Abstract
Background
Malignancy after transplantation is a leading cause of death among kidney transplant recipients. However, donor-derived malignancies are rare. We report a case of a high grade papillary urothelial carcinoma arising in a transplanted kidney.
Case presentation
A 62-year-old female who received a kidney transplantation more than 30 years ago presented with urinary tract infection, acute renal failure, and hydronephrosis of the transplant kidney. Anterograde nephrostogram showed a large filling defect in the lower pole of the transplant kidney and in the proximal 3–4 cm of the ureter. A biopsy from the renal pelvic mass showed a high grade urothelial carcinoma. She underwent an anterior exenteration, resection of both transplant and native kidneys and bilateral pelvic lymph node dissection. Pathologic examination showed a high grade papillary urothelial carcinoma which appeared to arise in the pelvis of the graft kidney, involve the graft ureter and native urinary bladder. The tumor had metastasized to one left obturator lymph node but spared the two native kidneys and ureters. Short tandem repeat (STR) analysis confirmed the tumor to be of donor origin. Next-generation sequencing identified amplification of TERT and loss of CDKN2A/CDKN2B in the primary tumor.
Conclusion
While it is known that transplant recipients have an increased risk of urothelial carcinoma compared to the general population, the lack of the well-documented risk factors, such as older age at transplantation, BK polyomavirus infection, and prolonged post-transplantation history and dissemination of the tumor in this case shed light on the de novo tumorigenesis of the graft kidney within the host microenvironment. Amplification of Telomerase reverse transcriptase (TERT) and loss of cyclin dependent kinase inhibitor 2A/2B (CDKN2A/CDKN2B) detected in the tumor by next gene sequencing suggests that they may play an important role in this case.
Keywords: High grade papillary urothelial carcinoma, Transplant kidney, TERT, CDKN2A/CDKN2B
Introduction
Kidney transplantation has been established as the treatment of choice for patients with end-stage renal disease [1, 2]. With advances in immunosuppressive regimens, transplant patients have a better quality of life and a significant survival benefit compared to those continuing on dialysis. However, transplant patients have a higher risk of developing secondary malignancies post transplantation, owing to their extended life expectancy and chronic immunosuppressive status [3]. Malignancy after transplantation has become the third leading cause of death in renal transplant recipients [4, 5]. Compared to the general population, post-transplant patients have a 7-fold increased risk of developing renal cell carcinoma (RCC) in the native kidney, where it portends a significantly worse prognosis than similar tumors arising outside of the transplantation setting. Risk factors include end-stage renal disease, longer time on dialysis, and older ages at transplant. Kidney transplant recipients also have an increased risk of developing urothelial carcinoma (UC) in the bladder and the upper genitourinary tract [6, 7], which is associated with infection with the BK polyomavirus. However, donor-derived UC is rarely reported [7–15].We herein report on a high grade papillary urothelial carcinoma arising in a donor renal allograft.
Case report
A 62-year-old female underwent living-related donor renal transplant in 1983 due to end stage kidney disease with primary renal hypertension. She was maintained on azathrothiozine and steroids. No episodes of rejection were reported post-transplant, and the transplant kidney functioned well. In 2017, the patient presented with a urinary tract infection. Medical evaluation revealed acute renal failure and hydronephrosis of the transplanted kidney. She was treated with Cefapime and urine cytology was positive for malignant cells. An anterograde nephrostogram showed a large filling defect in the lower pole of the transplant kidney as well as a filling defect in the proximal 3–4 cm of the ureter. At time of ureteroscopy, multiple biopsies from the renal pelvic mass demonstrated high grade urothelial carcinoma. Later on, the patient was found to have extensive bladder tumors; she underwent hysterectomy, bilateral salpingo-oophorectomy, nephrectomy of native kidneys and transplant kidney, cystectomy and bilateral pelvic lymph node dissections. Post operation follow-up, pelvic magnetic resonance imaging demonstrated a large liver mass which was confirmed by biopsy to be metastatic urothelial carcinoma. Immunotherapy was initiated and the liver metastasis responded well to the immunotherapy with dramatic reduction in size of liver mass. At last follow-up, the patient was well 44 months after her surgical resection.
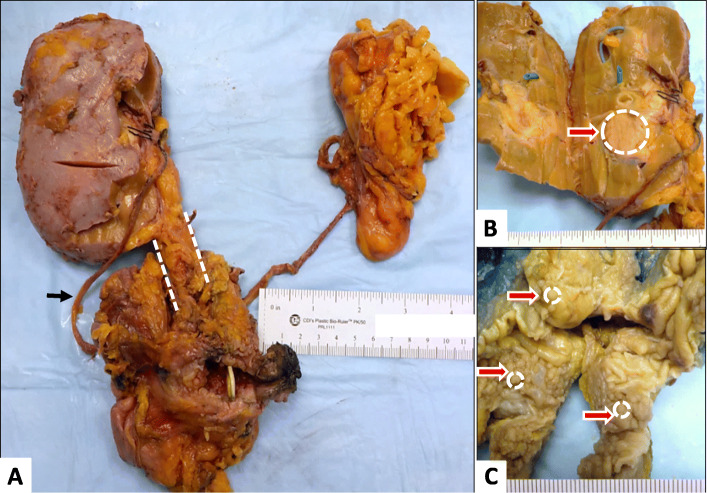
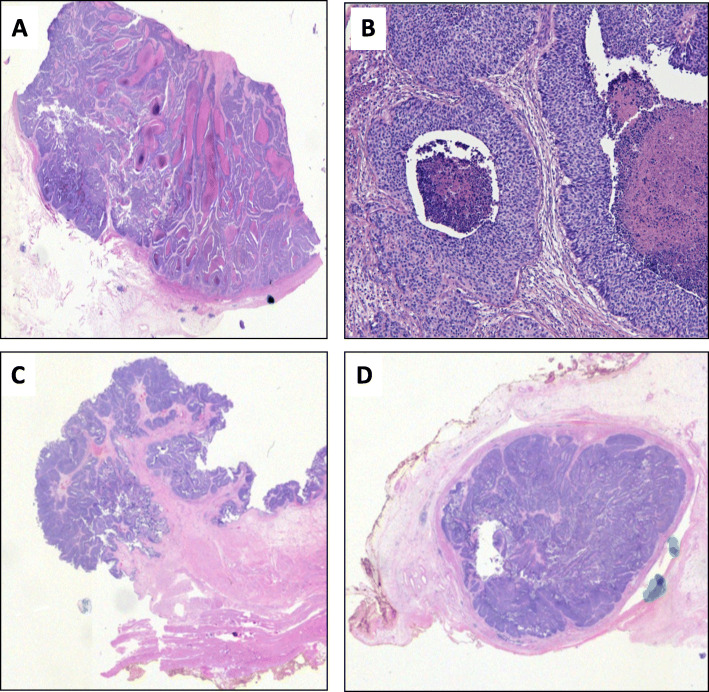
The Fig. 1a showed left native kidney, two native ureters, and one donor kidney connected to the native urinary bladder via a donor ureter. Upon sectioning, a 4.5 × 2.5 × 1.5 cm well-circumscribed flesh-colored mass was found in the renal pelvis of the graft kidney, a 1.2 × 1.4 × 2.5 cm flesh-colored mass encompassing the entire lumen of the proximal donor ureter, and over fifty tan discrete polypoid nodules, up to 1.5 cm, carpeting the wall of native bladder (Fig. 1b and c). The native kidneys were small (5.0 × 3.5 × 1.5 cm and 6.0 × 4.0 × 2.0 cm, respectively), with a thin cortex, poorly delineated corticomedullary junctions, and renal pelvises largely replaced by fatty tissue. No mass was identified in the native kidneys or ureters. Histology demonstrated high grade papillary UC involving the graft kidney, graft ureter and the native bladder. The carcinoma was predominantly non-invasive, however, focal invasion into subepithelial connective tissue was identified in the renal pelvis. Metastatic tumor was identified in one of thirteen obturator lymph nodes. The two native kidneys and ureters were negative for carcinoma (Fig. 2), demonstrating extensive thyroidization with tubular casts, calcium oxalate deposition, global and segmental glomerular sclerosis, interstitial fibrosis and chronic inflammationand arteriosclerosis. Subsequent to her surgical excision, a cystic lesion in the liver was biopsied, and confirmed to be metastatic urothelial carcinoma. The final pathological stage was therefore T1, N1, M1.
Fig. 1.
A, Gross photograph of the nephro-cystectomy specimen shows the bladder, donor kidney (left side of image) with ureter (dash line), right native ureter (black arrow), and left native kidneys (right side of image); B, Donor kidney with a renal pelvic mass; C, Native bladder with many nodules
Fig. 2.
H&E stain of the high-grade papillary UC of donor kidney (A&B), urinary bladder (C) and donor ureter (D)
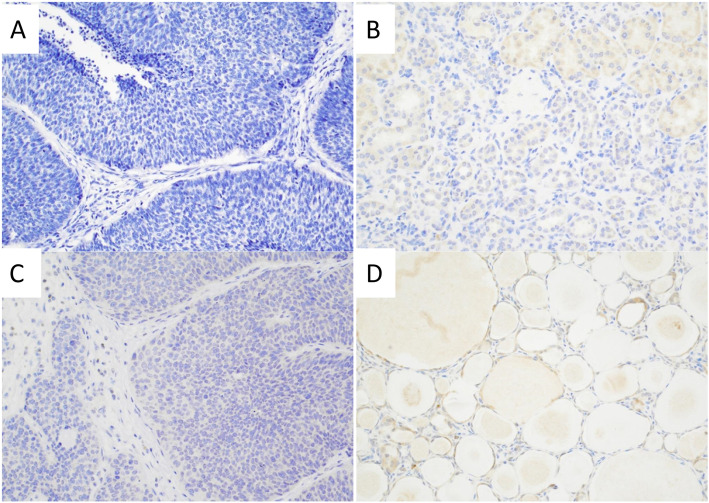
Since microsatellite instability (MSI) is commonly seen in upper urinary tract UC [16], we performed immunohistochemistry (IHC) and found that MLH1, MLH2, PMS2and MSH6 expression were retained in the UC of renal pelvis, donor ureter, and urinary bladder (not shown). BK polyomavirus has been shown as risk factors in post-transplant UC. However, both donor and recipient kidneys were negative for BK viral infection by SV40 IHC (Fig. 3). In order to determine whether the UC arose from native or donor tissue, STR analysis was performed on extracted DNA from the tumor as well as uninvolved native uterus as the control for recipient and representative apparent normal donor kidney as the control for donor. Of the 15 autosomal STRs, nine were informative. Review of these informative loci in the DNA extracted from the donor kidney tumor, ureter tumor, and bladder tumor indicated that 83, 84, and 80%, respectively, were of donor origin. These data would suggest that the tumor had drop metastasized to the bladder through the donor ureter. DNA extracted from the lymph node with evidence of metastasis showed only 8% donor origin, possibly due to low tumor cellularity in the lymph node sampled.
Fig. 3.
Immunostain for BK virus is negative in the UC in the renal pelvis (A), donor kidney (B), bladder (C), and in the non-neoplastic native kidney (D)
DNA extracted from the donor kidney tumor and the lymph node metastasis were submitted for mutation profiling of 147 genes. The results are provided in Table 1. The donor kidney tumor showed amplification of TERT and loss of CDKN2A/CDKN2B, which are genomic alterations found in approximately 7 and 25% of bladder cancers respectively (https://www.cbioportal.org). These were not detected in the lymph node metastasis. The renal pelvic tumor also showed a missense variant (p.N596S) in MSH2 at a low VAF (3.5%). This same variant was detected in the lymph node metastasis, but at a VAF of 37%. The lymph node metastasis also showed two other missense variants in MSH6 and FANCA with VAFs of 34 and 41% respectively. Interpretation of these variants would suggest these are of uncertain significance (VUS), potentially of germline origin. Given the low donor contribution in this lymph node specimen from the STR analyses (8%), it is suggested that these may be of recipient (native) germline origin. The low VAF MSH2 detected in the donor kidney tumor confirms the 7% recipient (native) contribution detected in this specimen. Thus, for the lymph node, no apparent somatic alterations were detected, possibly due to the overall low tumor cellularity of the specimen.
Table 1.
Mutation Profiling by Next Generation Sequencing (NGS)
| Specimen | Gene | Alteration | VAF | Coverage | Significance |
|---|---|---|---|---|---|
| Donor kidneytumor (50% estimated tumor cellularity, 83% donor) | |||||
| MSH2 | c.1787A > G; p.N596S | 3.5% | 1576x | Uncertain (germline) | |
| TERT | Amplification (18.8x) | Gain-of-function | |||
| CDKN2A | Loss (0.25x) | Loss-of-function | |||
| CDKN2B | Loss (0.23x) | Loss-of-function | |||
| Recipient lymph node with metastasis (50% estimated tumor cellularity, 8% donor) | |||||
| MSH2 | c.1787A > G; p.N596S | 37% | 1552x | Uncertain (germline) | |
| MSH6 | c.116G > A; p.G39E | 34% | 193x | Uncertain (germline) | |
| FANCA | c.1772G > A; p.R591Q | 41% | 3055x | Uncertain (germline) | |
Discussion
Malignancy is a leading cause of death in renal transplant recipients. Post-transplant malignancies most often develop within the first 5 years post transplantation. Papillary RCC is the most common tumor type, almost exclusively occurring within the native kidneys [17]. These patients are also at increased risk for urothelial carcinoma, arising in the bladder and/or native kidneys [6, 7]. Donor-derived urothelial carcinoma in graft kidneys are rarely reported. Ten cases of urothelial carcinoma in grafted kidneys have been reported [7–15]. Clinical-pathologic information was not published in two of the cases. The clinicopathological characteristics of the remaining 8 patients and our case are shown in Table 2. The nine patients (5 male, 4 female) had a median age at transplantation of 46 (range 28–58) years and mean time to development of UC after transplant of 132 (range 14–408) months. The tumor from the patient with the shortest transplant to carcinoma interval arose from her third grafted kidney. In this case, the UC was proven to be of donor origin by cytogenetic analysis. Five patients presented with urinary tract related symptoms (hematuria, UTI, or flank pain). Seven of the 9 tumors were high grade carcinoma. Only one case, in addition to ours, demonstrated regional lymph node or distant metastasis. Eight of the 9 patients were treated surgically. The mean follow-up time from UC diagnosis was 28.2 (range 1.9–94) months. Only the patient with extensive tumor dissemination and without surgical treatment died shortly after diagnosis.
Table 2.
Clinicopathological Characteristics of Urothelial Carcinoma in Graft Kidney
| Reference No | Age at Transplant | Gender/ KT Type | Immuno suppressant | Initial Presentation | Age at UC Diagnosis | UC Grade | UC Stage | Interval KT to UC (month) | Treatment | F/U (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| [14] | 41 | F/DDKT | Steroid, CsA, AZA, MMF | No symptom | 53 | High | T3NxMx | 147 | NUx | Alive (94) |
| [10] | 49 | F/DDKT | Steroid, FK, MMF | Fever, Flank Pain, Urinary symptoms | 61 | High | T3NxMx | 144 | NUx | Alive (24) |
| [10] | 57 | F/DDKT | Steroid, FK, MMF | No symptom | 59 | High | T3NxMx | 14 | NUx | Alive (12) |
| [11] | 46 | M/DDKT | Steroid, CsA, AZA | No symptom | 52 | Low | T2NxMX | 72 | Partial Nephrectomy | Alive (14) |
| [12] | 58 | M/DDKT | Steroid, FK, MMF | Gross Hematuria | 67 | High | T2N3M1 | 108 | CRTx | Dead (1.9) |
| [13] | 29 | M/LDKT | Steroid, CSA, MMF | Gross Hematuria | 40 | Low | T2NxMx | 132 | NUx + CRTx | Alive (24) |
| [8] | 23 | M/DDKT | N/A | Asymptomatic Microscopic Hematuria | 30 | High | T3NxMx | 84 | NUx | N/A |
| [9] | 57 | M/LDKT | FK Sirolimus | No symptom, | 69 | High | T3NxMx | 144 | NUx | Alive (12) |
| current case | 28 | F/LDKT | Steroid, AZA | UTI | 62 | High | T1N1M1 | 408 | NUx | Alive (44) |
Abbreviations: AZA Azathioprine, CRTx Chemoradiotherapy, CsA Cyclosporine A, DDKT Deceased-donor kidney transplantation, F Female, F/U Follow-up, FK Tacrolimus, KT Kidney transplantation, LDKT Living donor kidney transplantation, M Male, MMF Mycophenolate mofetil, N/A Not available, Nux Nephroureterectomy, UC Urothelial carcinoma, UTI Urinary tract infection
Our case of a rare donor-derived UC arising in a graft kidney is even more unusual in having arisen 34 years after transplantation, lacking other risk factors for secondary malignancy post-transplant, such as older age at transplantation and BK virus infection [18–21], and that the process spared her two native kidneys with end-stage renal disease were spared from the tumorigenesis, despite their 100-fold and 4.4-fold increased risk of developing RCC and UC, respectively, compared to the general population [3].
There are three possibilities regarding the UC origin in our case. One is that the tumor originated in the native bladder, the most common site of post-transplant UC, and spread retrograde to the donor ureter and graft kidney. The second possibility is that the primary tumor originated from the renal pelvis, and spread to the donor ureter and native bladder. This could be supported by the larger size of the tumor in the renal pelvis compared to other sites. The final possibility is the concurrent development of UC in the renal pelvis and urinary bladder. To answer these questions, we performed STR analysis, a molecular analysis of 16-STR widely used in medicine for establishing paternity and quality control in pathology. This test confirmed that the tumor in all three sites was of donor origin, supporting a renal pelvic origin, with drop metastasis to the ureter and urinary bladder.
Molecular analysis of the tumor showed a low VAF MSH2 mutation, which was not detected at the protein level by MSI IHC. This, suggests that MSI had a very limited role, if any, in this case. In contrast, amplification of telomerase reverse transcriptase (TERT) and loss of Cyclin Dependent Kinases Inhibitors (CDKN); CDKN2A/CDKN2B were detected in the tumor.
Telomerase reverse transcriptase (TERT) is the catalytic subunit of the enzyme telomerase and is essential for telomerase activity. Upregulation of TERT expression and resulting telomerase activity occurs in the large majority of malignancies, including urothelial carcinoma [22, 23]. This upregulation enables unlimited replication of cancer cells TERT activation in cancer occurs through a variety of mechanisms, including activating promoter mutations, alterations in promoter DNA methylation, chromatin remodeling, copy number alterations, and alternative splicing of TERT. Isharwal et al. [22] found 286 TERT promoter mutations and seven TERT gene amplifications in 276 tumors in a cohort of 398 UC patients. Tumors with TERT alterations had worse prognosis although, no association between TERT alterations and tumor stage or tumor grade was observed.
The CDKN2A and CDKN2B genes, encoding p16 and p15 respectively, are located on chromosome 9p21. Homozygous and heterozygous deletions occur at this locus in many primary human tumors, including urothelial carcinoma. CDKN2A and CDKN2B inhibit cyclin dependent kinase 4 (CDK4) and CDK6 which regulate cellular proliferation by preventing entry into the S phase of the cell cycle. Their inactivation may contribute to uncontrolled growth in human cancer. Deletion of CDKN2A/CDKN2B genes in urothelial carcinoma were found to be a common event and have been shown to be a crucial event in the progression from normal urothelium to carcinoma [24, 25].
Although the primary renal pelvic tumor only showed focal invasion into submucosal tissue, spread to the graft ureter, native bladder, lymph node and liver document the aggressive nature of the primary tumor. The aggressiveness in this case may be related to the patient’s immunosuppression. The role of TERT amplification and loss of CDKN2A/CDKN2B in such aggressive behavior is unknown and may require large cohort studies. Amplification of telomerase reverse transcriptase (TERT) and loss of CDKN2A/CDKN2B were not associated with UC grade and stage in tumors arising in the bladder. This may not be true in upper tract UC, as mutational differences exist between upper tract and bladder urothelial carcinomas [26].
Conclusion
While it is known that transplant recipients have an increased risk of urothelial carcinoma compared to the general population, the lack of the well-documented risk factors, such as older age at transplantation and BK polyomavirus infection, provide an opportunity to identify possible mechanisms of tumorigenesis of the graft kidney within the host microenvironment. Amplification of TERT and loss of CDKN2A/CDKN2B detected in the tumor by next gene sequencing suggests that they may play an important role in this case.
Acknowledgements
Not applicable.
Abbreviations
- STR
Short tandem repeat
- TERT
Telomerase reverse transcriptase
- CDKN
Cyclin dependent kinase inhibitor
- RCC
Renal cell carcinoma
- UC
Urothelial carcinoma
- MSI
Microsatellite instability
- VAF
Low variant allele frequency
Authors’ contributions
Joyce M. Chen; Data Collection, Manuscript writing. William Lam; Asha Reddy, Meenakshi Mehrotra; Performed short tandem repeat analysis and next gene sequencing. G. Kenneth Haines III; Jane Houldsworth; Qiusheng Si: Manuscript editing. The author(s) read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Declarations
Ethics approval and consent to participate
All ethical approval and consent procedures were approved by the Medical Ethical Committee of Icahn School of Medicine at Mount Sinai. According to the institution guidelines, a single case report with de-identified patient specific information, the patient’s singed privacy consent is not necessary.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest/competing interests in publishing the present manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998;9(11):2135–2141. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St. Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Stallone G, Infante B, Grandaliano G. Management and prevention of post-transplant malignancies in kidney transplant recipients. Clin Kidney J. 2015;8(5):637–644. doi: 10.1093/ckj/sfv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprangers B, Nair V, Launay-Vacher V, Riella LV, Jhaveri KD. Risk factors associated with post-kidney transplant malignancies: an article from the Cancer-kidney international network. Clin Kidney J. 2017;11(3):315–329. doi: 10.1093/ckj/sfx122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiberd BA, Rose C, Gill JS. Cancer mortality in kidney transplantation. Am J Transplant. 2009;9(8):1868–1875. doi: 10.1111/j.1600-6143.2009.02728.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers A, Ng JK, Glendinning J, et al. The management of transitional cell carcinoma (TCC) in a European regional renal transplant population. BJU Int. 2012;110(2):E34–E40. doi: 10.1111/j.1464-410X.2011.10777.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiang YJ, Yang PS, Wang HH, et al. Urothelial cancer after renal transplantation: An update. Transplant Proc. 2012;44(3):744–745. doi: 10.1016/j.transproceed.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 8.Gökçe Mİ, Kocaay AF, Aktürk S, Tüzüner A. Urothelial carcinoma of the allograft kidney developed in a renal transplant patient. Turk J Urol. 2016;42(3):213–215. doi: 10.5152/tud.2016.97415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega RM, Wolff DJ, Schandl CA, Drabkin HA. Urothelial carcinoma of donor origin in a kidney transplant patient. J Immunother Cancer. 2016;4(1):63–67. doi: 10.1186/s40425-016-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hevia V, Gómez V, Alvarez S, et al. Transitional cell carcinoma of the kidney graft: an extremely uncommon presentation of tumor in renal transplant recipients. Case Rep Transplant. 2013;2013:196528. doi: 10.1155/2013/196528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokos I, Pasini J, Stern-Padovan R, Mrsic S, Ries S. Conservative surgical treatment of low-grade urothelial carcinoma in the renal allograft recipient: a case report. Transplant Proc. 2006;38(5):1363–1365. doi: 10.1016/j.transproceed.2006.02.085. [DOI] [PubMed] [Google Scholar]
- 12.Cox J, Colli JL. Urothelial cancers after renal transplantation. Int Urol Nephrol. 2011;43(3):681–686. doi: 10.1007/s11255-011-9907-z. [DOI] [PubMed] [Google Scholar]
- 13.Hong YA, Hwang HS, Sul HJ, Kim SY, Chang YK. Transitional cell carcinoma involving graft kidney in a kidney transplant recipient: a case report. BMC Nephrol. 2017;18(1):299. doi: 10.1186/s12882-017-0715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu JW, Lee CN, Kang MY, et al. Incidences and oncological outcomes of urothelialcarcinoma in kidney transplant recipients. Cancer Manag Res. 2019;11:157–166. doi: 10.2147/CMAR.S185796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu MJ, Lian JD, Yang CR, et al. High cumulative incidence of urinary tract transitional cell carcinoma after kidney transplantation in Taiwan. Am J Kidney Dis. 2004;43(6):1091–1097. doi: 10.1053/j.ajkd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Roupret M, Azzouzi AR, Cussenot O. Microsatellite instability and transitional cell carcinoma of the upper urinary tract. BJU Int. 2005;96(4):489–492. doi: 10.1111/j.1464-410X.2005.05671.x. [DOI] [PubMed] [Google Scholar]
- 17.Karami S, Yanik EL, Moore LE, Pfeiffer RM, Copeland G, Gonsalves L, Hernandez BY, Lynch CF, Pawlish K, Engels EA. Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant. 2016;16(12):3479–3489. doi: 10.1111/ajt.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oikawa M, Hatakeyama S, Fujita T, Murakami R, Hagiwara K, Narita T, Noro D, Tanaka T, Tanaka Y, Tobisawa Y, Yoneyama T, Yoneyama T, Hashimoto Y, Koie T, Narumi S, Ohyama C. BK virus-associated urothelial carcinoma of a ureter graft in a renal transplant recipient: a case report. Transplant Proc. 2014;46(2):616–619. doi: 10.1016/j.transproceed.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Yan L, Salama ME, Lanciault C, Matsumura L, Troxell ML. Polyomavirus large T antigen is prevalent in urothelial carcinoma post-kidney transplant. Hum Pathol. 2016;48:122–131. doi: 10.1016/j.humpath.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Nickeleit V, Singh HK, Goldsmith CS, Miller SE, Kenan DJ. BK virus-associated urinary bladder carcinoma in transplant recipients: productive or nonproductive polyomavirus infections in tumor cells? Hum Pathol. 2013;44(12):2870–2871. doi: 10.1016/j.humpath.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kausman JY, Somers GR, Francis DM, Jones CL. Association of renal adenocarcinoma and BK virus nephropathy post transplantation. Pediatr Nephrol. 2004;19(4):459–462. doi: 10.1007/s00467-003-1407-7. [DOI] [PubMed] [Google Scholar]
- 22.Isharwal S, Audenet F, Drill F, et al. Prognostic value of TERT alterations, mutational and copy number alterations burden in urothelial carcinoma. Eur Urol Focus. 2019;5(2):201–204. doi: 10.1016/j.euf.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtis B, Zhuge J, Ojaimi C, Ye F, Cai D, Zhang D, Fallon JT, Zhong M. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol. 2016;21:7–11. doi: 10.1016/j.anndiagpath.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Brandau S, Böhle A. Bladder cancer. I. Molecular and genetic basis of carcinogenesis. Eur Urol. 2001;39(5):491–497. doi: 10.1159/000052494. [DOI] [PubMed] [Google Scholar]
- 25.Orlow I, Lacombe L, Hannon GJ, Serrano M, Pellicer I, Dalbagni G, Reuter VE, Zhang ZF, Beach D, Cordon-Cardo C. Deletion of the pl6 and pl5 genes in human bladder tumors. J Natl Cancer Inst. 1995;87(20):1524–1529. doi: 10.1093/jnci/87.20.1524. [DOI] [PubMed] [Google Scholar]
- 26.Audenet F, Isharwal S, Cha EK, Donoghue MTA, Drill EN, Ostrovnaya I, Pietzak EJ, Sfakianos JP, Bagrodia A, Murugan P, Dalbagni G, Donahue TF, Rosenberg JE, Bajorin DF, Arcila ME, Hechtman JF, Berger MF, Taylor BS, al-Ahmadie H, Iyer G, Bochner BH, Coleman JA, Solit DB. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res. 2019;25(3):967–976. doi: 10.1158/1078-0432.CCR-18-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.