Abstract
Traumatic brain injury (TBI) remains one of the leading causes of death and disability worldwide; more than 10 million people are hospitalized for TBI every year around the globe. While the primary injury remains unavoidable and not accessible to treatment, the secondary injury which includes oxidative stress, inflammation, excitotoxicity, but also complicating coagulation abnormalities, is potentially avoidable and profoundly affects the therapeutic process and prognosis of TBI patients. The endothelial glycocalyx, the first line of defense against endothelial injury, plays a vital role in maintaining the delicate balance between blood coagulation and anticoagulation. However, this component is highly vulnerable to damage and also difficult to examine. Recent advances in analytical techniques have enabled biochemical, visual, and computational investigation of this vascular component. In this review, we summarize the current knowledge on (i) structure and function of the endothelial glycocalyx, (ii) its potential role in the development of TBI associated coagulopathy, and (iii) the options available at present for detecting and protecting the endothelial glycocalyx.
Keywords: Traumatic brain injury, Coagulopathy, Endothelial glycocalyx
Introduction
Traumatic brain injury (TBI) is still one of the leading causes of death and disability worldwide with more than 10 million people hospitalized every year [1]. TBI refers to damage to the brain caused by any external mechanical force which may cause injury, for example rapid acceleration or deceleration, waves of shock, extrusion, impact, or penetration of projectiles and other objects [2]. Despite its high prevalence and cost for both, individuals and society, fundamental and underlying pathophysiology remain yet poorly understood and effective therapeutic options are still limited. Alterations of the coagulative system and disturbed coagulation function are common findings in patients with TBI; two out of three patients with severe TBI display abnormalities on conventional coagulation tests on admission which translate into poorer outcomes [3, 4]. TBI-associated coagulopathy may refer to both hypocoagulopathy with prolonged bleeding and hemorrhagic progression and hypercoagulopathy with an increased prothrombotic tendency, both of which can occur—either simultaneously or sequentially—after impact [5, 6].
Various mechanisms potentially linked to hemostatic disturbance after TBI have been studied, among which endothelial cell activation and glycocalyx shedding are currently given priority for further exploration [6, 7]. Poorer outcomes after isolated blunt TBI were associated with rapid increase in circulating biomarkers indicative for both endotheliopathy and coagulopathy according to the magnitude of injury. These profiles confirmed hemostatic failure, endothelial damage including damage to the inner endothelial layer (glycocalyx) injury, inflammation, and hyperfibrinolysis within the first 24 h after TBI [8]. Shedding of the glycocalyx seems capable to trigger systemic thrombin generation, activation of protein C, and hyperfibrinolysis in addition to potentially direct anticoagulative effects from endogenous heparinization. However, the shed glycocalyx may also increase circulating concentrations of damage-associated molecular patterns (DAMPs) including hyaluronan fragments and heparin sulfate. These DAMPs may then contribute to a hypercoagulative state through the production of inflammatory mediators and subsequent thrombin generation. Indeed, previous studies have shown glycocalyx disruption increases thrombin production [9].
In order to better understand the differences in occurrence and phenotype of TBI associated coagulopathy, it is necessary to further explore the structure and function of the glycocalyx, and to gain more in-depth knowledge about the potential mechanisms behind glycocalyx damage and down-stream consequences in the context TBI. In this review, we summarize the current knowledge on (i) structure and function of the endothelial glycocalyx, (ii) its potential role in the development of TBI associated coagulopathy, and (iii) the options available at present for detecting and protecting the endothelial glycocalyx.
Structure of the glycocalyx
The endothelial glycocalyx (EG) represents a layer of negatively charged, brush-like polysaccharide-protein complex structures on the surface of endothelial cells [10]. This layer of membrane-bound, carbohydrate-rich molecules covers the luminal surface of the endothelium along the entire vascular tree, mostly comprising glycoproteins and proteoglycans. Together with the underlying actin-rich endothelial cortex, 50 to 150 nm underneath the plasma membrane, EG is recognized as a vasoprotective nanobarrier and responsive hub; it presents highly dynamic and can adapt its nanomechanical properties (i.e., stiffness and height) in response to environmental changes [11]. The constant change between soft and stiff endothelial surfaces is imperative for proper functioning of the endothelium. Recently, EG has been defined as a critical component of the expanded neurovascular unit that influences the structure of the blood-brain barrier and plays various physiological functions, including an important role in maintaining normal neuronal homeostasis, which is related to maintaining the integrity of the blood vessel wall [12, 13]. However, the tiny structure and more nuanced functions of EG have been greatly ignored for a long time.
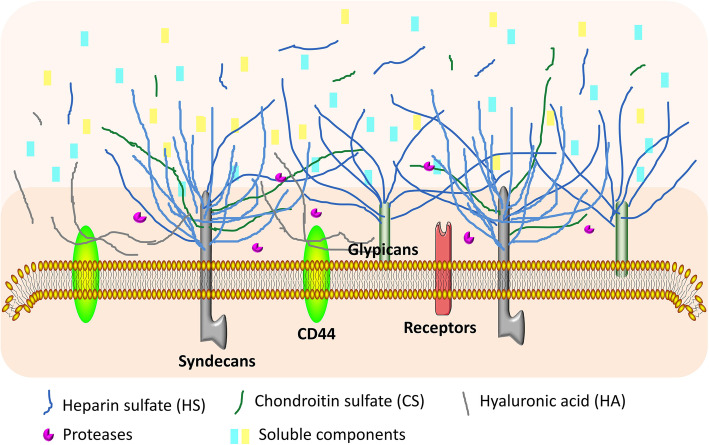
The concept of the thin endothelial layer was first proposed in 1940 [14], but it was not until 1966 that Luft and co-workers used an electron microscope to observe the glycocalyx structure of about 20 nm EG in rat intestinal mucosal blood vessels through ruthenium red staining [15]. With advanced observation technologies and research methods, the structure and function of glycocalyx have been gradually understood [16–18]. To date, it is believed that glycocalyx composition may vary according to tissue and cell type. The components of EG depend on plasma composition and local hemodynamic conditions and are composed of two layers [19, 20], e.g., (i) an inner-dense matrix layer: A relatively thin area of the glycocalyx (50–100 nm) composed primarily of “side chain”-glycosaminoglycans (GAGs) and “skeleton”-proteoglycans (PGs) directly bound to the plasma membrane and strictly glycocalyx, and (ii) an outer less-dense layer: a thicker area (0.5 μm) attached to the glycocalyx, which is a complex three-dimensional structure composed of soluble plasma components showing dynamic equilibrium (Fig. 1). The PGs skeleton bound to cell membrane and the GAG chain linked to the skeleton form a grid structure; incorporated in and on top of this grid are plasma and endothelium-derived soluble components and various proteins. Together, these components form the endothelial glycocalyx that functions as a barrier between blood plasma and the endothelium and exerts various roles in plasma and vessel wall homeostasis.
Fig. 1.
Schematic representation of the endothelial glycocalyx. Bound to the endothelial membrane are proteoglycans, with glycosaminoglycan side-chains (GAG-chain) and glycoproteins. Incorporated in and on top of this grid are plasma and endothelium-derived soluble components and various proteins. Together, these components form the endothelial glycocalyx that functions as a barrier between blood plasma and the endothelium and exerts various roles in plasma and vessel wall homeostasis
“Side chain”
Glycosaminoglycan (GAG) is considered as the most abundant component of the glycocalyx, mainly including hyaluronic acid (HA), heparin sulfate (HS), and chondroitin sulfate (CS). In the vascular system, HS accounts for 50–90% of the entire GAG. Quantitatively, HS and CS are usually present in a ratio of 4:1, which is altered under various stimuli, e.g., endothelial cell activation or injury. Except for HS, other GAGs are sulfated structures. Because GAGs contain a number of specific binding sites for plasma proteins, the enzymatic chemical modification and different sulfation patterns of sugar molecules can endow proteoglycans with unique physiological functions. These modification patterns may change over time under different pathophysiological stimuli, affecting vascular permeability and the binding activity of specific proteins.
“Skeleton”
Proteoglycan is usually considered as the most important “skeleton” molecule of the glycocalyx. It covalently binds to one or more GAGs through the core protein to form a kind of glycoconjugate, e.g., heparan sulfate proteoglycan (HSPG) [21, 22]. The type and number of glycosaminoglycan chains attached to different core proteins and whether they are bound to the cell membrane are not the same. Proteoglycan core proteins can be divided into membrane proteoglycans and extracellular matrix proteoglycans according to their localization in the cell microenvironment [22]. The former is represented by the syndecan family of transmembrane multi ligand proteoglycans connected with the cytoskeleton and the glypican family anchored on the endothelial cell membrane; the latter represents various secretory matrix proteoglycans. The syndecan family members expressed by endothelial cells (ECs) are Syndecan-1, -2, and -4. Its structure is divided into three segments: (i) the intracellular segment, (ii) the transmembrane domain, and (iii) the extracellular segment [23].
The extracellular matrix proteoglycans have three GAG attachment sites, wherein the endothelial cell syndecan-1 can bind to HS and CS. Glypican-1 is the only member of the glypican family that is expressed on endothelial cells [22]. It is different from syndecan-1 in that it only has an HS chain and no CS chain. HA is a non-sulfated secreted GAG, which is not linked to the PG core protein and binds to the receptor CD44. In addition, some glycoproteins are also considered as “skeletal structures” that connect the glycocalyx to the endothelial cell membrane, including endothelial cell adhesion molecules (E-selectin, P-selectin) and some glycoproteins with functions for coagulation and fibrinolysis. Their expression levels undergo significant alterations under stimulation [24].
“Dynamic soluble components”
Many soluble components, such as hyaluronic acid, albumin, thrombomodulin, extracellular superoxide dismutase, and antithrombin III, are embedded in the sieve structure formed by proteoglycans and glycoproteins [25, 26]. These soluble proteins and proteoglycans not only combine with each other to form a network to maintain the stability of the glycocalyx structure but also have significance in maintaining vascular permeability, anti-oxidation, and anti-coagulatio n[27, 28].
Function of glycocalyx
Natural barrier function
The glycocalyx is located at the interface between the blood flow and endothelial cells. Due to its particular spatial position and complex structure, it forms a natural mechanical barrier for endothelial cells, which can (i) reduce the risk for direct damage to endothelial cells by potentially harmful components in the blood stream, (ii) prevent receptor-mediated damage signal activation, and (iii) interaction between blood cells and the vascular wall. Under physiological conditions, the EG is much thicker than cell adhesion molecules in diameter, e.g., 0.2–0.5 μm in capillaries, 2–3 μm in arterioles, and 4.5 μm in carotid arteries [29, 30]. However, adhesion molecules such as P-selectin are only about 38 nm above the surface of endothelial cells. The GAGs and soluble component of glycocalyx seem to be capable to shield adhesion molecules and prevent the adhesion of erythrocytes, leukocytes, and platelets to endothelial cells [29, 31]. Glycocalyx can also serve as storage for functional proteins, such as galectins, which can be released and transferred to cells to perform their respective functions under stimulating conditions. In addition, there exists a buffer space between the glycocalyx and the blood vessel, and its colloid osmotic pressure is lower than the osmotic pressure of plasma and tissue gaps thereby acting as vascular barrier and preventing blood vessel leakage [32, 33].
Regulation of vascular permeability
The permeability of the glycocalyx to substances mainly depends on two aspects: (i) molecular weight and (ii) charge. Glycocalyx contains a large number of negatively charged residues such as sialic acid, sulfated polysaccharide, and uronic acid, and its complex network structure can act as a molecular sieve, which prevents negatively charged macromolecules, white blood cells, red blood cells, and platelets from passing through the blood vessel wall [32]. Vink and Duling [34] used a variety of plasma tracers to probe the barrier properties of the glycocalyx using combined fluorescence and brightfield intravital microscopy and found no permeation of the endothelial surface layer for either neutral or anionic dextrans ≥ 70 kDa, but for neutral Dextran 40 (40 kDa) and neutral free dye (rhodamine, 0.4 kDa) equilibrated with the endothelial surface layer within 1 min. In contrast, small anionic tracers of similar size (0.4–40 kDa) permeated the endothelial surface layer relatively slowly with half-times (τ50) between 11 and 60 min, depending on tracer size. Furthermore, fibrinogen (340 kDa) and albumin (67 kDa) moved slowly into the endothelial surface layer at comparable rates. The glycocalyx is semi-permeable to albumin which has amphoteric characteristics and can be tightly combined with the glycocalyx, then reduces its permeability [32]. These findings suggest that multiple factors may influence the permeability of the barrier, including molecular size, weight, charge, and structure.
In addition to the glycocalyx itself, the adsorbed plasma components may also play a role for microvascular permeability. It has been shown that a variety of soluble plasma components and selected synthetic substances can reduce the permeability of endothelial cells, including albumin, orosomucoid (ORM), hydroxyethyl starch (hetastarch, HES), and cationized ferritin among others [35]. Haraldsson and Rippe [36] have assessed the perfusion properties of rat skeletal muscle microvessels and found that when serum was added to the perfusion solution containing albumin, even if the serum concentration was only 10%, the permeability of the perfusion solution was reduced fourfold. In another set of studies in which orosomucoid was added to the albumin solution, it was found that compared with albumin alone, the permeability was reduced. However, the degree of permeability change was different from that when serum was added, indicating that not a single component can maintain low permeability, but most of them are negatively charged substances and glycoproteins with a molecular weight > 10 kDa.
Effect on hemodynamics
Within the circulatory system, local blood viscosity and hematocrit, at least to some extent, may also seem to be regulated by glycocalyx. It has been shown that in capillaries with a diameter of 5 μm, provided a 0.5-μm glycocalyx layer, the flow resistance may be increased threefold and the hematocrit can be reduced by at least 30% [37]. Compared with a smooth glass tube, even at the same red blood cell velocity, the presence of glycocalyx will reduce the flow volume of plasma [37]. Based upon hemodynamics and hematocrit within the physiological microvascular network, and considering the elasticity of the glycocalyx, Pries and co-workers [38] have added the actual glycocalyx thickness to the ideal model of mesenteric microvascular and found that the apparent viscosity of blood increased twofold, which laid the foundation for establishing a rheological model in vitro to be closer to physiological conditions in vivo.
Mechanoreceptor properties
The endothelial glycocalyx may act as a mechanoreceptor on endothelial cells lining every blood vessel wall which are consistently exposed to the mechanical forces generated by the blood flow [39]. An increased shear stress may increase nitric oxide (NO) production, which, in turn, leads to vessel dilation and reduced adherence of leukocytes and platelets [30]. Higher shear stress is also known to increase albumin uptake and alter glycocalyx properties, e.g., increased thickness [40]. When the glycocalyx is intact, shear stress can be transmitted to the actin cytoskeleton or directly to the cell membrane through the core protein, thereby mediating cell signal transduction [41, 42]. Damage to the glycocalyx impairs these mechanisms and perturbs the endothelial response to mechanical stress. Besides physical stress, the binding of ligands and enzymes to the glycocalyx may also induce signal transduction and enzymatic modification [43, 44]. The activity of growth factors, such as fibroblast growth factor (FGF) and epithelial growth factor, depend on the interactions with the glycocalyx. EG repair would be promoted by activation of endothelial growth factor signaling by highly-sulfated HS fragments released into the circulation during EG degradation [44]. The mechanism of glycocalyx participation in mechanical force transmission can be linked to both the decentralized and centralized mechanisms of mechanotransduction put forth by Davies and co-workers [45]. Syndecans that contain both HS and CS have an established association with the cytoskeleton and can decentralize the signal through multiple site distribution within the cell, e.g., nucleus, organelles, focal adhesions, and intercellular junctions [42]. These findings suggest that the glycocalyx may act as a receptor for physical and chemical signals, thereby inducing the physiological responses of the vascular endothelium.
Anticoagulantive function
The maintenance of its antithrombotic property is a key feature of the glycocalyx. The intact endothelium exerts range of anticoagulant properties, including production and release of NO, prostacyclin, and tissue factor pathway inhibitor (TFPI) [46]. Endothelial cells may also secrete heparan sulfate, which augments the anticoagulant action of antithrombin. Reportedly, circulating antithrombin in plasma may bind to heparan sulfate located on the luminal surface and in the basal membrane of the endothelium [27]. Thrombomodulin is another endothelium-bound protein with anticoagulant and anti-inflammatory functions that is triggered in response to systemic procoagulant stimuli [47]. Together with these critical anticoagulative functions, the glycocalyx plays an essential role in maintaining blood flow in the microcirculation. After TBI, the disruption of the glycocalyx loses its protective effects from the endothelial surface, allowing leukocyte and platelet adhesion and microthrombi formation [48, 49]. Anticoagulative substances, for example antithrombin III (AT III), heparin cofactor II and thrombomodulin, are lost after glycocalyx destruction, resulting in an imbalance between pro- and anticoagulation [50]. Furthermore, when the glycocalyx is shed, the anticoagulant activity of heparin and chondroitin sulfate in the side chain structure is retained, which may release into the blood circulation and induce autoheparinization [51]. Typically, the excessive activation of coagulation and pathological hyperfibrinolysis lead to disseminated intravascular coagulation (DIC), and the disruption of the glycocalyx contributes to this state. Gonzalez and co-workers have linked syndecan-1 levels not only to the severity of TBI but also to the development of coagulopathy [49].
Evaluating the endothelial glycocalyx
Experimental studies
Direct visualization and corresponding techniques are crucial for the assessment and characterization of the glycocalyx. The glycocalyx can be labeled through specific markers that attach to one or more of its components, making them fluorescent or detectable. The first imaging studies of the glycocalyx based upon transmission electron microscopy (TEM) date back to the year 1966 and have used ruthenium red; since then, many other attempts were made to visualize the glycocalyx using TEM and improved staining protocol [52, 53]. To date, electron microscopy is still the gold standard for visualization, even though many other research techniques have been applied for the detection of the glycocalyx. An alternative method, e.g., intravital microscopy, enables the observation of capillary blood flow in living animals. Brightfield and fluorescent microscopy can demonstrate the virtual diameter of capillaries in vivo, which are comparable to anatomical diameters [54]. The functional diameter of capillaries can also be estimated by measuring the diameter of deformed erythrocytes flowing through capillaries. The thickness of the EG can be calculated from the difference between functional diameters and anatomical diameters of capillaries [55]. In addition, the double tracer dilution method by using two tracers respectively label erythrocytes and endothelial cells to quantify the virtual volume of EG within the circulatory system [56]. Although the intravital microscopy and double tracer dilution method do not damage the integrity of the glycocalyx, they cannot be used to study cells in vitro. This can be performed by the application and development of flow cytometry and laser confocal fluorescence microscopy. However, there are still many limitations that need to be further surmounted.
While the techniques mentioned above may be beneficial for quantitative and qualitative assessment of EG, they do not completely represent the EG´s functional aspect. At present, controversy exists in the literature concerning the thickness of the glycocalyx, which can be interfered with by many factors, such as the different techniques applied, EG observed in vivo or in vitro, and even dimensions vary for different vascular beds, different flow rates, different organs, and in different species [52]. Hall and co-workers [53] have shown that the brain capillary glycocalyx thickness may differ in different sections of brain capillaries after TBI. Moreover, the in vitro model of cultured EG could not express glycocalyx with the same thickness as the glycocalyx found in vitro. Chappell and co-workers [57], by using electron microscopy of fixed umbilical vein endothelial cells, showed that the average thickness of the glycocalyx was 878 nm, while cultured human umbilical vein endothelial cells (HUVECs) showed that the thickness of glycocalyx was only 29 to 118 nm. Earlier studies have also suggested a disparity in vascular permeability between cultured ECs and the in situ microvascular wall, as evidenced by a twofold increase in the flux of albumin across cultured cells compared to that in capillaries [56], which may be attributed, at least in part, to differences in the structure of the overlying glycocalyx. Obviously, in vitro models failed to replicate the in vivo structure of the glycocalyx; whether the in vitro model can reflect the function of the glycocalyx in vivo is also worth pondering.
Clinical studies
Rapid and “point-of-care” (POC) assessment of the EG may be of high clinical value in the care and outcome for TBI patients and potentially would open new therapeutic windows of opportunity [58]. However, the direct visualization of the glycocalyx in humans remains extremely difficult in vivo, mostly due to its fragility. At present, indirect methods are predominantly used to evaluate glycocalyx in humans.
A variety of noninvasive microscopic camera techniques have been used to visualize sublingual microcirculation in patients and to assess the glycocalyx in human sublingual microvasculature by recording perfused boundary regions (PBR) which refer to the penetration extent of flowing red blood cells (RBCs) into the EG [59–61]. Examples of these techniques include orthogonal polarization spectral (OPS), sidestream dark field (SDF), or incident dark field (IDF) imaging, There have also been put forward a range of other comprehensive approaches to bedside for in vivo sublingual microcirculation image capture and analysis techniques in the human clinical setting, but the microcirculatory imaging systems and the availability of automated software for the analysis of glycocalyx thickness still need further improvement for facilitating glycocalyx studies in humans. In addition, as the dynamic equilibrium with the glycocalyx components in plasma, humoral markers have been assessed for their ability to estimate glycocalyx shedding in vivo; these markers include syndecan-1 (also known as CD138), HA, HS, and CS [58]. However, none of these markers have been found to be endothelial-specific, thereby limiting their use in clinical research. In addition, the comparability of results is complicated by the lack of reliable enzyme-linked immunosorbent assay kits, and interpreting the results of such assays remains difficult because of the rapid, or yet unknown, turnover rate of these markers and the impact of hepatic uptake in their metabolism. After the tight link between the endothelial and RBCs glycocalyx was demonstrated, it has been suggested that routine clinical evaluation of erythrocyte glycocalyx might be a suitable method for evaluating the EG [61].
Damage and protection of the glycocalyx in TBI
Damage to the glycocalyx can be found in the pathological sequelae of many acute and chronic diseases, such as trauma [62], sepsis [50], diabetes [63], atherosclerosis [64], nephropathy [65], and tumors [66]. It has been shown that the glycocalyx layer becomes thin, followed by destruction and shedding of its components. Traumatic brain injury unifies both the common characteristics of trauma and its particularities. Blood brain barrier (BBB) disruption is a major pathophysiological feature of TBI and is associated with neuroinflammatory events contributing to brain edema and cell death [67]. Both astrocytes and microglia can rapidly respond to injury by increasing the production of multiple factors that may have a significant effect on BBB function [68]. EG influences the structure of the BBB, which is related to maintaining the integrity of the blood vessel wall [12]. However, the primary injury of TBI leads to damaged endothelial cells and EG shedding, thus leading to a loss of barrier integrity and subsequent elevated permeability [49]. BBB breakdown not only triggers leukocyte recruitment and the migration of inflammatory cells activating astrocytes, but it also causes the release of pro-inflammatory cytokines, cytotoxic proteases, and reactive oxygen species (ROS) to activate microglia, affecting neuronal activit y[67]. Moreover, local inflammation occurs, expanding the site of injury, exacerbating the damage neutrophils into the damaged area of the CNS and inflammatory mediators are released into the systemic circulation. This set of events promotes the onset of a vicious cycle, which helps further damage to the BBB and even systemic secondary injury [67, 69].
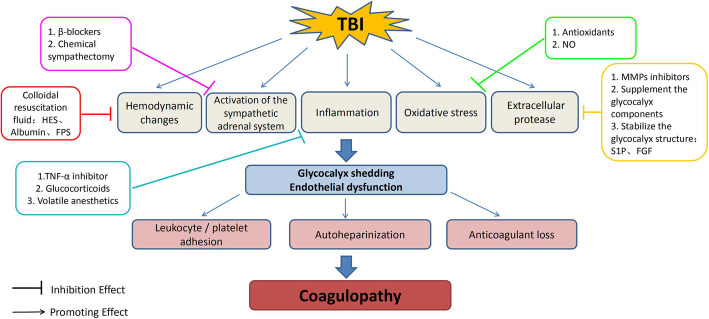
In the following, we will summarize potential damage mechanisms and options to restore glycocalyx function under the unique characteristics of TBI (Table 1, Fig. 2).
Table 1.
The possible factors of EG damage in TBI coagulopathy
| Factors | Types |
|---|---|
| Natriuretic peptide | ANP, BNP, CNP |
| Catecholamine | NE, Epi |
| Inflammatory mediators | TNF-α, IL-1β, IL-6, IL-8, IL-10 |
| Angiopoietin | Ang-2 |
| Chemotactic factors | fMLP |
| Reactive species | ROS, RNS |
| Histone deacetylase | HDAC |
| Extracellular proteases |
MMP-1, MMP-2, MMP-7, MMP-9, MMP-14, MMP-16, ADAM-10, ADAM-15, ADAM-17 ADAMTS-1, ADAMTS-4 Heparanase, hyaluronidase, thrombin, plasmin, elastase, lysosomal cathepsin B, tryptase β |
EG endothelial glycocalyx, TBI traumatic brain injury, ANP atrial natriuretic peptide, BNP brain natriuretic peptide, NE norepinephrine, Epi epinephrine, TNF tumor necrosis factor, IL interleukin, Ang angiopoietin, fMLP N-formylmethionyl-leucyl-phenylalanine, ROS reactive oxygen species, RNS reactive nitrogen species, HDAC histone deacetylase, MMP matrix metalloproteinase, ADAM a disintegrins and metalloproteinase, ADAMT a disintegrin and metalloproteinase with thrombospondin motif
Fig. 2.
Summary of the main mechanisms of EG injury induced TBI coagulopathy and the potential treatment for protecting glycocalyx. After TBI, there are many pathological changes can induce glycocalyx damage and the imbalance between coagulation and anticoagulation, thereby causing coagulopathy. Effects of different treatment measures (various colors) are pointed out with a closed tip arrow, at every stage where benefits have been observed. HES, hydroxyethyl starch; FPS, fresh frozen plasma; NO, nitric oxide; S1P, sphingosine-1 phosphate; FGF, fibroblast growth factor
Hemodynamic changes
Under physiological conditions, the vascular EG, as a mechanical sensor of blood flow, can reduce shear forces on cellular surfaces to a negligible level [70]. However, under pathological conditions, the shear force acting on the surface of endothelial cells can affect the structure of the glycocalyx by (i) changing its molecular composition, (ii) affecting the biosynthesis of new components, or (iii) activating proteases and lyases synthesized by endothelial cells [71–73]. Trauma patients often suffer from extensive tissue damage, hemorrhagic shock, and insufficient tissue perfusion, resulting in hemodynamic change. It has been shown that the decrease in plasma colloidal osmolality is related to the magnitude of the injury and the increase in syndecan-1 shedding, which leads to an increase in the demand for blood transfusion and resuscitation [74]. Fluid resuscitation is often used in critically ill patients to increase cardiac output and reverse pathological hypoperfusion, thereby preventing organ damage. Experimental and clinical evidence, however, suggests that fluid resuscitation only exerts short-term hemodynamic improvements, but may lead to long-term abnormal cardiovascular function, microcirculatory dysfunction, and increased demand for vasopressor therapy, with all significantly related mortality [75, 76]. These derangements are mainly due to increased release of atrial natriuretic peptide (ANP) following mechanical pressure caused by high blood volume, which mediates glycocalyx shedding increased vascular permeabilit y[77]. In addition to ANP, B- (BNP) and C-type (CNP) natriuretic peptide can also induce damage to the glycocalyx, which may explain the long-term adverse outcomes in clinical studies when recombinant human BNP (nesiritide) or ANP (carperitide) have been administered to improve cardiac function or reduce myocardial infarction injur y[78]. However, there is no proven mechanism yet described by which natriuretic peptides cause glycocalyx shedding. The concentration-dependent glycocalyx shedding provided by ANP in the experimental setting suggests an activation of natriuretic peptide receptors (NPRs).
Hemodynamic changes after TBI frequently show a close relation to the extent and magnitude of brain injury [79]. In fact, the change in systemic blood volume remains the most common factor for secondary injury in TBI; this includes the reduction in absolute blood volume, for example in hemorrhagic shock, as well as the reduction in relative blood volume, for example due to peripheral vasodilation and reductions in effective blood volume, which may cause mean arterial pressure to decline [80, 81]. Among patients with severe TBI, about one in three patients had hypotension and hypoxemia prior to rescue and resuscitation. Even on the intensive care unit (ICU), more than 25% of patients with severe TBI suffer from ongoing or periodic hypotension [82]. Besides, TBI can indirectly induce hypotension and subsequent coronary hypoperfusion through sympathetic overactivation and stress-induced cardiac dysfunction, thereby exacerbating systemic hemodynamic changes. In experimental combined TBI and hemorrhagic shock models, shedding of glycocalyx was determined and closely related to the occurrence of hemocoagulative dysfunction including coagulopathy [48]. Unfortunately, the correlation between hemodynamic changes in TBI and damage to the glycocalyx was not analyzed in further detail.
Potential therapeutic options
Given that changes in blood volume can be associated with increased glycocalyx degradation, protecting and restoring the vascular EG may be an important goal for clinical management. Therefore, the type of fluid resuscitation and the appropriate fluid volume are of yet to be specified significance to maintain the glycocalyx integrity. Currently, colloidal resuscitation is recommended to protect the integrity of the glycocalyx. Margraf and co-workers have reported that 6% hydroxyethyl starch (HES 130/0.4) diminishes glycocalyx degradation and decreases vascular permeability during systemic and pulmonary inflammation in mice as compared to resuscitation with crystalloids [83]. Sphingosine-1 phosphate (S1P) is a sphingolipid in plasma that plays a critical role in the cardiovascular and immune systems. Serum albumin and high-density lipoprotein (HDL) carry ~ 90% of the S1P, and both elicit the release of S1P from RBCs. The S1P receptor most abundantly expressed on ECs is S1P1, and S1P can protect the glycocalyx via the S1P1 receptor. Albumin, a colloid commonly used for volume resuscitation, has been considered to have protective effects on the glycocalyx because it may carry S1P derived from red blood cells into endothelial cells and mediate glycocalyx recovery by inhibiting matrix metalloproteinases (MMPs) activity [84, 85]. A series of other preclinical studies have found that, compared with the control group and lactated Ringer’s solution (LRS) resuscitation group, fresh frozen plasma (FFP) may exert protective effect on glycocalyx degradation in experimental animals when exposed to hemorrhagic shock [86, 87]. Therefore, a more in-depth understanding of the vascular EG may help to refine fluid resuscitation programs and strategies for critically ill patients with TBI. It also has been suggested that B-type receptor inhibitors, for example anantin, or inhibitors of natriuretic peptide receptor signal transduction may have a protective effect on glycocalyx. No such drugs have yet been approved for human use so far.
Activation of the sympathetic adrenal system
Preclinical and clinical studies have shown that catecholamine neurotransmitters are in disorder in the presence of central degenerative diseases and brain injury. TBI often leads to excessive activation of the sympathetic nervous system, which is manifested by the increased activity of catecholamine synthetases such as tyrosine hydroxylase and the surge of catecholamine transmitters (e.g., dopamine, norepinephrine and epinephrine) not only aggravates brain tissue damage but also relates closely to the pathological events that lead to secondary brain and systemic injury [88–90]. At present, a large body of evidence has shown that the sympathetic adrenal system is triggered in trauma including TBI, septic shock, ischemia-reperfusion and acute myocardial infarction, leading to endothelial cell injury, glycocalyx shedding, and abnormal blood coagulation [8, 89]. This may be interpreted as an adaptive response at first, which is conductive to the transformation from a hypercoagulable to a hypocoagulable state in order to maintain microvessels open and to promote blood perfusion and oxygen delivery within various tissues/organs [90]. In addition to effects on the systemic circulatory system, sustained high catecholamine levels can also directly damage endothelial cells, leading also to shedding of the glycocalyx including its components. Martin and co-workers [91] have directly used different titrations of norepinephrine (NE) and epinephrine (Epi) to stimulate HUVECs under simulated conditions of shock and found that catecholamines may cause glycocalyx damage and endothelial activation under stress conditions in a dose-dependent manner. However, the current research on correlation of catecholamines and glycocalyx shedding is limited to descriptive and analyses only with specific damage. Mechanisms still need to be further explored.
Potential therapeutic options
Many experimental studies have found that treatment with nonselective β-blockers (BBs) (e.g., propranolol and clonidine) can reduce cerebral edema, blood-brain barrier permeability, and glycocalyx damage at 24 h in animals exposed to TBI [92]. Clinical studies also confirmed that early use of β-blockers after TBI could (i) protect end organs that are vulnerable to catecholamine damage, (ii) improve microcirculation, and (iii) increase survival [93, 94]. Similarly, chemical sympathectomy has also been shown to reduce inflammation, glucocorticoid shedding, and disturbed hemostasis in experimental animals with acute traumatic coagulopathy [95]. However, clinical guideline has yet been established for the use of BBs in TBI patients.
Inflammation
In most tissues, including the central nervous system, inflammatory responses are elicited after both trauma and ischemia [96]. Glycocalyx acts as the first barrier to endothelial injury; studies have demonstrated the correlation between inflammatory mediators (such as TNF-α, IL-1β, IL-6, and IL-10) and glycocalyx shedding [97]. Many clinical syndromes of systemic inflammation, including sepsis, major surgery, trauma, atherosclerosis, and persistent hyperglycemia, etc., can cause diffuse and persistent damage to the glycocalyx [31]. The combination of TBI and shock results in an immediate activation of both coagulation and complement systems with subsequent EG shedding, protein C activation, and inflammation [48]. Many studies have shown that adherent leukocytes can directly damage the endothelium; however, in the absence of leukocyte-endothelial cell adhesion, Henry and co-workers have treated endothelial cells with TNF-α for 20 min and observed glycocalyx shedding [98]. It may be that TNF-α increases endothelial-derived free radicals or activates surface-bound proteases and phospholipases, thereby causing glycocalyx damage. Angiopoietin 2 (Ang-2) is a protein secreted by endothelial cells under inflammatory stimulation which can inhibit the anti-inflammatory signal induced by Ang-1 activation of the tyrosine kinase-2 (Tie2) receptor which is also considered a key mediator of glycocalyx degradation. Lukasz and co-workers [99] have used Ang-2 to treat HUVECs and mice, which can cause rapid degradation of the EG both in vivo and in vitro, indicating that inhibition of Ang-2 and activation of Tie2 may be potential therapeutic targets for glycocalyx protection. In addition, n-formyl-met-leu-phe (fMLP), a potent neutrophil chemotactic peptide, can also cause glycocalyx shedding and intercellular adhesion molecule 1 (ICAM-1) exposure by binding to specific G protein-coupled receptors on endothelial cells, thus promoting leukocyte adhesion in rat mesenteric capillaries [100]. During inflammation, the signal of glycocalyx repair mediated by FGF is significantly suppressed [44]. Therefore, enhancing the glycocalyx repair signal may be a potential treatment method to restore the glycocalyx layer and improve its function.
Potential therapeutic options
Data from experimental animal and cell experiments indicate that glycocalyx injury occurs within hours after inflammation, but it takes 5–7 days to return to normal. Therefore, inhibition of inflammatory response may represent a promising target for glycocalyx protection and restoration. TNF-α is one of the critical mediators in both acute and chronic inflammation. The clinical use of TNF-α inhibitor, Etanercept, an analog of TNF-α receptor, can significantly reduce endotoxin-induced glycocalyx component shedding, coagulation activation, and vascular dysfunction [101]. Since the vascular permeability of macromolecules is significantly increased during inflammation, glucocorticoids are usually used to prevent edema and swelling between tissues. Antithrombin is an important inhibitor of the coagulation system and has specific anti-inflammatory effects on endothelial cells. In an isolated heart model, Chappell and co-workers found that hydrocortisone and antithrombin can significantly reduce glycocalyx shedding after ischemia/reperfusion and TNF-α-induced inflammation [102, 103]. Glucocorticoids can also stabilize mast cells and prevent degranulation, thereby reducing inflammation on the glycocalyx. Although glucocorticoids have positive effects, the exact mechanism through which they act on EG is still unclear. Other volatile anesthetics, such as sevoflurane and isoflurane [104, 105], have also been found to have anti-inflammatory effects, which may potentially attenuated EG destruction caused by inflammation. These suggest that sedative and analgesic drugs with anti-inflammatory effects used in the clinical management of TBI may be beneficial to protect glycocalyx and improve microcirculation.
Oxidative stress
Oxidative stress refers to the process of tissue damage caused by an imbalance between the oxidation and the antioxidant system when the body is subjected to various harmful stimuli [106]. As early as 1986, Kontos and co-workers have shown that a large number of free radicals are generated after TBI [106, 107]. Free radicals are by-products of the body metabolism, which are promptly eliminated by the antioxidant mechanism under physiologic conditions. After TBI, due to the decrease in local cerebral blood flow in affected areas, the oxidation level within brain tissue cells is enhanced, and the antioxidant capacity and the ability to scavenge free radicals are weakened, automatically leading to the accumulation of free radicals. Severe TBI patients with shock can also experience systemic ischemia and tissue hypoxia, which will eventually cause a vicious circle.
Oxidative stress can occur in cardiopulmonary surgery [73], severe trauma [108], massive blood loss [31], hypoxia [109], and ischemia-reperfusion [110]. In all cases, the EG covering the microvasculature is attacked and damaged by reactive oxygen and nitrogen species, ROS and RNS respectively, which can cause EG injury and increase in plasma syndecan-1 and HS levels [110]. At present, the detrimental effects of oxidative stress on glycocalyx are mostly derived from various experimental models and clinical studies of ischemia/reperfusion and hypoxia [111]. In vivo studies have shown that the EG shed rapidly after ischemia-reperfusion [112, 113], and the injury of the glycocalyx was also confirmed in patients who experienced local ischemia during clinical vascular surgery [73]. The mechanisms behind these observations are still unclear. Potential mediators may include excessive free radicals, complement activation, TNF-α expression, mast cell degranulation, and protease (especially metalloproteinase) activation. Ali and co-workers have reported that MMP is the primary cellular protease involved in the degradation of EG induced by oxidative stress in vitro [114]. After oxidative stress, histone deacetylase (HDAC) mediates the upregulation of MMPs and the downregulation of tissue inhibitors of metalloproteinases (TIMPs), leading to the shedding of GAGs and extracellular superoxide dismutase (SOD) and aggravating oxidative stress injury [115].
Potential therapeutic options
Several studies have shown that antioxidants can protect tissues and organs from oxidative stress damage [116, 117]. It may be speculated this holds also true in the context of glycocalyx damage. As an important signal molecule of vascular cells, low-dose NO can prevent oxidative damage. Bruegger and co-workers [112] found that the use of NO prior to myocardial reperfusion can reduce coronary artery leakage and edema formation, and has a glycocalyx protective effect. The premise of this protective effect is that the glycocalyx is not degraded in advance.
Extracellular protease
The endothelial glycocalyx, a poly glycoprotein complex covering the surface of the endothelial cell, is a component to the extracellular matrix (ECM) and is in direct contact to various components within the blood stream, including a range of proteases. A large number of studies have confirmed the effect of various “sheddases” on the glycocalyx in different pathological settings, and different “sheddases” may have selective targeting effects on various components of the glycocalyx (Table 2) [64, 114, 118]. The most common is the superfamily of MMPs, with more than 20 members, including “classical” MMPs, membrane-bound MMPs (MT-MMPs), disintegrins and metalloproteinases (ADAMs), and disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) [119]. One of the most in-depth studies of these enzymes is their ability to catalyze the cleavage of the extracellular domain of transmembrane proteins and reduce their cell surface levels [120–122]. The hypothesis that MMPs can cleave the EG and promote the shedding of different components under pathological conditions has been fully supported [123–126]. MMPs were stored in the endothelial vesicles in an inactive form and could be released rapidly by activated endothelial cells under appropriate stimulation. The activity of MMPs is regulated by a group of endogenous proteins, called TIMPs, which bind to the active sites of activated MMPs to prevent their activation. Besides MMPs, heparanase [127] and hyaluronidase [53] can specifically cleave HS and HA components in glycocalyx. According to limited reports, thrombin [128], plasmin [129], elastase [130], tryptase [131], and cathepsin B [132], which can induce glycocalyx shedding, are also potential “sheddases.”
Table 2.
Shaddases of EG and the studies in TBI
| Candidate sheddases | EG components | Studies in TBI |
|---|---|---|
| MMPs | ||
| MMP-1 | Syndecan-1,HS | Neuroinflammation |
| MMP-2 | Syndecan-1,-2,CS | BBB dysfunction, neuroinflammation, apoptosis |
| MMP-7 | Syndecan-1,-2,CS | Neuroinflammation |
| MMP-9 | Syndecan-1,-2,CS | BBB dysfunction, neuroinflammation, apoptosis |
| MMP-14(MT1-MMP) | Syndecan-1 | |
| MMP-16(MT3-MMP) | Syndecan-1 | |
| ADAMs | ||
| ADAM-10 | EMCN | Poor synaptic recovery |
| ADAM-15 | CD44 | |
| ADAM-17 | Syndecan-1,-4, EMCN | Neuroinflammation, neural progenitor cell migration, neurogenesis |
| ADAMTSs | ||
| ADAMTS-1 | Syndecan-4 | Neuroinflammation |
| ADAMTS-4 | Syndecan-4 | Neuroinflammation |
| Heparanase | HS | |
| Hyaluronidase | HA | BBB dysfunction, behavioral decrements, neuroinflammation |
| Thrombin | Syndecan-1,-4 | BBB dysfunction, apoptosis, cerebral ischemia, excitotoxicity |
| Plasmin | Syndecan-4 | Cerebral hemorrhage, inflammation, coagulopathy |
| Elastase | Syndecan-1,-4 | Neuroinflammation, oxidative stress, apoptosis |
| Lysosomal cathepsin B | Syndecan-1, HS | Neuroinflammation, apoptosis, neurodegeneration |
| Tryptase β | Syndecan-1, HS | Neuroinflammation, apoptosis |
EG endothelial glycocalyx, TBI traumatic brain injury, MMPs matrix metalloproteinases, MT-MMP membrane-bound MMP, ADAMs a disintegrins and metalloproteinases, ADAMTS a disintegrin and metalloproteinase with thrombospondin motifs, HS heparin sulfate, HA hyaluronic acid, CS chondroitin sulfate, EMCN endomucin, CTF C-terminal fragments
At present, many proteases have been reported to play important roles in various nervous system diseases and injuries, with their expression up-regulated and enzyme activity increased after TBI [133]. These proteases are responsible for the cleavage of transmembrane proteins in neurons, glial cells, and endothelial cells, resulting in the release of the extracellular domain, which mediates neural function injury as well as repair after TBI. In the adult central nervous system (CNS), the expression level of most MMPs is low or uncertain [134–138], only 17 out of 30 ADAMs [133, 139–141] play a role in the brain, and the literature on the expression of ADAMs in CNS pathology is very limited. Most of the “sheddases” of the glycocalyx display expression and activity changes in the context of TBI [53, 130, 142–152], but research has yet mainly focused on inflammatory response and neural repair processes (Table 2). The relationship between these “sheddases” of glycocalyx injury and secondary coagulopathy after TBI needs further study.
Potential therapeutic options
Dane and co-workers [153] have reported that the glomerular EG damage induced by intravenous injection of hyaluronidase takes 4 weeks to return to physiologic levels. Therefore, it is important to take measures to prevent or reduce glycocalyx damage of vascular endothelial cells early when injury factors are acting.
Specific inhibitors of “sheddases” can be selected to prevent degradation of the glycocalyx/endothelial surface layer. Although many studies have shown that nonselective inhibitors of metalloproteinases such as phenanthroline, doxycycline can reduce damage promoted by inflammation and oxidative stress on the glycocalyx [154, 155]; early administration of tranexamic acid (TXA) can inhibit the activities of ADAM-17, TNF-α, and MMP-9 and may protect the glycocalyx-vascular barrier, reduce the occurrence of post-traumatic bleeding, and improve survival [156]. However, the currently available MMP inhibitors are not selective for specific protease subtypes and do not have the same inhibitory activity against other members of the family. In addition, combined with the similar structure of MMP members and the complexity of the activation pathway, the continued inhibitory effect of MMPs must be considered. In addition, in combination with the similarity of the structure of MMPs and the complexity of the activation pathway, the duration of MMP inhibition and the later role of these proteases in CNS repair after brain injury must be considered. All these aspects have led to a very limited application and efficacy of MMP inhibitors in clinical research so far. In addition, Grundman and co-workers [157] have found that antithrombin III can bind tightly to the glycocalyx, by promoting the release of prostacyclin, inhibiting other “sheddases” activation pathways, and reducing glycocalyx damage.
The specific supplementation of glycocalyx components may represent another therapeutic avenue. For example, Broekhuizen and co-workers have reported that after 8 weeks of treatment with sulodexide, a mixture of glycocalyx GAG precursors, glycocalyx damage to the sublingual and retinal vascular beds was reduced, and capillary albumin leakage trended towards normal in diabetic patients [158]. Hayashida and co-workers have found that intraperitoneal injection of HS can reduce the level of inflammatory proteins underlying glycocalyx damage in liver and lung tissues of mice with endotoxemia [159]. Supplementation of heparin analogs can also reduce the plasma levels of syndecan-1 and thus alleviating glycocalyx injury [160].
A final therapeutic concept that needs further exploration is the stabilization of the glycocalyx structure. S1P is a sphingolipid synthesized by red blood cells, which can regulate the synthesis of glycocalyx components by binding to receptors on the surface of endothelial cells. Adamson and co-workers [85] have found that a certain amount of S1P (100–300 nmol/L) may promote and maintain EG stability. In addition, HS fragments produced by glycocalyx shedding in the circulation can activate FGF, which binds to FGF receptors to activate glycocalyx repair molecules by initiating signal transduction pathways, such as exostosin-1 (a kind of the enzyme responsible for the synthesis of heparan sulfate), and mediates the physiological repair of the glycocalyx.
Conclusion
There is increasing interest and awareness for the potential role of vascular EG damage in the pathophysiological sequelae after TBI. Selecting the endothelial glycocalyx as a therapeutic target to prevent and limit glycocalyx damage and to protect the vascular endothelial barrier function provides a new concept for both TBI and coagulation management. The specific mechanisms by which damage to the glycocalyx is promoted are still unknown and need further exploration. The majority of studies yet conducted in the field have been experimental animal studies whose results clearly need verification in the clinical arena.
Acknowledgements
Not applicable.
Abbreviations
- TBI
Traumatic brain injury
- DAMPs
Damage-associated molecular patterns
- EG
Endothelial glycocalyx
- GAGs
Glycosaminoglycans
- PGs
Proteoglycans
- HA
Hyaluronic acid
- HS
Heparin sulfate
- CS
Chondroitin sulfate
- HSPG
Heparan sulfate proteoglycan
- EC
Endothelial cells
- HES
Hydroxyethyl starch
- NO
Nitric oxide
- HUVECs
Human umbilical vein endothelial cells
- RBCs
Red blood cells
- ANP
Atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- CNP
C-type natriuretic peptide
- S1P
Sphingosine-1 phosphate
- MMPs
Matrix metalloproteinases
- BBs
β-blockers
- Ang-2
Angiopoietin 2
- Tie2
Tyrosine kinase-2
- FGF
Fibroblast growth factor
- TIMPs
Tissue inhibitors of metalloproteinases
- MT-MMPs
Membrane-bound MMPs
- ADAMs
A disintegrins and metalloproteinases
- ADAMTSs
A disintegrin and metalloproteinase with thrombospondin motifs
- CNS
Central nervous system
Authors’ contributions
Zhimin Zou: collected and organized literature and was a major contributor in writing the manuscript. Li Li: supplemented the literature and was major contributor in drawing the figures and tables. Nadine Schäfer: modified the manuscript’s format. Qiaobing Huang: advised the basic clue of writing. Marc Maegele and Zhengtao Gu: revised and improved the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Nature Science Fund of China [NO.81901997], Guangdong Natural Science Foundation of China [NO.2019A1515010295; NO.2020A1515010092], Certificate of China Postdoctoral Science Foundation Grant [NO.2019 M652960; NO.2019 M652965], Youth Scientific Research Staring Foundation for the Third Affiliated Hospital of Southern Medical University [NO. 2019Q006], and Scientific Research Staring Foundation for Talent Introduction for Southern Medical University (to MM).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhimin Zou and Li Li contributed equally to this work.
Contributor Information
Zhimin Zou, Email: zou9190@126.com.
Li Li, Email: li_435319585@126.com.
Nadine Schäfer, Email: Nadine.Schaefer@uni-wh.de.
Qiaobing Huang, Email: bing@smu.edu.cn.
Marc Maegele, Email: Marc.Maegele@t-online.de.
Zhengtao Gu, Email: guzhengtao@126.com.
References
- 1.Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci. 2018;62(5):535–541. doi: 10.23736/S0390-5616.18.04532-0. [DOI] [PubMed] [Google Scholar]
- 2.Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–238. doi: 10.1016/j.mcna.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Hoyt DB. A clinical review of bleeding dilemmas in trauma. Semin Hematol. 2004;41(1 Suppl 1):40–43. doi: 10.1053/j.seminhematol.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien) 2008;150(2):165–175. doi: 10.1007/s00701-007-1475-8. [DOI] [PubMed] [Google Scholar]
- 5.Maegele M, Schochl H, Menovsky T, Marechal H, Marklund N, Buki A, Stanworth S. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16(8):630–647. doi: 10.1016/S1474-4422(17)30197-7. [DOI] [PubMed] [Google Scholar]
- 6.Samuels JM, Moore EE, Silliman CC, Banerjee A, Cohen MJ, Ghasabyan A, Chandler J, Coleman JR, Sauaia A. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J Trauma Acute Care Surg. 2019;86(4):686–693. doi: 10.1097/TA.0000000000002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Zhang F, Dong JF. Coagulopathy induced by traumatic brain injury: systemic manifestation of a localized injury. Blood. 2018;131(18):2001–2006. doi: 10.1182/blood-2017-11-784108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Battista AP, Rizoli SB, Lejnieks B, Min A, Shiu MY, Peng HT, Baker AJ, Hutchison MG, Churchill N, Inaba K, et al. Sympathoadrenal activation is associated with acute traumatic coagulopathy and endotheliopathy in isolated brain injury. Shock. 2016;46(3S):96–103. doi: 10.1097/SHK.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M, Van DPT. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–S34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 10.Jedlicka J, Becker BF, Chappell D. Endothelial glycocalyx. Crit Care Clin. 2020;36(2):217–232. doi: 10.1016/j.ccc.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Cosgun ZC, Fels B, Kusche-Vihrog K. Nanomechanics of the endothelial glycocalyx: from structure to function. Am J Pathol. 2020;190(4):732–741. doi: 10.1016/j.ajpath.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhao F, Zhong L, Luo Y. Endothelial glycocalyx as an important factor in composition of blood-brain barrier. Cns Neurosci Ther. 2021;27(1):26–35. doi: 10.1111/cns.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012;32(7):1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danielli JF. Capillary permeability and oedema in the perfused frog. J Physiol. 1940;98(1):109–129. doi: 10.1113/jphysiol.1940.sp003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966;25(6):1773–1783. [PubMed] [Google Scholar]
- 16.Broekhuizen LN, Mooij HL, Kastelein JJ, Stroes ES, Vink H, Nieuwdorp M. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr Opin Lipidol. 2009;20(1):57–62. doi: 10.1097/MOL.0b013e328321b587. [DOI] [PubMed] [Google Scholar]
- 17.Van Teeffelen JW, Brands J, Stroes ES, Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med. 2007;17(3):101–105. doi: 10.1016/j.tcm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9(1):121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 19.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng. 2014;16(1):505–532. doi: 10.1146/annurev-bioeng-071813-104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janczyk P, Hansen S, Bahramsoltani M, Plendl J. The glycocalyx of human, bovine and murine microvascular endothelial cells cultured in vitro. J Electron Microsc (Tokyo) 2010;59(4):291–298. doi: 10.1093/jmicro/dfq007. [DOI] [PubMed] [Google Scholar]
- 21.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7). 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed]
- 22.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99(9):2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park PW. Isolation and functional analysis of syndecans. Methods Cell Biol. 2018;143:317–333. doi: 10.1016/bs.mcb.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieve I, Munster-Kuhnel AK, Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul Pharmacol. 2018;100:26–33. doi: 10.1016/j.vph.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Fridén V, Oveland E, Tenstad O, Ebefors K, Nyström J, Nilsson UA, Haraldsson B. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 2011;79(12):1322–1330. doi: 10.1038/ki.2011.58. [DOI] [PubMed] [Google Scholar]
- 26.Adamson RH, Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol. 1992;445(1):473–486. doi: 10.1113/jphysiol.1992.sp018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Agostini AI, Watkins SC, Slayter HS, Youssoufian H, Rosenberg RD. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. J Cell Biol. 1990;111(3):1293–1304. doi: 10.1083/jcb.111.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schott U, Solomon C, Fries D, Bentzer P. The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med. 2016;24(1):48. doi: 10.1186/s13049-016-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 31.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286(5):H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 32.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40(4):828–839. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 34.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278(1):H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 35.Rehm M, Zahler S, Lötsch M, Welsch U, Conzen P, Jacob M, Becker BF. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100(5):1211–1223. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Haraldsson B, Rippe B. Higher albumin clearance in rat hindquarters perfused with pure albumin solution than with serum as perfusate. Acta Physiol Scand. 1984;122(1):93–95. doi: 10.1111/j.1748-1716.1984.tb07486.x. [DOI] [PubMed] [Google Scholar]
- 37.Lipowsky HH, Gao L, Lescanic A. Shedding of the endothelial glycocalyx in arterioles, capillaries, and venules and its effect on capillary hemodynamics during inflammation. Am J Physiol Heart Circ Physiol. 2011;301(6):H2235–H2245. doi: 10.1152/ajpheart.00803.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pries AR, Secomb TW. Microvascular blood viscosity in vivo and the endothelial surface layer. Am J Physiol Heart Circ Physiol. 2005;289(6):H2657–H2664. doi: 10.1152/ajpheart.00297.2005. [DOI] [PubMed] [Google Scholar]
- 39.Zeng Y, Zhang XF, Fu BM, Tarbell JM. The role of endothelial surface glycocalyx in mechanosensing and transduction. Adv Exp Med Biol. 2018;1097:1–27. doi: 10.1007/978-3-319-96445-4_1. [DOI] [PubMed] [Google Scholar]
- 40.Ueda A, Shimomura M, Ikeda M, Yamaguchi R, Tanishita K. Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287(5):H2287–H2294. doi: 10.1152/ajpheart.00808.2003. [DOI] [PubMed] [Google Scholar]
- 41.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a "bumper-car" model. Proc Natl Acad Sci U S A. 2004;101(47):16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93(10):e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 43.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H452–H458. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Haeger SM, Suflita MA, Zhang F, Dailey KL, Colbert JF, Ford JA, Picon MA, Stearman RS, Lin L, Liu X, Han X, Linhardt RJ, Schmidt EP. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol. 2017;56(6):727–737. doi: 10.1165/rcmb.2016-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75(3):519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott I, Miyagi Y, Miyazaki K, Heeb MJ, Mueller BM, Rao LV, Ruf W. Reversible regulation of tissue factor-induced coagulation by glycosyl phosphatidylinositol-anchored tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2000;20(3):874–882. doi: 10.1161/01.ATV.20.3.874. [DOI] [PubMed] [Google Scholar]
- 47.Helms J, Clere-Jehl R, Bianchini E, Le Borgne P, Burban M, Zobairi F, Diehl JL, Grunebaum L, Toti F, Meziani F, Borgel D. Thrombomodulin favors leukocyte microvesicle fibrinolytic activity, reduces NETosis and prevents septic shock-induced coagulopathy in rats. Ann Intensive Care. 2017;7(1):118. doi: 10.1186/s13613-017-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sillesen M, Rasmussen LS, Jin G, Jepsen CH, Imam A, Hwabejire JO, Halaweish I, DeMoya M, Velmahos G, Johansson PI, Alam HB. Assessment of coagulopathy, endothelial injury, and inflammation after traumatic brain injury and hemorrhage in a porcine model. J Trauma Acute Care Surg. 2014;76(1):12–19. doi: 10.1097/TA.0b013e3182aaa675. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez RE, Cardenas JC, Cox CS, Kitagawa RS, Stensballe J, Holcomb JB, Johansson PI, Wade CE. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand J Trauma Resusc Emerg Med. 2018;26(1):102. doi: 10.1186/s13049-018-0565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17(2):283–294. doi: 10.1111/jth.14371. [DOI] [PubMed] [Google Scholar]
- 51.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73(1):60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 52.Ando Y, Okada H, Takemura G, Suzuki K, Takada C, Tomita H, Zaikokuji R, Hotta Y, Miyazaki N, Yano H, Muraki I, Kuroda A, Fukuda H, Kawasaki Y, Okamoto H, Kawaguchi T, Watanabe T, Doi T, Yoshida T, Ushikoshi H, Yoshida S, Ogura S. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8(1):17523. doi: 10.1038/s41598-018-35976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall AA, Mendoza MI, Zhou H, Shaughness M, Maudlin-Jeronimo E, McCarron RM, Ahlers ST. Repeated low intensity blast exposure is associated with damaged endothelial glycocalyx and downstream behavioral deficits. Front Behav Neurosci. 2017;11:104. doi: 10.3389/fnbeh.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kataoka H, Ushiyama A, Akimoto Y, Matsubara S, Kawakami H, Iijima T. Structural behavior of the endothelial glycocalyx is associated with pathophysiologic status in septic mice: an integrated approach to analyzing the behavior and function of the glycocalyx using both electron and fluorescence intravital microscopy. Anesth Analg. 2017;125(3):874–883. doi: 10.1213/ANE.0000000000002057. [DOI] [PubMed] [Google Scholar]
- 55.Nieuwdorp M, Meuwese MC, Mooij HL, Ince C, Broekhuizen LN, Kastelein JJ, Stroes ES, Vink H. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Appl Physiol (1985) 2008;104:845–852. doi: 10.1152/japplphysiol.00440.2007. [DOI] [PubMed] [Google Scholar]
- 56.Michel CC, Curry FR. Glycocalyx volume: a critical review of tracer dilution methods for its measurement. Microcirculation. 2009;16(3):213–219. doi: 10.1080/10739680802527404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009;104(11):1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 58.Cerny V, Astapenko D, Brettner F, Benes J, Hyspler R, Lehmann C, Zadak Z. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit Rev Clin Lab Sci. 2017;54(5):343–357. doi: 10.1080/10408363.2017.1379943. [DOI] [PubMed] [Google Scholar]
- 59.Massey MJ, Shapiro NI. A guide to human in vivo microcirculatory flow image analysis. Crit Care. 2016;20:35. doi: 10.1186/s13054-016-1213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donati A, Damiani E, Domizi R, Romano R, Adrario E, Pelaia P, Ince C, Singer M. Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res. 2013;90:86–89. doi: 10.1016/j.mvr.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Oberleithner H. Vascular endothelium leaves fingerprints on the surface of erythrocytes. Pflugers Arch. 2013;465(10):1451–1458. doi: 10.1007/s00424-013-1288-y. [DOI] [PubMed] [Google Scholar]
- 62.Naumann DN, Hazeldine J, Midwinter MJ, Hutchings SD, Harrison P. Poor microcirculatory flow dynamics are associated with endothelial cell damage and glycocalyx shedding after traumatic hemorrhagic shock. J Trauma Acute Care Surg. 2018;84(1):81–88. doi: 10.1097/TA.0000000000001695. [DOI] [PubMed] [Google Scholar]
- 63.Dogne S, Flamion B, Caron N. Endothelial glycocalyx as a shield against diabetic vascular complications: involvement of hyaluronan and hyaluronidases. Arterioscler Thromb Vasc Biol. 2018;38(7):1427–1439. doi: 10.1161/ATVBAHA.118.310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper S, McDonald K, Burkat D, Leask RL. Stenosis hemodynamics disrupt the endothelial cell glycocalyx by MMP activity creating a proinflammatory environment. Ann Biomed Eng. 2017;45(9):2234–2243. doi: 10.1007/s10439-017-1846-0. [DOI] [PubMed] [Google Scholar]
- 65.Garsen M, Lenoir O, Rops AL, Dijkman HB, Willemsen B, van Kuppevelt TH, Rabelink TJ, Berden JH, Tharaux PL, van der Vlag J. Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol. 2016;27(12):3545–3551. doi: 10.1681/ASN.2015091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mensah SA, Nersesyan AA, Ebong EE. Endothelial glycocalyx-mediated intercellular interactions: mechanisms and implications for atherosclerosis and cancer metastasis. Cardiovasc Eng Technol. 2020. [DOI] [PMC free article] [PubMed]
- 67.Price L, Wilson C, Grant G. Blood–brain barrier pathophysiology following traumatic brain injury. 2016. [PubMed] [Google Scholar]
- 68.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2(4):492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cash A, Theus MH: Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int J Mol Sci. 2020;21(9):3344. [DOI] [PMC free article] [PubMed]
- 70.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355(1):228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grimm J, Keller R, de Groot PG. Laminar flow induces cell polarity and leads to rearrangement of proteoglycan metabolism in endothelial cells. Thromb Haemost. 1988;60(3):437–441. doi: 10.1055/s-0038-1646986. [DOI] [PubMed] [Google Scholar]
- 72.Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- 73.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17):1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 74.Rahbar E, Baer LA, Cotton BA, Holcomb JB, Wade CE. Plasma colloid osmotic pressure is an early indicator of injury and hemorrhagic shock. Shock. 2014;41(3):181–187. doi: 10.1097/SHK.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 75.Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon AC, Hoe LS, Pedersen S, Fauzi MH, Pimenta LP, van Haren F, Anstey CM, Cullen L, Tung JP, Shekar K, Maitland K, Fraser JF. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198(8):1043–1054. doi: 10.1164/rccm.201801-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, McMurtry SA, Colbert JF, Lindsell CJ, Angus DC, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019;23(1):259. doi: 10.1186/s13054-019-2534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, Conzen P, Becker BF, Rehm M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18(5):538. doi: 10.1186/s13054-014-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108:347. doi: 10.1007/s00395-013-0347-z. [DOI] [PubMed] [Google Scholar]
- 79.Chaikittisilpa N, Vavilala MS, Lele AV, Moore AE, Bethel J, Krishnamoorthy V. Early cardiovascular function and associated hemodynamics in adults with isolated moderate-severe traumatic brain injury: a pilot study. J Clin Neurosci. 2019;69:97–103. doi: 10.1016/j.jocn.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 80.El-Menyar A, Goyal A, Latifi R, Al-Thani H, Frishman W. Brain-heart interactions in traumatic brain injury. Cardiol Rev. 2017;25(6):279–288. doi: 10.1097/CRD.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 81.Zeiler FA, Ercole A, Beqiri E, Cabeleira M, Aries M, Zoerle T, Carbonara M, Stocchetti N, Smielewski P, Czosnyka M, Menon DK. Cerebrovascular reactivity is not associated with therapeutic intensity in adult traumatic brain injury: a CENTER-TBI analysis. Acta Neurochir (Wien) 2019;161(9):1955–1964. doi: 10.1007/s00701-019-03980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ostrowski SR, Sørensen AM, Windeløv NA, Perner A, Welling KL, Wanscher M, Larsen CF, Johansson PI. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: a prospective study. Scand J Trauma Resusc Emerg Med. 2012;20(1):27. doi: 10.1186/1757-7241-20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Margraf A, Herter JM, Kühne K, Stadtmann A, Ermert T, Wenk M, Meersch M, Van Aken H, Zarbock A, Rossaint J. 6% Hydroxyethyl starch (HES 130/0.4) diminishes glycocalyx degradation and decreases vascular permeability during systemic and pulmonary inflammation in mice. Crit Care. 2018;22(1):111. doi: 10.1186/s13054-017-1846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacob M, Rehm M, Loetsch M, Paul JO, Bruegger D, Welsch U, Conzen P, Becker BF. The endothelial glycocalyx prefers albumin for evoking shear stress-induced, nitric oxide-mediated coronary dilatation. J Vasc Res. 2007;44(6):435–443. doi: 10.1159/000104871. [DOI] [PubMed] [Google Scholar]
- 85.Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306(3):H363–H372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nikolian VC, Dekker SE, Bambakidis T, Higgins GA, Dennahy IS, Georgoff PE, Williams AM, Andjelkovic AV, Alam HB. Improvement of blood-brain barrier integrity in traumatic brain injury and hemorrhagic shock following treatment with valproic acid and fresh frozen plasma. Crit Care Med. 2018;46(1):e59–e66. doi: 10.1097/CCM.0000000000002800. [DOI] [PubMed] [Google Scholar]
- 87.Nienaber U, Innerhofer P, Westermann I, Schochl H, Attal R, Breitkopf R, Maegele M. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma-associated haemorrhage and massive transfusion. Injury. 2011;42(7):697–701. doi: 10.1016/j.injury.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 88.Meyfroidt G, Baguley IJ, Menon DK. Paroxysmal sympathetic hyperactivity: the storm after acute brain injury. Lancet Neurol. 2017;16(9):721–729. doi: 10.1016/S1474-4422(17)30259-4. [DOI] [PubMed] [Google Scholar]
- 89.Mautes AE, Müller M, Cortbus F, Schwerdtfeger K, Maier B, Holanda M, Nacimiento A, Marzi I, Steudel WI. Alterations of norepinephrine levels in plasma and CSF of patients after traumatic brain injury in relation to disruption of the blood-brain barrier. Acta Neurochir (Wien) 2001;143(1):51–57. doi: 10.1007/s007010170138. [DOI] [PubMed] [Google Scholar]
- 90.Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care. 2016;4(1):29. doi: 10.1186/s40560-016-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin JV, Liberati DM, Diebel LN. Disparate effects of catecholamines under stress conditions on endothelial glycocalyx injury: An in vitro model. Am J Surg. 2017;214(6):1166–1172. doi: 10.1016/j.amjsurg.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 92.Genét GF, Bentzer P, Hansen MB, Ostrowski SR, Johansson PI. Effects of propranolol and clonidine on brain edema, blood-brain barrier permeability, and endothelial glycocalyx disruption after fluid percussion brain injury in the rat. J Trauma Acute Care Surg. 2018;84(1):89–96. doi: 10.1097/TA.0000000000001708. [DOI] [PubMed] [Google Scholar]
- 93.Schroeppel TJ, Fischer PE, Zarzaur BL, Magnotti LJ, Clement LP, Fabian TC, Croce MA. Beta-adrenergic blockade and traumatic brain injury: protective? J Trauma. 2010;69(4):776–782. doi: 10.1097/TA.0b013e3181e981b8. [DOI] [PubMed] [Google Scholar]
- 94.Patel MB, McKenna JW, Alvarez JM, Sugiura A, Jenkins JM, Guillamondegui OD, Pandharipande PP. Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH After TBI Study): study protocol for a randomized controlled trial. Trials. 2012;13(1):177. doi: 10.1186/1745-6215-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu L, Yu WK, Lin ZL, Tan SJ, Bai XW, Ding K, Li N. Chemical sympathectomy attenuates inflammation, glycocalyx shedding and coagulation disorders in rats with acute traumatic coagulopathy. Blood Coagul Fibrinolysis. 2015;26(2):152–160. doi: 10.1097/MBC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 96.Cederberg D, Siesjö P. What has inflammation to do with traumatic brain injury? Childs Nerv Syst. 2010;26(2):221–226. doi: 10.1007/s00381-009-1029-x. [DOI] [PubMed] [Google Scholar]
- 97.Kolářová H, Ambrůzová B, Svihálková ŠL, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediat Inflamm. 2015;2014:694312. doi: 10.1155/2014/694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diebel LN, Liberati DM, Martin JV. Acute hyperglycemia increases sepsis related glycocalyx degradation and endothelial cellular injury: A microfluidic study. Am J Surg. 2019;217(6):1076–1082. doi: 10.1016/j.amjsurg.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 99.Lukasz A, Hillgruber C, Oberleithner H, Kusche-Vihrog K, Pavenstädt H, Rovas A, Hesse B, Goerge T, Kümpers P. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res. 2017;113(6):671–680. doi: 10.1093/cvr/cvx023. [DOI] [PubMed] [Google Scholar]
- 100.Lipowsky HH. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann Biomed Eng. 2012;40(4):840–848. doi: 10.1007/s10439-011-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MH, Hayden A, Levi M, Meijers JC, Ince C, Kastelein JJ, Vink H, Stroes ES. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202(1):296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 102.Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104(1):78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 103.Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, Becker BF. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res. 2009;83(2):388–396. doi: 10.1093/cvr/cvp097. [DOI] [PubMed] [Google Scholar]
- 104.Annecke T, Rehm M, Bruegger D, Kubitz JC, Kemming GI, Stoeckelhuber M, Becker BF, Conzen PF. Ischemia-reperfusion-induced unmeasured anion generation and glycocalyx shedding: sevoflurane versus propofol anesthesia. J Invest Surg. 2012;25(3):162–168. doi: 10.3109/08941939.2011.618524. [DOI] [PubMed] [Google Scholar]
- 105.Chappell D, Heindl B, Jacob M, Annecke T, Chen C, Rehm M, Conzen P, Becker BF. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology. 2011;115(3):483–491. doi: 10.1097/ALN.0b013e3182289988. [DOI] [PubMed] [Google Scholar]
- 106.Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G, Radak Z, Calabrese EJ, Cuzzocrea S. Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal. 2013;19(8):836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 107.Kontos HA, Wei EP. Superoxide production in experimental brain injury. J Neurosurg. 1986;64(5):803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- 108.Kaur P, Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr Neuropharmacol. 2018;16(8):1224–1238. doi: 10.2174/1570159X15666170613083606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Odorcyk FK, Ribeiro RT, Roginski AC, Duran-Carabali LE, Couto-Pereira NS, Dalmaz C, Wajner M, Netto CA. Differential Age-Dependent Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis Induced by Neonatal Hypoxia-Ischemia in the Immature Rat Brain. Mol Neurobiol. 2021;58:2297–308. [DOI] [PubMed]
- 110.van Golen RF, Reiniers MJ, Vrisekoop N, Zuurbier CJ, Olthof PB, van Rheenen J, van Gulik TM, Parsons BJ, Heger M. The mechanisms and physiological relevance of glycocalyx degradation in hepatic ischemia/reperfusion injury. Antioxid Redox Sign. 2014;21(7):1098–1118. doi: 10.1089/ars.2013.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;290(6):H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 112.Bruegger D, Rehm M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Conzen P, Becker BF. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in guinea pig hearts. Crit Care. 2008;12(3):R73. doi: 10.1186/cc6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kurzelewski M, Czarnowska E, Beresewicz A. Superoxide- and nitric oxide-derived species mediate endothelial dysfunction, endothelial glycocalyx disruption, and enhanced neutrophil adhesion in the post-ischemic guinea-pig heart. J Physiol Pharmacol. 2005;56(2):163–178. [PubMed] [Google Scholar]
- 114.Ali MM, Mahmoud AM, Le Master E, Levitan I, Phillips SA. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am J Physiol Heart Circ Physiol. 2019;316(3):H647–H663. doi: 10.1152/ajpheart.00090.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gilles S, Zahler S, Welsch U, Sommerhoff CP, Becker BF. Release of TNF-alpha during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc Res. 2003;60(3):608–616. doi: 10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 116.Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang WC, Cao HL, Wang YZ, Li TT, Hu HY, Wan Q, Li WZ. Inhibition of nitric oxide synthase aggravates brain injury in diabetic rats with traumatic brain injury. Neural Regen Res. 2021;16(8):1574–1581. doi: 10.4103/1673-5374.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80(3):389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11(1):1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 120.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 121.Sammel M, Peters F, Lokau J, Scharfenberg F, Werny L, Linder S, et al. Differences in shedding of the interleukin-11 receptor by the proteases ADAM9, ADAM10, ADAM17, Meprin α, Meprin β and MT1-MMP. Int J Mol Sci. 2019;20(15). 10.3390/ijms20153677. [DOI] [PMC free article] [PubMed]
- 122.Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13(9):649–665. doi: 10.1038/nri3499. [DOI] [PubMed] [Google Scholar]
- 123.Ramnath R, Foster RR, Qiu Y, Cope G, Butler MJ, Salmon AH, Mathieson PW, Coward RJ, Welsh GI, Satchell SC. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor α: a contributor to endothelial cell glycocalyx dysfunction. Faseb J. 2014;28(11):4686–4699. doi: 10.1096/fj.14-252221. [DOI] [PubMed] [Google Scholar]
- 124.Manon-Jensen T, Multhaupt HA, Couchman JR. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. Febs J. 2013;280(10):2320–2331. doi: 10.1111/febs.12174. [DOI] [PubMed] [Google Scholar]
- 125.Yang X, Meegan JE, Jannaway M, Coleman DC, Yuan SY. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res. 2018;114(13):1752–1763. doi: 10.1093/cvr/cvy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pruessmeyer J, Martin C, Hess FM, Schwarz N, Schmidt S, Kogel T, Hoettecke N, Schmidt B, Sechi A, Uhlig S, Ludwig A. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285(1):555–564. doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen S, He Y, Hu Z, Lu S, Yin X, Ma X, Lv C, Jin G. Heparanase mediates intestinal inflammation and injury in a mouse model of sepsis. J Histochem Cytochem. 2017;65(4):241–249. doi: 10.1369/0022155417692536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272(23):14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 129.Schmidt A, Echtermeyer F, Alozie A, Brands K, Buddecke E. Plasmin- and thrombin-accelerated shedding of syndecan-4 ectodomain generates cleavage sites at Lys(114)-Arg(115) and Lys(129)-Val(130) bonds. J Biol Chem. 2005;280(41):34441–34446. doi: 10.1074/jbc.M501903200. [DOI] [PubMed] [Google Scholar]
- 130.Semple BD, Trivedi A, Gimlin K, Noble-Haeusslein LJ. Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain. Neurobiol Dis. 2015;74:263–280. doi: 10.1016/j.nbd.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Annecke T, Fischer J, Hartmann H, Tschoep J, Rehm M, Conzen P, Sommerhoff CP, Becker BF. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischaemia/reperfusion. Br J Anaesth. 2011;107(5):679–686. doi: 10.1093/bja/aer269. [DOI] [PubMed] [Google Scholar]
- 132.Annecke T, Chappell D, Chen C, Jacob M, Welsch U, Sommerhoff CP, Rehm M, Conzen PF, Becker BF. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth. 2010;104(4):414–421. doi: 10.1093/bja/aeq019. [DOI] [PubMed] [Google Scholar]
- 133.Zhang S, Kojic L, Tsang M, Grewal P, Liu J, Namjoshi D, Wellington CL, Tetzlaff W, Cynader MS, Jia W. Distinct roles for metalloproteinases during traumatic brain injury. Neurochem Int. 2016;96:46–55. doi: 10.1016/j.neuint.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 134.Roberts DJ, Jenne CN, Léger C, Kramer AH, Gallagher CN, Todd S, Parney IF, Doig CJ, Yong VW, Kubes P, Zygun DA. Association between the cerebral inflammatory and matrix metalloproteinase responses after severe traumatic brain injury in humans. J Neurotrauma. 2013;30(20):1727–1736. doi: 10.1089/neu.2012.2842. [DOI] [PubMed] [Google Scholar]
- 135.Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X, Mori T, Jung JC, Fini ME, Lo EH. Secretion of matrix metalloproteinase-2 and -9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J Neurotrauma. 2002;19(5):615–625. doi: 10.1089/089771502753754082. [DOI] [PubMed] [Google Scholar]
- 137.Sifringer M, Stefovska V, Zentner I, Hansen B, Stepulak A, Knaute C, Marzahn J, Ikonomidou C. The role of matrix metalloproteinases in infant traumatic brain injury. Neurobiol Dis. 2007;25(3):526–535. doi: 10.1016/j.nbd.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 138.Jia F, Pan YH, Mao Q, Liang YM, Jiang JY. Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. J Neurotrauma. 2010;27(6):1059–1068. doi: 10.1089/neu.2009.1067. [DOI] [PubMed] [Google Scholar]
- 139.Warren KM, Reeves TM, Phillips LL. MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J Neurotrauma. 2012;29(10):1922–1940. doi: 10.1089/neu.2012.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mohamedi Y, Fontanil T, Cobo T, Cal S, Obaya AJ. New insights into ADAMTS metalloproteases in the central nervous system. Biomolecules. 2020;10(3). 10.3390/biom10030403. [DOI] [PMC free article] [PubMed]
- 141.Lemarchant S, Dunghana H, Pomeshchik Y, Leinonen H, Kolosowska N, Korhonen P, Kanninen KM, García-Berrocoso T, Montaner J, Malm T, Koistinaho J. Anti-inflammatory effects of ADAMTS-4 in a mouse model of ischemic stroke. Glia. 2016;64(9):1492–1507. doi: 10.1002/glia.23017. [DOI] [PubMed] [Google Scholar]
- 142.Washington PM, Lee C, Dwyer M, Konofagou EE, Kernie SG, Morrison BR. Hyaluronidase reduced edema after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2019:271678X–19882780X. [DOI] [PMC free article] [PubMed]
- 143.Xing G, Ren M, Verma A. Divergent temporal expression of hyaluronan metabolizing enzymes and receptors with craniotomy vs. controlled-cortical impact injury in rat brain: a pilot study. Front Neurol. 2014;5:173. doi: 10.3389/fneur.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84(1):3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 145.Faraoni D, Van Der Linden P. A systematic review of antifibrinolytics and massive injury. Minerva Anestesiol. 2014;80(10):1115–1122. [PubMed] [Google Scholar]
- 146.Draxler DF, Awad MM, Hanafi G, Daglas M, Ho H, Keragala C, Galle A, Roquilly A, Lyras D, Sashindranath M, Medcalf RL. Tranexamic acid influences the immune response, but not bacterial clearance in a model of post-traumatic brain injury pneumonia. J Neurotrauma. 2019;36(23):3297–3308. doi: 10.1089/neu.2018.6030. [DOI] [PubMed] [Google Scholar]
- 147.Postl LK, Bogner V, van Griensven M, Beirer M, Kanz KG, Egginger C, Biberthaler P, Kirchhoff C. Polymorphonuclear (PMN) elastase in patients after severe traumatic brain injury. Eur J Med Res. 2018;23(1):44. doi: 10.1186/s40001-018-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luo CL, Chen XP, Yang R, Sun YX, Li QQ, Bao HJ, Cao QQ, Ni H, Qin ZH, Tao LY. Cathepsin B contributes to traumatic brain injury-induced cell death through a mitochondria-mediated apoptotic pathway. J Neurosci Res. 2010;88(13):2847–2858. doi: 10.1002/jnr.22453. [DOI] [PubMed] [Google Scholar]
- 149.Yu J, Zhu H, Taheri S, Monday WL, Perry S, Kindy MS. Reduced neuroinflammation and improved functional recovery after traumatic brain injury by prophylactic diet supplementation in mice. Nutrients. 2019;11(2). 10.3390/nu11020299. [DOI] [PMC free article] [PubMed]
- 150.Hook V, Yoon M, Mosier C, Ito G, Podvin S, Head BP, Rissman R, O'Donoghue AJ, Hook G. Cathepsin B in neurodegeneration of Alzheimer’s disease, traumatic brain injury, and related brain disorders. Biochim Biophys Acta Proteins Proteom. 1868;2020:140428. doi: 10.1016/j.bbapap.2020.140428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lozada A, Maegele M, Stark H, Neugebauer EM, Panula P. Traumatic brain injury results in mast cell increase and changes in regulation of central histamine receptors. Neuropathol Appl Neurobiol. 2005;31(2):150–162. doi: 10.1111/j.1365-2990.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 152.Ahmad A, Crupi R, Impellizzeri D, Campolo M, Marino A, Esposito E, Cuzzocrea S. Administration of palmitoylethanolamide (PEA) protects the neurovascular unit and reduces secondary injury after traumatic brain injury in mice. Brain Behav Immun. 2012;26(8):1310–1321. doi: 10.1016/j.bbi.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 153.Dane MJ, van den Berg BM, Avramut MC, Faas FG, van der Vlag J, Rops AL, Ravelli RB, Koster BJ, van Zonneveld AJ, Vink H, Rabelink TJ. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol. 2013;182(5):1532–1540. doi: 10.1016/j.ajpath.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 154.Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res. 2013;90:80–85. doi: 10.1016/j.mvr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gerber E, Bredy A, Kahl R. Ortho-phenanthroline modulates enzymes of cellular energy metabolism. Toxicology. 1996;110(1-3):85–93. doi: 10.1016/0300-483X(96)03331-8. [DOI] [PubMed] [Google Scholar]
- 156.Diebel ME, Martin JV, Liberati DM, Diebel LN. The temporal response and mechanism of action of tranexamic acid in endothelial glycocalyx degradation. J Trauma Acute Care Surg. 2018;84(1):75–80. doi: 10.1097/TA.0000000000001726. [DOI] [PubMed] [Google Scholar]
- 157.Grundmann S, Fink K, Rabadzhieva L, Bourgeois N, Schwab T, Moser M, Bode C, Busch HJ. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83(6):715–720. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 158.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(12):2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114(14):3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lipowsky HH, Lescanic A. Inhibition of inflammation induced shedding of the endothelial glycocalyx with low molecular weight heparin. Microvasc Res. 2017;112:72–78. doi: 10.1016/j.mvr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.