Abstract
Cigarette smoking is the most preventable risk factor related to cardiovascular morbidity and mortality. Tobacco usage has declined in recent years; however, the use of alternative nicotine delivery methods, particularly e-cigarettes, has increased exponentially despite limited data on their short- and long-term safety and efficacy. Due to their unique properties, the impact of e-cigarettes on cardiovascular physiology is not fully known. Here, we summarize both preclinical and clinical data extracted from short- and long-term studies on the cardiovascular effects of e-cigarette use. Current findings support that e-cigarettes are not a harm-free alternative to tobacco smoke. However, the data are primarily derived from acute studies. The impact of chronic e-cigarette exposure is essentially unstudied. To explore the uniqueness of e-cigarettes, we contemplate the cardiovascular effects of individual e-cigarette constituents. Overall, data suggest that exposure to e-cigarettes could be a potential cardiovascular health concern. Further preclinical research and randomized trials are needed to expand basic and clinical investigations before considering e-cigarettes safe alternatives to conventional cigarettes.
Keywords: Electronic cigarette, Cardiovascular disease, Nicotine, Aerosol, Smoking, Toxicity
Key points
E-cigarette use has increased dramatically in the past decade, particularly among teens and young adults.
The general public perception is that e-cigarettes are harm-free and safer alternatives to traditional tobacco cigarettes.
In contrast, there is growing evidence that e-cigarettes and their aerosol constituents, nicotine, carbonyl compounds, particulate matter, metals, and flavourings, can have adverse effects on the cardiovascular system.
While there is paucity of data, recent studies have also suggested that e-cigarette use is associated with inflammation, oxidative stress, and haemodynamic imbalance leading to increased cardiovascular diseases risk.
Additional experimental and clinical studies investigating acute and chronic use of e-cigarettes are needed to establish their cardiovascular safety profile and their potential as a smoking cessation aid.
1. Introduction
Traditional cigarette smoking continues to be a major public issue as it has continuously been shown to have many pathogenic and negative effects on the heart. Presently, cardiovascular diseases (CVDs) are the number one cause of death among Americans, killing approximately 630 000 persons annually.1 Despite strong efforts to reduce smoking, traditional smoking continues to be one of the most preventable risk factors for heart disease as 15% of the population use combustible cigarettes.2 Resultantly, marketing for alternatives to tobacco smoking and nicotine replacements has skyrocketed in recent years. More specifically, since being introduced in 2006, electronic cigarettes (e-cigarettes) have become more popular due to their perceived safety when compared with traditional cigarette smoking. These public perceptions of safety have made e-cigarettes not only appealing to smokers attempting to quit but have also gained popularity among non-smokers, who constitute a substantial segment of the e-cigarette market. From 2011 to 2015, the use of e-cigarettes among middle school and high school students grew from 1.5% to 16%, making it the most used tobacco product by teens and young adults.3 As of 2018, the National Youth Tobacco Survey reported that 20.8% of the same population reported current use of e-cigarettes.4 While e-cigarettes have generally been advertised to contain less toxic constituents than traditional cigarettes, they do contain a variety of potentially toxic compounds that have yet to be thoroughly studied in preclinical and clinical research.5
In this review, we discuss recent, relevant studies from the available literature, focusing on the components of and potential cardiovascular risks associated with exposure to e-cigarette vapour. In their recent work, Benowitz and Fraiman6 evaluated a related topic with a focus on the cardiovascular effects of e-cigarettes vs. tobacco cigarettes. Here, we focus on evaluating and extensively discussing data from preclinical and epidemiological studies regarding the cardiovascular effects of acute (short-term) and chronic (long-term) e-cigarette exposure (summarized in Table 1). Furthermore, we have reviewed various constituents of e-cigarettes for their potential cardiovascular toxicity and disease burden. Few studies have reported no adverse effects associated with e-cigarette usage, while others suggest that e-cigarettes could increase a users’ risk of CVDs. Heightened concern over the safety profile of e-cigarettes from these studies displays an urgent need for additional studies to establish and better understand the acute and chronic health consequences of e-cigarette use.
Table 1.
Preclinical and clinical studies showing acute and chronic cardiovascular effects of e-cigarette exposure
| Acute exposure studies | ||
|
| ||
| Cytotoxicity | ||
|
| ||
| Type of study | Exposure | Finding |
|
| ||
| Preclinical | Human umbilical vein endothelial cells (HUVECs) incubated in first-generation e-cigarette aerosol extract (EAE) or 3R4F cigarette smoke extract (CSE) for 24–72 h. | Reactive oxygen species, DNA damage, and cell death observed in HUVECs following exposure to either EAE and CSE.7 |
| HUVECs incubated with 11 different e-cigarette vapours from disposable or refillable devices for 48 h. | HUVECs displayed high cytotoxicity, cell proliferation inhibition, and changes in cell morphology following exposure to various e-cigarette vapours of varying flavour and nicotine content.8 | |
| Cardiomyoblast cells incubated in varying concentrations of e-cigarette (EC) or cigarette smoke (CS) liquid extracts for 24 h. | 4 of 20 EC extracts at 100% and 50% concentrations and CS extracts at concentrations diluted to 12.5% showed cytotoxic effects on cardiomyoblasts.9 | |
| Murine fibroblasts incubated in varying concentrations of EC or CS liquid extracts for 24 h. | 1 of 21 EC extracts at 100% concentration and CS extracts at concentrations diluted to 12.5% showed cytotoxic effects on fibroblasts.10 | |
| HUVECs incubated with e-cigarette or 3R4F cigarette aqueous aerosol extracts (AqE) for 20 h. | Scratch wound assay showed e-cigarette AqEs had no significant effect on migration of HUVECs, while cigarette AqE induced complete inhibition at >20% concentration.11 | |
|
| ||
| Inflammation, oxidative stress, and platelet activity | ||
|
| ||
| Preclinical | Primary rat lung endothelial cells exposed to e-cigarette liquid or condensed vapour or CS extract. | E-cigarette liquid and vapour and CS extract exposure significantly increased permeability associated with inflammation and oxidative stress in RLECs.12 |
| Human coronary artery endothelial cells (HCAECs) exposed to cigarette smoke extract (CSE) or e-cigarette aerosol extract (eCAE) and lysed after 16 h. | HCAECs displayed oxidative stress (activation of NFR2 and up-regulation of cytochrome p450) following exposure to CSE but not eCAE.13 | |
| Male mice exposed to 200 puffs of menthol flavoured e-cigarette vapour per day for 5 days. | Significant platelet hyperactivity was demonstrated through decreased bleeding and occlusion times and increased aggregation and dense and α granule secretion when compared with air exposed mice.14 | |
| Platelets drawn from 50 healthy volunteers exposed to tobacco smoke extracts, e-cigarette vapour extracts, or pure nicotine. | Enhanced expression of pro-inflammatory gC1qR and cC1qR and increased platelet activation, aggregation, and adhesion potential was observed following exposure.15 | |
| Clinical | Blood drawn from 16 healthy seldom smokers either exposed to or not exposed to 10 puffs of e-cigarette vapour (ECV) from a second-generation device for 10 min was measured for endothelial progenitor cells (EPCs) and microvesicles (MVs). | Blood levels of EPCs significantly increased following exposure to ECV indicative of endothelial activation or stress.16 |
| Blood drawn from 40 healthy subjects (20 smokers and 20 non- smokers) before and after smoking a traditional cigarette and 1 week later before and after an e-cigarette (rechargeable cigalike). | Increases in levels of soluble NOX2-derived peptide and 8-iso-prostaglandin F2α and decreases in NO bioavailability, vitamin E levels, and FMD were observed indicating oxidative stress and altered vascular function.17 | |
| Cardiovascular function | ||
|---|---|---|
| Preclinical | Cleavage stage zebrafish embryos exposed continuously to nicotine, e-cigarette, or tobacco extracts in fish water for 3 days. Human embryonic stem cells exposed to the same extracts during directed differentiation (14 days). | Developmental defects including severe heart malformation, pericardial oedema, and reduced heart function observed following exposure in zebrafish. Additionally, e-cigarette exposure decreased cardiac transcription factors in cardiac progenitor cells and decreased sarcomeric gene (MLC2v and MYL6) expression in cardiomyocytes.18 |
| Clinical | 23 chronic smokers (≥10 cigarettes per day for ≥12 months) used 50 puffs (at 30 s intervals) of one of five e-cigarettes (rechargeable cigalikes) or one cigarette. | Significantly lower nicotine plasma levels, blood pressure, heart rate, and no increase in exhaled CO were observed when compared with cigarette use.19 |
| 15 traditional smokers randomized to three groups of using 10 puffs of e-cigarettes (third-generation vapourizer) containing nicotine, e-cigarettes without nicotine, or traditional cigarettes over three separate exposures (≥48 h between). | Individuals using nicotine-containing e-cigarettes experienced short-term elevations in peripheral SBP and DBP and PWV (a measure of arterial stiffness) when compared with nicotine free e-cigarette use.20 | |
| 24 smokers on four occasions used either a tobacco cigarette (TC) for 5 min, EC for 5 min (unspecified type), EC for 30 min, or nothing for 60 min. | Significant increases in blood pressure and PWV following 30 min EC vaping were observed, similar to those measured after 5 min of TC smoking.21 | |
| 25 occasional tobacco smokers randomized to three groups of using 25 puffs of e-cigarettes (last-generation high powered device) containing nicotine, e-cigarettes without nicotine, or a sham (making the motion with the device turned off) over three separate exposures. | Only nicotine-containing e-cigarettes resulted in increased PWV, augmentation index, blood pressure and heart rate, as well as impaired acetylcholine mediated vasodilation and increased plasma myeloperoxidases (a measure of oxidative stress).22 | |
| 36 heavy (15 cigarettes per day for ≥5 years) smokers (SM) smoked one cigarette and 40 e-cigarette (≥1 month) users (ECIG) used a second-generation device with 11 mg/mL e-liquid for 7 min. | No changes were observed in the ECIG group compared with baseline measurements. Significantly higher mitral annulus diastolic velocity and diastolic strain rate, and lower isovolumetric relaxation time and myocardial performance index were reported following e-cigarette use compared with tobacco cigarette use.23 | |
| 33 not current tobacco or electronic cigarette users (within 1 year) used 60 puffs of a cigalike device with nicotine e-liquid, with nicotine-free e-liquid and a sham control (no e-liquid) over three exposures (4 weeks between). | Increased cardiac sympathetic nerve activity was observed following use of e-cigarettes with nicotine but not in nicotine free e-cigarettes.24 | |
| 105 smokers (10 cigarettes a day for ≥12 months) were randomized to clinically confined exclusive cigalike e-cigarette use, dual (both e-cigarette and tobacco cigarette) use and complete cessation groups for 5 days. | Significant reductions in blood pressure and heart rate were observed in exclusive e-cigarette use and cessation groups. Small non-statistically significant improvements in lung function were observed compared with baseline for all groups.25 | |
| 15 female smokers (≥5 cigarettes a day for at least 2 years) smoked 10–12 puffs of a tobacco cigarette and 15 puffs of an e-cigarette (second-generation device) on two separate exposures (1 day between). | No significant increases in arterial stiffness (SI and RI) or blood pressure and heart rate were measured following e-cigarette exposure.26 | |
|
| ||
| Chronic exposure studies | ||
|
| ||
| Cardiovascular function, inflammation, and oxidative stress | ||
|
| ||
| Preclinical | Female mice were exposed to e-cigarette vapour, cigarette smoke or filtered air for 4 h/day, 5 days/week for 8 months. | Increased aortic stiffness (measured by PWV) and impaired vascular reactivity responses were observed in e-cigarette vapour and tobacco smoke groups.27 |
| Female mice underwent nose exposure to e-cigarette vapour (24 mg/mL nicotine e-liquid) or air 1 h/day, 5 days/week for 6 months. | Increased systemic inflammation, 2.75 greater levels of heart fibrosis, along with increased SBP and upward trending DBP were observed in mice exposed to e-cigarette vapour when compared with those exposed to air.28 | |
| 20 male mice were randomized into two groups and exposed to e-cigarette smoke (ECS) or filtered air (FA) for 3 h/day, 5 days/week for 12 week. | ECS mice demonstrated increased DNA mutagenic adducts, γ-OH-PdG and O6-medG, in various tissues including the heart when compared with FA.29 | |
| Clinical | Heart rate variability and oxidative stress measures in the participants plasma were taken on 23 habitual e-cigarette (unspecified type) users (most days for at least 1 year) and 19 non-tobacco or electronic cigarette users. | Habitual e-cigarette use was associated with a shift towards sympathetic predominance and increased systemic oxidative stress when compared with non-users.30 |
| Fluorodeoxyglucose positron emission tomography/computer tomography (FDG-PET/CT) was performed on nine habitual tobacco cigarette smokers, e-cigarette (unspecified type) users (most days for at least 1 year) or non-users (27 total). | Increased FDG uptake in the spleen and aortic tissues, indicating activation of the Splenocardiac Axis, was observed in both habitual e-cigarette and cigarette users.31 | |
| Using the National Health Interview Surveys (NHIS) of 2014 and 2016 the cross-sectional association between e-cigarette (unspecified type) use, cigarette smoking, and myocardial infarction (MI) was examined. | Daily e-cigarette users were found to be 1.79 times more likely, when accounting for other risk factors such as cigarette smoking, to experience a MI than individuals who had never used e-cigarettes.32 | |
| 145 smokers (at least 10 tobacco cigarettes/day for at least 5 year) were randomized to three arms receiving either 2.4%, 1.8%, or 0% nicotine cigalike e-cigarettes for 52 weeks. | No change in HR was observed, but statistically significant lowering of blood pressure after switching to e-cigarettes was measured after 52 weeks.33 | |
2. Design of e-cigarettes
Since the e-cigarette was first introduced, its design has diversified greatly with very little regulation.34 Common forms are first-generation disposable ‘cigalikes’, second-generation rechargeable e-cigarettes, and third-generation tanks, pens, and personalized large tank devices. Recently, the development of pod-based devices such as the popularized ‘JUUL’ has significantly changed how e-cigarettes are marketed and now constitute the largest portion of the e-cigarette market.35 These pod devices are believed to have shown success because of their discrete design, high nicotine concentrations and flavourings making them especially appealing to youth.36 Despite the diversity of their shape, all e-cigarettes function similarly, typically consisting of three major parts: a lithium battery power source, a cartridge containing e-liquid and an atomizer or heating device that vapourizes the liquid. When a user inhales through the mouthpiece, the airflow triggers the atomizer and the e-liquid is vapourized, producing a smoke-like vapour. Each additional generation has been designed to hold larger amounts of e-liquid, require less airflow to produce vapour and run at increased heating voltages, allowing users to intake much larger and more concentrated puff volumes for longer periods.
3. Potential cardiovascular toxicity of e-cigarette constituents
The e-liquids used in e-cigarettes typically contain a mixture of propylene glycol and glycerine as solvents with added nicotine and flavourings34 , 37; however, the concentrations of these and other compounds present in e-cigarette vapour remains controversial. There are many variables that play a role in e-cigarette vapour composition, including the propylene glycol to glycerine ratio, manufacturer of the e-liquid, type of e-cigarette, coil temperature, air flow rate,38 nicotine content, and the flavourings added to the e-liquids. That being said, a majority of analyses confirm the presence of nicotine, carbonyl compounds, particulate matter, metals, and flavouring compounds in significant concentrations.39 The potential cardiovascular effects associated with these compounds and their additive effects are summarized in Figure 1.
Figure 1.
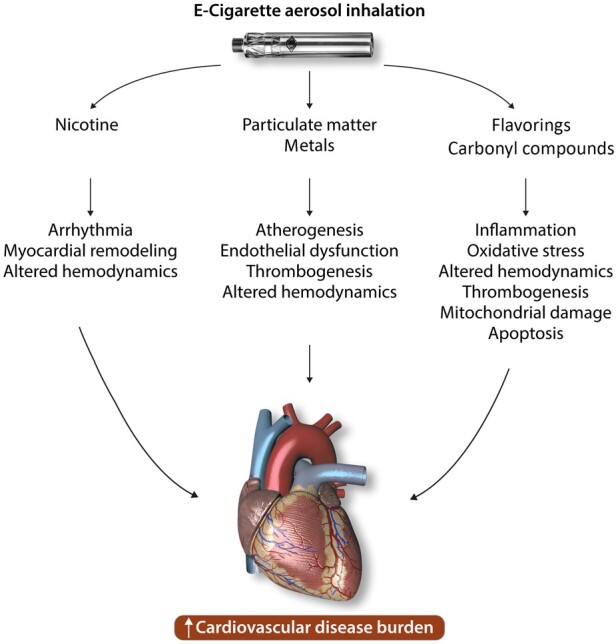
Potential adverse cardiovascular effects induced by various constituents of e-cigarette aerosol.
3.1 Nicotine
Nicotine has been shown to induce a multitude of effects on the cardiovascular system. Nicotine systemically binds to nicotinic cholinergic receptors, which are present in the autonomic ganglia and adrenal gland resulting in release of norepinephrine and epinephrine. The resulting sympathetic nervous stimulation causes predictable plasma nicotine concentration-dependent increases in haemodynamic measurements.19 , 40 Through persistent sympathetic stimulation, nicotine has been shown to cause fibrosis which is associated with arrhythmias41 and myocardial remodelling in animal models.42 A study conducted in pregnant rats exposed to nicotine aerosol inhalation demonstrated reduction and irregular fluctuations in uterine artery blood flow, which is associated with cardiac arrhythmias and fluctuations in systemic blood pressure.43 Additionally, mice subjected to subcutaneous nicotine for 4 weeks demonstrated significant hypertrophy and remodelling in models of systemic hypertension.44 In vitro and in vivo studies comparing cigarettes with normal and low nicotine content suggest that nicotine may modulate platelet activation by decreasing aggregation and therefore decreasing thrombogenesis.45 This claim is controversial as other studies suggest that there is no effect on platelet activity.46 Some studies have shown that e-cigarettes produce lower plasma nicotine levels than traditional cigarettes,19 while others have shown comparable levels believed to be a result of newer e-cigarette models and e-liquids.40 Most e-liquids contain approximately 0–36 mg/mL of free-based nicotine, however, pod devices like the JUUL use tobacco leaf protonated nicotine salts at concentrations above 50 mg/mL35 and have been almost entirely unstudied. Differences in the distribution kinetics of nicotine in e-cigarettes have created discrepancies in its effects on the cardiovascular system, so further studies are essential to determine if nicotine delivery of e-cigarettes produces similar toxicity to that of regular cigarettes.47
3.2 Carbonyl compounds and oxidizing agents
The main carbonyl compounds found in e-cigarette vapour48 (formaldehyde, acetaldehyde, and acrolein) are believed to result from the thermal decomposition of propylene glycol and glycerine49 and some flavourings50 and are known to cause oxidative stress and inflammation.51 Concentrations of these carbonyl compounds are voltage dependent, yielding higher concentrations when a higher battery voltage and higher coil temperatures are used, causing increased thermal degradation products.52 , 53
Exposure to acrolein, a reactive α,β-unsaturated aldehyde, has been shown to cause oxidative stress that can lead to accelerated atherosclerosis. Acrolein can modify A-I protein in HDL, impairing its antiatherogenic function.54 It also oxidizes thioredoxins, an essential protein in the regulation of oxidation and reduction balance in endothelial cells, leading to dysfunction and cell death, and further contributing to atherogenesis.55 One study found that intravenous acrolein depressed myofilament Ca2+ responsiveness and caused myocardial dysfunction in a closed-chest mouse model, likely through the modification of proteins involved in myocardial contractility and metabolism.56 The inhalation of acrolein has also been shown to enhance platelet activation and decrease bleeding time in exposed mice, suggesting a prothrombotic state57 making them susceptible for an increased risk of thrombosis.
Aside from the classification of formaldehyde as a carcinogen, it has been shown to elicit a number of effects on the cardiovascular system. Rat hearts exposed to formaldehyde solution showed decreases in left ventricular end-systolic pressure, heart rate, and cardiac output indicating acute pumping failure.58 Additionally, formaldehyde gas-exposed mice exhibit a significant increase in platelet counts,59 as well as increased oxidative stress in cardiac tissue.60
In a rat model of acetaldehyde administration, moderate to high doses not only decreased the number and density of myocardial mitochondria but also caused significant damage.61 Additionally, acetaldehyde has been found to promote alcoholic cardiomyopathy through mitochondrial dysfunction in mice.62 Systemic changes resulting from the inhalation of acetaldehyde are likely different, however, the effects of e-cigarette exposure on cardiac mitochondria should still be examined.
3.3 Particulate matter
Ultrafine and fine particulate matter (PM0.1, PM2.5) found in significant concentrations in e-cigarettes,63 , 64 are known to elicit a number of cardiovascular effects and contribute to a number of health conditions including atherosclerosis, thrombosis, coronary heart disease, and hypertension. Mechanistically, these effects occur through both direct and indirect pathways.65 PM0.1 and PM2.5 can pass through the alveolar–endothelial interface into the systemic circulation directly affecting the heart, vasculature, and other organs. Specifically, the mechanisms by which PM directly induces cardiovascular effects are through oxidative stress and direct calcium ion channel interference.66 Activation of ANS and pulmonary inflammation results in PM-induced indirect cardiovascular effects.67 The thrombogenic effects of PM are believed to arise from the promotion of clot formation after entering the systemic circulation as well as from pulmonary inflammation caused from prolonged exposure.65 Additionally, PM2.5 has been shown to cause direct dysfunction of vascular endothelial cells,68 a contributing factor to the development of CVDs, particularly atherosclerosis.
3.4 Metals
The presence of a large number of metals in e-cigarette vapour has been demonstrated by previous studies69 , 70; however, these studies are surrounded by discrepancies in the types and concentrations of the metals present in the vapour. Based on a current review of metals found in e-cigarette vapours, even the highest reported metal exposures based on extreme daily e-cigarette use (1200 puffs/day), generally, still fall below exposure guidelines.71 The authors concluded that overall exposure to metals from e-cigarette use is not expected to be of significant health concern for smokers switching to e-cigarettes but is an unnecessary source of exposure for never-smokers. However, metals have been shown to act as a catalyst in the oxidative reactions of cellular proteins by the compounds in cigarette smoke (CS),72 so their role related to other e-cigarette constituents is likely similar.
3.5 Flavourings
Based on comprehensive internet searches of English-language websites, Zhu et al.73 stated that over 7700 unique flavours were identified across all brands of e-cigarettes. The concentration of these molecules in e-liquid was reported to range from 0 to 36 mg/mL.74 Many of the chemicals are deemed safe via ingestion75; however, limited data are available on their effects following chronic inhalation. For example, cinnamaldehyde is considered safer via oral administration; however, inhalation induces dysfunction of pulmonary immune cells through alterations of proinflammatory cytokines, a theorized mechanism for adverse cardiac function.65 , 76 In a recent study, monocytes treated with flavouring chemicals and flavoured e-liquid without nicotine caused dose-dependent cytotoxicity. The authors concluded that the flavourings used in e-juices can trigger an inflammatory response in monocytes, mediated by reactive oxygen species (ROS) production.77 In another study, freshly isolated endothelial cells from participants treated with different flavouring compounds impaired nitric oxide production and induced inflammation consistent with endothelial dysfunction.78 A multitude of studies have shown proinflammatory and cytotoxic responses in the lungs, with certain flavour molecules also known to cause pulmonary disease via inhalation.79 , 80 Given the general lack of knowledge, but potential inflammatory and toxic effects of inhalation of flavouring additives, studies analysing cardiac-specific alterations in response to e-cigarette flavours are imperative for determining health risks.
3.6 Synergistic/additive effects
It should be pointed out that exposure to individual e-cigarette aerosol constituents in isolation as described above does not occur for a user. Instead, exposure to all of these compounds in e-cigarette vapour likely has various additive and synergistic effects. For example, the oxidative stress and inflammatory pathways of acrolein, PM, and flavouring compounds could all contribute to a greater risk of developing atherosclerosis. Similarly, increases in platelet activity demonstrated by exposure to acrolein and PM could be joint contributors to potential prothrombotic issues for the user. Therefore, while individual constituents found in e-cigarette aerosol have been shown to individually induce negative effects on the cardiovascular system, the extent to which they act (as agonists or antagonists to one another) likely effects the overall harmfulness of e-cigarette use.
4. Experimental and epidemiological studies on the cardiovascular effects of e-cigarette use
4.1 Acute exposure effects
While chronic e-cigarette exposure studies are generally limited, more studies evaluating their short-term impacts on the cardiovascular system have been conducted. Acute study exposures are typically one-time exposures, while chronic studies typically include multiple exposures over a significant period of time or observe measures in populations of long-term e-cigarette users. Different durations and populations used in current studies are examined in Table 1. Large discrepancies in findings of acute exposure studies have resulted due to a variety of factors, including differences in methods of measurement, device voltage, e-liquid (nicotine content and flavouring) used, and the amount and duration of vapour inhaled.81 These discrepancies create difficulty in drawing absolute conclusions about the short-term impacts of e-cigarettes on the cardiovascular system, however, current evidence suggests that they are not without harm.82
4.1.1 Preclinical studies
4.1.1.1 Cytotoxicity and inflammatory effects
Human umbilical vein endothelial cells (HUVECs) treated with e-cigarette aerosol demonstrated an induction of ROS, DNA damage, reduced cell viability, apoptosis and necrosis, inhibition of cell proliferation, and alterations in cell morphology.7 , 8 Farsalinos et al.9 evaluated the cytotoxic potential of 20 e-liquid samples on cultured H9C2 cells. Their results demonstrated three e-cigarette extracts were cytotoxic at 100% and 50% extract concentrations and one was only cytotoxic at 100% concentration. A similar study was conducted by Romagna et al.10 using 21 e-liquid samples on mammalian fibroblasts, where only one vapour extract was cytotoxic at 100% extract concentration. Both studies demonstrated e-cigarette vapour cytotoxicity to be far less than CS, likely due to the lower number of toxic compounds present in e-cigarette vapour.9 , 10 Primary lung microvascular endothelial cells treated with e-cigarette solution, condensed e-cigarette vapour, or CS with and without nicotine demonstrated that nicotine-free e-cigarette liquids and vapours and nicotine-containing e-cigarette and cigarette extracts caused significantly greater endothelial permeability compared with nicotine free-CS extract (10–20%). These effects were associated with oxidative stress and inflammation12 and suggest that e-cigarettes can induce cytotoxic and inflammatory effects. However, caution must be used in interpreting these in vitro studies for their extrapolation to the in vivo setting as both the concentrations and contents of e-liquids and vapours and their effects likely differ once heated, aerosolized, and metabolized in vivo.
4.1.1.2 Cardiac development
In a recent study, human embryonic stem cells and a zebrafish model were used as tools to study the effects of e-cigarettes on cardiac differentiation and development. The authors concluded that e-cigarette exposure decreased expression of cardiac transcription factors in cardiac progenitor cells, suggesting a persistent delay in differentiation. They also found decreased expression of MLC2v and MYL6 (sarcomeric genes). In zebrafish, the authors observed developmental defects such as severe heart malformation, pericardial oedema, and reduced heart function as evidenced by decreased contractile proteins cmlc2 and tnnt2, the transcription factor mef2ca, and the junctional protein cx43, responsible for heart electromechanical conduction.18 It is important to note that this study used a cigarette extract group, e-cigarette extract group, and nicotine extract as a control exposure group. Significant effects were observed in groups exposed to cigarette and e-cigarette extracts, but not in the nicotine controls. This suggests that the cardiac developmental effects observed in the study resulted from the constituents other than nicotine present in e-cigarette aerosol.
4.1.1.3 Platelet function
In a 1-week exposure study, platelets from mice exposed to e-cigarette aerosol showed significant hyperactivity through enhanced aggregation and dense α granule secretion, increased resistance to inhibition by prostacyclin and decreased bleeding and occlusion times when compared with controls.14 The effect on platelet function in this study was proposed to be caused by either the PM or acrolein present in e-cigarette aerosol,14 which have previously been shown to individually increase platelet function. In another study, platelets from healthy volunteers exposed to e-cigarette vapour extract showed significant up-regulation in proinflammatory markers gC1qR and cC1qR and deposition of C3b similarly to traditional tobacco smoke extracts. Additionally, platelet activation, aggregation, and adhesion were also significantly enhanced after exposure to e-cigarette vapour extracts.15 Taken together, this hyperactivity shows a pro-thrombotic state that arises following short-term exposure to e-cigarettes, suggesting chronic exposure may increase the risk of thrombogenic events.
4.1.2 Clinical studies
4.1.2.1 Heart rate and blood pressure/haemodynamic function
Use of e-cigarettes causes significant short-term elevations in heart rate and systolic blood pressure (SBP) and diastolic blood pressure (DBP). The magnitude of this effect was originally shown to be much less when compared with conventional cigarettes.19 Increased sympathetic nerve activity (SNA) has also been reported after e-cigarette use (with nicotine) but not nicotine-free e-cigarette use,24 which predisposes users to increased cardiovascular risk.83 The acute increases in SNA, blood pressure, and heart rate are typically attributed to the vasoactivity of nicotine. Aside from the addictive properties, the effects of nicotine likely result from its role in sympathetic nervous system activation causing increased heart rate, blood pressure, and myocardial contractility.47 The lower magnitude of effect on heart rate and blood pressure observed in e-cigarette users vs. traditional cigarette users has been attributed to the lower plasma nicotine concentrations that result from e-cigarette use. However, newer model devices with higher voltages, lower resistances, and higher e-liquid nicotine concentrations produce blood-nicotine levels comparable to traditional cigarettes.84 More recent studies have demonstrated that acute e-cigarette use is associated with increased SBP, DBP, and arterial stiffness similar to tobacco cigarettes.20–22 Extrapolation of this more recent acute exposure data suggests that the chronic elevations in haemodynamic function and arterial stiffness may induce the adverse cardiovascular effects and increased CVD risk.39
4.1.2.2 Oxidative stress, endothelial dysfunction, and vascular injury
Levels of endothelial progenitor cells (EPCs) in blood samples of healthy volunteers were significantly increased 1 h following exposure to e-cigarette vapour (10 puffs for 10 min) and returned to baseline values after 24 h.16 EPCs play a key role in endothelial repair and therefore elevated levels serve as a biomarker of vascular injury.85 In a recent study,17 following e-cigarette exposure, users showed increases in serum soluble NOX2 derived peptide and 8-iso-prostaglandin F2α, and decreases in serum nitric oxide bioavailability, indicating an increase in oxidative stress. Flow mediated dilation (FMD), a conventional measure of vascular function in humans,86 was also found to be significantly reduced after exposure, showing that participants experienced significant endothelial dysfunction. Similar decreases in FMD have been shown to be associated with coronary artery disease.17 These results were believed to arise from the multitude of compounds found in e-cigarette aerosol that can elicit negative effects on inflammatory status including nicotine, PM, and flavourings.17 A more recent study of 25 occasional smokers found elevated myeloperoxidase levels following acute exposure to e-cigarettes, indicative of oxidative stress22 and positively correlated with risk of experiencing a CVD event.87 Interestingly, the authors observed elevated myeloperoxidases after vaping nicotine containing e-cigarettes, but not after nicotine-free vaping suggesting that nicotine is possibly responsible for the observed effect.
4.2 Chronic exposure effects
4.2.1 Preclinical studies
4.2.1.1 Cardiac function
Only three studies using mouse models have evaluated the effects of chronic e-cigarette exposure on the cardiovascular system.27–29 Olfert et al.27 demonstrated that chronic exposure to e-cigarettes (conducted over 8 months and equivalent to ∼25 years in humans) even at relatively low levels leads to significantly increased arterial stiffness, reduced vascular relaxation to vasodilators, and enhanced responses to vascular constrictor agents. These findings are associated with impaired cardiac function and development of CVD. No impact on heart rate, stroke volume, and cardiac output was reported; however, modest decreases in fractional shortening and ejection fraction were observed compared with exposure to traditional CS. It was extrapolated that the degree of vascular dysfunction caused by chronic e-cigarette exposure in this study when compared with other well-known CVD risk factors was similar to traditional cigarette smoking.27 Various other short-term studies support this effect that e-cigarette exposure can have on endothelial dysfunction,16 , 17 which is closely associated with accelerated atherosclerosis and CVD.88 While the mechanism for this result was not examined, the authors proposed it to be a result of the similar PM levels in e-cigarettes and traditional cigarettes. In another recent study,28 increased systemic inflammation, greater levels of heart fibrosis, along with increased SBP and an upward trending DBP in mice exposed for 3–6 months to e-cigarette vapour was shown, compared with those exposed to air. The e-cigarette vapour used in this study was composed of 50/50 propylene glycol/glycerine solvent mix with 24 mg/mL of nicotine, indicating that the adverse effects resulted from nicotine, the solvents and their byproducts of aerosolization and metabolism. Additionally, mice exposed for 3 months to e-CS demonstrated increased DNA mutagenic adducts γ-OH-PdG and O6-medG formation in various tissues including the heart.29 The authors suggested that the observed effect was due to the small amount of nicotine (∼10%) which is metabolized into nitrosamines, capable of inducing DNA damage.29 This important study clearly suggests that chronic e-cigarette use may put the user at an increased risk of both the development of cancers and various CVDs.
4.2.2 Clinical studies
4.2.2.1 Cardiac sympathetic activity, inflammation, and oxidative stress
A cross-sectional case–control study by Moheimani et al.30 analysed habitual e-cigarette users to identify the adverse effects of e-cigarette use during a year-long study. Their findings demonstrated that e-cigarette use was associated with a shift in cardiac autonomic balance towards sympathetic predominance. Compared with non-user controls, LDL oxidizability (indicative of the susceptibility of apolipoprotein B-containing lipoproteins to oxidation) and markers of oxidative stress were also significantly increased in e-cigarette users. Both of these effects of chronic e-cigarette use are associated with increased cardiovascular risk. A recent study by Boas et al.31 reported activation of the splenocardiac axis (an inflammatory signalling network initiated by increased SNA which underlies the development of atherosclerosis and acute myocardial infarction) following chronic exposure to both traditional cigarettes and e-cigarettes. Using 18F-flurorodeoxyglucose positron emission tomography/computer tomography, investigators demonstrated increased metabolic activity of haematopoietic and vascular tissues through increased 18F-flurorodeoxyglucose uptake in spleen and aortic tissue of habitual (most days for ≥1 year) e-cigarette and traditional cigarette users (not dual-users). This activation is suggestive of an underlying mechanism by which e-cigarette use may lead to an increased risk of cardiovascular events. Overall, this increased chronic use risk is supported by a recent study analysing data from the National Health Interview Study (NHIS) of 2014 and 2016 which found that daily e-cigarette users were 1.79 times more likely, when accounting for other risk factors such as cigarette smoking, to experience myocardial infarction than individuals who had never used e-cigarettes.32 It is necessary to note that due to this being an observational study, it is potentially limited by non-random misclassification bias.89 Even since the systematic review of e-cigarettes by Skotsimara et al. was published in 2019, this study remains the only evidence available directly associating e-cigarette use and cardiovascular risk, suggesting the need for confirmation and validation from other studies.
5. The ongoing controversy/e-cigarette debate
There is an existing argument in considering e-cigarettes as a safe alternative to traditional cigarettes as some studies have reported no adverse effects. Such findings give rise to the controversy regarding risk associated with e-cigarette use and advocates for its use as a tool for smoking cessation.
5.1 Preclinical studies
To investigate the effect of e-cigarettes on stress response, Teasdale et al.13 exposed human coronary artery endothelial cells to cigarette smoke extract (CSE) or e-cigarette aerosol extract (eCAE). Their results demonstrated activation of NRF2 and up-regulation of cytochrome p450 following CSE exposure but the effect was not observed following eCAE exposure. The investigators concluded that eCAE does not induce the same stress response as CSE and therefore could be used as a substitute for conventional cigarettes as it is likely to reduce immediate tobacco-related cardiovascular effects. Another study performed on HUVECs to measure cell migration (scratch wound assay) reported that CS had an inhibitory effect on endothelial migration while no significant inhibition of HUVEC migration was measured following e-cigarette exposure. The scratch wound assay enables a comparative assessment between conventional cigarettes and e-cigarettes in vitro and demonstrates the usefulness and versatility of this assay for the assessment of e-cigarettes.11 The evidence shown by these in vitro models suggests e-cigarettes could be effective as a harm-reduction alternative to traditional cigarettes by reducing oxidative stress, inflammation, and cytotoxicity. However, these findings are in direct contrast to the majority of studies to date, so caution should be used when interpreting findings for intervention in smokers.
5.2 Clinical studies
Fifteen healthy chronic smoking (≥5 cigarettes a day for ≥2 years) participants were subjected to smoking a traditional cigarette or e-cigarette to evaluate the effect on arterial stiffness. The use of e-cigarettes resulted in no change in the stiffness index and reflection index, coupled with an insignificant increase in SBP and DBP and heart rate.26 Another study conducted in 105 clinically confined smokers investigated the effect of acute e-cigarette on cardiovascular functions (SBP and DBP and heart rate), respiratory function (FVC, FEV1, and exhaled CO and NO), and adverse events.25 The subjects were randomized into groups that either completely or partially switched from conventional cigarettes to e-cigarettes or completely discontinued using tobacco and nicotine products altogether. The authors reported that the use of e-cigarettes for 5 days under the various study conditions (subjects who had completely or partially switched from conventional cigarettes to e-cigarettes or completely discontinued using tobacco and nicotine products altogether) did not lead to higher blood pressure or heart rate values, negative respiratory health outcomes, or serious adverse health events. Instead, lower blood pressure and heart rate and better lung function were reported in participants who switched to e-cigarettes or ceased smoking altogether. Similarly, a recent study conducted by Farsalinos et al.33 demonstrated that smokers who reduce or quit smoking by switching to e-cigarettes may lower their SBP in the long term, and this reduction is apparent in smokers with elevated BP. An additional study by Farsalinos et al.23 conducted on 40 electronic cigarette users demonstrated higher mitral annulus diastolic velocity and diastolic strain rate, and lower isovolumetric relaxation time and myocardial performance index after seven minutes of e-cigarette use compared with chronic smokers who smoked one tobacco cigarette. The observed differences were significant even after adjusting for changes in heart rate and blood pressure. These studies used their findings to advocate for the potential of e-cigarettes as a safer alternative to tobacco cigarettes and use as a cessation tool.
5.3 Limitations
Limitations of clinical studies impact their applicability to the general public. Many studies have low statistical power and enroll volunteers who were previous or current smokers, impacting the validity of results to cover all profiles of e-cigarette use such as the large population of never-smoking youth who are being introduced to vaping. The primary research question addressed in the majority of studies is the efficacy and safety of c-cigarette use as an aid to quit traditional cigarette smoking. As shown in Table 1, there is very little replicability amongst current studies in terms of methods and exposure protocol for evaluating the effects of e-cigarettes, making it difficult to compare results and draw concrete conclusions. Additionally, the wide variety of flavourings, device models, and populations that vape further decrease the replicability across studies and hinders the ability to accurately determine the safety profile of e-cigarette use. The generalizability of many findings is limited as many older and even some recent studies continue to use older e-cigarette models, while the majority of users now use pod-based devices not yet studied in an exposure model.
5.4 Current/future studies
From available NIH data (clinicaltrials.gov) and the search term ‘electronic cigarette’, there appear to be multiple major clinical studies ongoing evaluating the cardiovascular effects of e-cigarette use. These studies vary but include evaluations of both acute and chronic use. Measurements include biomarkers for inflammation, oxidative stress, endothelial function, platelet function, blood pressure, heart rate, myocardial function, and arterial stiffness. Data regarding current in vitro and in vivo studies is unavailable however our laboratory is currently assisting with this goal in various mouse model exposures. Measures of cardiomyocyte function, ventricular function, heart tissue fibrosis, and inflammatory and oxidative stress markers are being conducted on various populations including adolescent and adult mice exposed to e-cigarette aerosol (±nicotine) for one week (acute) up to 3 months (chronic). Current clinical and preclinical studies still lack consistency using various devices, e-liquids, and exposure protocols; however, they will hopefully provide more valuable data to fill many of the gaps in and build upon the current knowledge presented from the studies in this review.
6. Conclusion
While the current but still limited literature suggests that e-cigarette use may lead to fewer negative cardiovascular effects than conventional cigarettes, our review supports that there is not sufficient data to conclusively make these resolutions. These studies have expanded the existing knowledge on e-cigarettes and their potential in the development of CVDs, but whether or not these results can be translated into clinical practice is still a cause for concern. The studies presented in this review have shown that e-cigarettes can induce negative cardiovascular effects through various mechanisms such as oxidative stress, inflammation, DNA damage, arterial stiffness, and altered haemodynamics and platelet activity (shown in Figure 2). Individually and in combination with one another, these effects suggest pathways that chronic e-cigarette use could increase the development of CVD. As a result, great caution and hesitation should remain concerning e-cigarette use until its health risk profile is better established. Therefore, additional high-quality randomized controlled trials are needed to conclusively establish the safety and efficacy of e-cigarettes. The focus of future studies should continue to be placed on investigating both the long- and short-term effects of e-cigarette exposure and their potential role in CVD development. Currently, a majority of studies evaluating the effects of e-cigarettes on the cardiovascular system focus on the addition of nicotine to e-liquid and heavily neglect to include flavourings as a variable. Flavouring molecules add significant variations to e-cigarette aerosol composition and while most are deemed safe when ingested orally, little is known of their systemic effects following inhalation. Recognizing this gap in knowledge of the chronic inhalation of flavourings, additional studies analysing cardiovascular specific alterations in response to common e-cigarette flavours must be conducted to truly determine potential risks. Experimental studies should also further explore other populations including cardiovascular developmental effects of e-cigarette exposure during the in utero period to examine their perceived safety as a cigarette cessation method during pregnancy. With the addition of these future experimental and clinical trial data determining the general safety profile of e-cigarettes, regulatory guidelines defining their proper use and public perception can be better formulated.
Figure 2.
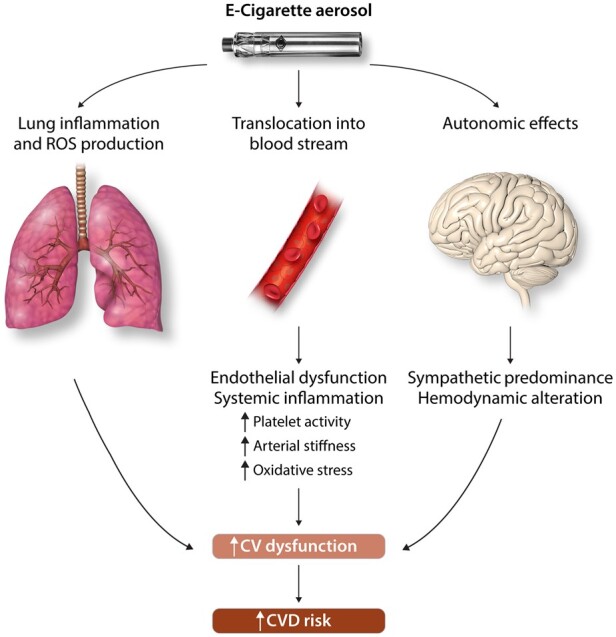
Mechanisms of e-cigarette induced cardiovascular dysfunction.
Conflict of interest: none declared.
Funding
The authors are supported by National Institutes of Health [AG057046, ES019923 to L.E.W.] and [HL139348 to P.J.M. and L.E.W.].
References
- 1. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief 2017;293:1–8. [PubMed] [Google Scholar]
- 2. Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, King BA. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep 2016;65:361–367. [DOI] [PubMed] [Google Scholar]
- 4. Gentzke AS, Creamer M, Cullen KA, Ambrose BK, Willis G, Jamal A, King BA. Vital signs: tobacco product use among middle and high school students—United States, 2011-2018. MMWR Morb Mortal Wkly Rep 2019;68:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glantz SA, Bareham DW. E-cigarettes: use, effects on smoking, risks, and policy implications. Annu Rev Public Health 2018;39:215–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 2017;14:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson C, Majeste A, Hanus J, Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci 2016;154:332–340. [DOI] [PubMed] [Google Scholar]
- 8. Putzhammer R, Doppler C, Jakschitz T, Heinz K, Förste J, Danzl K, Messner B, Bernhard D. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One 2016;11:e0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farsalinos K, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health 2013;10:5146–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol 2013;25:354–361. [DOI] [PubMed] [Google Scholar]
- 11. Taylor M, Jaunky T, Hewitt K, Breheny D, Lowe F, Fearon IM, Gaca M. A comparative assessment of e-cigarette aerosols and cigarette smoke on in vitro endothelial cell migration. Toxicol Lett 2017;277:123–128. [DOI] [PubMed] [Google Scholar]
- 12. Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, Kranz WD, Carroll CJ, Ray BD, Bittman R, Goodpaster J, Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol 2015;309:L175–L187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teasdale JE, Newby AC, Timpson NJ, Munafò MR, White SJ. Cigarette smoke but not electronic cigarette aerosol activates a stress response in human coronary artery endothelial cells in culture. Drug Alcohol Depend 2016;163:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qasim H, Karim ZA, Silva‐Espinoza JC, Khasawneh FT, Rivera JO, Ellis CC, Bauer SL, Almeida IC, Alshbool FZ. Short-term E-cigarette exposure increases the risk of thrombogenesis and enhances platelet function in mice. J Am Heart Assoc 2018;7:e009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W, Rubenstein DA. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets 2016;27:694–702. [DOI] [PubMed] [Google Scholar]
- 16. Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, Lundbäck M. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 2016;255:179–185. [DOI] [PubMed] [Google Scholar]
- 17. Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AGM, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 2016;150:606–612. [DOI] [PubMed] [Google Scholar]
- 18. Palpant NJ, Hofsteen P, Pabon L, Reinecke H, Murry CE. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PloS One 2015;10:e0126259.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol 2015;71:24–34. [DOI] [PubMed] [Google Scholar]
- 20. Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, Dalhoff K, Mortensen K, Droemann D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: a randomized, double-blinded pilot study. Vasc Med 2018;23:419–425. [DOI] [PubMed] [Google Scholar]
- 21. Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, Stefanadis C, Tousoulis D. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol 2016;67:2802–2803. [DOI] [PubMed] [Google Scholar]
- 22. Chaumont M, de Becker B, Zaher W, Culié A, Deprez G, Mélot C, Reyé F, Van Antwerpen P, Delporte C, Debbas N, Boudjeltia KZ, van de Borne P. Differential effects of E-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: a randomized crossover trial. Sci Rep 2018;8:10378.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: comparison with the effects of regular cigarettes. BMC Cardiovasc Disord 2014;14:78.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moheimani RS, Bhetraratana M, Peters KM, Yang BK, Yin F, Gornbein J, Araujo JA, Middlekauff HR. Sympathomimetic effects of acute E-cigarette use: role of nicotine and non-nicotine constituents. J Am Heart Assoc 2017;6:e006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Ruiz CD, O'Connell G, Graff DW, Yan XS. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regul Toxicol Pharmacol 2017;87:36–53. [DOI] [PubMed] [Google Scholar]
- 26. Szołtysek-Bołdys I, Sobczak A, Zielińska-Danch W, Bartoń A, Koszowski B, Kośmider L. Influence of inhaled nicotine source on arterial stiffness. Przeglad Lekarski 2014;71:572–575. [PubMed] [Google Scholar]
- 27. Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, Sullivan DP, Breit MJ, Wu Z, Klinkhachorn P, Mandler WK, Erdreich BH, Ducatman BS, Bryner RW, Dasgupta P, Chantler PD. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985) 2018;124:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, Breen EC. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol 2018;314:R834–R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee HW, Park SH, Weng MW, Wang HT, Huang WC, Lepor H, Wu XR, Chen LC, Tang MS. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA 2018;115:E1560–E1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol 2017;2:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boas Z, Gupta P, Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Czernin J, Middlekauff HR. Activation of the “Splenocardiac Axis” by electronic and tobacco cigarettes in otherwise healthy young adults. Physiol Rep 2017;5:e13393.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alzahrani T, Pena I, Temesgen N, Glantz SA. Association between electronic cigarette use and myocardial infarction. Am J Prev Med 2018;55:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farsalinos K, Cibella F, Caponnetto P, Campagna D, Morjaria JB, Battaglia E, Caruso M, Russo C, Polosa R. Effect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettes. Intern Emerg Med 2016;11:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N. Electronic cigarettes: a policy statement from the American Heart Association. Circulation 2014;130:1418–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang J, Duan Z, Kwok J, Binns S, Vera LE, Kim Y, Szczypka G, Emery SL. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019;28:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. King BA, Gammon DG, Marynak KL, Rogers T. Electronic cigarette sales in the United States, 2013-2017. JAMA 2018;320:1379–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 2014;12:CD010216. [DOI] [PubMed] [Google Scholar]
- 38. Korzun T, Lazurko M, Munhenzva I, Barsanti KC, Huang Y, Jensen RP, Escobedo JO, Luo W, Peyton DH, Strongin RM. E-cigarette airflow rate modulates toxicant profiles and can lead to concerning levels of solvent consumption. ACS Omega 2018;3:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems; Eaton DL, Kwan LY, Stratton K. Public Health Consequences of E-Cigarettes. Washington (DC: ): National Academies Press (US; ); 2018. [PubMed] [Google Scholar]
- 40. St Helen G, Havel C, Dempsey DA, Jacob P, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016;111:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. D'Alessandro A, Boeckelmann I, Hammwhöner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol 2012;19:297–305. [DOI] [PubMed] [Google Scholar]
- 42. Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB J 2012;26:4778–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shao XM, López-Valdés HE, Liang J, Feldman JL. Inhaled nicotine equivalent to cigarette smoking disrupts systemic and uterine hemodynamics and induces cardiac arrhythmia in pregnant rats. Sci Rep 2017;7:16974.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Colombo ES, Davis J, Makvandi M, Aragon M, Lucas SN, Paffett ML, Campen MJ. Effects of nicotine on cardiovascular remodeling in a mouse model of systemic hypertension. Cardiovasc Toxicol 2013;13:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Girdhar G, Xu S, Bluestein D, Jesty J. Reduced-nicotine cigarettes increase platelet activation in smokers in vivo: a dilemma in harm reduction. Nicotine Tob Res 2008;10:1737–1744. [DOI] [PubMed] [Google Scholar]
- 46. Ljungberg LU, Persson K, Eriksson AC, Green H, Whiss PA. Effects of nicotine, its metabolites and tobacco extracts on human platelet function in vitro. Toxicol In Vitro 2013;27:932–938. [DOI] [PubMed] [Google Scholar]
- 47. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med 2016;26:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, Fu X-A. Aldehyde detection in electronic cigarette aerosols. ACS Omega 2017;2:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, Shusterman D, Mendell M, Kumagai K. A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLoS One 2017;12:e0169811.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khlystov A, Samburova V. Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ Sci Technol 2016;50:13080–13085. [DOI] [PubMed] [Google Scholar]
- 51. Shields PG. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: a focus on inflammation. Cancer Epidemiol Biomarkers Prev 2017;26:1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kosmider L. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014;16:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 2014;11:11192–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shao B, Fu X, McDonald TO, Green PS, Uchida K, O'Brien KD, Oram JF, Heinecke JW. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem 2005;280:36386–36396. [DOI] [PubMed] [Google Scholar]
- 55. Szadkowski A, Myers CR. Acrolein oxidizes the cytosolic and mitochondrial thioredoxins in human endothelial cells. Toxicology 2008;243:164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luo J, Hill BG, Gu Y, Cai J, Srivastava S, Bhatnagar A, Prabhu SD. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am J Physiol Heart Circ Physiol 2007;293:H3673–H3684. [DOI] [PubMed] [Google Scholar]
- 57. Sithu SD, Srivastava S, Siddiqui MA, Vladykovskaya E, Riggs DW, Conklin DJ, Haberzettl P, O'Toole TE, Bhatnagar A, D'Souza SE. Exposure to acrolein by inhalation causes platelet activation. Toxicol Appl Pharmacol 2010;248:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takeshita D, Nakajima-Takenaka C, Shimizu J, Hattori H, Nakashima T, Kikuta A, Matsuyoshi H, Takaki M. Effects of formaldehyde on cardiovascular system in in situ rat hearts. Basic Clin Pharmacol Toxicol 2009;105:271–280. [DOI] [PubMed] [Google Scholar]
- 59. Zhang Y, Liu X, McHale C, Li R, Zhang L, Wu Y, Ye X, Yang X, Ding S. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One 2013;8:e74974.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Güleç M, Songur A, Sahin S, Ozen OA, Sarsilmaz M, Akyol O. Antioxidant enzyme activities and lipid peroxidation products in heart tissue of subacute and subchronic formaldehyde-exposed rats: a preliminary study. Toxicol Ind Health 2006;22:117–124. [DOI] [PubMed] [Google Scholar]
- 61. Jin Y-Z, Wang G-F, Wang Q, Zhang X-Y, Yan B, Hu W-N. Effects of acetaldehyde and L-carnitine on morphology and enzyme activity of myocardial mitochondria in rats. Mol Biol Rep 2014;41:7923–7928. [DOI] [PubMed] [Google Scholar]
- 62. Brandt M, Garlapati V, Oelze M, Sotiriou E, Knorr M, Kröller-Schön S, Kossmann S, Schönfelder T, Morawietz H, Schulz E, Schultheiss H-P, Daiber A, Münzel T, Wenzel P. NOX2 amplifies acetaldehyde-mediated cardiomyocyte mitochondrial dysfunction in alcoholic cardiomyopathy. Sci Rep 2016;6:32554.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut 2014;184:523–529. [DOI] [PubMed] [Google Scholar]
- 64. Sosnowski TR, Odziomek M. Particle size dynamics: toward a better understanding of electronic cigarette aerosol interactions with the respiratory system. Front Physiol 2018;9:853.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tanwar V, Katapadi A, Adelstein JM, Grimmer JA, Wold LE. Cardiac pathophysiology in response to environmental stress: a current review. Curr Opin Physiol 2018;1:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nelin TD, Joseph AM, Gorr MW, Wold LE. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett 2012;208:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mills NL, TöRnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 2005;112:3930–3936. [DOI] [PubMed] [Google Scholar]
- 68. Dai J, Chen W, Lin Y, Wang S, Guo X, Zhang QQ. Exposure to concentrated ambient fine particulate matter induces vascular endothelial dysfunction via miR-21. Int J Biol Sci 2017;13:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 2013;8:e57987.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jörres RA, Fromme H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health 2014;217:628–637. [DOI] [PubMed] [Google Scholar]
- 71. Farsalinos KE, Voudris V, Poulas K. Are metals emitted from electronic cigarettes a reason for health concern? A risk-assessment analysis of currently available literature. Int J Environ Res Public Health 2015;12:5215–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bernhard D, Csordas A, Henderson B, Rossmann A, Kind M, Wick G. Cigarette smoke metal-catalyzed protein oxidation leads to vascular endothelial cell contraction by depolymerization of microtubules. FASEB J 2005;19:1096–1107. [DOI] [PubMed] [Google Scholar]
- 73. Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014;23 (Suppl 3):iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res 2015;17:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sears CG, Hart JL, Walker KL, Robertson RM. Generally recognized as safe: uncertainty surrounding E-cigarette flavoring safety. Int J Environ Res Public Health 2017;14:1274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, Jaspers I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol 2017;313:L278–L292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol 2018;8:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A, Hamburg NM. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol 2018;38:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control 2016;25:ii81–ii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gerloff J, Sundar IK, Freter R, Sekera ER, Friedman AE, Robinson R, Pagano T, Rahman I. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol 2017;3:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ. Impact of electronic cigarettes on the cardiovascular system. J Am Heart Assoc 2017;6:e006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bhatnagar A. Cardiovascular perspective of the promises and perils of E-cigarettes. Circ Res 2016;118:1872–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk in Communities. Circulation 2000;102:1239–1244. [DOI] [PubMed] [Google Scholar]
- 84. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep 2015;4:4133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El-Chami MF, Bhakta S, Winchester DE, Al-Mallah MH, Sanchez Shields M, Deedwania P, Mehta LS, Phan BA, Benowitz NL. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the American College of Cardiology. J Am Coll Cardiol 2015;66:1378–1391. [DOI] [PubMed] [Google Scholar]
- 86. Loffredo L, Carnevale R, Perri L, Catasca E, Augelletti T, Cangemi R, Albanese F, Piccheri C, Nocella C, Pignatelli P, Violi F. NOX2-mediated arterial dysfunction in smokers: acute effect of dark chocolate. Heart 2011;97:1776–1781. [DOI] [PubMed] [Google Scholar]
- 87. Wong ND, Gransar H, Narula J, Shaw L, Moon JH, Miranda-Peats R, Rozanski A, Hayes SW, Thomson LE, Friedman JD, Berman DS. Myeloperoxidase, subclinical atherosclerosis, and cardiovascular disease events. JACC Cardiovasc Imaging 2009;2:1093–1099. [DOI] [PubMed] [Google Scholar]
- 88. Steyers CM, Miller FJ. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 2014;15:11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Skotsimara G, Antonopoulos AS, Oikonomou E, Siasos G, Ioakeimidis N, Tsalamandris S, Charalambous G, Galiatsatos N, Vlachopoulos C, Tousoulis D. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol 2019;26:1219–1228. [DOI] [PubMed] [Google Scholar]


