Abstract
Background:
The economic outcome research of approved tyrosine kinase inhibitors for treating the chronic phase of chronic myeloid leukemia in developing is scarce. The aim of this study was to assess the cost-effectiveness of dasatinib and nilotinib for newly diagnosed chronic myeloid leukemia patients.
Methods:
A decision tree model was developed linking clinical effectiveness (defined as major molecular response) and/or complete cytogenetic response, utility, and cost data over a 12-month period. Patients are recruited from Qatar Cancer Registry. The probability of primary clinical outcome is calculated from DASISION (dasatinib) and ENESTnd (nilotinib) trials. Direct healthcare costs were derived from the national healthcare payer system, whereas adverse effects data were derived from local incident reporting system.
Results:
In the first-line treatments of chronic myeloid leukemia patients, nilotinib has greater major molecular response (39% nilotinib vs 12% dasatinib) and complete cytogenetic response (24% nilotinib vs 16% dastinib) response outcomes, and more adverse effects than dasatinib (13.3% vs 4%). Moreover, nilotinib is more cost-effective with annual costs (USD63,589.59) and after 12 months of follow-up. Despite the lower acquisition annual cost of dasatinib (USD59,486.30), the incremental cost-effectiveness ratio of nilotinib (vs dasatinib) per major molecular response/complete cytogenetic response achieved was USD15,481.10 per year. There were no cases in both arms that progressed to accelerated or blast phase. At a threshold of 3 times gross domestic product per capita of Qatar and according to World Health Organization recommendation, the nilotinib use is still cost-effective.
Conclusion:
Upfront therapy of chronic myeloid leukemia–chronic phase patients by nilotinib plan appears to be more cost-effective than dasatinib.
Keywords: cancer, chronic myeloid leukemia, tyrosine kinase inhibitors, nilotinib, dasatinib, leukemia
Introduction
Chronic myeloid leukemia (CML) is a malignant disease that shows an acquired genetic anomaly as the final trigger in a chronic myeloproliferative syndrome. CML is characterized by a translocation between chromosomes 9 and 22, giving rise to the formation of the so-called Philadelphia chromosome (Ph) and the development of a new gene, BCR-ABL. 1,2 It represents approximately 10 to 15% of all cases of leukemia. 3 Although there is no clear evidence suggesting a particular ethnicity to develop CML, a few reports have showed lower incidence rates among Asian populations. 4,5 A review established evidence showed that the incidence of CML was 0.92 per 100,000 populations and no significant regional variation was found between Europe compared to other countries. 6 Additionally, CML is more commonly seen among men and white race population 7 with an average diagnosis age of 55 years. 8 In the United States, for example, the CML in chronic phase (CP) incidence is 1 to 1.5 cases per 100,000 inhabitants. 9,10
The treatment options depend on the phase of the disease, either chronic, accelerated or blast phase. 11 The patient’s age, other prognostic factors and the availability of a stem cell donor are also important factors in choosing a treatment. The tyrosine kinase inhibitor (TKI) is currently the standard treatment for the management of CMP-CP. Imatinib was the first TKIs in CML and has been widely used due to its efficacy and safety profiles compared to other second-generation TKIs including nilotinib, dasatinib, bosutinib, and ponatinib. 11
Resistance to imatinib, defined as a lack of complete hematological response after 3 months of treatment or the lack of cytogenetic response after 6 months of treatment or the lack of a major cytogenetic response (Ph-positive cells > 35%) after 12 months of treatment, 11 may be due to imatinib administration or acquired during treatment as shown in previous studies. 11,12 In such case of resistance, other treatment options should be considered including dasatinib and nilotinib. 13
In Qatar, dasatinib and nilotinib are licensed as formulary medications in Hamad Medical Corporation (HMC), the main public healthcare provider in the country, for the treatment of adults with CML-CP with resistance or intolerance to prior therapy. 14 Also, they have been used as upfront line treatment modalities to treat the same population. While many studies evaluated the cost-effectiveness of second generation TKIs versus the first-generation TKIs, there are relatively few studies that analyzed the cost-effectiveness of dasatinib versus nilotinib for treatment of CML-CP. Therefore, the current study sought to evaluate the cost-effectiveness of nilotinib versus dasatinib as an upfront therapy for the management of CMP-CP to create an informative analysis of both HMC-approved treatment options for decision makers in Qatar healthcare authorities as well to CML treating physicians.
Materials and Methods
Study Design
This study was a retrospective data review of CML patients at National Center for Cancer Care & Research (NCCCR), HMC. The eligible patients were treated with dasatinib or nilotinib between January 2011 until December 2018. It used the CHEERS guideline.
Study Setting
The study was undertaken from the perspective of NCCCR, HMC (i.e. Qatar healthcare system perspective). HMC is the principal public healthcare provider in the State of Qatar. NCCCR is part of HMC and the premier and the only specialized, tertiary hospital for cancer care in the country. It looks after cancer patients who require ongoing treatments such as chemotherapy and radiotherapy.
Ethical Considerations
The study was approved by HMC-IRB (ID #: MRC-01-19-343). The study was a retrospective data review, thus information of patients was gathered through the electronic medical record of selected patients (Cerner®). There was no enrollment of human subjects, hence no informed consent was necessary.
Study Population
Study population included all patients who received dasatinib and nilotinib as upfront therapy for CML-CP collected between 1/11/2011-31/12/2018. CML-CP patients were treated as outpatients as treatment options were administered orally. Patients were included in the study if: (i) patients with confirmed diagnosis of CML-CP during the study period and resident in the contributing registration areas; (ii) patients with good performance status that qualifies to either drugs as decided by treating clinicians; and (iii) age of 18 years and older. On the other hand, patients were excluded if: (i) with incomplete records; (ii) without a confirmed diagnosis /advanced stage of the neoplastic disease (accelerated or blast crisis phase); and (iii) did not complete a 12-month follow-up since the initiation of any of comparator TKIs.
Sample Size
The sample size was based on previously published study, 15 on the basis of major molecular response (MMR) rates of 25% and 50% at 12 months after random assignment to either treatments and a significance level of 0.05 at the final analysis (2-sided). Sixty patients in each treatment arm needed to be included to detect a 25% difference with a power of 80% (χ2 test). A total of 120 patient records was included in the study.
Outcome Measure
The following are the target outcomes in this study: (i) achieving MMR and/or complete cytogenetic response (CCyR) at the end of 12-month period of initiating either of treatment modalities and (ii) overall medical cost of managing CML-CP patients during 1-year period. Further, the study defined: (i) a complete cytogenetic response (CCyR) “as absence of the Ph chromosome among at least 20 cells in metaphase in a bone marrow” 16 ; (ii) MMR is reached if the standardized BCR-ABL:ABL genes ratio is less than 0.1%, which is equivalent to a 4.5 log reduction from the 100% baseline for CML-naive patients. 16 Further, Sokal score was used as a prognostic tool to predict response of these medications to therapy. 17 Sokal risk calculation is needed to plan the treatment regimen for CML patients. It is based on spleen size, blood counts and blood differential. It is widely used as prognostic tool for CML survival and treatment response prediction.
Model Structure
A model was developed utilizing decision tree model analysis. All the patients were assumed to start with either of the following treatments for CML-CP: (i) nilotinib 600 mg daily (nilotinib strategy) or (ii) dasatinib 100 mg daily (dasatinib strategy). The following responses to medical treatment after an initial 12-month treatment period was used to predict disease progression: (i) no response to treatment (NR) and (ii) achieved a CCyR and/or MMR. The model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA). Incremental cost-effectiveness ratios (ICERs) were calculated and expressed as cost per additional MMR and/or CCyR gained. ICER calculation was carried out using such model to estimate the cost-effectiveness of the comparators. Incremental cost-effectiveness ratio was calculated using the following formula:
where C1 is the cost of the dasatinib; C0 is the cost of the nilotinib; E1 and E0 are the consequences i.e. MMR and/or CCyR, respectively. Costs for drugs and non-drug disease management that were obtained from central cashier system are incorporated and summed up using Microsoft Excel®. For AEs, incident reporting system was used and then gathered in excel sheet. Costs were measured in Qatari riyal while clinical outcomes were measured in MMR and CCyR.
The time horizon for this study was 1 year, and it was chosen because the median follow-up available in the literature for MMR and CCyR outcomes was at a 12-month period. Costs and effects were not discounted, given the 1-year time horizon to conduct this analysis. Moreover, there was no change in drug pricing during the studied period (2011-2018). Drug acquisition is done through drug supply and by contracts with local providers who may negotiate enlisting of new medications, returned expiry medications, etc. as part of their business model. Accordingly, fixed price of medications—in this case—is at expense of other benefits, which is out-of-scope of this analysis.
Economic Model Variables
(i) Clinical data
For each treatment option, base case primary and secondary clinical outcome measures and their durations was populated into the model. The choice of a 12-month period was due to the evidence that the degree of short-term molecular and cytogenetic responses at certain follow-up times was correlated with CML prognosis. Patients who achieved a CCyR or MMR were shown to have high remission and overall survival (OS) rates. Patients with newly diagnosed CML were adopted from Qatar Cancer Registry. The probability of primary clinical outcomes (MMR and CCyR) were calculated from DASISION (dasatinib) 18 and ENESTnd (nilotinib) trials, 19 which were applied for the percentage of responders and non-responders independently, whereas adverse drug events (ADEs) data were derived from local incident reporting system.
(ii) Cost data
The cost-effectiveness was performed from Qatar healthcare system perspective, thus direct medical costs aggregated by the hospital in relation to different treatment strategies were included. Both treatment options were given as ambulatory with no other incurred costs. Direct medical costs included cost of medications, management of ADEs, laboratory tests during physician visitations (exchange rate: USD1 = QAR3.65). Direct healthcare costs were derived from the national healthcare payer system. The frequency of physician visits to CML-CP patients are quarterly in their first year of treatment and it get longer with more stable disease state. CML patients are routinely treated by hematology consultants and specialists.
Statistical Analysis
Baseline characteristics were analyzed using SPSS program version 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). Normality test was done to check for type of data distribution. Frequencies (%), mean (standard deviation, SD) and median (interquartile range, IQR) were used to describe the data. Chi-square test was used for categorical data, and the student’s t-test and Mann–Whitney test were used for continuous data (alpha level = 0.05).
Sensitivity Analysis
Due to potential uncertainty associated with our economic model, variability in the values of many variables included in our economic analysis, related to deterministic and probabilistic input data were tested to assess the robustness of the model and the resultant outcomes against any uncertainty. Moreover, with such robustness, one would assume an increased generalizability of the study results. This was carried out by including these variables in isolation first and then the all input variables were included in the final model.
Univariate sensitivity analyses were initially conducted to evaluate individual inputs on outcome estimates. 10% of uncertainty were used for clinical probabilities. Furthermore, the CML clinician’s panel opinion was prompted to provide a range of possible approximations for the proportion of patients who may develop ADEs in both treatment arms. Costs of treatment were allowed to vary between ±20%. The costs of any incurred diagnostic testing were allowed to vary between zero and twice the base case estimation. Time to response was varied between 1 and 12 months. Multivariate sensitivity analysis was also conducted to assess the uncertainty in the model input parameters simultaneously. The model was re-run up to 10,000 times.
Four scenarios were considered in our model. These 4 scenario analyses were designed to reflect on different median time to MMR/CCyR responders or suboptimal responders and/or non-responders, assuming an estimate for median follow-up of 12 months in the first year of diagnosis. The combinations of median follow-up in these 4 scenarios were based on the possibility that there was either no overlap between these scenarios and they were exclusively independent. The subsequent scenarios assessed the reach to full response of both dasatinib and nilotinib, yet with and without developing ADEs that did not lead to discontinuation of these drugs or the loss of remission of such drugs and the development to progressed disease that necessitates other treatment modalities.
Results
Patient’s Profile
There were 110 patients in the final analysis; 33 patients (30.0%) on nilotinib, 25 patients (22.7%) on dasatinib, while 52 patients (47.3%) on imatinib. Out of these, 65.5% were males (n = 72), mean (sd) age of 46.6 years old (13.5) and median (IQR) of 45.0 (34.8-60.0). A majority of the patients were non-Qatari (n = 97; 88.2%). Based on the 2 study groups, 33 patients were on nilotinib and out of these, 22 (66.7%) were males, 11 (33.3%) were females and mean (sd) age of 45.8 (13.0). For the dasatinib group, 22 patients (88.0%) were males, 3 patients (12.0%) were females and mean (sd) age of 46.3 (14.1). Further statistical analyses showed that there were no significant differences between the 2 studied groups in terms of gender (P = 0.060) and age (P = 0.981). Based on the baseline Sokal score calculation for both treatment arms, they are comparable between dasatinib and nilotinib across different risk strata; low, intermediate and high. In high risk patients, it was 28% vs 39.4%; 44% vs 36.4% in the intermediate risk patients; and 28% vs 24.2% in low risk patients in dasatinib and nilotinib treatment arms, respectively.
Economic Analysis
Analysis was conducted at a period of 1 year. Table 1 gives an overview of patient success probabilities (response), total annual cost of each strategy, and attained ICER of those strategies. An incremental cost-effectiveness analysis of the dasatinib-first strategy and the nilotinib-first strategy was then planned. Nilotinib achieved a higher success of achieving MMR/CCyR of 55% compared to 28% with dasatinib, associated with a cost saving of QAR 121,044 (33,244 USD) per additional case of success. The ICER of the dasatinib-first strategy yielded negative ICER; thus, the strategy was dominant and cost-effective.
Table 1.
Base-Case Results.
| Base case | Comparator | |
|---|---|---|
| Treatments | Dasatinib | Nilotinib |
| Expected annual cost | $64,045.02 | $58,695.35 |
| Patient success (MMR/CCyR) | 0.28 | 0.55 |
| CE ratio | $228,732 | $107,688 |
| Incremental cost | −$5,349.67 | |
| Incremental effect | 0.27 | |
| ICER | −$20,183.48 |
Following responses in both CML patients’ groups, higher MMR (39% nilotinib vs 12% dasatinib) and CCyR (24% nilotinib vs 16% dasatinib) response outcomes were in the nilotinib treatment arm compared to dasatinib, and more ADEs than dasatinib (13.3% vs 4%). Nilotinib was more cost-effective with annual costs USD58,695 (QAR217,125) and after 12 months of follow-up. Despite the slight higher acquisition annual cost of dasatinib i.e. USD64,045 (QAR232,102), the ICER of nilotinib versus dasatinib per MMR/CCyR achieved was USD20,183 (QAR73,483) per patient per year in favor of nilotinib therapy. There were no cases in both arms that progressed to accelerated or blast phase. At a threshold of 3 times GDP per capita and according to WHO recommendation, the nilotinib use is still cost-effective in Qatar’s context.
Base-Case Analysis
Compared to the dasatinib, nilotinib had an economic advantage of USD20,183.48 per patient and a 55% successful cure rate, compared to 28% (Table 1).
Sensitivity Analysis
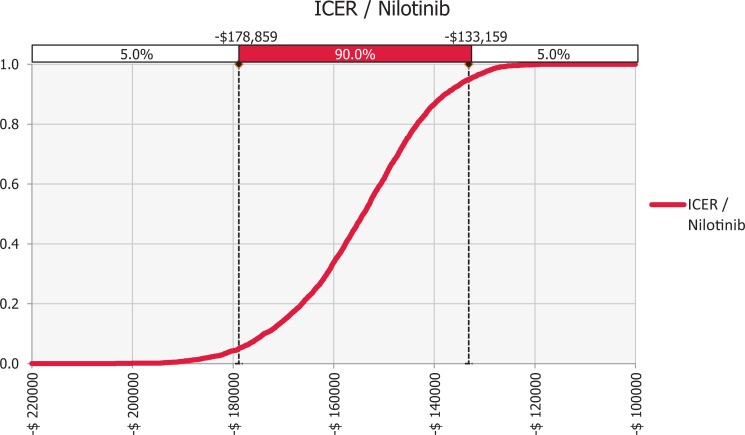
There was a potential uncertainty associated with economic model inputs obtained from medical records. Hence, variations in the values of key variables of the economic analysis, related to probabilistic input data were analyzed to assess the robustness of the baseline study conclusion against any uncertainty and to increase the generalizability of results. Multivariate probabilistic analysis with uncertainty of ±5% was performed for all model inputs. The model was re-run up to 10,000 times, with a different simulated input value in each run. The sensitivity analysis was run using a triangular type of value distribution of Monte Carlo value distribution @Risk-7.5® (Palisade Corporation, NY, USA). Variables and their uncertainty ranges are listed in Figure 1.
Figure 1.
Probability curve of first-line nilotinib strategy.
One-way sensitivity analysis indicated that the model was insensitive to changes in all the model’s variables.
Probabilistic Sensitivity Analyses
The probabilistic curve illustrates that nilotinib achieves cost saving over dasatinib in 100% of cases, as seen in Figure 2.
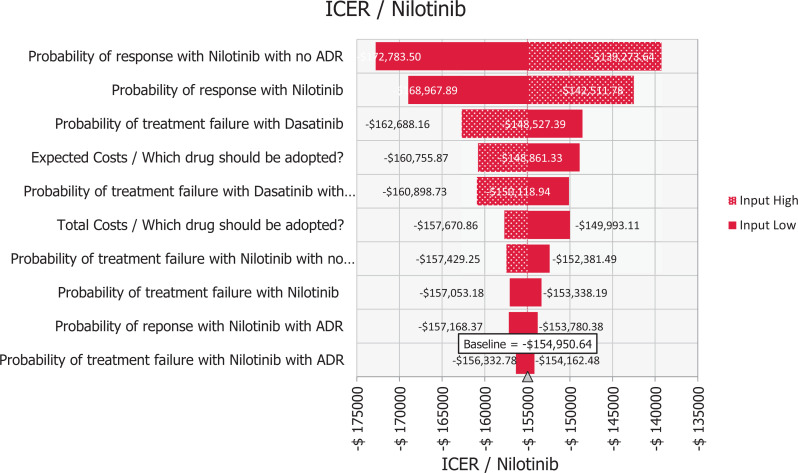
Figure 2.
Tornado diagram of nilotinib versus dasatinib first-line treatment for CML.
The tornado diagram in Figure 1 shows that the study outcome was mostly influenced by the uncertainty in the response rate (MMR/CCyR) with no ADE in the nilotinib group, while the uncertainty in the failure with ADEs in nilotinib group had the lowest impact on the outcome. Consequently, the probabilistic sensitivity analyses suggested that the nilotinib-first therapy was more cost-effective compared with the dasatinib-first therapy in 100% of 10,000 Monte Carlo simulations. The probability curve of the dominant option i.e. nilotinib was generated to present the probabilities of cost-effectiveness as shown in Figure 2. The nilotinib-first strategy showed being a cost-effectiveness option in the simulations.
Discussion
In this report, we assessed the cost-effectiveness of 2 new generation treatment strategies in Qatari patients with newly diagnosed CML in the chronic phase. The main finding identified nilotinib as the more cost-effective strategy in terms of incremental costs per additional MMR/CCyR response gained. Analysis results showed that the nilotinib-first strategy was a better option because it cost less and better MMR/CCyR response than the dasatinib. Of note, in our study, when it comes to MMR for both medications, our patient population is different than those studied in landmark studies in Europe and North America. Most of them are Asian and Middle Eastern Arabs which creates the need to study the effect of these medications on these ethnic groups. The need for cost-effective for both medications is inevitable. In the US for example, a study investigated the price trend of cancer medications between 1995 to 2013 and an annual increase in price of 10% was reported. 8,18 Another report expected that cancer care in the United States would reach $173 billion by 2020. 7 Consequently, the move to value-based oncology (VBO) is evitable. 12 To enhance patient access and evaluate economic impact of applying both medications, cost-effectiveness analyses are useful in quantifying possible cost associated with the new therapies through making use of the best available evidence.
Sasaki et al (2016) studied the safety and efficacy of nilotinib and dasatinib in newly diagnosed chronic-phase chronic myelogenous leukemia. 20 Their findings suggested that nilotinib and dasatinib produce comparable response rates and tolerability among CML-CP patients with or without pre-existing liver and/or renal dysfunction. Another study by Takasashi et al (2016) reported that dasatinib and nilotinib offer similar response and survival outcomes in propensity score matched cohort of newly diagnosed CML-CP patients. 21 Based on 2 discontinuation studies, nilotinib is the only TKI that has been approved for the treatment-free remission label. 22,23
Pavey and coworkers (2012) assessed the quality evidence for the clinical effectiveness and cost-effectiveness of dasatinib, nilotinib and standard-dose imatinib. 24 These drugs were used for the first-line treatment of Philadelphia chromosome-positive CML. Between dasatinib and nilotinib, the former was not cost-effective if decision thresholds of £30,000 per QALY (quality-adjusted life years) were used, compared with nilotinib. Li et al (2018) indicated a different outcome. In their study for first-line treatment in Chinese patients who were first diagnosed with CML-CP, the nilotinib-first strategy exhibited a lower utility and highest price compared to dasatinib. 25 The most common ADEs reported are hematologic toxicities e.g. thrombocytopenia and neutropenia, and non-hematologic toxicities e.g. gastrointestinal discomfort and rash. Those ADEs were somehow comparable between both arms, yet none of such ADEs necessitated the need to stop TKIs administration.
Strengths and Limitations
To our knowledge, this is among the first study to address the surrogate outcomes of second generation TKIs in CML-CP patients and the cost-effectiveness of each if applied as upfront for those patients at first diagnosis during first year of diagnosis in Qatar. It also reflects real world data of CML patients in Qatar. Moreover, with good quality registry data in Qatar, there was no missing data.
Nevertheless, the study had several limitations. Firstly, our tree decision model was a simplification of real practice. The treatments strategies and practice algorithms were derived from our local CML expert clinicians, which are in accordance to European LeukemiaNet Guidelines. 26 Individualized treatment modalities for certain CML patients were not included in our model. All cost inputs into our model were derived exclusively for the Qatar population, which may limit the generalizability to other settings or countries. Although Qatar may have a small population, it shares almost the same structure as those of neighboring gulf states; Saudi Arabia, United Emirates, Bahrain, Kuwait and Oman. This, in turn, makes the study of value to them in terms of generalizability of outcome(s). Moreover, a regional study in Middle Eastern countries would be interesting, yet logistically tedious. However, efforts are underway to conduct such study. The costs included were from the governmental healthcare system in Qatar. Moreover, the NCCCR is the main center for cancer care in Qatar, with nationwide coverage of all CML patients; hence, treatments and costs included represent the real practice in the country. Secondly, no utilities’ inputs were available for Qatar setting, which is a major limitation when comparing QALYs and future utility studies on Qatari population are warranted. Thirdly, the use of surrogate markers; MMR and CCyR as outcome in our study were based on expert panel opinion on TKIs final patient outcome i.e. overall survival. Though the use of such markers represents a viable biological response for these medications, it may not be enough to establish such final outcome. Accordingly, an empirical evidence to associate such surrogates and the survival at relevant follow-up time is needed. Finally, the cost of the ADEs of both TKI medications was not exhaustive of all possible ADEs, and again they were based on the expert panel decision that no ADEs to either of medications have led to discontinuation of medications and accordingly no costs have incurred. Moreover, the non-hematologic toxicities discussed were minor during the study and again did not lead to stopping the medications. The objective of the study was to analyze the cost and outcome during the first year of initiating the medication because it may give an idea about the overall performance of these drugs on CML patients on long term. Of note, it would be better to study the effects of long term ADEs which may change the overall analysis and finding of our study. Thus, further study to include this concern is warranted.
This is because the ADEs of either TKIs differed between individuals and almost 95% of ADEs arisen during the first year of treatment initiation. However, a sensitivity analysis was performed to check the robustness of variables included in the model.
Conclusions
In summary, nilotinib was observed as a cost-effective strategy versus dasatinib as first-line treatment for CML-CP in first year of diagnosis. Decision was built in the context of Qatar healthcare system perspective. These results are intended merely to provide clinicians and healthcare policy makers in Qatar with better CML treatment decision making. In the present paper, the model used includes different compilation of clinical outcome data from different studies. This in turn mandates the future need to carry out other studies once data become available on evidence-based patient-relevant outcome e.g. survival or change in drug prices as generic drugs become available in the country.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211001796 for Assessment of Dasatinib Versus Nilotinib as Upfront Therapy for Chronic Phase of Chronic Myeloid Leukemia in Qatar: A Cost-Effectiveness Analysis by Ahmad Adel, Dina Abushanab, Anas Hamad, Mohammad Abdulla, Mohamed Izham and Mohamed Yassin in Cancer Control
Footnotes
Data Availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Hamad Medical Corporation, Medical Research Center.
ORCID iD: Mohamed Yassin, MD  https://orcid.org/0000-0002-1144-8076
https://orcid.org/0000-0002-1144-8076
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Yassin MA, Hamad AA, Hussein RM, et al. Dasatinib versus nilotinib as upfront therapy for treatment naïve chronic myeloid leukemia chronic phase. Medicine: Case Reports and Study Protocols. 2021;2(2):e0061. [Google Scholar]
- 2. Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J Hematol Oncol. 2018;11(1):84. Published June 20, 2018. doi:10.1186/s13045-018-0624-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Granatowicz A, Piatek CI, Moschiano E, El-Hemaidi I, Armitage JD, Akhtari M. An overview and update of chronic myeloid leukemia for primary care physicians. Korean J Fam Med. 2015;36(5):197–202. doi:10.4082/kjfm.2015.36.5.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendizabal AM, Garcia-Gonzalez P, Levine PH. Regional variations in age at diagnosis and overall survival among patients with chronic myeloid leukemia from low and middle income countries. Cancer Epidemiol. 2013;37(3):247–254. [DOI] [PubMed] [Google Scholar]
- 5. Au WY, Caguioa PB, Chuah C, et al. Chronic myeloid leukemia in Asia. Int J Hematol. 2009;89(1):14–23. [DOI] [PubMed] [Google Scholar]
- 6. Hamad Y. Incidence of chronic myeloid leukemia: systematic review and meta-analysis. A Thesis Submitted to the Faculty of the College of Health Science, Qatar University. 2019. [Google Scholar]
- 7. American Cancer Society. Leukemia-chronic myeloid (myelogenous). American Cancer Society; 2009: p. 40.
- 8. Cortes J. Natural history and staging of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:569–584. [DOI] [PubMed] [Google Scholar]
- 9. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 10. Al-Dewik NI, Jewell AP, Yassin MA, El-Ayoubi HR, Morsi HM. Molecular monitoring of patients with chronic myeloid leukemia (CML) in the state of Qatar: optimization of techniques and response to imatinib. QScience Connect. 2014;2014(1):24. [Google Scholar]
- 11. Bitencourt R, Zalcberg I, Louro ID. Imatinib resistance: a review of alternative inhibitors in chronic myeloid leukemia. Rev Bras Hematol Hemoter. 2011;33(6):470–475. doi:10.5581/1516-8484.20110124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Dewik NI, Morsi HM, Samara MM, et al. Is adherence to imatinib mesylate treatment among patients with chronic myeloid leukemia associated with better clinical outcomes in Qatar? Clin Med Insights Oncol. 2016;10:CMO-S32822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valent P. Imatinib-resistant chronic myeloid leukemia (CML): current concepts on pathogenesis and new emerging pharmacologic approaches. Biologics. 2007;1(4):433–448. [PMC free article] [PubMed] [Google Scholar]
- 14. Turkina A, Wang J, Mathews V, et al. TARGET: a survey of real-world management of chronic myeloid leukaemia across 33 countries. Br J Haematol. 2020;190(6):869–876. doi.org/10.1111/bjh.16599 [DOI] [PubMed] [Google Scholar]
- 15. Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Tolerability-adapted imatinib 800mg/d versus 400mg/d versus 400mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29(12):1634–1642. [DOI] [PubMed] [Google Scholar]
- 16. Alattar ML, Kantarjian HM, Jabbour E, et al. Clinical significance of complete cytogenetic response (CCyR) and major molecular response (MMR) achieved with different treatment modalities used as frontline therapy in chronic myeloid leukemia (CML) chronic phase (CP). Blood. 2011;118(21):745. doi.org/10.1182/blood.V118.21.745.745 [Google Scholar]
- 17. Waheed S, Zaidi U, Maqsood S, Borhany M, Shamsi T. Sokal risk score is a useful predictor of response to Nilotinib Therapy. J Hematol Transfus. 2017;5(4):1075. [Google Scholar]
- 18. Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2011;119(5):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomized ENESTnd trial. Lancet Oncol. 2011;12(9):841–851. [DOI] [PubMed] [Google Scholar]
- 20. Sasaki K, Lahoti A, Jabbour E, et al. Clinical safety and efficacy of nilotinib or dasatinib in patients with newly diagnosed chronic-phase chronic myelogenous leukemia and pre-existing liver and/or renal dysfunction. Clin Lymphoma Myeloma Leuk. 2016;16(3):152–162. doi:10.1016/j.clml.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi K, Kantarjian HM, Yang Y, et al. A propensity score matching analysis of dasatinib and nilotinib as a frontline therapy for patients with chronic myeloid leukemia in chronic phase. Cancer. 2016;122(21):3336–3343. doi:10.1002/cncr.30197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radich JP, Deininger M, Abboud CN, et al. Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(9):1108–1135. doi:10.6004/jnccn.2018.0071 [DOI] [PubMed] [Google Scholar]
- 23. Hochhaus A, Saussele S, Rosti G, et al. ESMO guidelines committee. Ann Oncol. 2018;29(Suppl 4):iv261. [DOI] [PubMed] [Google Scholar]
- 24. Pavey T, Hoyle M, Ciani O, et al. Dasatinib, nilotinib and standard-dose imatinib for the first-line treatment of chronic myeloid leukaemia: systematic reviews and economic analyses. Health Technol Assess. 2012;16(42):iii–iv, 1–277. doi:10.3310/hta16420.) [DOI] [PubMed] [Google Scholar]
- 25. Li N, Zheng B, Cai H, et al. Cost effectiveness of imatinib, dasatinib, and nilotinib as first-line treatment for chronic-phase chronic myeloid leukemia in China. Clin Drug Investig. 2018;38(1):79–86. doi:10.1007/s40261-017-0587-z [DOI] [PubMed] [Google Scholar]
- 26. Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–984. doi.org/10.1038/s41375-020-0776-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211001796 for Assessment of Dasatinib Versus Nilotinib as Upfront Therapy for Chronic Phase of Chronic Myeloid Leukemia in Qatar: A Cost-Effectiveness Analysis by Ahmad Adel, Dina Abushanab, Anas Hamad, Mohammad Abdulla, Mohamed Izham and Mohamed Yassin in Cancer Control