Abstract
Objective:
We investigated the clinical value of the Controlling Nutritional Status score in evaluating the prognosis of patients with non-muscle invasive bladder cancer.
Methods:
We conducted a retrospective analysis of the clinical data of 88 patients with non-muscle invasive bladder cancer who underwent transurethral resection of bladder tumor or partial cystectomy between January 2011 and May 2015 in a single center. The patients were divided into groups base on high (>1) and low (≤1) Controlling Nutritional Status score.
Results:
Clinical and demographic data of the patient groups were analyzed using the Kaplan-Meier method and log-rank test to generate survival curves. Univariate and multivariate analyses were conducted using the Cox proportional hazard model. Among the participants, the male-to-female ratio was 70:18 and median age was 64.5 years (range, 25-84 years). The numbers of patients with Controlling Nutritional Status score of 0, 1, 2, 3, 4, 5, and 6 were 26 (29.55%), 21 (23.86%), 20 (22.73%), 12 (13.64%), 5 (5.68%), 1 (1.14%), and 3 (3.41%), respectively. The 5-year recurrence rate was 29 out of 88 patients (32.95%). The recurrence-free survival of the high-score group was significantly lower than that of the low-score group (P < 0.001). On univariate analysis, age, smoking history, Controlling Nutritional Status score, depth of tumor invasion, pathological grade, and tumor diameter were related to the prognosis of patients with non-muscle invasive bladder cancer. On multivariate analysis, the Controlling Nutritional Status score (hazard ratio, 4.938; 95% confidence interval, 1.392-17.525; P = 0.013) was an independent factor affecting the recurrence-free survival of patients with non-muscle-invasive bladder cancer.
Conclusion:
Therefore, the Controlling Nutritional Status score could be a simple, cost-effective, and reliable predictor of prognoses among of patients with non-muscle-invasive bladder cancer.
Keywords: non-muscle invasive bladder cancer, controlling nutritional status score, nutritional status, prognosis, prediction index
Introduction
Bladder cancer is one of the most common malignant tumors in urology, approximately 75% of which comprise non-muscle invasive bladder cancer (NMIBC). 1 The classic surgical treatment for NMIBC is transurethral resection of bladder tumor (TURBT), however, the 5-year recurrence rate of NMIBC is high (30%∼80%). 2 Nearly 40% of the patients with recurrent bladder cancer develop muscle invasive bladder cancer, which requires a radical cystectomy (RC), which seriously affects the quality of life. 3 The prognosis of NMIBC is closely related to tumor grade, tumor stage, tumor size, tumor number, tumor recurrence time and frequency, and whether in situ carcinoma. The pathological grade and stage of the tumor are the most important factors affecting the prognosis factor. However, the prognosis of patients with NMIBC is still varies substantially. Therefore, it is necessary to find effective and accurate clinical indicators for evaluating the prognosis and guiding treatment. Recent studies have shown that tumor cell proliferation, invasion, metastasis, and angiogenesis are all affected by inflammation. 4 Moreover, the prognosis of patients with malignancies is strongly associated with general nutritional status and immune- inflammatory response. 5,6
The Controlling Nutritional Status (CONUT) score is a new index used to evaluate the preoperative nutritional status of patients in recent years. 7 It is calculated based on serum albumin level, peripheral blood lymphocyte count and cholesterol level. The CONUT score is simple to use and the cost-effective. In recent years, many reports have indicated that the CONUT score is a new prediction index of the prognosis of patients with malignancies, and it has been proven to be associated with the prognosis of patients with lung cancer, colorectal cancer, liver cancer and gastric cancer. Miyake et al studied the impact of nutritional indicators including the CONUT score on the prognosis of patients who underwent radical cystectomy (RC). 8 However, to the best of our knowledge, no studies have yet investigated the relationship between the CONUT score and prognosis of NMIBC underwent bladder-preserving surgery. This study aimed to analyze the clinical data of NMIBC patients to explore the clinical value of the preoperative CONUT score in the prognostic assessment of NMIBC.
Materials and Methods
Materials of Patients
We conducted a retrospective analysis of 88 patients diagnosed with NMIBC for the first time through TURBT or partial cystectomy between January 2011 and May 2015 in Xiaoshan Hospital of Zhejiang Province. All postoperative pathological diagnoses were NMIBC and complete the clinical follow-up information was present for all patients. The main exclusion criteria were as follows: (1) preoperative infections that affect blood routine results, and patients with a history of other tumors, autoimmune diseases, and hematological diseases; (2) patients with chronic obstructive pulmonary disease, heart failure, liver insufficiency, renal failure, etc. The latter will affect the prognosis of surgery; (3) those who received radiotherapy and chemotherapy before surgery; (4) those who were treated with antibiotics, immunosuppressants, and glucocorticoids during the perioperative period.
Methods
Preoperative lymphocyte count, albumin levels, and cholesterol levels were collected, and the CONUT score was calculated (Table 1). The CONUT score was calculated using peripheral blood lymphocyte count, serum albumin levels and cholesterol levels (Table 1).
Table 1.
Assessment of Undernutrition Status by the CONUT Score.
| Undernutrition degree | None | Light | Moderate | Severe |
|---|---|---|---|---|
| Serum albumin (g/dL) | ≥35 | 30-34.9 | 25-29 | <29 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocyte count (/mm3) | ≥1600 | 1200-1599 | 800-1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) | ≥180 | 140-179 | 100-139 | <100 |
| Score | 0 | 1 | 2 | 3 |
Simultaneously, we collected general data, such as age, gender, body mass index (BMI), tumor diameter (diameters of multiple tumors were added together), infiltration depth and pathological grades and other clinical and pathological indicators for each group of patients. Due to the small number of deaths from NMIBC, we use recurrence-free survival (RFS) as the endpoint and followed patients until tumor recurrence or death. If there was no recurrence or death, the final follow-up was estimated to 5 years after the operation, and the data at the end of the follow-up was included in the statistical analysis.
Statistical Method
The optimal cut-off value of the CONUT score was acquired using X-tile software v3.6.1 (Yale University). 9 SPSS 23.0 was used for all statistical analyses. Normally distributed data was analyzed using the t test, and the count data comparisons were analyzed using the χ2 test. The Kaplan-Meier method and log-rank test were used for survival analysis, and survival curves were drawn at the same time. The Cox proportional hazard model was used for univariate and multivariate analyses. In order to determine the independent risk factors affecting RFS of patients with NMIBC, all important variables in univariate analysis were included in the multivariate analysis. P < 0.05 was considered statistically significant.
Results
Clinicopathologic Characteristics of Patients
A total of 88 patients were enrolled (Table 2), with a male to female ratio of 70:18 and a median age of 64.5 years (range, 25-84 years). There were 54 patients with BMI <24 (61.36%) and 34 patients with BMI ≥24 (38.64%). Forty-eight patients had a history of smoking. There were 64 patients with stage Ta cancer (72.73%), 24 patients with stage T1 cancer (27.27%). There were 53 patients with low grade cancer (60.23%), 35 patients with high grade cancer (39.77%). There were 55 patients (62.50%) with a tumor diameter < 3 cm and 33 patients (37.50%) with a tumor diameter ≥ 3 cm. The numbers of patients with CONUT score ranging from 0 to 6 were 26 (29.55%), 21 (23.86%), 20 (22.73%), 12 (13.64%), 5 (5.68%), 1 (1.14%), and 3 (3.41%), respectively. The 5-year recurrence rate was 29 out of 88 patients (32.95%).
Table 2.
Clinicopathologic Characteristics of Patients.
| Factors | Value or number of patients (n = 88) |
|---|---|
| Age (years) | |
| Median | 64.5 |
| Range | 25-84 |
| Gender | |
| Male | 70 (79.55%) |
| Female | 18 (20.45%) |
| BMI | |
| <24 | 54 (61.36%) |
| ≥24 | 34 (38.64%) |
| Smoking history | 48 (54.55%) |
| T Stage | |
| Ta | 64 (72.73%) |
| T1 | 24 (27.27%) |
| Pathological grade | |
| Low-grade papillary urothelial carcinoma | 53 (60.23%) |
| High-grade papillary urothelial carcinoma | 35 (39.77%) |
| Tumor diameter (cm) | |
| <3 | 55 (62.50%) |
| ≥3 | 33 (37.50%) |
| CONUT score | |
| 0 | 26 (29.55%) |
| 1 | 21 (23.86%) |
| 2 | 20 (22.73%) |
| 3 | 12 (13.64%) |
| 4 | 5 (5.68%) |
| 5 | 1 (1.14%) |
| 6 | 3 (3.41%) |
| Recurrence rate | 29/88 (32.95%) |
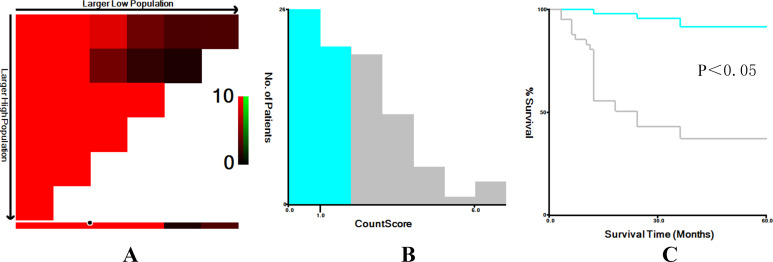
The Optimal Cut-off Value of CONUT Score
X-tile software (v3.6.1) was used to determine the cut-off value of CONUT score as 1 (Figure 1). Accordingly, all patients with NMIBC were divided into high- (>1) and low- score (≤1) groups.
Figure 1.
The optimal cut-off value for CONUT score was determined using X-tile software. The optimal cut-off value highlighted by the black circles (A) is shown in histograms of the entire cohort (B) and along with the Kaplan-Meier plots (C).
Correlation Between CONUT Score and Basic Clinical Data and Pathological Parameters of Patients
When the clinical data and pathological parameters of the study group were divided into high- and low-score groups according to the cut-off value of CONUT score of 1, significant differences were found between the high and low CONUT groups in term of the depth of invasion, pathological grade, tumor diameter, recurrence rate, peripheral blood lymphocyte count, serum albumin levels, and cholesterol levels (P < 0.05; Table 3). There was no significant difference in other clinicopathological features between the 2 groups.
Table 3.
Comparison of Basic Clinical Data Between the 2 Groups.
| Factors | CONUT score | P value | |
|---|---|---|---|
| Low (n = 67) | High (n = 21) | ||
| Age (years) | 60.55 ± 12.46 | 71.19 ± 11.81 | <0.001 |
| BMI | 23.23 ± 3.05 | 22.24 ± 2.68 | 0.186 |
| Male | 51 | 19 | 0.155 |
| Smoking history | 31 | 17 | 0.005 |
| T Stage | |||
| Ta | 55 | 8 | <0.001 |
| T1 | 12 | 13 | |
| Pathological grade | |||
| Low-grade | 49 | 4 | <0.001 |
| High-grade | 18 | 17 | |
| Tumor diameter (cm) | |||
| <3 | 52 | 3 | <0.001 |
| ≥3 | 15 | 18 | |
| Recurrence rate | 13/67 | 16/21 | <0.001 |
| Serum albumin (g/dL) | 43.45 ± 3.57 | 37.58 ± 2.91 | <0.001 |
| Total lymphocyte count (/mm3) | 1.80 ± 0.41 | 1.07 ± 0.38 | <0.001 |
| Total cholesterol (mg/dL) | 177.60 ± 20.85 | 148.80 ± 21.15 | <0.001 |
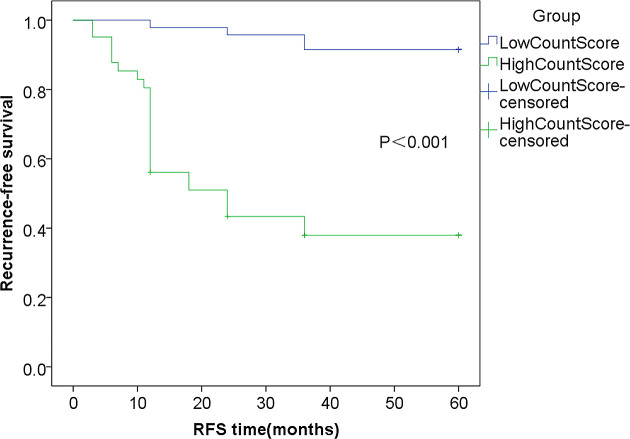
Survival Analysis
The Kaplan-Meier curve analysis of the 2 groups is shown in Figure 2. The RFS in the high-score group was significantly lower than that in low-score group (P < 0.001).
Figure 2.
Kaplan-Meier curve analysis of RFS in the high and low CONUT score groups.
Analysis of Prognostic Factors
The univariate Cox regression model showed that age, smoking history, CONUT score, depth of invasion, pathological grade and tumor diameter were related to the prognosis of NMIBC patients. Multivariate Cox regression analysis indicated that in addition to tumor diameter and pathological grade, the CONUT score (hazard ratio, 4.938; 95% confidence interval, 1.392-17.525; P = 0.013) was an independent factor influencing the 5-year RFS rate of patients with NMIBC (Table 4).
Table 4.
Univariate and Multivariate Analyses of Prognostic Factors.
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P a | |
| Age (<65, ≥65) | 2.757 | 1.254-6.060 | 0.012 | 1.133 | 0.458-2.805 | 0.787 |
| Gender (male, female) | 1.314 | 0.561-3.076 | 0.529 | |||
| BMI (<24, ≥24) | 0.536 | 0.237-1.211 | 0.134 | |||
| Smoking (yes, no) | 2.528 | 1.118-5.741 | 0.026 | 2.027 | 0.840-4.891 | 0.116 |
| T Stage (Ta, T1) | 3.686 | 1.772-7.665 | <0.001 | 0.588 | 0.203-1.703 | 0.327 |
| Pathological Grade (High, Low) | 5.756 | 2.532-13.084 | <0.001 | 3.423 | 1.050-11.156 | 0.041 |
| Size of Tumor (<3cm, ≥3cm) | 6.013 | 2.649-13.653 | <0.001 | 3.051 | 1.209-7.701 | 0.018 |
| CONUT Score (≤2, >2) | 6.890 | 3.253-14.592 | <0.001 | 4.938 | 1.392-17.525 | 0.013 |
a The bold value indicates that this data is statistically significant in multivariate analysis (P < 0.05).
Discussion
To the best of our knowledge, our study is the first to evaluate the value of the CONUT score in determining the prognosis of patients with NMIBC. We compared the CONUT score and clinicopathological parameters concurrently. We found that in addition to there being a significant difference (P < 0.05) between the clinicopathological features of patients with low and high CONUT scores, there was also a considerable difference between the recurrence rates of the 2 groups (8.51% vs. 60.98%) (Figure 2). Therefore, CONUT score can help clinical workers distinguish high-risk patients in timely fashion, leading to early and reasonable intervention measures after surgery.
Several studies have shown that nutritional status and immune-inflammatory response are closely related to the prognosis of patients with malignancies. 5,6 It is reported that 32% of cancer patients have a low nutritional status that may be consequent to tumor-related anorexia, inflammation, and metabolic changes. 10 Malnutrition can lead to muscle atrophy and muscle function damage by impairing the immune function, delaying activity, damaging cardiopulmonary function, and increasing the risk of adverse surgical outcomes. 11 -13
The CONUT score is designed to evaluate the nutritional status of patients conveniently and objectively. Serum albumin level is one such reliable indicator of nutritional status and immune-inflammatory response that is closely related to the survival rate of cancer patients. 14,15 The tumor-associated inflammatory response can produce a large number of inflammatory factors, such as C-reactive protein and cytokines. These inflammatory factors can regulate the albumin synthesis. 16 In addition, some studies have reported that hypoalbuminemia is associated with immune impairment and immune tolerance, which promotes the proliferation of tumor cells and disease progression. 17 Lymphocytes, as members of the inflammatory cell family, play a significant role in the immune response to cancer, as they induce apoptosis, inhibit tumor growth and metastasis, and mediate cytotoxicity. For example, CD4+ T lymphocytes and natural killer cells are the key inflammatory cells in cellular immunity. In addition, studies have shown that when comparing patients with low and high blood lymphocyte counts, the decrease in lymphocyte count indicates a decline in immune function, an insufficient immune response to cancer cells, and the formation of a microenvironment suitable for the proliferation and metastasis of cancer cells, leading to worse clinical prognosis. 18,19 The difference between the CONUT score and other prognostic indicators is that the serum cholesterol level is included in the evaluation system. Cholesterol, as an important component of cell membrane, is potentially associated with tumor cell proliferation, metastasis, and immune response. 20,21 Muldoon et al showed that the total number of lymphocytes, total T lymphocytes, and CD8+ T lymphocytes in the circulating blood of tumor patients with hypocholesterolemia were less than those with hypercholesterolemia. 22 In addition, cholesterol is shown to increase the antigen-presenting function of monocytes, thus accelerating the recognition of tumor cells by immune cells, and indirectly affecting the immune response of the tumor microenvironment. 23 Therefore, cholesterol is a powerful prognostic factor that possibly increases the ability of the CONUT score to evaluate the clinical outcomes of patients with malignant tumors.
The CONUT score is related to the prognosis of patients with various cancers. Ishihara et al indicated that the preoperative CONUT score can predict the survival rate of patients with localized upper urinary tract cancer. 24 Miyake et al did not find that the CONUT score had a significant impact on the prognosis of patients underwent RC, however, the muscle loss and nutritional deterioration were significantly associated with the prolonged hospital stay after RC. 8 Our study shows that the CONUT score is an independent risk factor for the prognosis of patients with NMIBC. The reason for this difference may be that the study by Miyake et al included patients with T1-stage tumors and muscle-invasive bladder cancer, and overall survival was used as the prognostic evaluation index, whereas our study included patients with NMIBC and used RFS as the prognostic evaluation index.
Patients with a high CONUT score, on account of poor nutritional status, have an impaired immune-inflammatory response, more active micrometastasis and residual cancer cells, and a higher risk of recurrence. Therefore, patients with a high CONUT score need to be followed up more closely than patients with a low CONUT score. For NMIBC patients with a high CONUT score, it is necessary to increase the frequency of cystoscopy or abdominal computed tomography (CT), or even extend the follow-up period. Meanwhile, early and appropriate nutritional intervention for patients with a high CONUT score can significantly improve the treatment tolerance and survival rate, and improve the prognosis. Since the 3 evaluation indexes of CONUT score are commonly used and easy to detect in clinical practice, the CONUT score has the advantage of being simple, cost-effective and reliable in predicting prognosis, thereby providing clinicians with prognostic information and guiding the development of individualized treatment plans.
Conclusion
The CONUT score is an independent risk factor affecting RFS of patients with NMIBC, and it is simple to use, rapid, and cost-effective. It is calculated before surgery or initial treatment, and may help determine the need for individualized intervention. This study has several limitations. Other nutritional prognostic indicators were not included in this study, so it is not possible to determine whether the CONUT score is the best nutritional indicator for evaluating the prognosis of patients with NMIBC. In addition, this study was a retrospective study, and the sample sizes were limited. Our results need to be confirmed by further prospective studies with larger sample sizes.
Abbreviations
- BMI
body mass index
- CONUT
Controlling Nutritional Status
- CT
computed tomography
- NMIBC
non-muscle invasive bladder cancer
- RC
radical cystectomy
- RFS
recurrence-free survival
- TURBT
transurethral resection of bladder tumor
Footnotes
Authors’ Note: (I) Conception and design: Yi Fan, Jiaguo Huang, Liwei Zhao, Kai Wang; (II) Administrative support: None; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: Yi Fan, Jiaguo Huang, Ji Sun, Shengcheng Tai, Runmiao Hua, Yufu Yu; (V) Data analysis and interpretation: Yi Fan, Jiaguo Huang, Liwei Zhao; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. As our study is a retrospective analysis, ethics statement is not required.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yi Fan, MD  https://orcid.org/0000-0002-2397-2465
https://orcid.org/0000-0002-2397-2465
References
- 1. Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur Urol. 2017;71(3):447–461. [DOI] [PubMed] [Google Scholar]
- 2. Ogihara K, Kikuchi E, Yuge K, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel biomarker for predicting worse clinical outcomes in non-muscle invasive bladder cancer patients with a previous history of smoking. Ann Surg Oncol. 2016;23(Suppl 5):1039–1047. [DOI] [PubMed] [Google Scholar]
- 3. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–475; discussion 475-467. [DOI] [PubMed] [Google Scholar]
- 4. Zheng RR, Huang M, Jin C, et al. Cervical cancer systemic inflammation score: a novel predictor of prognosis. Oncotarget. 2016;7(12):15230–15242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. [DOI] [PubMed] [Google Scholar]
- 6. Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla E, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199–211. [DOI] [PubMed] [Google Scholar]
- 7. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for Controlling Nutritional Status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 8. Miyake M, Morizawa Y, Hori S, et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer. 2017;17(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. [DOI] [PubMed] [Google Scholar]
- 10. Gangadharan A, Choi SE, Hassan A, et al. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget. 2017;8(14):24009–24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arora NS, Rochester DF. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis. 1982;126(1):5–8. [DOI] [PubMed] [Google Scholar]
- 12. Heymsfield SB, Bethel RA, Ansley JD, Gibbs DM, Nutter DOJAHJ. Cardiac abnormalities in cachectic patients before and during nutritional repletion. Am Heart J. 1978;95(5):584–594. [DOI] [PubMed] [Google Scholar]
- 13. Tsai S. Importance of lean body mass in the oncologic patient. Nutr Clin Pract. 2012;27(5):593–598. [DOI] [PubMed] [Google Scholar]
- 14. Ayhan A, Günakan E, Alyazıcı İ, Haberal N, Altundağ Ö, Dursun P. The preoperative albumin level is an independent prognostic factor for optimally debulked epithelial ovarian cancer. Arch Gynecol Obstet. 2017;296(5):989–995. [DOI] [PubMed] [Google Scholar]
- 15. McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41(1-2):64–69. [DOI] [PubMed] [Google Scholar]
- 16. Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(6 Suppl 4): S118–S125. [DOI] [PubMed] [Google Scholar]
- 17. Cengiz O, Kocer B, Sürmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit. 2006;12(6): CR240–CR247. [PubMed] [Google Scholar]
- 18. Orhan A, Vogelsang RP, Andersen MB, et al. The prognostic value of tumour-infiltrating lymphocytes in pancreatic cancer: a systematic review and meta-analysis. Eur J Cancer. 2020;132:71–84. [DOI] [PubMed] [Google Scholar]
- 19. Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ducimetiere P, Cambien F, Jacqueson A, Richard J. Serum retinol and the inverse relationship between serum cholesterol and cancer. Br Med J (Clin Res Ed). 1982;284(6320):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams RR, Sorlie PD, Feinleib M, Mcnamara PM, Dawber TR. Cancer incidence by levels of cholesterol. JAMA. 1981;245(3):247–252. [PubMed] [Google Scholar]
- 22. Muldoon MF, Marsland A, Flory JD, Rabin BS, Whiteside TL, Manuck SB. Immune system differences in men with hypo- or hypercholesterolemia. Clin Immunol Immunopathol. 1997;84(2):145–149. [DOI] [PubMed] [Google Scholar]
- 23. Wu B, Teng L, He D, Yu DD, Jiang F. Dose-response relation between serum total cholesterol levels and overall cancer risk: evidence from 12 prospective studies involving 1,926,275 participants. Int J Food Sci Nutr. 2019;70(4):432–441. [DOI] [PubMed] [Google Scholar]
- 24. Ishihara H, Kondo T, Yoshida K, et al. Preoperative controlling nutritional status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urol Oncol. 2017;35(9):539. e539-539 e516. [DOI] [PubMed] [Google Scholar]