Abstract
Objective:
Esophageal carcinosarcoma (ECS) is a rare malignant tumor that accounts for only 0.5%-2.8% of all esophageal malignancies. As most current studies are case reports, the relationship between clinical features and prognosis remains controversial.
Methods:
We investigated the clinical features and prognosis of 24 patients with ECS in a single center from 2006 to 2018. There were 18 male and 6 female patients aged 52-82 years with a median age of 62.5 years. In addition, we included 9 studies on ECS from PubMed and a literature review.
Results:
The median follow-up time of the 24 patients was 70.5 (range, 10-156)months. The 3-year and 5-year survival rates were 83.3% and 70.8%, respectively. Among the 24 patients, none of the 10 (41.7%) stage T1 cancer patients had lymph node metastasis; however, lymph node metastasis was noted in 8 (57.1%) stage T2-4 cancer patients. The literature review revealed that 211 patients had a 5-year survival rate of 11.8%-68.2%, and 54.5%-95.8% study participants had early stage ECS. Although the information provided in the literature review is limited, it appears to be a characteristic of the early stage of the disease and predicts better prognosis when ECS is diagnosed, which is similar to the result of the current study.
Conclusion:
Our results indicate that ECS has a favorable prognosis, even among patients with early stage ECS who undergo radical esophagectomy with lymph node dissection. Because of the low incidence of ECS, further studies with more cases need to investigate this rare malignancy.
Keywords: esophageal cancer, carcinosarcoma, cancer survival, solid tumor, cancer
Introduction
Esophageal cancer mainly includes esophageal adenocarcinoma(EAC) and esophageal squamous cell carcinoma(ESCC) and ranks seventh and sixth with respect to tumor incidence (572,000 new cases) and total tumor mortality (509,000 deaths), respectively. 1 Carcinosarcoma is a rare malignant tumor consisting of carcinomatous and sarcomatous components, which was first proposed by Virchow in 1865. 2 Carcinosarcoma usually occurs in different parts of the uterus, breast, thyroid, lung and gastrointestinal system. 3 Esophageal carcinosarcoma (ECS) is a relatively rare malignant tumor, accounting for 0.5%-2.8% 4 of all esophageal malignancies, and it was first described by Hansemannl in 1904. 5 Multiple designations such as carcinosarcoma, pseudosarcoma and pseudosarcomatous carcinoma have been assigned to this neoplastic disorder, which reflects the controversy and differing views regarding histogenesis and biology of ECS, as well as whether the spindle cell component is epithelial or mesenchymal in origin. 6
ECS usually presents as a large intraluminal polypoid mass located in the middle third of the esophagus with a large age interval; however, the prognosis of ECS appears to be better than that of other esophageal malignancies, probably owing to its early diagnosis and exogenous growth into the lumen, rather than deep invasion. 7
In the past 2 decades, most studies on ECS have been case reports, with a total of less than 200 cases. Only a few studies have retrospectively evaluated local medical databases covering large time spans to identify patients with a diagnosis of carcinosarcoma. 4,8 -15 Owing to the rarity of ECS, the relationship between clinical features and prognosis of ECS remains controversial.
In this retrospective study, we enrolled 24 patients with ECS who were admitted to the Tumor Hospital of Nantong University in the past 13 years, and summarized existing literatures on ECS. The aim was to explore the clinical features that influenced the prognosis of patients with this rare malignancy.
Methods
Patient Selection
From January 2006 to December 2018, a total of 8864 patients were diagnosed with esophageal malignancies based on histopathological evidence at the Tumor Hospital of Nantong University. All diagnoses were confirmed using endoscopy before anti-tumor treatment. Based on the examination of morphological and immunohistochemical results by an experienced pathologist, 24 (0.27%) patients with ECS were enrolled in the study. Owing to the retrospective nature of the study, the Ethics Review Committee of the Affiliated Tumor Hospital of Nantong University gave a written exemption. The following data were obtained from patients’ medical records: sex, age (when diagnosed), smoking history, drinking history, length of lesion, lesion location, lymph node metastasis, clinical stage (according to the 8th edition of the American Joint Committee on Cancer [AJCC] Esophageal Squamous Cell Carcinoma Staging Manual), treatment, tumor recurrence date, and date of death (if applicable). Subsequent information was taken from patients’ medical records or obtained by telephone interviews. We also searched the PubMed database using keywords such as “Esophageal,” “Carcinosarcoma,” and “Sarcomatoid Carcinoma” to identify relevant research and conduct a literature review.
Statistical Analysis
In the survival analysis, the starting point was defined as the time of pathological diagnosis. The endpoints of follow-up were defined as the date of death of the patient or the date of follow-up of the surviving patient. Survival rates were calculated using the Kaplan-Meier method. All data were analyzed using SPSS 23.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Demographic, Patient, Tumor, and Treatment Characteristics
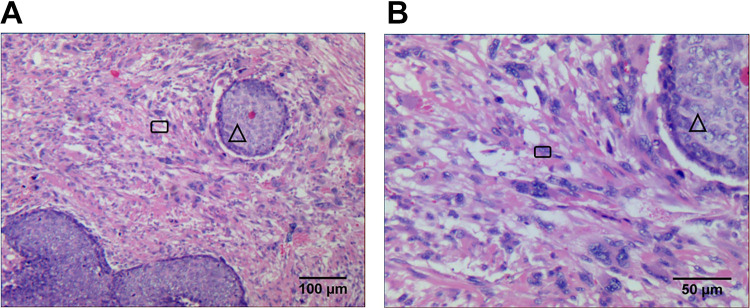
The clinical characteristics of the 24 patients with pathologically confirmed ECS (Figure 1) are shown in Table 1. The mean age of the patients was 63.8 (range, 52-82; median age, 62.5) years. Among the 24 patients with ECS, 10 (41.7%) had stage T1 ECS and 14 (58.3%) had stage T2-4 ECS; furthermore, 16 (66.7%) patients did not have lymph node metastasis, while 8 (33.3%) had lymph node metastasis. Lymph node metastasis was not observed seen in all stage T1 ECS patients, lymph node metastasis occurred in 8 (57.1%) stage T2-4 ECS patients, and it did not occur in 6 (42.9%) stage T2-4 ECS patients. Among the 24 patients, 10 (41.7%) had stage I ECS, 6 (25%) had stage II ECS, 6 (25%) had stage III ECS, and 2 (8.3%) had stage IV ECS. Twelve (50%) patients underwent surgery only, 7 (29.2%) underwent surgery and other treatments, 4 (16.7%) received radiotherapy and chemotherapy, and 1 (4.2%) received radiotherapy alone.
Figure 1.
HE pictures of esophageal carcinosarcoma (1A × 100 times, 1B × 200 times; cancer nest, △; sarcoma, □ ).
Table 1.
Clinical Features of 24 Patients With Esophageal Carcinosarcoma.
| Case | Gender | Age | Smoking | Drinking | Location | Length (cm) | Treatment | cTNM |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 63 | No | No | Middle | 8 | OP | T1N0M0 |
| 2 | Male | 60 | No | No | Middle | 3 | OP | T3N1M0 |
| 3 | Female | 52 | No | No | Lower | 6 | CT+OP | T1N0M0 |
| 4 | Male | 58 | No | No | Upper | 7.5 | RT+CT | T3N1M0 |
| 5 | Male | 64 | No | No | Middle | 5 | OP+RT+CT | T1N0M0 |
| 6 | Male | 56 | No | No | Middle | 4.5 | RT+CT | T2N3M0 |
| 7 | Female | 75 | No | No | Middle | 8.5 | OP | T3N1M0 |
| 8 | Female | 65 | No | No | Middle | 6 | OP+CT | T2N0M0 |
| 9 | Male | 70 | Yes | No | Middle | 8.3 | OP+RT+CT | T3N1M0 |
| 10 | Male | 58 | Yes | Yes | Upper | 2.5 | RT+CT | T3N0M0 |
| 11 | Male | 82 | Yes | Yes | Middle | 5.5 | OP | T1N0M0 |
| 12 | Male | 62 | No | No | Middle | 4 | OP | T1N0M0 |
| 13 | Male | 62 | Yes | Yes | Upper | 6 | RT | T2N0M0 |
| 14 | Male | 69 | No | No | Upper | 5.5 | OP | T1N0M0 |
| 15 | Male | 68 | No | No | Middle | 5 | OP | T1N0M0 |
| 16 | Male | 75 | No | No | Upper | 8.3 | OP | T2N0M0 |
| 17 | Male | 78 | No | No | Upper | 5.6 | OP | T1N0M0 |
| 18 | Male | 59 | Yes | Yes | Middle | 7 | OP | T3N0M0 |
| 19 | Female | 55 | No | No | Middle | 7.5 | RT+CT | T3N0M0 |
| 20 | Female | 69 | No | No | Middle | 7 | OP+RT+CT | T3N1M0 |
| 21 | Female | 54 | No | No | Upper | 6 | OP | T1N0M0 |
| 22 | Male | 54 | No | No | Middle | 6 | OP+RT | T3N1M1 |
| 23 | Male | 65 | Yes | No | Middle | 3.5 | OP+CT | T3N1M0 |
| 24 | Male | 58 | Yes | Yes | Middle | 5 | OP | T1N0M0 |
Abbreviations: OP, Operation; CT, Chemotherapy; RT, Radiotherapy.
Patients Outcomes
In this study, when follow-up was discontinued, at a median time of 70.5 (range, 10-156) months, the 24 patients had a survival rate of 54.2%, and 11 (45.8%) patients dying of the disease. The 3-year and 5-year survival rates of the 24 ECS patients were 83.3% and 70.8%, respectively (Figure 2). With respect to treatment, the 3-year and 5-year survival rates of patients who underwent surgery were 85% and 75%, respectively, and those of patients who received radiotherapy ± chemotherapy were 75% and 50%, respectively. The 3-year and 5-year overall survival(OS) rates of patients who received the treatment regimen including radiotherapy were 88.9% and 55.6%, respectively, whereas the rates of patients who did not receive radiotherapy were 80%. The 3-year and 5-year survival rates of patients who received the treatment regimen including chemotherapy were 88.9% and 63.6%, respectively, whereas the rates of patients who did not receive chemotherapy were 76.9%.
Figure 2.
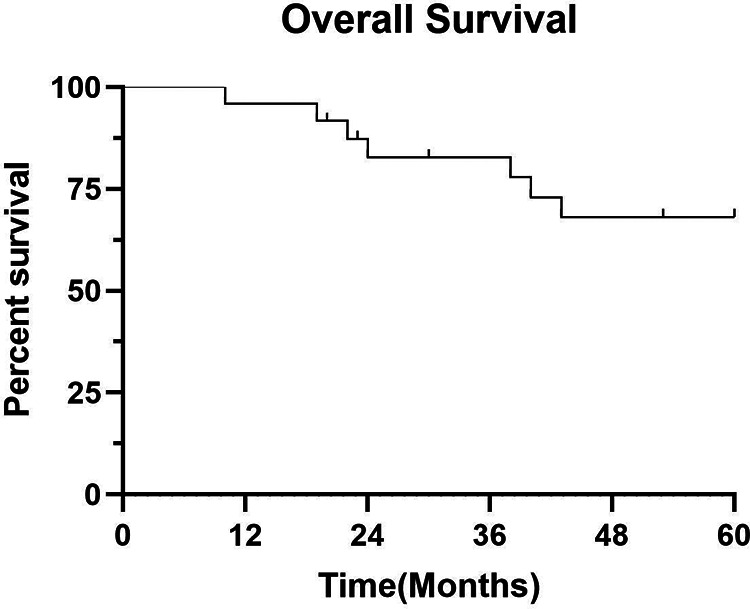
Overall survival curve of 24 patients with esophageal carcinosarcoma.
Clinical Prognostic Factors
Clinical factors and survival status are shown in Table 2. Univariate analysis revealed there was a statistically significant difference between the survival distribution of patients with stage T1 ECS and T2-4 stage ECS (Figure 3A). The 5-year survival rates of patients with stage T1 ECS and stage T2-4 were 90% and 57.1%, respectively. There was a statistically significant difference in the survival distribution of patients with stage I, stage II, stage III, and stage IV ECS according to the cTNM stage system (Figure 3B). The 5-year survival rates of patients with stage I, stage II, stage III, and stage IV ECS were 90.0%, 33.3%, 66.7%, and 100%, respectively.
Table 2.
Univariate Analysis of OS in 24 Patients With Esophageal Carcinosarcoma.
| Features | n | Death (%) | |
|---|---|---|---|
| Gender | |||
| Male | 18 | 8 (44.4%) | |
| Female | 6 | 3 (50.0%) | |
| Age | |||
| ≤60 | 10 | 5 (50.0%) | |
| >60 | 14 | 6 (42.9%) | |
| Smoking | |||
| No | 17 | 8 (47.1%) | |
| Yes | 7 | 3 (42.9%) | |
| Drinking | |||
| No | 19 | 9 (47.4%) | |
| Yes | 5 | 2 (40.0%) | |
| Location | |||
| Upper | 7 | 4 (57.1%) | |
| Middle | 16 | 7 (43.7%) | |
| Lower | 1 | 0 (0%) | |
| Length | |||
| ≤5 cm | 8 | 3 (37.5%) | |
| >5 cm | 16 | 8 (50.0%) | |
| Treatment | |||
| OP | 12 | 4 (33.3%) | |
| NSOP | 7 | 4 (57.1%) | |
| RT ± CT | 5 | 3 (60.0%) | |
| Lymph node metastasis | |||
| No | 16 | 7 (43.7%) | |
| Yes | 8 | 4 (50.0%) | |
| T | |||
| T1 | 10 | 2 (20.0%) | |
| T2-4 | 14 | 9 (64.3%) | |
| cTNM | |||
| Ⅰ | 10 | 2 (20.0%) | |
| Ⅱ | 6 | 5 (83.3%) | |
| Ⅲ | 6 | 2 (50.0%) | |
| Ⅳ | 2 | 1 (50.0%) | |
Abbreviations: OP, Operation; CT, Chemotherapy; RT, Radiotherapy; NSOP, Non-simple operation; CT, Chemotherapy; RT, Radiotherapy.
Figure 3.
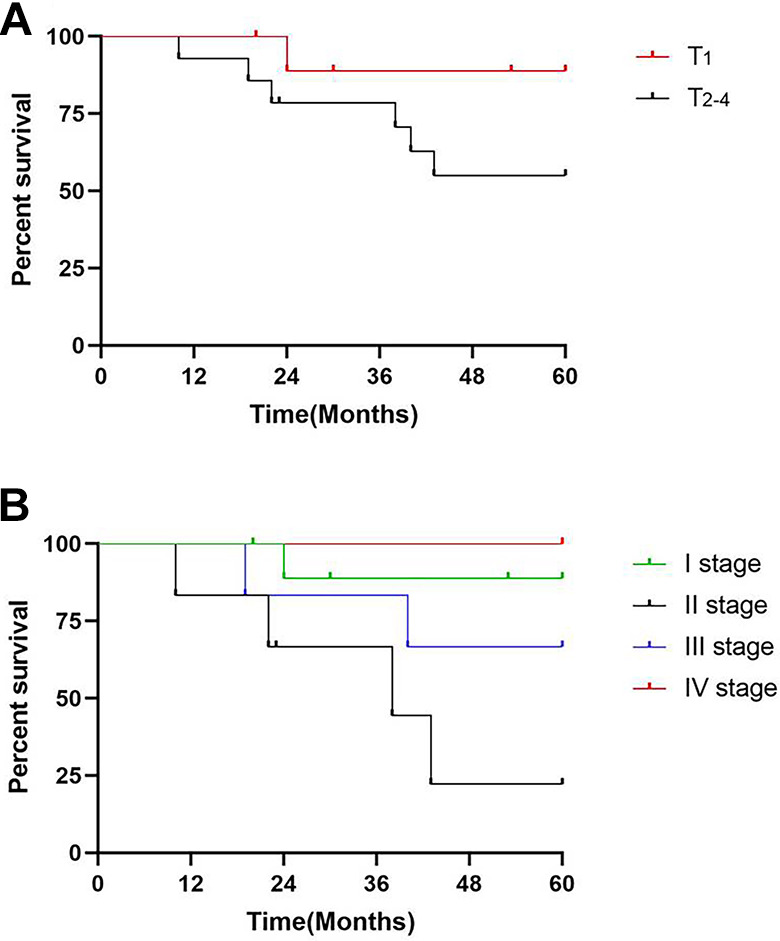
Kaplan-Meier survival curve analysis of 24 patients with esophageal carcinosarcoma. A, T1 and T2-4 survival distribution was statistically different. B, The cTNM stage was negatively correlated with survival distribution in 24 patients with esophageal carcinosarcoma.
Research and Clinical Features of ECS From Published Literature
Nine published studies on ECS survival analysis from countries such as China, Italy, and Japan were included. The data from the selected studies are shown in Table 3. From 1967 to 2013, 211 patients were included in these studies. Most studies reported a 5-year survival rate ranging from 11.80% to 68.20%. The incidence of ECS was relatively low 0.32%-2.4%. Among the reported cases, 54.5%-95.8% patients had the early stage ECS, accounts for 54.5-95.8%, surgery was the main treatment, and many comprehensive treatments such as chemotherapy and radiotherapy were available.
Table 3.
Nine Studies on Previous Survival Analysis of Esophageal Carcinosarcoma.
| Researcher | Patient inclusion time | N | Incidence rate | Treatment | 5-year survival rate | Median survival (month) |
|---|---|---|---|---|---|---|
| Wang J 8 | 1998.8-2013.8 | 24 | 0.43% | OP (24/24) | 52.40% | – |
| Zhang BH 9 | 1967.1-2008.12 | 32 | 0.35% | OP (27/32) | 57% | – |
| OP+RT (1/32) | ||||||
| OP+CT (1/32) | ||||||
| OP+RT+CT (3/32) | ||||||
| Kuo CJ 10 | 1976.1-2007.12 | 12 | 0.36% | OP (7/12) | – | 11.5 |
| CCRT( 2/12) | ||||||
| CCRT+OP( 2/12) | ||||||
| CT (1/12) | ||||||
| Yu Z 11 | 1990.1-2005.12 | 22 | 0.39% | OP (22/22) | 68.20% | – |
| Chen PC 12 | 2000.1-2009.12 | 31 | – | OP (22/31) | 33.40% | 40 |
| NCRT+OP+CT( 1/31) | ||||||
| OP+RT+CT (8/31) | ||||||
| Wang L 4 | 2000.1-2011.1 | 33 | 0.66% | OP (23/33) | 48% | 43.5 |
| OP+CT (4/33) | ||||||
| OP+RT (3/33) | ||||||
| RT+CT (1/33) | ||||||
| TCM (2/33) | ||||||
| Sano A 13 | – | 20 | – | OP (19/20) | 60% | – |
| NT (1/20) | ||||||
| Cavallin F 14 | 1980.1-2011.12 | 17 | 0.32% | OP (7/17) | 11.80% | 12 |
| NT (1/17) | ||||||
| LT (2/17) | ||||||
| ES (1/17) | ||||||
| GS (2/17) | ||||||
| OP+RT (2/17) | ||||||
| RT+OP+RT (1/17) | ||||||
| OP+CT (1/17) | ||||||
| Lyomasa 15 | 1971-1988 | 20 | 2.40% | OP (14/20) | 26.70% | – |
| OP+CT (6/20) |
Abbreviations: TCM, Traditional Chinese medicine; NT, No treatment; LT, Laser therapy; ES, Endoscopic stent; GS, Gastrostomy; OP, Operation; CT, Chemotherapy; RT, Radiotherapy.
Discussion
ECS contains both carcinomatous and sarcomatous components. In histological studies, the 2 components are mixed and often dominated by sarcomatoid components. There is also a transition and migration between the 2 components.
The concept of etiology and tumorigenesis is conflicting, that Enrile et al proposed that sarcomatoid spindle cells are produced in response to cancer, 16 Iwaya et al assumed that 2 separate stem cells are transformed independently or simultaneously into malignant cells to form a separate tumor (true carcinosarcoma), 17 and Taniyama et al revealed that individual components are derived from a single common progenitor cell. 18 This last theory posits that the sarcoma component is derived from the differentiation of cancer components. 19,20
Because of the rare incidence of ECS, from the initial name to now, most of the literature mainly consists of reports. In this study, we summarized the survival analysis of 9 large studies of previous ECS through a literature review. The 5-year survival rate reported in the reviewed cases was between 11.80% and 68.20% (Table 3). Limited case reports showed that the age range of patients with ECS was wide (44-86 years), with an average age of 70 years. 21 Most cases of ECS occurred in middle-aged and older men, and the ratio of male to female patients was 3.7:1. 22 Additionally, patients with ECS were more likely to be smokers or alcoholics. Our study followed up 24 patients with a 5-year survival rate of 54.2% and a median follow-up time of 70.5 months. Compared with the 5-year survival rate of patients with ESCC (< 20%) 23 and that of patients with EAC (20.1%), 24 the 5-year survival rate of patients with ECS was significantly higher. Patients with ECS have a symptom of dysphagia at the earlier stage, and the survival outcome of patients with ECS is generally better than that of patients with typical esophageal cancer of the same size. 4,25,26 In the current study, 24 patients with ECS also had showed progressive dysphagia as their first symptom. It has been reported that the doubling time of ECS is 2.2 months, while that of ESCC is 5 months. 27 Patients with carcinosarcoma exhibit symptoms earlier in the disease course than those with ESCC. 28
The prognosis of ECS compared with that of ESCC is controversial. Currently, no TNM staging is dedicated to ECS, while the latest NCCN guidelines have different clinical stages of ESCC and EAC owing to differences in prognosis. 29 In this study, we found that the cancerous part of the 24 patients with carcinosarcoma was squamous cell carcinoma. Therefore, we use the staging of ESCC and found that it could better distinguish the prognosis of patients. There was a statistically significant difference in survival distribution among between different clinical stages, indicating that this staging was more reasonable for ECS (i.e., using the AJCC eighth edition of the ESCC for ECS). In addition, our prognostic data were different for ESCC and EAC. The survival rate of patients with ECS was significantly higher than that of patients with ESCC and EAC. In addition, because of the small sample size of our study, we could include only 2 stage IV ECS patients. The OS curves in Figure 3B show that the prognosis of stage IV ECS patients is better than that of other stage ECS patients. Therefore, we believe that we need to further expand the sample size and conduct more in-depth research in the future.
In the subgroup analysis, we found no lymph node metastasis in any of the 10 patients with stage T1 ECS; however, lymph node metastasis occurred in 8 (57.1%) of 14 patients with stage T2-4 ECS. This finding is consistent with that of the report on ESCC, which revealed that when the tumor is confined to the submucosal layer, the incidence of esophageal lymph node metastasis in the mediastinum and lower mediastinum is very low. 30 However, when the tumor invades or penetrates the muscle layer and mediastinum, the incidence of paraesophageal lymph node metastasis increases. 31,32 We also found that in the current study, 10 (41.7%), 6 (25%), 6 (25%), and 2 (8.3%) patients had stage I, stage II, stage III, and stage IV ECS, respectively. At the time of diagnosis, the carcinomatous component of ECS is usually in its early stage, while the carcinomatous component tends to metastasize and thus has a low rate of lymph node metastasis. 33 ECS is clearly a unique esophageal malignancy. Our study found that the risk of lymph node metastasis after ECS invasion of the esophageal muscle layer is greatly increased. The existing ESCC staging may be used to guide the clinical practice of ECS. The probability of lymph node metastasis in patients with stage T1 ECS is extremely low, and the prognosis is significantly better; this finding is noteworthy. Comprehensive treatment for patients with stage T2 ECS and above may be needed, which has certain guiding significance for our future clinical research. In the future, a clinical research with a large sample population is needed to further confirm the above mentioned findings.
ECSs have been treated according to the guidelines used for esophageal carcinomas, and it is difficult to develop a specific standard treatment strategy based on high-quality clinical trials owing to the low incidence of ECS. However, the growth pattern of most ECSs is grossly polypoid, and they do not invade as deeply as ESCC. Therefore, ECS-related symptoms occur much earlier in the disease course, thereby allowing earlier diagnosis and treatment, thus, the prognosis of ECS is better than that of other esophageal cancer subtypes according to the literature review and the current study. A surgical resection with regional lymph node dissection was the traditional treatment for ECSs without distant metastasis, 25,34 Zhang et al 35 reported that the percentage of stage T1/2 lesions was higher in the ECS group than in the ESCC group (67.6% vs 29.7%, P < .001), which was similar to the findings reported by Wang et al 4 (20/33, 60.6%), However, our study revealed that surgical patients in our center had a 5-year survival rate of 75%. Endoscopic techniques, with the advantages of minimal invasion and preservation of the esophagus, have also been applied for the resection of ECS. 34 Li et al reported that a 55-year-old man with ECS had a tumor measuring 26 × 5 × 4 cm and underwent endoscopic resection to diagnose and relieve the obstruction. One month later, the patient gained 6 kg in weight and no obstruction was found on endoscopy. The subsequent radical surgery with lymph node dissection was successfully performed. The tumor was the largest endoscopically resected ECS reported to date. 36 Radiotherapy is also an option for patients who can not tolerate surgery. Kimura et al reported that palliative radiotherapy alone (45 Gy /15 FR) could achieve complete pathological response in an 89 year old patient with a tumor diameter (T2N0M0) of 80 mm. 37 Our study showed that patients receiving radiotherapy and chemotherapy or radiotherapy alone had a 5-year survival rate of 60%. Moreover, several studies have shown that for advanced stage disease, some chemotherapy regimens including DCF (docetaxel, cisplatin, and 5-fluorouracil) 26 and DP (docetaxel +cisplatin), 4 have shown good efficacy.
However, this study had some limitations mainly owing to the rarity of ECS that are common among similar studies. This study was a single-center retrospective study that included a limited number of patients. The study was limited by the technology at the time, and there was no further study of T staging based on esophageal ultrasound. The study also covered an extended time period, leading to a possible bias related to changes in diagnostic procedure and treatment strategies.
Conclusion
Through this retrospective case analysis of 24 ECS patients at a single center for over 13 years and a comparison of literature review, we found that ECS has favorable prognosis, even in patients with early stage ECS who undergo radical esophagectomy with lymph node dissection. Endoscopic techniques combined with other treatments is an alternative treatment option. Because the low incidence of ECS prevents researchers from gathering adequate-sized samples, future collaborative efforts are needed to investigate this rare malignancy.
Abbreviations
AJCC, American Joint Committee on Cancer; EAC, Esophageal Adenocarcinoma; ECS, Esophageal Carcinosarcoma; ESCC, Esophageal Squamous Cell Carcinoma; OS, Survival Analysis.
Footnotes
Authors’ Note: Shusen Chen, Yu Shi, and Zhengjing Lu contributed equally to this work. XY and JC contributed to the study’s conception and design. XDC made clear the pathology. MWW and LFC acquired the data. SSC and BXY contributed to the analysis and interpretation of the data. SSC, YS and ZJL drafted the manuscript. All authors participated in the discussion and approved the final manuscript. Data are available on reasonable request, but data sharing would be subjected to additional ethics approval. Due to the retrospective nature of the study, the Ethics Review Committee of the Affiliated Tumor Hospital of Nantong University gave a written exemption. Not commissioned; externally peer reviewed.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Key Research and Development Program of Social Development of Jiangsu Province (BE2017679).
ORCID iD: Xi Yang, MD, PhD  https://orcid.org/0000-0001-6125-7641
https://orcid.org/0000-0001-6125-7641
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Virchow R. Die krankhaften Geschwülste. vol II. August Hirschwald. 1864-1865; 181-182. [Google Scholar]
- 3. Robey-Cafferty SS, Grignon DJ, Ro JY, et al. Sarcomatoid carcinoma of the stomach. A report of three cases with immunohistochemical and ultrastructural observations. Cancer. 1990;65(7):1601-1606. doi:10.1002/1097-0142(19900401)65:7<1601:: aid-cncr2820650725>3.0.co;2-n [DOI] [PubMed] [Google Scholar]
- 4. Wang L, Lin Y, Long H, et al. Esophageal carcinosarcoma: a unique entity with better prognosis. Ann Surg Oncol. 2013;20(3):997-1004. doi:10.1245/s10434-012-2658-y [DOI] [PubMed] [Google Scholar]
- 5. Hansemann V. Das gleichzeitige Vorkommen verschiedenartiger Geschulste bie derselben Person. J Clin Cancer Res Clin Oncol. 1904;1(1-5):183-198. [Google Scholar]
- 6. Au JT, Sugiyama G, Wang H, et al. Carcinosarcoma of the oesophagus—a rare mixed type of tumor. J Surg Case Rep. 2010;2010(7):7. doi:10.1093/jscr/2010.7.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu LT, Sun CF, Wu LH, Chang ZR, Liu TH. Clinical and pathological characteristics of carcinosarcoma of the esophagus: report of four cases. Ann Thorac Surg. 1984;37(3):197-203. doi:10.1016/s0003-4975(10)60324-4 [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Wei D, Fan J, Zhu X. [Clinical analysis of 24 cases of esophageal carcinosarcoma]. Zhonghua Zhong Liu Za Zhi. 2014;36(8):633-635. [PubMed] [Google Scholar]
- 9. Zhang BH, Yang WJ, Wang YG, Zhang HT. [Clinical manifestation and prognosis of the surgical treatment of esophageal carcinosarcoma]. Zhonghua Wai Ke Za Zhi. 2012;50(3):256-259. [PubMed] [Google Scholar]
- 10. Kuo CJ, Lin TN, Lin CJ, et al. Clinical manifestation of esophageal carcinosarcoma: a Taiwan experience. Dis Esophagus. 2010;23(2):122-127. doi:10.1111/j.1442-2050.2009.00976.x [DOI] [PubMed] [Google Scholar]
- 11. Yu Z, Cheng BC, Chang S, et al. [Clinicopathological analysis of esophageal carcinosarcoma: a report of 22 cases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2008;11(3):235-237. [PubMed] [Google Scholar]
- 12. Chen PC, Chen QX, Ni XH, Zhou XM, Mao WM. [Clinicopathological features and prognostic analysis of esophageal sarcomatoid carcinoma]. Zhonghua Zhong Liu Za Zhi. 2012;34(4):287-290. doi:10.3760/cma.j.issn.0253-3766.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 13. Sano A, Sakurai S, Kato H, et al. Clinicopathological and immunohistochemical characteristics of esophageal carcinosarcoma. Anticancer Res. 2009;29(8):3375-3380. [PubMed] [Google Scholar]
- 14. Cavallin F, Scarpa M, Alfieri R, et al. Esophageal carcinosarcoma: management and prognosis at a single Italian series. Anticancer Res. 2014;34(12):7455-7459. [PubMed] [Google Scholar]
- 15. Iyomasa S, Kato H, Tachimori Y, Watanabe H, Yamaguchi H, Itabashi M. Carcinosarcoma of the esophagus: a twenty-case study. Jpn J Clin Oncol. 1990;20(1):99-106. [PubMed] [Google Scholar]
- 16. Enrile FT, De Jesus PO, Bakst AA, Baluyot R. Pseudosarcoma of the esophagus (polypoid carcinoma of esophagus with pseudosarcomatous features). Cancer. 1973;31(5):1197-1202. doi:10.1002/1097-0142(197305)31:5<1197:: aid-cncr2820310523>3.0.co;2-9 [DOI] [PubMed] [Google Scholar]
- 17. Iwaya T, Maesawa C, Tamura G, et al. Esophageal carcinosarcoma: a genetic analysis. Gastroenterology. 1997;113(3):973-977. doi:10.1016/s0016-5085(97)70194-x [DOI] [PubMed] [Google Scholar]
- 18. Taniyama K, Sasaki N, Mukai T, et al. Carcinosarcomas of the esophagus. Pathol Int. 1995;45(4):297-302. [DOI] [PubMed] [Google Scholar]
- 19. Freitas J, Almeida J, Silva AO, Costa O, Carvalho M, de Freitas AF. [Circadian patterns of heart rate variability in patients with dysautonomia]. Rev Port Cardiol. 1997;16(3):313-315. Padrao circadiano da variabilidade da frequencia cardiaca em doentes com disautonomia. [PubMed] [Google Scholar]
- 20. Matsumoto T, Fujii H, Arakawa A, et al. Loss of heterozygosity analysis shows monoclonal evolution with frequent genetic progression and divergence in esophageal carcinosarcoma. Hum Pathol. 2004;35(3):322-327. doi:10.1016/j.humpath.2003.02.001 [DOI] [PubMed] [Google Scholar]
- 21. Ziauddin MF, Rodriguez HE, Quiros ED, Connolly MM, Podbielski FJ. Carcinosarcoma of the esophagus—pattern of recurrence. Dig Surg. 2001;18(3):216-218. doi:10.1159/000050133 [DOI] [PubMed] [Google Scholar]
- 22. Matsusaka T, Watanabe H, Enjoji M. Pseudosarcoma and carcinosarcoma of the esophagus. Cancer. 1976;37(3):1546-1555. doi:10.1002/1097-0142(197603)37:3<1546:: aid-cncr2820370344>3.0.co;2-i [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Lu Y, Ren Y, et al. Starvation-induced suppression of DAZAP1 by miR-10b integrates splicing control into TSC2-regulated oncogenic autophagy in esophageal squamous cell carcinoma. Theranostics. 2020;10(11):4983-4996. doi:10.7150/thno.43046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haiyu Z, Xiaofeng P, Xiangqiong M, et al. Incidence and survival changes in patients with esophageal adenocarcinoma during 1984-2013. Biomed Res Int. 2019;2019:7431850. doi:10.1155/2019/7431850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hashimoto M, Kitagami H, Niwa H, et al. Prognosis and prognostic factors of esophageal spindle cell carcinoma treated by esophagectomy: a retrospective single-institution analysis. Esophagus. 2019;16(3):292-299. doi:10.1007/s10388-019-00667-y [DOI] [PubMed] [Google Scholar]
- 26. Yoshimoto T, Kobayashi S, Kanetaka K, et al. Preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil for locally advanced esophageal carcinosarcoma: a case report and review of the literature. Surg Case Rep. 2018;4(1):1-7. doi:10.1186/s40792-018-0425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sasajima K, Taniguchi Y, Morino K, et al. Rapid growth of a pseudosarcoma of the esophagus. J Clin Gastroenterol. 1988;10(5):533-536. [DOI] [PubMed] [Google Scholar]
- 28. Ohtaka M, Kumasaka T, Nobukawa B, et al. Carcinosarcoma of the esophagus characterized by myoepithelial and ductal differentiations. Pathol Int. 2002;52(10):657-663. [DOI] [PubMed] [Google Scholar]
- 29. Rice TW, Ishwaran H, Blackstone EH, et al. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29(8):913-919. doi:10.1111/dote.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizutani M, Murakami G, Nawata S, Hitrai I, Kimura W. Anatomy of right recurrent nerve node: why does early metastasis of esophageal cancer occur in it? Surg Radiol Anat. 2006;28(4):333-338. doi:10.1007/s00276-006-0115-y [DOI] [PubMed] [Google Scholar]
- 31. Tachimori Y, Nagai Y, Kanamori N, Hokamura N, Igaki H. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus. 2011;24(1):33-38. doi:10.1111/j.1442-2050.2010.01086.x [DOI] [PubMed] [Google Scholar]
- 32. Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus. 2016;13(1):1-7. doi:10.1007/s10388-015-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madan AK, Long AE, Weldon CB, Jaffe BM. Esophageal carcinosarcoma. J Gastrointest Surg. 2001;5(4):414-417. [DOI] [PubMed] [Google Scholar]
- 34. Yabuuchi Y, Tanaka M, Ono H. Carcinosarcoma of the esophagus with rapid morphological change. Am J Gastroenterol. 2018;113(5):642. doi:10.1038/s41395-018-0013-z [DOI] [PubMed] [Google Scholar]
- 35. Zhang B, Xiao Q, Yang D, et al. Spindle cell carcinoma of the esophagus: a multicenter analysis in comparison with typical squamous cell carcinoma. Medicine (Baltimore). 2016;95(37):e4768. doi:10.1097/MD.0000000000004768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y, Guo LJ, Ma YC, Ye LS, Hu B. Endoscopic palliative resection of a giant 26-cm esophageal tumor: a case report. World J Clin Cases. 2020;8(19):4624-4632. doi:10.12998/wjcc.v8.i19.4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kimura K, Hayashi Y, Otani K, et al. Esophageal carcinosarcoma that disappeared pathologically by palliative radiotheraphy alone. Clin J Gastroenterol. 2019;12(3):247-253. doi:10.1007/s12328-019-00933-7 [DOI] [PubMed] [Google Scholar]