee guidelines. Twelve male and 12 female skeletally mature New Zealand white rabbits (West Oregon Rabbit Company), 5–6 months of age and weighing from 4.0–4.5 kg, were used. According to an established protocol,14 the rabbits were given one intramuscular injection of 20 mg/kg methylprednisolone acetate (MPSL; Depo-Medrol® single-dose vial 40 mg/ml; Pfizer Inc.) into the right gluteus medius muscle to initiate steroid-associated osteonecrosis. Rabbits of both genders were further divided into two groups (n = 6 in each group): the disease control group and the CD group.
In the disease control group, rabbits did not receive any treatment and were euthanized at 12 weeks after MPSL injection.
In the CD group, a CD of the femoral head on the left hip was performed 4 weeks after MPSL injection. In brief, rabbits were anesthetized by administering 40 mg/kg ketamine and 4 mg/kg xylazine. Analgesia was administered by injection of 0.15 mg/kg buprenorphine SR. Additional inhalation anesthesia was performed with isoflurane. A 20-mm skin incision was made over the proximal lateral thigh, and the vastus lateralis muscle was dissected to expose distal end of the third trochanter. A small hole was created at the distal end of the third trochanter with a 2-mm-diameter round burr. Under fluoroscopic guidance, a 0.9-mm C-wire was inserted from the hole towards the center of the femoral head as a guidewire, then a bone tunnel was created using a 3-mm-diameter cannulated drill bit with a power drill to within 2 mm from the surface of the femoral head (Figure 1). The length of bone tunnel was approximately 30 mm. The muscle and wound were closed using nonabsorbable suture. Antibiotics were given for the first 2 days after surgery. There were no complications after surgery. Rabbits were kept in cages and allowed free activities after surgery. At 8 weeks postoperatively (at 12 weeks after MPSL injection), rabbits were euthanized with a lethal dose of sodium pentobarbital. The entire femurs were resected, and the soft tissues were removed.
FIGURE 1.
Core decompression surgery. A 20-mm lateral skin incision was made, and the vastus lateralis muscle was dissected to expose distal end of the third trochanter. A small hole was created at the distal end of the third trochanter with a 2-mm-diameter round burr. Under fluoroscopic guidance, a 0.9-mm C-wire was inserted from the hole towards the center of the femoral head as a guidewire (A), then a bone tunnel was created using a 3-mm-diameter cannulated drill bit with power drill up to 2 mm from the surface of the femoral head (B,C). The length of bone tunnel was approximately 30 mm
2.2 |. Micro-computed tomography analysis
The proximal femurs were scanned using a micro-computed tomography (CT; Skyscan 1176; Bruker) with 20 μm resolution at 2016 × 1344, Al 1 mm, 85 kV, 200 μA, with two average frames at every 0.4° angle step. The 1.6 version of NRecon software was used to reconstruct data. Reconstructed data were further analyzed by GEMS MicroView software (eXploreMicroView v.2.5, Analysis Plus; GE Healthcare). For the analysis, bone mineral density (BMD, mg/mm3) and bone volume fraction (BVF) of outside and inside the region of interest (ROI) in the femoral head were evaluated. In the CD group, the ROI was defined as a 3-mm-diameter cylindrical area which was positioned inside the CD area. In the disease control group, the ROI was similar to that inside the CD area; this area was defined as 3 mm diameter × 4 mm length and was centrally positioned at the bottom of 6 mm thickness of femoral head (Figure 2). The total volume (TV, mm3), bone volume (BV, mm3), and bone mineral content (BMC, mg), BMD, and BVF inside the ROI were measured. Alternatively, the area outside the ROI was defined as the entire region of femoral head excluding the ROI. An 11-mm-diameter cylindrical area (6 mm length) was positioned to cover the entire of the femoral head, and TV, BV, and BMC were measured. BMD and BVF of the outside ROI were determined by calculating the data for the entire femoral head minus the data inside the ROI. A threshold value of bony tissue was determined by a phantom.
FIGURE 2.
Micro-CT analysis. Inside and outside the ROI in the disease control and CD groups. In the CD group, the ROI was defined as a 3-mm-diameter cylindrical area which was positioned inside the CD area. In the disease control group, the ROI was similar to that inside the CD area; this area was defined as 3-mm-diameter X 4-mm length and was centrally positioned at the bottom of 6 mm thickness of femoral head. The TV, BV, BMC, BMD, and BVF (TV/BV) inside the ROI were measured. Alternatively, the area outside the ROI was defined as the entire region of femoral head excluding the ROI. An 11-mm-diameter cylindrical area (6 mm length) was positioned to cover the entire of the femoral head, and TV, BV, and BMC were measured. BMD and BVF of the outside ROI were determined by calculating the data for the entire femoral head minus the data inside the ROI. BMC, bone mineral content; BMD, bone mineral density; BV, bone volume; BVF, bone volume fraction; CD, core decompression; CT, computed tomography; ROI, region of interest; TV, total volume
2.3 |. Biomechanical analysis
Specimens were stored in a −80°C freezer until the testing day. Specimens were defrosted and maintained moist with phosphate-buffered saline throughout the preparation and testing. The testing was performed at room temperature. An indentation test was performed first. Specimens were cut 60 mm distal to femoral head. The remaining diaphyseal bone was aligned vertically in an aluminum block and then potted with polymethylmethacrylate (PMMA). PMMA was also placed on the inferior surface of femoral neck to prevent bone deflection during indentation testing.
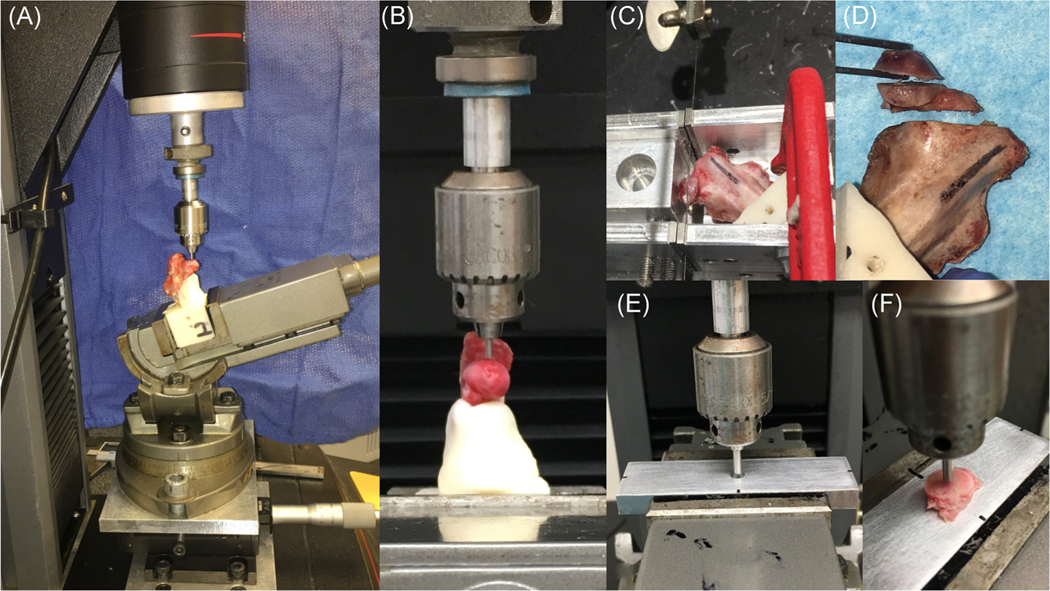
Mechanical testing was performed on a Materials Testing System fitted with a 2-kN load cell (5944; Instron Corporation). Specimens were secured in a swivel vise with the diaphyseal axis oriented 17° from vertical in the frontal plane, with the resulting load vector on the femoral head directed 17° lateral (Figure 3A,B). The loading direction approximates the frontal plane angle at peak load during a variety of daily activities in humans15. Although this loading direction may not be physiologic for rabbits, it was chosen to approximate loading that would cause femoral head collapse in cases of ONFH in humans. Forces were transmitted to the femoral head via an aluminum cylinder with a machined spherical recess. The 1.6 mm diameter of the indenter was used to indent on a flat surface location of femoral head, in line with the Ligamentum Teres in the sagittal plane. Following the application of a 1 N compressive preload, specimens were loaded at a displacement rate of 10 mm/min until 0.5 mm displacement or 300 N to avoid the fracture at femoral neck. Load and displacement data were recorded at 100 Hz. Stiffness was calculated from the linear portion of load versus displacement curve.
FIGURE 3.
Test setup for indentation testing. (A,B) First, the surface of femoral heads was indented using a 1.9-mm diameter of the indenter via a simulated acetabulum at a 17° angle, directed laterally, in the frontal plane. (C,D) Then, specimens were cut perpendicularly along with the bone tunnel into three pieces using handmade saw guide: the proximal (4 mm thickness), middle (4 mm thickness), and distal fragments. (E,F) Finally, the middle segments at bone tunnel or center of the middle fragment were indented vertically using a 2.3 mm diameter of the indenter
After testing, specimens were cut into three pieces: proximal, middle, and distal fragments (Figure 3C,D). The proximal and middle fragments were cut into 4 mm thicknesses using a 0.5-mm hand saw with a handmade saw guide set perpendicularly along with the bone tunnel. The proximal fragment was used for histological analysis and the middle fragment was used for another indentation test. The 2.3 mm-diameter of the indenter was positioned to push vertically at the bone tunnel or center of the bone specimen (Figure 3E,F). The loading protocol and measurement of stiffness were the same as the first indentation test.
2.4 |. Histological analysis for the osteonecrotic area
The proximal fragments of specimens were processed for histology. The segments were fixed in 4% paraformaldehyde (pH 7.4), decalcified in 0.5 M ethylenediaminetetraacetic acid (pH 7.4), and then embedded in optimal cutting temperature compound. An 8-μm longitudinal frozen section parallel to the direction of the drill hole/femoral neck axis was obtained and stained with hematoxylin and eosin (H&E). The images of the H&E stained sections were viewed under a microscope (BZ-X 710 digital microscope; Keyencen). To assess the changes in ONFH, a total of tenfields outside ROI were randomly selected under ×200 magnification.16 In the CD group, the ROI was defined as the CD area. In the disease control group, the ROI was defined as the center of a 3 mm × 2 mm area which was assumed to be the CD area. The percentage of empty lacunae outside ROI was evaluated using MATLAB program, as previously described.16
2.5 |. Histochemical and immunohistochemical analysis for osteogenesis and angiogenesis
Sections were stained for alkaline phosphatase (ALP), tartrate-resistant acid phosphatase (TRAP), and CD31 (platelet endothelial cell adhesion molecule 1). For the ALP staining, sections were incubated with ALP substrate solution (1-StepTM NBT/BICP Substrate Solution; Thermo Fisher Scientific) at 37°C overnight. For the TRAP staining, the TRAP Histochemical Staining Kit (Sigma-Aldrich) was used. The images of the stained sections were observed under ×40 magnification for the ALP staining and ×100 magnification for the TRAP staining. The percentage of ALP and TRAP positive staining area outside and inside the ROI was quantitatively evaluated. The total positive staining area and the entire of section area were measured using ImageJ, and the percentage of positive staining area was calculated. The color threshold of each parameter was determined by two investigators by consensus.
For CD31 staining, sections were rehydrated and treated with proteinase K (S3020; DakoCytomation). Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide treatment. Sections were incubated with a mouse monoclonal anti-rabbit CD31 antibody (diluted 1:800, NB600-562; Novus Biologicals) at 4°C overnight. The bound antibodies were detected by secondary antibodies (Histofine Simple Stain MAX-PO (M), 414132F; Nichirei Bioscience) and visualized by 3,3′-diaminobenzidine solution (34002; Thermo Fisher Scientific). The sections were then counterstained with hematoxylin. For analysis of CD31 staining, five fields under ×200 magnification inside and outside the ROI were randomly selected, and the CD31 positive-stained microvessels were counted by two investigators individually. The definition of outside and inside the ROI in ALP, TRAP, and CD31 staining was as same as for the H&E staining.
2.6 |. Statistical analysis
All data were expressed as mean ± standard deviation. Data were compared between the disease control and CD groups for both genders. In addition, data were also compared between the males and the females in each group. Nonpaired t tests for parametric data and the Mann–Whitney U test for nonparametric data were used. The internal reliability of the number of CD31 positive-stained microvessels between two investigators was evaluated by intraclass correlation coefficients (ICC). All plotting of data and statistical analyses were performed using GraphPad Prism 8.1 (GraphPad Software). Statistical significance was set p < .05. Power analysis calculated using previous data16 indicated that a sample size of six per group would provide 80% statistical power to detect significant differences between the groups (α = .05, β = .20) using analysis of t tests.
3 |. RESULTS
All treated animals lost weight; one male rabbit died at 3 weeks and four female rabbits (one at 2 weeks and three at 3 weeks) died of respiratory tract infection. This mortality rate is not unexpected for this MPSL injection protocol.14,17–19 These five rabbits were replaced by new rabbits. No rabbit died after surgery.
3.1 |. BMD and BVF outside the ROI in the CD group were significantly higher than in the disease control group
Representative micro-CT reconstruction images of the proximal femur and micro-CT analysis are shown in Figure 4. New bone formation was seen inside the ROI, and abundant trabecular bone outside the ROI in the femoral head was observed in the CD group. BMD and BVF inside the ROI showed no differences between the disease control and CD groups for both genders. In contrast, BMD outside the ROI in the CD group was significantly higher than in the disease control group in both genders (the disease control group vs. the CD group: 1094 ± 48 vs. 1160 ± 44 mg/mm3, p < .05 in females; 1102 ± 63 vs. 1171 ± 47 mg/mm3, p < .05 in males). Similarly, BVF outside the ROI in the CD group was significantly higher than in the disease control group for both genders (the disease control group vs. the CD group: 0.48 ± 0.04 vs. 0.56 ± 0.04, p < .001 in females; 0.48 ± 0.04 vs. 0.55 ± 0.05, p < .001 in males). However, the differences in both BMD and BVF between the two groups were small. There were no gender differences between the disease control groups or the CD groups.
FIGURE 4.
Representative micro-CT reconstruction images of the proximal femur (A) and BMD and BVF inside and outside the ROI (B,C). (A) New bone formation is seen inside the ROI, and abundant trabecular bone outside the ROI in the femoral head was observed in the CD group. (B) Inside the ROI, both BMD and BVF showed no differences between the disease control and CD groups for both genders. (C) Outside the ROI, both BMD and BVF in the CD group were significantly higher than in the disease control group in both genders. There were no gender differences of both BMD and BVF in both two groups. *p < .05, **p < .01 by unpaired t test. BMD, bone mineral density; BVF, bone volume fraction; CD, core decompression; CT, computed tomography; ROI, region of interest
3.2 |. No differences in stiffness of femoral head surface after CD in either gender
The results of mechanical testing are shown in Figure 5. In the indentation test for the femoral head surface, the stiffness was 738.6 ± 175.8 N/mm in the disease control group, 648.3 ± 167.8 N/mm in the CD group in females, 807.0 ± 133.1 N/mm in the disease control group, and 752.3 ± 89.1 N/mm in the CD group in males. No significant differences in the stiffness were found between the control and CD groups in both genders and between genders in each two group. For indentation testing of the middle segment, the stiffness in the disease control group was significantly higher than in the CD group in females (the disease control group vs. the CD group: 1045.0 ± 149.2 vs. 623.9 ± 371.9 N/mm, p < .05). Similarly, the stiffness in the disease control group tended to be higher than in the CD group in males (the disease control group vs. the CD group: 823.3 ± 195.0 vs. 552.1 ± 304.3 N/mm, p = .08). In addition, the stiffness in females tended to be higher than in the males in the disease control group (p = .06). There was no significant difference between genders in the CD group.
FIGURE 5.
Results of indentation testing. (A) In the indentation test for the surface of femoral head, no significant differences in the stiffness were found between two groups for both genders, or between genders. (B) In the indentation testing for the middle segment, the stiffness in the disease control group was significantly higher than in the core decompression (CD) group in females (p < .05). Similarly, the stiffness in the disease control group tended to be higher than in the CD group in males (p = .08). In addition, the stiffness in the female tended to be higher than in the male in the disease control group (p = .06). There was no gender difference in the CD group. *p < .05 by unpaired t test
3.3 |. The percentage of empty lacunae in the CD group was higher than in the disease control group
Representative photomicrographs of H&E staining are shown in Figure 6A–D. Interestingly, 77% of the fields outside the ROI in the all groups did not contain any bone marrow, and the remaining fields contained very scant bone marrow. The disease control group in both genders showed normal osteocytes and occasional empty lacunae in the trabecular bone both inside and outside the ROI; pyknotic nuclei or malformed osteocytes with peripherally displaced nuclei were seen in the region of the subchondral bone. In contrast, the CD groups in both genders showed new bone formation with normal osteocytes inside the ROI, whereas the empty lacunae in the trabecular bone was found outside the ROI, and osteocytes with pyknotic or abnormal nuclei were seen in the region of the subchondral bone. The percentage of empty lacunae in the CD group was significantly higher than in the disease control group in both genders (the disease control group vs. the CD group: 41.8 ± 15.2% vs. 23.6 ± 10.9%, p < .001 in females; 46.9 ± 10.0% vs. 15.0 ± 8.2%, p < .001 in males; Figure 6E). There were no significant differences between males and females in the disease control group and in the CD group.
FIGURE 6.
Representative H&E staining photomicrographs of the femoral heads (A–D) and the percentage of empty lacunae (E). The disease control group in both genders showed normal osteocytes and few empty lacunae in the trabecular bone both inside and outside the ROIs; pyknotic nuclei and malformed osteocytes with peripherally displaced nuclei were seen in the region of subchondral bone (arrow head) (A,C). In the CD group, new bone formed inside the ROI, whereas the large number of empty lacunae was observed outside the ROI for both genders (B,D). (E) The percentage of empty lacunae in the CD group was significantly higher than in the disease control group for both genders. There were no gender differences in both two groups. The ROI: yellow dashed line, scale bar = 50 μm. *p < .05, ***p < .005 by Mann–Whitney U test. CD, core decompression; H&E, hematoxylin and eosin; ROI, region of interest
3.4 |. The percentage of ALP positive staining area and number of CD31 positive-stained microvessels inside and outside ROI in the CD group was higher than in the disease control group in males
Representative photomicrographs of the ALP staining are shown in Figure 7. In males, the positive ALP staining area both outside and inside the ROIs in the CD group was significantly higher than in the disease control group (p < .05). In addition, in the disease control group, the ALP positive staining area both outside and inside the ROIs in females was significantly higher than in males (p < .05).
FIGURE 7.
Representative ALP staining photomicrographs of the femoral heads (A–D) and percentage of ALP positive-stained area (E,F). In males, positive ALP stained areas both outside and inside the ROIs in the CD group was significantly higher than in the disease control group (p < .05). In addition, in the disease control group, the ALP positive staining areas both outside and inside the ROIs in females was significantly higher than in males (p < .05). The ROI: yellow dashed line, scale bar = 100 μm. *p < .05 by unpaired t test. ALP, alkaline phosphatase; CD, core decompression; ROI, region of interest
Representative photomicrographs of the TRAP staining are shown in Figure 8. The TRAP staining cells were seen within the subchondral bone and trabecular bone. There were no significant differences in the percentage of TRAP positive staining area between the disease control and CD groups and between the genders.
FIGURE 8.
Representative TRAP staining photomicrographs of femoral heads (A–D) and percentage of TRAP positive-stained area (E,F). TRAP staining cells were seen in the subchondral and trabecular bone. No significant differences in the percentage of TRAP positive-stained area were confirmed between two groups and between genders. The ROI: yellow dashed line, scale bar = 100 μm. CD, core decompression; ROI, region of interest; TRAP, tartrate-resistant acid phosphatase
Representative photomicrographs of the CD31 staining are shown in Figure 9. ICC of number of CD31 positive-stained microvessels between two investigators was found to be r = .713 (95% confidence interval: 0.63–0.78, p < .0001). The number of CD31 positive stained microvessels both outside and inside the ROIs in the CD group was significantly higher than in the disease control group in males (p < .05). In addition, in the disease control group, the number of CD31 positive stained microvessels both outside and inside the ROIs in females was significantly higher than in males (p < .05). Moreover, in the CD group, the number of CD31 positive stained microvessels outside the ROIs in females was significantly higher than in males (p < .05).
FIGURE 9.
Representative photomicrographs of CD31 staining of the femoral heads (A–D) and the number of CD31 positive-stained microvessels (E,F). The number of CD31 positive-stained microvessels both outside and inside the ROIs in the CD group was significantly higher than in the disease control group in males (p < .05). In the disease control group, the number of CD31 positive-stained microvessels both outside and inside the ROIs in females was significantly higher than in males (p < .05). In the CD group, the number of CD31 positive stained microvessels outside the ROIs in females was significantly higher than in males (p < .05). The ROI: yellow dashed line, scale bar = 100 μm. *p < .05, **p < .01, ***p < .005 by Mann–Whitney U test. CD, core decompression; ROI, region of interest
4 |. DISCUSSION
A recent review article summarized previous studies that focused on the efficacy of CD treatment in the area inside the drill track.5 However, it is essential to evaluate the entirety of the femoral head in ONFH, as there may be multiple lesions that increase the potential for collapse of femoral head. The current study investigated the efficacy of CD both inside and outside the CD area in the femoral head in rabbits with steroid induced disease, a clinically important objective.
For the area outside the CD area, micro-CT analysis showed that BMD and BVF in the CD group were higher than in the disease control group for both genders. Furthermore, the ALP positive staining area and the number of CD31 positive stained microvessels in the CD group were higher than in the disease control group in males. Wang et al.20 used a model of steroid-associated ONFH in rabbits and demonstrated that the decreased blood flow in femoral head was improved after CD. Their and our data suggest that CD enhances both osteogenesis and angiogenesis and increases the morphological properties outside the CD area in the femoral head. However, our study also showed that the percentage of empty lacunae in the femoral head in the CD group was higher than in the disease control group in both genders. These data indicate that CD also injures local cells within the femoral head and causes limited cell necrosis. Xie et al.21 created a bone tunnel in the distal femora in both normal rabbits and those who received systemic corticosteroids; histomorphological analysis around the bone tunnel and the subchondral bone at 12 weeks after CD showed empty lacunae in the trabecular bone in the rabbits receiving corticosteroids, whereas a normal distribution and morphology of osteocytes were found in healthy rabbits. Corticosteroids are known to inhibit osteogenesis22; in vitro studies revealed that human and rabbit mesenchymal stem cells in steroid-associated ONFH demonstrated decreased osteogenic ability.23,24 These findings suggest that CD provokes an injury–repair response in the area outside the CD, and that this repair response is impaired in steroid-associated ONFH, resulting in the necrotic lesion in the femoral head. Furthermore, the differences in BMD and BVF between the disease control group and CD group were small. The indentation test showed no difference in femoral head stiffness between the disease control and CD groups for both genders. These data point to the need for additional biological and mechanical strategies to improve the outcome for early-stage steroid-associated ONFH.
Inside the CD area, new trabecular bone with normal cells was confirmed histologically, and both angiogenesis and osteogenesis were increased in males. However, micro-CT analysis showed no differences in BMD and BVF between the disease control and CD groups, and the bone tunnel after CD demonstrated inferior mechanical properties in both genders. Therefore, similar to the observations outside the CD, the findings of impaired bone healing within the CD tunnel support the need for additional strategies to improve the biological and mechanical parameters of bone in steroid-associated ONFH.
Several clinical9–11 and rabbit12,13 studies reported that males develop steroid-associated ONFH more often than females, and that these gender differences may be related to cytochrome P450A activity, which metabolizes corticosteroids. However, to our knowledge, there have been no studies to document the gender differences of osteogenesis and angiogenesis after steroid injection. In our study, for the disease control group, we are the first to observe that males are more susceptible to a single injection of MPSL by significantly suppressed osteogenesis and angiogenesis, compared to females. This gender difference in response to exogenous corticosteroids in rabbits is consistent with and may partially explain the higher incidence of ONFH in males than in females. After CD, osteogenesis and angiogenesis were increased in males, but no differences were observed between the disease control and CD groups in females. Furthermore, the stiffness inside the ROI in females was higher than in males in the disease control group. This suggests potential gender differences in the susceptibility or potential for repair after steroid-associated osteonecrosis.
Steroid-associated ONFH in rabbits was used as a model of early-stage ONFH in humans. Several noninvasive methods have been described to develop steroid-associated ONFH in rabbits including corticosteroids only, corticosteroids with lipopolysaccharide (LPS), or allogeneic serum.25,26 However, the ideal animal model has not been established yet. Although the pathological definition of ONFH is not standardized,15 the most commonly used findings include diffuse empty lacunae or pyknotic nuclei of osteocytes within the bone trabeculae, accompanied by surrounding bone marrow cell necrosis.14 On the basis of these findings, Qin et al.27 reported a protocol for steroid-associated ONFH model in rabbits with a single dose 10 μg/kg of LPS, following by three injections of 20 mg/kg of MPSL. The induction rate of osteonecrotic lesions in this protocol was up to 93% at 2–6 weeks26,28; however, only 29% of cases had osteonecrotic lesions in the femoral head.27 The protocol that we used included a single injection of 20-mg/kg MPSL, which has been reported to induce osteonecrotic lesions as high as 80% at 4 weeks,14,18,29 but the osteonecrotic lesions may not be uniformly observed in the femoral head.14 However, Kawai et al.30 evaluated the effect of corticosteroids inthe femoral head in rabbitshistologically; healthy rabbits without any steroid treatments had bone marrow containing hematopoietic cells; empty lacunae were occasionally seen, but the majority of osteocytes had round or oval nuclei. On the other hand, steroid-treated rabbits showed that bone marrow has been replaced by enlarged fat cells without hematopoietic cells, and pyknotic nuclei or malformed osteocytes with peripherally displaced nuclei were concentrated in the region of the subchondral bone. In addition, Yamamoto et al.14 demonstrated that the repair process including progressive revascularization with granulation tissue, osteoblastic repair, or appositional bone formation was seen after 6 weeks; furthermore, 25% of the femurs showed that the necrotic marrow was almost wholly replaced by reparative tissue at 10 weeks. In our study, the endpoint in our study was 12 weeks after steroid injection (8 weeks after CD); 77% of the fields in the femoral head did not contain any bone marrow, and the remaining fields contained very scant bone marrow. Furthermore, pyknotic nuclei or malformed osteocytes with peripherally displaced nuclei were confirmed within the region of subchondral bone in the all groups. Thus, although we did not evaluate the specimens histologically at 4 weeks after steroid injection, the above findings indicated that the osteonecrotic changeshad occurred in the femoral head. Therefore, the model used in the present study should be viewed as CD after corticosteroid injection, early in the disease of ONFH. In addition, to accurately assess the repair processes associated with ONFH with and without CD, we evaluated the number of empty lacunae.
There are several limitations in this study. First, even though we used a lower overall dose of MPSL, 25% (4/16) of female and 8% (1/13) of male rabbits died or had to be euthanized due to severe weight loss and respiratory tract infection. The mortality rate of the protocol that we used was as high as 18%.14,17–19 Addition of LPS would have undoubtedly increased the number of deaths. Therefore, the protocol using a single injection of MSPL is employed in the current study with respect to safety and efficacy, although further study will be needed for improvement in overall survivorship. There were no gender differences for the efficacy of CD, but the mortality rate in male rabbits was substantially less than in females. Moreover, all rabbits died during the initial 2–3 weeks after injection. Thus, for logistical reasons, we continue our studies on steroid-associated ONFH using male rather than rabbits, and perform clinical, laboratory, and radiographic examinations during the critical period after MPSL injection. Second, no radiological examinations to detect ONFH were performed at 4 weeks after MPSL injection, before surgery. Previous studies have shown that at least 6 weeks are necessary to see the initial onset of changes in ONFH using magnetic resonance imaging.31,32 Thus, examination using plain radiographs at 4 weeks is of little value to evaluate the osteonecrotic lesions in the early stages of ONFH. Finally, rabbits are quadrupedal, and hip biomechanics are different from humans. In our study, a protocol of indentation testing for the surface of femoral head was designed to simulate physiological loading of the proximal femur in humans, which is similar to the general anatomical area affected by ONFH in rabbits.33
In conclusion, the current animal study demonstrated that CD increased BMD and BVF in both genders and promoted osteogenesis and angiogenesis in the femoral head preferentially in males. These findings are probably due to the CD procedure provoking an injury–repair response in the area outside the CD area. Increased mechanical strength in the femoral head was not observed after the CD due to the remaining cortical shell at the early stage of ONFH in the study period. These data point to the need for additional biological, mechanical strategies, and therapeutic windows to improve the outcome for early-stage steroid-associated ONFH.
ACKNOWLEDGMENTS
This study was supported in part by the National Institutes of Health Grants (R01AR072613, R01AR057837, U01AR069395, R01AR074458, R01AR073145, S10OD02349701, and R01AR063717 from NIAMS). The authors would like to thank the Stanford Osteonecrosis Study Group (Seyedsina Moeinzadeh, Chi-Wen Lo, Ning Zhang, Monica Romero-Lopez, Takeshi Utsunomiya, and Masaya Ueno) for their help. They would also like to thank the staff of the Veterinary Department at Stanford University for their help and advice.
Funding information
National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Numbers: R01AR057837, R01AR063717, R01AR072613, R01AR073145, R01AR074458, S10OD02349701, U01AR069395
REFERENCES
- 1.Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am. 2015;97:1604–1627. [DOI] [PubMed] [Google Scholar]
- 2.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. [DOI] [PubMed] [Google Scholar]
- 3.Amanatullah DF, Strauss EJ, Di Cesare PE. Current management options for osteonecrosis of the femoral head: part II, operative management. Am J Orthop. 2011;40:E216–E225. [PubMed] [Google Scholar]
- 4.Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J. 2017;99-B:1267–1279. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama M, Lin T, Pan CC, et al. Cell-based and scaffold-based therapies for joint preservation in early-stage osteonecrosis of the femoral head: a review of basic research. JBJS Rev. 2019;7:e5. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi S, Fukushima W, Yamamoto T, et al. Temporal trends in characteristics of newly diagnosed nontraumatic osteonecrosis of the femoral head from 1997 to 2011: a hospital-based sentinel monitoring system in Japan. J Epidemiol. 2015;25:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai SW, Wu PK, Chen CF, et al. Etiologies and outcome of osteonecrosis of the femoral head: etiology and outcome study in a Taiwan population. J Chin Med Assoc. 2016;79:39–45. [DOI] [PubMed] [Google Scholar]
- 8.Cui L, Zhuang Q, Lin J, et al. Multicentric epidemiologic study on six thousand three hundred and ninety five cases of femoral head osteonecrosis in China. Int Orthop. 2016;40:267–276. [DOI] [PubMed] [Google Scholar]
- 9.Guo KJ, Zhao FC, Guo Y, Li FL, Zhu L, Zheng W. The influence of age, gender and treatment with steroids on the incidence of osteonecrosis of the femoral head during the management of severe acute respiratory syndrome: a retrospective study. Bone Joint J. 2014;96-B:259–262. [DOI] [PubMed] [Google Scholar]
- 10.Oinuma K. Osteonecrosis in patients with systemic lupus erythematosus develops very early after starting high dose corticosteroid treatment. Ann Rheum Dis. 2001;60:1145–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagasawa KTY, Koarada S, Horiuchi T, et al. Very early development of steroid-associated osteonecrosis of femoral head in systemic lupus erythematosus: prospective study by MRI. Lupus. 2005;14: 385–390. [DOI] [PubMed] [Google Scholar]
- 12.Ikemura S, Yamamoto T, Nishida K, Motomura G, Iwamoto Y. Gender difference in the development of steroid-induced osteonecrosis in rabbits. Rheumatology. 2010;49:1128–1132. [DOI] [PubMed] [Google Scholar]
- 13.Ikemura S, Yamamoto T, Motomura G, et al. Cytochrome P4503A activity affects the gender difference in the development of steroid-induced osteonecrosis in rabbits. Int J Exp Pathol. 2014;95:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, Irisa T, Sugioka Y, et al. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40:2055–2064. [DOI] [PubMed] [Google Scholar]
- 15.Parajuli S, Fowler JR, Balasubramanian E, et al. Problems with the pathological diagnosis of osteonecrosis. Skeletal Radiol. 2016;45: 13–17. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama M, Nabeshima A, Pan CC, et al. The effects of a functionally-graded scaffold and bone marrow-derived mononuclear cells on steroid-induced femoral head osteonecrosis. Biomaterials. 2018;187:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyanishi K, Yamamoto T, Irisa T, et al. A high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a potential risk factor for corticosteroid-induced osteonecrosis in rabbits. Rheumatology. 2001;40:196–201. [DOI] [PubMed] [Google Scholar]
- 18.Motomura G, Yamamoto T, Irisa T, Miyanishi K, Nishida K, Iwamoto Y. Dose effects of corticosteroids on the development of osteonecrosis in rabbits. J Rheumatol. 2008;35:2395–2399. [DOI] [PubMed] [Google Scholar]
- 19.Kuribayashi M, Fujioka M, Takahashi KA, et al. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010;81: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang GJ, Dughman SS, Reger SI, Stamp WG. The effect of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am. 1985; 67:121–124. [PubMed] [Google Scholar]
- 21.Xie XH, Wang XL, Zhang G, et al. Impaired bone healing in rabbits with steroid-induced osteonecrosis. J Bone Joint Surg Br. 2011;93: 558–565. [DOI] [PubMed] [Google Scholar]
- 22.Patschan D, Loddenkemper K, Buttgereit F. Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone. 2001;29:498–505. [DOI] [PubMed] [Google Scholar]
- 23.Houdek MT, Wyles CC, Packard BD, Terzic A, Behfar A, Sierra RJ. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-induced osteonecrosis of the femoral head. J Arthroplasty. 2016;31:893–898. [DOI] [PubMed] [Google Scholar]
- 24.Qin L, Yao D, Zheng L, et al. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials. 2015;59: 125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Gong H, Lu S, Deasey MJ, Cui Q. Animal models of steroid-induced osteonecrosis of the femoral head—a comprehensive research review up to 2018. Int Orthop. 2018;42:1729–1737. [DOI] [PubMed] [Google Scholar]
- 26.Xie XH, Wang XL, Yang HL, Zhao DW, Qin L. Steroid-associated osteonecrosis: epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview). J Orthop Translat. 2015;3:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin L, Zhang G, Sheng H, et al. Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone. 2006; 39:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng H, Zhang G, Wang YX, et al. Functional perfusion MRI predicts later occurrence of steroid-associated osteonecrosis: an experimental study in rabbits. J Orthop Res. 2009;27:742–747. [DOI] [PubMed] [Google Scholar]
- 29.Miyanishi K, Yamamoto T, Irisa T, et al. Effects of cyclosporin A on the development of osteonecrosis in rabbits. Acta Orthop. 2006;77: 813–819. [DOI] [PubMed] [Google Scholar]
- 30.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopic study. J Bone Joint Surg Am. 1985;67: 755–763. [PubMed] [Google Scholar]
- 31.Kubo T, Yamazoe S, Sugano N, et al. Initial MRI findings of non-traumatic osteonecrosis of the femoral head in renal allograft recipients. Magn Reson Imaging. 1997;15:1017–1023. [DOI] [PubMed] [Google Scholar]
- 32.Fujioka MKT, Nakamura F, Shibatani M, et al. Initial changes of nontraumatic osteonecrosis of femoral head in fat suppression images: bone marrow edema was not found before the appearance of band patterns. Magn Reson Imaging. 2001;19:985–991. [DOI] [PubMed] [Google Scholar]
- 33.Bergmann G, Bender A, Dymke J, Duda G, Damm P. Standardized loads acting in hip implants. PLoS One. 2016;11:e0155612. [DOI] [PMC free article] [PubMed] [Google Scholar]