Abstract
Cervical cancer is a common female cancer. It is strongly associated with human papillomavirus (HPV) infection. However, HPV infection alone is not sufficient to induce cervical cancer because its development is dependent on the coexistence of several factors that enable the virus to overcome the host immune system. These include individual genetic background, environmental factors, or diet, including dietary selenium intake. Selenium is an essential trace element with antiviral properties and has been shown to exert antitumor effects. Surprisingly, the role of selenium in cervical cancer has not been studied as intensively as in other cancers. Here, we have summarized the existing experimental data on selenium and cervical cancer. It may be helpful in evaluating the role of this nutrient in treatment of the mentioned malignancy as well as in planning further studies in this area.
Keywords: selenium, selenium compounds, cervical cancer, HPV
Introduction
Cervical cancer is the fourth most common female cancer worldwide, with an estimated 570,000 new cases and 310,000 deaths in 2018 (Globocan 2018). The main risk factor associated with this disease is infection with human papillomavirus (HPV; mostly types 16 and 18), which is found in the majority of cases. 1 However, HPV infection alone is not sufficient to induce cervical cancer. Certain factors, such as individual genetic variation and environmental factors or diet, may influence the risk of this disease. 2 Recent retrospective epidemiological studies suggest that there may be a relationship between selenium (Se) status and cervical cancer. 3 Se is an essential trace element that plays some important functions in the human organism through selenoproteins. These functions are related to the antioxidant system, redox signaling, thyroid hormone metabolism, immune system, and many others. 4 Notably, studies have shown that Se possesses antiviral activity. 5 At the same time, Se has been implicated in human cancers. 6 Scientific research has focused on the association between Se and cancer at different sites, including the head and neck, esophagus, colon, liver, skin, lung, breast, bladder, and prostate. 7 However, little attention has been paid to the role of Se in cervical cancer. Considering the antiviral activity of Se, 5 it is important to investigate its possible therapeutic effect in cervical cancer. Since this type of cancer is a challenging clinical problem due to its high metastatic potential and resistance to therapy, effective treatment methods are highly required. Altough the possible preventive role of Se in cervical cancer is still under question (no association was shown in one prospective study 8 and there was no possibility of concluding the causative relationship based on case-control studies), 3,9 -11 data from laboratory experiments conducted in cells and animals show that Se may be used in cervical cancer therapy. However these observations need to be further confirmed in clinical studies. In this short review we summarized the existing experimental data on Se and cervical cancer. This may be helpful in evaluating the therapeutic potential of this nutrient in the mentioned malignancy as well as in planning further research.
Methods
We searched literature in electronic databases including PubMed and Scopus until the 15th of September 2020. Search terms included a combination of terms “selenium” with “cervical cancer” or “HPV” or “CIN1” (cervical intraepithelial neoplasia grade 1). Titles and abstracts were further screened in order to select experimental studies that aimed to assess the cancer therapeutic effects of Se or Se compounds in patients with cervical cancer or CIN1, or in different laboratory cervical cancer models (in vitro and in vivo). At least two authors independently selected the literature. Finally, the selected experimental studies discussed in this review included 19 in vitro studies, 7 in vivo studies, and 2 human randomized placebo-controlled trials.
Laboratory Studies on Se and Cervical Cancer
In vitro studies that investigated the anticancer activity of Se in cervical cancer were conducted mainly on the HeLa cell line (a human cervical carcinoma cell line which contains HPV18 DNA and was derived from a 31-year old patient). 12 Some authors have assessed the effects of Se on cervical cancer using the HeLa derivative - Hep2 cells 13 or SiHa cells (containing HPV 16 DNA). 14 In vivo models of cervical cancer include: a xenograft mouse model (tumors induced by HeLa cell transplantation), MCA (3-methylcholanthrene) -induced cervical cancer, and HPV induced cervical cancer. Of these three, the last one seems to most accurately mimic the in vivo carcinogenesis of patients with cervical cancer in terms of similar trace element concentrations. 15 In this section, we discuss both the in vitro and in vivo data that shed more light on the cancer therapeutic potential of Se and its possible mechanisms of action in cervical cancer development, taking into consideration different forms of Se, including both organic and inorganic compounds, and with a special emphasis on Se at the nanoscale (Tables 1 and 2).
Table 1.
In Vitro Studies on Se Compounds in Cervical Cancer.
| References | Cell line | Se compound (dose, time of treatment) | Effect of Se exposure | Mechanisms of action |
|---|---|---|---|---|
| Rudolf et al, 2008 13 | Hep2 | Sodium selenite 5 µmoL/L and 50 µmol/L 24h |
Caspase independent apoptosis (in a time and dose dependent manner); affected mitochondria dynamics |
Oxidative stress induction; suppressed DNA synthesis; DNA damage; activation of p53 and p38 pathways; increased expression of p21 and Bax; AIF and Smac/Diablo release from mitochondria |
| Fu et al, 2011 16 | HeLa | Sodium selenite 40 µmol/L 24h |
apoptosis |
Oxidative stress; decreased mitochondrial membranę potential; decreased expression of: SOD1, Prx2a, Prx4, Prx 6, QPRTase, DDT,YWHA, eEF2, ubiquitin and antiapoptotic protein Bcl2; increased expression of cofilin 1, hnRNP A2/B1 and PHB |
| Sun et al, 2017 17 | HeLa | SeMet 3 µM, 12-72h Alone or in combination with SAM |
No effect on cell proliferation; inhibition of cell migration; inhibition of cell adhesion, enhanced by combination with SAM |
inhibition of AKT activation |
| Sun et al, 2017 17 | HeLa | MeSeCys 3 µM, 12-72h Alone or in combination with SAM |
No effect on cell proliferation; inhibition of cell migration and adhesion, enhanced by combination with SAM |
Inhibition of AKT activation, enhanced by combination with SAM |
| Sun et al, 2017 17 | HeLa | MeSeA (alone or in combination with SAM) 3 µM 12-72h |
Inhibition of cell proliferation (alone or in combination with SAM); inhibition of cell migration and adhesion, enhanced by combination with SAM |
Inhibition of ERK and AKT activation, enhanced by combination with SAM |
| Zhao et al, 2016 18 | HeLa, SBP1 knock downed HeLa |
SeMet 1-5 µM 24h |
Increased expression of SBP1 in a dose-dependent manner; increase in Se was dependent on SBP1 |
Not studied |
| Tolen et al, 2015 19 | HPV-18 exposed mouse trophoblast cells HPV-18 exposed mouse ICM cells |
SeMet 0.5 µM, 5 µM (coexposure with HPV-18) 48h |
protection of trophoblast cells from HPV induced nuclear shrinkage and apoptosis; increased apoptosis in IMC cells when coexposed to HPV |
not studied |
| Rizvi et al, 2015 20 | HeLa | Bis(3,5-bis(trifluoromethyl)phenyl) 10-50 µM 24h |
Nuclear condensation with blebbing and fragmentation of nuclei; increased GPx1 activity |
Binding to DNA |
| Yadav and Singh, 2019 21 | HeLa | C31H35O4SeRuCl 100 µM 48h |
cytotoxic effect | binding to DNA |
| Guo et al, 2013 22 | HeLa | Sucrose Se ester 1-5 ppm 72h |
Apoptosis in dose dependent manner; DNA fragmentation |
Not studied |
| Luo et al, 2012 23 | HeLa | Se-NPs synthetised in the lab 10-40 µM 24h |
Dose-dependent growth inhibition; shrunken cell morphology with truncated lamellipodia |
Cell cycle arrest at S phase |
| Srivastava and Kowshik, 2016 24 | HeLa | Se-NPs from bacteria 2.5 – 100 µg/mL 24h |
Dose-dependent increase in ROS and apoptotic index; caspase dependent apoptosis |
Activation of caspase dependent apoptotic pathway |
| Zheng et al, 2011 25 | HeLa | Se-NPs combined with sialic acid 0-40 µg//mL, 72h |
Cancer selectiveness; apoptosis |
Caspase 3 activation and cleavage of PARP |
| Yuwen et al, 2016 26 | HeLa | Se-NPs MoSe2 nanodots + laser irradiation 10-40 µg/mL 12h |
Dose-dependent cytotoxic effect | Not studied |
| Bidkar et al, 2017 27 | HeLa | Se-NPs combined with drug (PTX) | Apoptosis | G2/M cell cycle arrest; perturbation of mitochondrial membrane potential; increased production reactive oxygen species; activation of effector caspases |
| Xia et al, 2018 28 | HeLa | Se-NPs combined with FA and drug (DOX) |
Supressed migration and invasion; apoptosis |
not studied |
| Xia et al, 2020a 29 | HeLa | Se-NPs combined with HA and drug (DOX) 0.5-16 µg/mL DOX 48h |
apoptosis | Increased expression of proapoptotic factors: Bam, Bax, Bak, Bad; decreased expression of antiapoptotic Bcl-2 protein |
| Li et al, 2016 30 | HeLa | Se-NPs combined with drug (DOX) Bi2Se3@PDA/DOX/HAS 2-200 µg/mL 48h additional combination with irradiation 5 -100 µg/mL 12h |
Dose-dependent cytotoxicity higher as compared to drug alone and additionally enhanced by combining treatment with irradiation | Not studied |
| Xia et al, 2020b 31 | HeLa | Se-NPs combined with arginylglycylaspartic acid peptide and Derlin1-siRNA 6-200 nM 48h |
Suppressed invasion, migration and proliferation; apoptosis |
ROS overproduction; mitochondrial dysfunction |
Abbreviations: AIF, Apoptosis Inducing Factor; AKT, Protein Kinase B; Bad, Bcl-2 Associated Agonist of Cell Death; Bak, Bcl-2 Homologous Antagonist/killer; Bax, Bcl-2-like protein 4; DDT, D-dopachrome Tautomerase; DOX, Doxorubicin; eEF2, Eukaryotic Translation Elongation Factor 2; ERK, Extracellular Regulated Kinase; FA, Folic Acid; GPx1, Glutathione Peroxidase; HA, Hyaluronic Acid; hnRNP A2/B1, Heterogeneous Nuclear Ribonucleoprotein A2/B1; HPV, Human Papillomavirus; HAS, Human Serum Albumin; ICM, Inner Cell Mass; MeSeA, Methylseleninic Acid; Scpso PARP, Poly (ADP-ribose) Polymerase; PHB, Prohibitin; Prx2a, Peroxiredoxin 2a; Prx4, Peroxiredoxin 4; Prx6, Peroxiredoxin 6; PTX, Paclitaxel; QPRTase, Quinolinate Phosphoribosyl Transferase; ROS, Reactive Oxygen Species; SAM, S-adenosyl-methionine; SBP1, Selenium Binding Protein 1; SeMet, Selenomethione; SE-NPs, Selenium Nanoparticles; SOD1, Superoxide Dismutase 1; YWHA, Tyrosine 3/tryptophan 5 Monooxygenase Activation Protein.
Table 2.
In Vivo Studies on Se Compounds Used in the Experimental Studies on Cervical Cancer.
| References | In vivo model | Se compound or Se containing product (dose, time of treatment) | The effect of Se | Mechanisms of action |
|---|---|---|---|---|
| Hussain and Rao, 1992 32 | MCA induced cervical cancer in mice | Sodium selenite 1 ppm exposure 1 week before and 12 weeks after MCA insertion |
Decreased tumor incidence | Not studied |
| Guo et al, 2013 22 | HeLa injected BALB/c mice | Sucrose Se ester 1, 2, 4, 8 mg/kg/day 7 days |
Increased survival time 0.75, 1.06, 1.67, and 0.50 for doses 1, 2, 4, and 8 mg/kg, respectively |
Not studied |
| Xia et al, 2020a 29 | HeLa injected BALB/c mice | Se-NPs modified with HA and drug (DOX) 2 mg/kg DOX 21 days | supressed tumor growth | Inhibition of cancer cells proliferation (as shown by decreased expression of Ki67 protein); induction of apoptosis |
| Li et al, 2016 30 | HeLa injected BALB/c mice | Bi2Se3NPs modified with drug (DOX) 0.81 mg/kg DOX and 25 mg/kg Bi2Se3
12 days additional combination with 10 min irradiation |
Tumor growth inhibition maintained also after the therapy | Not studied |
| Xia et al, 2020b 31 | HeLa injected BALB/c mice | Se-NPs combined with arginylglycylaspartic acid peptide and Derlin1-siRNA 0.2 mg/kg of the eqivalent siRNA per day, 21 days | Decreased tumor volume; no toxicity observed in heart, liver, spleen, lung, and kidney | Apoptosis; increased expression of pp53, caspase-3 and Bak |
| Ji et al, 2014 33 | MCA induced cervical cancer in mice | Sodium selenite enriched mushrooms (Se-CS) Se content in mycella: 4789 µg/g, daily dose not known, 90 days | Decreased tumor incidence by 40% | Improved immune function (as shown by alleviation of MCA induced decrease of thymus and spleen); decreased oxidative stress |
| Zhang et al, 2012 34 | MCA induced cervical cancer in mice | Aqueous suspension of pulverized mace of Myristica fragrans Houtt 10 mg of mace/day 90 days | Decreased tumor incidence from 100% to 10% | Not studied |
Abbreviations: Bak, Bcl-2 homologous antagonist/killer; DOX, doxorubicin; HA, hyaluronic acid; MCA, 3-methylcholanthrene; SE-NPs, selenium nanoparticles.
Sodium Selenite
Among the inorganic forms of Se, sodium selenite (Na2SeO3) was the only one tested in studies on cervical cancer (Tables 1–3). It is an excellent example of Se compounds with redox activities. Its ability to generate reactive oxygen species underlies its toxicity in cancer cells. 35
Table 3.
Randomized Placebo Controlled Trials Investigating the Effect of Se Supplementation on Clinical Outcomes in Cervical Intraepithelial Neoplasia or Cervical and Uterine Cancer.
| Author | Subjects | Intervention | Major results |
|---|---|---|---|
| Karamali et al, 2015 36 | 58 women with cervical intraepithelial neoplasia grade 1 (CIN1) | 200 µg of Se (form: Se yeast), n = 26 Placebo, n = 26, Time: 6 months | Higher CIN1 regression |
| Muecke et al, 2014 37 | 81 patients with cervical (n = 11) and uterine (n = 70) cancer | 500 µg of Se on days of RT, 300 µg of Se on days without RT, (form: sodium selenite), n = 39; Placebo, n = 42; Time: 6 weeks | No effect on disease free survival and overall survival; reduced number and severity of RT induced diarrhea |
Abbreviations: CIN1, cervical intraepithelial neoplasia 1; RT, radiotherapy.
Rudolf et al exposed Hep2 cells to sodium selenite at 5 and 50 µmol/L for 24h. 13 The authors observed that Se induced oxidative stress in the cells, which resulted in suppressed DNA synthesis, DNA damage, and subsequent activation of the p53 pathway. Along with these changes, the Se activated p38 pathway led to the accumulation of proapoptotic Bax protein and release of AIF and Smac/Diablo proteins from mitochondria, which together led to caspase-independent apoptosis. 13 Proteomic changes upon sodium selenite treatment were investigated by Fu et al in HeLa cells treated with the compound at a concentration of 40 µmol/L for 24h. 16 It was shown that selenite altered the expression of 13 proteins, including proteins involved in redox balance, apoptosis, signal transduction, mRNA transcription, protein translation, degradation, and translocation. Increased generation of ROS and decreased mitochondrial membrane observed along with downregulation of antioxidant proteins led the authors to conclude that sodium selenite induced apoptosis in HeLa cells via a ROS-mediated mitochondrial pathway. 16
Hussain and Rao conducted a study in mice to assess the chemopreventive properties of sodium selenite in vivo in chemically induced cervical cancer. 32 In this model, a cotton thread containing 600 µg of MCA was inserted into the canal of the mouse uterine cervix to induce tumors. Sodium selenite was administered through drinking water at 1 ppm 1 week before and 12 weeks after carcinogen insertion. Tumor incidence in mice was decreased upon Se administration in this study from 72% to 37%. 32
Organic Se Compounds
Organic selenium compounds may be of natural and synthetic origin and include compounds largely differing in chemical structure, cellular uptake, metabolic pathways and biological effects. Some of them were shown to exert antitumor activity in vitro and in vivo, and because of their lower toxicity and higher cancer cell selectivity compared to sodium selenite, they are considered to be better candidates for anticancer agents. 38 Organic Se compounds tested in cervical cancer experimental models included natural selenoamino acids or synthetic compounds.
The potential antitumor activity of a combination of organic Se compounds with a methyl group donor, S-adenosyl-methionine (SAM), was investigated in HeLa cells by Sun et al. 17 The authors tested three organic Se compounds, two of which, selenomethionine (SeMet) and methylselenocysteine (MeSeCys), are found naturally in the food, and the third one, methylseleninic acid (MeSeA), is of synthetic origin. The anticancer effect was studied at the level of cell proliferation, migration and adhesion, with further assessment of the levels of activated AKT and ERK proteins, which are both related to cell proliferation and migration. MeSeA alone (3 µM) inhibited both ERK and AKT activation and suppressed proliferation, migration and adhesion in HeLa cells, and all these effects were enhanced by combination with SAM (3 µM). Two other compounds, SeMet (3 µM) and MeSeCys (3 µM), significantly inhibited AKT alone and this inhibition was enhanced by SAM in the case of MeSeCys. No effect on cell proliferation was shown in the case of MeSeCys and SeMet either alone or in combination with SAM. However, both MeSeCys and SeMet inhibited cell migration alone (with the enhancing effect of combination with SAM observed for MeSeCys) and adhesion (with the enhancing effect of combination with SAM observed for the two compounds). 17 Altogether, this study shows that among the three tested organic Se compounds, MeSeA exerted the most promising tumor-suppressive effects in HeLa cells.
Zhao et al observed that exposure of HeLa cells to SeMet at concentrations of 1-5 µM for 24h increased the expression of selenium-binding protein 1 (SBP1). 18 SBP1 is a protein that binds covalently to Se (in contrast to selenoproteins to which Se is incorporated co-translationally as a consequence of decoding selenocysteine UGA codon). Human studies have shown that SBP1 is often underexpressed in cancer. This suggests a tumor-suppressive function of this protein. 39 Zhao et al showed in their study that the knockdown of SBP1 in HeLa affected intracellular Se levels in these cells in response to SeMet treatment (the dose-dependent increase was lower compared to that in the control cells). 18
Interesting observations regarding SeMet and HPV coexposure were made by Tolen et al. 19 These authors did not investigate the cervical cancer model. They used HeLa cell lysates to isolate HPV-18 DNA, which they further used to infect mouse embryos. The embryos were exposed to HPV in the presence of SeMet at 0,5 µM and 5 µM, and it was observed that SeMet at both concentrations prevented nuclear shrinkage caused by the virus and decreased the number of apoptotic cells as compared to cells exposed only to HPV. On the other hand, co-exposure to HPV18 and SeMet increased apoptosis in the second type of cells associated with the trophoblast (inner cell mass), showing that the effect of SeMet on HPV-exposed cells depends on the specific cell type. 19
Apart from methylseleninic acid, there are few other synthetic organoselenium compounds studied, including bis(3,5-bis(trifluoromethyl)phenyl)selane, synthesized as an example of an organometallic candidate for cervical cancer chemotherapeutic drugs. 20 HeLa cells exposed to this compound at 10-50 µM were shown to undergo dose-dependent changes typical for late apoptosis, including nuclear condensation with blebbing and nuclei fragmentation. Yadav and Singh investigated the anticancer properties of different organometallic ruthenium complexes with Se and compared them to those of organometallic ruthenium complexes with sulfur. 21 Within the studied complexes, the complex containing Se (C31H35O4SeRuCl) seemed to inhibit the proliferation of HeLa cells most efficiently. Sucrose selenium ester, synthesized from sucrose and selenium oxychloride, was shown to inhibit proliferation of HeLa cells as well as three other cancer cell lines (bladder carcinoma cell line 5637, human malignant melanoma cell line A375, and gastric carcinoma cell line MGC-803). 22 The inhibitory effect was dose-dependent and associated with the induction of apoptosis. Importantly sucrose-Se was not toxic in the normal human liver cell line HL-7702. Furthermore, in the in vivo experiment, sucrose selenium ester was shown to increase survival time in mice injected with HeLa cells. 22
Se Nanoparticles
Special interest in the therapeutic potential of Se in cancer has been paid in recent years to Se nanoparticles (Se-NPs). Nanoparticles (particles with at least one dimension less than 100 nm) are generally attractive because of their unique biomedical features, allowing them to function as very effective drug carriers. Thus combining nanotechnology with the anticancer effects of Se offers very promising possibilities for cancer treatment. 40 Most importantly, Se-NPs have been shown to exert lower toxicity in vivo as compared to SeMet and Se-methylselenocysteine 41,42 and to be less toxic to normal cells than selenium dioxide (SeO2). 43 They also seem to possess the same or even stronger biological activity in terms of increasing the activity of antioxidant enzymes as compared to other Se compounds. 41,42,44,45
Several in vitro studies in HeLa cells have been conducted to assess the effect of Se-NPs. Luo et al observed dose-dependent inhibition of HeLa upon Se-NPs, which was associated with S phase cell cycle arrest. 23 A dose-dependent cytotoxic effect in HeLa cells was also observed by Srivastava et al who treated cells with Se-NPs obtained from marine bacterium (Idiomarina sp.) PR58-8. 24 Cytotoxic effects were accompanied by an increase in reactive oxygen species and apoptotic index, resulting from caspase-dependent apoptosis. Notably, bacterial SeNPs were not toxic to normal HaCat cells. 24
Different modifications of Se-NPs by adding additional factors to their structure improved their therapeutic utility specifically in cervical cancer treatment. For example, Zheng et al added sialic acid (SA) to Se-NPs to enhance their cancer-targeting and cell-penetrating abilities. The combination with SA significantly increased the cellular uptake of Se-NPs in HeLa cells as compared to SeNPs alone. 25 Apart from dose-dependent cytotoxicity in HeLa cells, Se-NPs decorated with SA (SA-Se-NPs) were shown to be non-toxic in normal human kidney HK-2 cells. The mechanism underlying the cytotoxic effect of SA-Se-NPs in HeLa was apoptosis induced by activation of caspase-3 and subsequent cleavage of PARP. 25
Se-NPs have also been shown to be effective photothermal therapy (PTT) agents. PTT agents are factors that induce high temperature in the cell upon irradiation and consequently lead to cell death. The combination of PTT with molybdenum diselenide (MoSe2) nanodots was shown to exert cytotoxic effects in HeLa cells, suggesting that MoSe2 nanodots are a novel effective PTT agent. This study also shows a broad spectrum of general anticancer potential in the case of Se-NPs, which results not only from its biological activity but also from its physical properties. 26
Nevertheless, the most promising property of Se-NPs relates to their drug-carrying ability. Effective combinations of Se-NPs with drugs in cervical cancer treatment were shown in HeLa cells and a xenograft mouse model. For example, paclitaxel (PTX)-loaded Se-NPs induced apoptosis in HeLa cells (but also in various other cancer cell lines). The authors indicated that the combination of Se-NPs with the drug led to G2/M cell cycle arrest, perturbation of mitochondrial membrane potential, increased production of reactive oxygen species, and activation of effector caspases. 27 More complex Se-NP-based drug delivery systems include additional decoration, for example, with folic acid (FA) or hyaluronic acid (HA). These molecules were added to the system in order to enhance the tumor-targeting ability of the drug since tumor cells were shown to express high levels of cell surface receptors for FA and HA. 46,47 Se-NPs modified with FA increased, for example, the anticancer effects of doxorubicin (DOX) in HeLa cells. 28 Xia et al tested the antitumor activity of HA-Se@DOX - DOX-loaded Se-NPs decorated with HA. HA-Se@DOX was shown in this study to exert very good cytotoxic effects both in vitro and in vivo. HeLa cell proliferation was inhibited much more effectively by HA-Se@DOX than by DOX alone or Se@DOX. Moreover, HA-Se@DOX was shown to suppress tumor growth and induce apoptosis of HeLa cancer cells in vivo in a xenograft mouse model. The identified mechanisms of apoptosis induced by HA-Se@DOX included the Bcl-2 signaling pathway (as shown in vitro) and activation of the caspase-3 protein (as shown in vivo). 29 Interesting results were obtained by Li et al, 30 who used bismuth selenide (Bi2Se3) nanoparticles combined with human serum albumin (HSA) and doxorubicin. This novel compound, Bi2Se3@PDA/DOX/HSA, induced apoptosis in HeLa cells and was more cytotoxic than DOX alone. Furthermore, when the treatment was combined with irradiation, a superior synergistic effect was observed. Thermochemotherapy was much more effective in destroying HeLa cells than the combination of irradiation with DOX alone or NPs without DOX. The combination of Bi2Se3@PDA/DOX/HSA with irradiation was also the most effective treatment in Balb/C female mice (which developed tumors induced by HeLa cell injection) compared to other tested therapies, including laser alone, laser + DOX, Bi2Se3 + laser, or Bi2Se3@PDA/DOX/HSA alone. 30
Apart from drugs, nanoparticles have been shown to be good delivery platforms for small interfering RNAs (siRNAs). These are sequence-specific molecules with the ability to silence genes, which are very promising candidates for novel anticancer drugs. Using siRNA technology for therapeutic purposes is particularly attractive due to the ability to target specific genes and have less toxic effects than traditional chemotherapy. 48 Combining siRNA with nanotechnology is expected to become the gold standard in cancer therapy in the near future. An example of using Se-NPs combined with siRNA in cervical cancer treatment was presented recently by Xia et al. 31 In their study a tumor-targeting gene delivery vehicle, RGDfC-Se@siRNA was constructed. For this purpose, Se-NPs were loaded with RGDfC peptide and Derlin1-siRNA. RGDfC peptide (arginylglycylaspartic acid peptide) was used here to enhance the tumor-targeting ability of the compound since it binds to membrane protein, integrin alpha V beta 3, shown to be overexpressed in cancer cells. Similarly, the product of the siRNA target gene, Derlin1, is a protein that is overexpressed in cancer. This combination resulted in a specific cellular uptake of the compound in HeLa and effective downregulation of Derlin1 at both the mRNA and protein levels. Furthermore, RGDfC-Se@siRNA suppressed the invasion, migration, and proliferation of HeLa cells by inducing apoptosis, probably by ROS-mediated mitochondrial dysfunction. In vivo effect assessment showed that RGDfC-Se@siRNA decreased tumor volume in a HeLa tumor-bearing mouse model and was at the same time not toxic to the major animal organs. 31
Mushrooms and Plants Rich in Selenium
Ji et al analyzed the in vivo activity of a combination of two potential anticancer factors: selenium and Cordyceps Sinensis (CS). 33 CS are mushrooms used in traditional Chinese medicine and have been shown to exert anticancer effects in vitro. 49 Using a mouse model of MCA-induced uterine cervical cancer, the authors observed that treatment of animals with sodium selenite-enriched mushrooms (Se-CS) decreased the prevalence of the disease by 40%. This effect was accompanied by alleviation of the thymus and spleen decrease induced by MCA, which may suggest that Se-CS act by modulating immune function. Se-CS was also shown to improve the antioxidant defense system in MCA-treated mice. 33
Zhang et al investigated the effect of seeds from the herb Myristica fragrans Houtt, which they found to be a rich source of Se (Se was most abundant among seven different elements that were analyzed by the authors, including zinc, magnesium, iron, calcium, manganese, lead, and Se content in this herb was found to be 35 µg/g of mace). 34 Supplementation with an aqueous suspension of pulverized mace from the herb reduced the incidence of cervical cancer in mice exposed to MCA from 100% to 10%. The observed protective effect could have been attributed to trace elements in mace, including Se, but also to other unknown bioactive compounds.
On the other hand, Se fortification had no effect on the cytotoxic activity of kale and kohlrabi sprouts in SiHa cells as similar cytotoxic effect was observed in both fortified and unfortified sprouts. 14
Human Studies on Se and Cervical Cancer—Randomized Controlled Trials
As far as we are concerned, no clinical trials on Se treatment efficacy in cervical cancer have been conducted so far. Considering that some mechanisms of Se (such as the antiviral activity) may overlap both in treatment and prevention, it is important to examine the outcomes of supplementation trials conducted for preventive purposes (Table 3). Such a chemopreventive potential of Se was suggested by observational retrospective studies showing decreased Se status in cervical cancer patients or subjects infected with HPV as compared to healthy controls. 3,9 -11 Although these studies do not provide evidence on the causative relationship between low Se status and increased risk of cervical cancer, and the only observational cohort study of relevant interest did not indicate any association between Se status and cervical cancer risk, 8 some promising data were revealed in the supplementation trial conducted by Karamali et al. 36 This randomized, double-blind placebo-controlled trial aimed to assess the possible preventive activity of Se against the progression of cervical intraepithelial neoplasia 1 (CIN1), which is a premalignant lesion, usually associated with HPV infection. 50 In this study, 26 women with CIN1 were supplemented with 200 µg for six months and notably, Se supplementation was associated with higher CIN1 regression (88% vs. 56% in the placebo group, P = 0.01). Additionally, Se was also shown to exert beneficial antioxidant and metabolic effects in the supplemented group. 36
Little attention has been paid to the effects of Se supplementation on clinical outcomes in cervical cancer patients (Table 3). Muecke et al seemed to be the only authors who investigated this issue. 37,51,52 They conducted a study of 81 patients with cervical cancer (n = 11) and uterine cancer (n = 70) described as Se deficient (blood Se <84 µg/L) who were randomly assigned to receive either Se or placebo during radiotherapy. The subjects were supplemented with Se at a dose of 500 µg on the days of radiotherapy and 300 µg on the days without radiotherapy. Adjuvant Se supplementation increased Se status in patients and was associated with a lower incidence of radiation-induced diarrhea. 51,52 However, Se supplementation had no effect on disease-free survival and overall survival in this group. 37 It should be noted that the majority of patients in the above study were diagnosed with uterine cancer (endometrial cancer), which, unlike cervical cancer, appears not to be associated with HPV infection. 53 The important issue to be taken into account in terms of analyzing the Se effects in humans, especially when comparing different studies, is the chemical form of the used supplement. In the study by Karamali et al, Se yeast was used, 36 whereas Muecke et al used sodium selenite. 51 This fact could have an impact on the final outcome of the study because different Se compounds generate different biological effects. 54,55
Summary, Concluding Remarks, and Research Gaps
Experimental studies conducted in cells and mouse models of cervical cancer indicate that different Se compounds exert anticancer effects in cervical cancer induced by HPV or chemical carcinogen. Of all the tested Se compounds, the most promising anticancer effects in cervical cancer seem to be associated with Se-NPs, which when combined with antineoplastic drugs and factors enhancing their tumor-targeting ability, were highly selective towards cancer cells and effective at relatively low doses. An additional combination of Se-NPs with thermal therapy or with siRNA technology further expanded the therapeutic possibilities of Se in cervical cancer. The most recently investigated combinations of Se-NP platforms with immunotherapy based on the ability of Se-NPs to deliver therapeutic mRNAs have not been tested in cervical cancer yet and await evaluation in this type of malignancy. 56 -58 Importantly, future studies should be conducted on different cervical cancer cell lines since most of the existing evidence is concerned only with HeLa cells. Most importantly, Se-NP drug delivery platforms were shown to be non-toxic to normal cells or tissues. This is a critical issue because depending on its dose and chemical form, Se can be extremely toxic, 35 as clearly indicated in humans by cases of intoxication from misformulated dietary supplements. 59 Moreover, long-term Se exposure at dose 200 µg (considered to be non-toxic) may be associated with adverse health effects in humans, including diabetes 60,61 and cancer. 62 Altogether, these facts should be taken into account when using Se for therapeutic purposes.
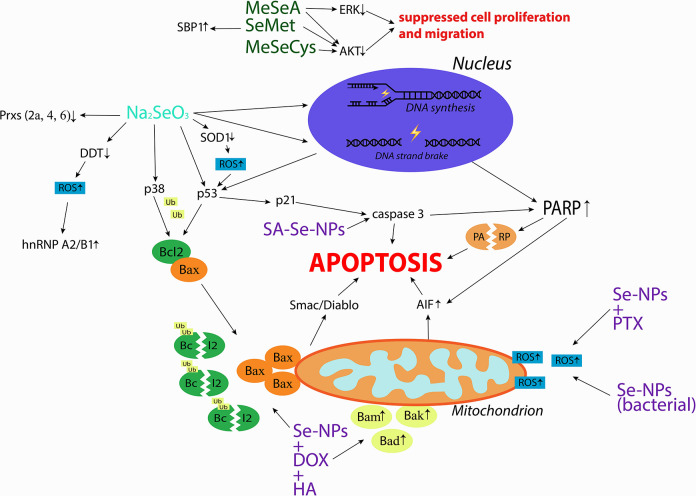
Laboratory evidence on specific mechanisms of action of Se in cervical cancer is scarce (Figure 1). According to the in vitro observations, Se compounds (both organic and inorganic compounds, as well as Se-NPs), were able in most cases to induce oxidative stress and apoptosis, both of which are considered to underlie antitumor effects of Se in general. 63 Considering that HPV infection is a major cause of cervical cancer in humans, it is urgent to investigate the mechanisms specifically related to molecular changes in this type of infection. These changes are linked to HPV oncoproteins, mainly E6 and E7, whose increased expression leads to inhibition of tumor suppressor proteins, p53 and pRb (retinoblastoma protein), and alteration of multiple signaling pathways involved in cell proliferation and differentiation. Thus, studies on Se action in cervical cancer should focus on pathways related to Wnt/beta-catenin, PI3K/AKT, ERK/MAPK, JAK/STAT, AP-1 and NF-kB signaling, as well as epithelial-mesenchymal transition (EMT) pathway, 64 Similarly, it is of interest to investigate Se effects on factors directly interacting with HPV like YY1 (Yin Yang 1) transcription factor, 65 or on other markers involved in cervical cancer development and progression (like specific miRNAs, receptors or proteins). 66 Such mechanistic studies are warranted also because of substantial research gaps in the effects of Se on DNA viruses as studies which have been conducted so far, concerned mainly with the impact of Se on RNA viruses. 67
Figure 1.
Mechanisms of action investigated in studies on Se compounds effects in cervical cancer cells. Organic Se compounds are indicated in green, inorganic Se compound is indicated in blue, Se nanoparticles are indicated in purple. AIF, Apoptosis Inducing Factor; AKT, Protein Kinase B; Bad, Bcl-2 Associated Agonist of Cell Death; Bak, Bcl-2 Homologous Antagonist/killer; Bax, Bcl-2-like protein 4; Bcl-2, B-cell lymphoma 2; DDT, D-dopachrome Tautomerase; DOX, Doxorubicin; ERK, Extracellular Regulated Kinase; HA, Hyaluronic Acid; hnRNP A2/B1, Heterogeneous Nuclear Ribonucleoprotein A2/B1; MeSeA, Methylseleninic Acid; MeSeCys, Methylselenocysteine; PARP, Poly (ADP-ribose) Polymerase; Prx2a, Peroxiredoxin 2a; Prx4, Peroxiredoxin 4; Prx6, Peroxiredoxin 6; PTX, Paclitaxel; ROS, Reactive Oxygen Species; SA, Sialic Acid; SBP1, Selenium Binding Protein 1; SeMet, Selenomethione; SE-NPs, Selenium Nanoparticles; SOD1, Superoxide Dismutase 1; Ub, Ubiquitin. 13,17,20-21,34-35,37, 41.
Analysis of human cervical cancer tissues as well as human cervical cancer cell lines (all with HPV) indicated the downregulation of selenium-dependent glutathione peroxidase 3 gene, which may further suggest possible role of selenoproteins in cervical cancer and HPV infection. 68 This issue has not been discussed in this review because most studies concerning Se and cervical cancer have focused on Se compounds in terms of their cytotoxic properties. Nevertheless, the activity and functions of selenoproteins are also considered to be responsible for the anticancer effect of Se. 6 Hence, more interest should be gained in the role of selenoproteins in cervical cancer.
Overall, the existing experimental evidence on possible therapeutic potential of Se in cervical cancer is limited. However, because the available data is promising, it should be further explored, especially with respect to the specific mechanisms of action exerted by this element in HPV infection. A more in-depth investigation of the antiviral activity of Se is also of high interest in terms of its possible chemopreventive effect in cervical cancer, a topic that was not addressed in this review. The fact that cervical cancer is a preventable malignancy makes it important to identify potential nutritional chemopreventive strategies to reduce its incidence and mortality rates. In this context, the hypothesis that high or adequate Se status may decrease the risk of cervical cancer by protecting against HPV infection is very attractive. However, it currently lacks solid scientific evidence. Epidemiological studies indicate a decreased Se status in cervical cancer patients, but the causative nature of this relationship is still not clear. Therefore, more data from human studies (prospective studies and supplementation trials) are needed to verify the hypothesis that higher Se status may prevent HPV infection and/or the development of cervical cancer. Certainly, the role of Se in cervical cancer treatment (and prevention) requires further research addressing the issues discussed in this review It would also be important in the light of the scientific discussion about the general role of Se in cancer and the many still unresolved issues in this area. 69
Acknowledgment
The authors would like to thank Nataliia Pavlenko for graphical support in preparing this manuscript.
Authors' Note: Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Nofer Institute of Occupational Medicine—grant number IMP 14.5/2020; Postdoctoral Scientific Development Fund of Heilongjiang Province—grant number LBH-Q17096; China Postdoctoral Science Foundation—grant number 2015M571440; National Natural Science Foundation of China—grant number 81773368.
ORCID iD: Ewa Jablonska, PhD  https://orcid.org/0000-0002-3946-3964
https://orcid.org/0000-0002-3946-3964
Edyta Reszka, PhD  https://orcid.org/0000-0003-2153-4864
https://orcid.org/0000-0003-2153-4864
Kateryna Tarhonska, MSc  https://orcid.org/0000-0003-2940-0449
https://orcid.org/0000-0003-2940-0449
References
- 1. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi:10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 2. Haverkos HW. Multifactorial etiology of cervical cancer: a hypothesis. MedGenMed. 2005;7(4):57. [PMC free article] [PubMed] [Google Scholar]
- 3. He D, Wang Z, Huang C, Fang X, Chen D. Serum selenium levels and cervical cancer: systematic review and meta-analysis. Biol Trace Elem Res. 2017;179(2):195–202. doi:10.1007/s12011-017-0982-6 [DOI] [PubMed] [Google Scholar]
- 4. Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739–777. doi:10.1152/physrev.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. doi:10.3390/nu11092101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends Bioch Sci. 2014;39(3):112–120. doi:10.1016/j.tibs.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinceti M, Filippini T, Wise LA. Environmental selenium and human health: an update. Curr Environ Health Rep. 2018;5(4):464–485. doi:10.1007/s40572-018-0213-0 [DOI] [PubMed] [Google Scholar]
- 8. Batieha AM, Armenian HK, Norkus EP, Morris JS, Spate VE, Comstock GW. Serum micronutrients and the subsequent risk of cervical cancer in a population-based nested case-control study. Cancer Epidemiol Biomarkers Prev. 1993;2(4):335–339. [PubMed] [Google Scholar]
- 9. Okunade KS, Dawodu OO, Salako O, Osanyin GE, Okunowo AA, Anorlu RI. Comparative analysis of serum trace element levels in women with invasive cervical cancer in Lagos, Nigeria. Pan Afr Med J. 2018;31:194. doi:10.11604/pamj.2018.31.194.14425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abulizi G, Zhang YY, Mijiti P, et al. Serum Se, Ni, and As are associated with HPV infection and CIN2+ among Uyghur women in rural China. BMC Cancer. 2018;18(1):925. doi:10.1186/s12885-018-4734-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Obhielo E, Ezeanochie M, Olokor OO, Okonkwo A, Gharoro E. The Relationship between the serum level of selenium and cervical intraepithelial neoplasia: a comparative study in a population of Nigerian women. Asian Pac J Cancer Prev: APJCP. 2019;20(5):1433–1436. doi:10.31557/APJCP.2019.20.5.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lucey BP, Nelson-Rees WA, Hutchins GM. Henrietta Lacks, HeLa cells, and cell culture contamination. Arch Pathol Lab Med. 2009;133(9):1463–1467. doi:10.1043/1543-2165-133.9.1463 [DOI] [PubMed] [Google Scholar]
- 13. Rudolf E, Rudolf K, Cervinka M. Selenium activates p53 and p38 pathways and induces caspase-independent cell death in cervical cancer cells. Cell Biology Toxicol. 2008;24(2):123–141. doi:10.1007/s10565-007-9022-1 [DOI] [PubMed] [Google Scholar]
- 14. Zagrodzki P, Pasko P, Galanty A, et al. Does selenium fortification of kale and kohlrabi sprouts change significantly their biochemical and cytotoxic properties? J Trace Elem Med Biol. 2020;59:126466. doi:10.1016/j.jtemb.2020.126466 [DOI] [PubMed] [Google Scholar]
- 15. Ji J, Liu J, Liu H, Wang Y. Comparison of serum and tissue levels of trace elements in different models of cervical cancer. Biol Trace Elem Res. 2014;159(1-3):346–350. doi:10.1007/s12011-014-9981-z [DOI] [PubMed] [Google Scholar]
- 16. Fu L, Liu Q, Shen L, Wang Y. Proteomic study on sodium selenite-induced apoptosis of human cervical cancer HeLa cells. J Trace Elem Med Biol. 2011;25(3):130–137. doi:10.1016/j.jtemb.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 17. Sun L, Zhang J, Yang Q, et al. Synergistic effects of SAM and selenium compounds on proliferation, migration and adhesion of HeLa cells. Anticancer Res. 2017;37(8):4433–4441. doi:10.21873/anticanres.11838 [DOI] [PubMed] [Google Scholar]
- 18. Zhao C, Zeng H, Wu RT, Cheng WH. Loss of selenium-binding protein 1 decreases sensitivity to clastogens and intracellular selenium content in HeLa cells. PLoS One. 2016;11(7):e01586 50. doi:10.1371/journal.pone.0158650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tolen JA, Duerksen-Hughes P, Lau K, Chan PJ. Selenium attenuates HPV-18 associated apoptosis in embryo-derived trophoblastic cells but not inner cell mass in vitro. Int J Reprod Med. 2015;2015:562567. doi:10.1155/2015/562567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizvi MA, Zaki M, Afzal M, et al. Nuclear blebbing of biologically active organoselenium compound towards human cervical cancer cell (HeLa): in vitro DNA/HSA binding, cleavage and cell imaging studies. Eur J Med Chem. 2015;90:876–888. doi:10.1016/j.ejmech.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 21. Yadav S, Singh JD. Synthesis and preliminary biological evaluation for the anticancer activity of organochalcogen (S/se) tethered chrysin-based organometallic Ru(II)(eta(6)-p-cymene) complexes. J Biomol Struct Dyn. 2019;37(13):3337–3353. doi:10.1080/07391102.2018.1513867 [DOI] [PubMed] [Google Scholar]
- 22. Guo P, Zhao P, Liu J, et al. Preparation of a novel organoselenium compound and its anticancer effects on cervical cancer cell line HeLa. Biol Trace Elem Res. 2013;151(2):301–306. doi:10.1007/s12011-012-9563-x [DOI] [PubMed] [Google Scholar]
- 23. Luo H, Wang F, Bai Y, Chen T, Zheng W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf B Biointerfaces. 2012;94:304–308. doi:10.1016/j.colsurfb.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 24. Srivastava P, Kowshik M. Anti-neoplastic selenium nanoparticles from idiomarina sp. PR58-8. Enzyme Microb Technol. 2016;95:192–200. doi:10.1016/j.enzmictec.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 25. Zheng JS, Zheng SY, Zhang YB, et al. Sialic acid surface decoration enhances cellular uptake and apoptosis-inducing activity of selenium nanoparticles. Colloids Surf B Biointerfaces. 2011;83(1):183–187. doi:10.1016/j.colsurfb.2010.11.023 [DOI] [PubMed] [Google Scholar]
- 26. Yuwen L, Zhou J, Zhang Y, et al. Aqueous phase preparation of ultrasmall MoSe2 nanodots for efficient photothermal therapy of cancer cells. Nanoscale. 2016;8(5):2720–2726. doi:10.1039/c5nr08166a [DOI] [PubMed] [Google Scholar]
- 27. Bidkar AP, Sanpui P, Ghosh SS. Efficient induction of apoptosis in cancer cells by paclitaxel-loaded selenium nanoparticles. Nanomedicine (Lond). 2017;12(21):2641–2651. doi:10.2217/nnm-2017-0189 [DOI] [PubMed] [Google Scholar]
- 28. Xia Y, Xu T, Zhao M, et al. Delivery of doxorubicin for human cervical carcinoma targeting therapy by folic acid-modified selenium nanoparticles. Int J Mol Sci. 2018;19(11):3582. doi:10.3390/ijms19113582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xia Y, Xiao M, Zhao M, et al. Doxorubicin-loaded functionalized selenium nanoparticles for enhanced antitumor efficacy in cervical carcinoma therapy. Mater Sci Eng C Mater Biol Appl. 2020;106:110100. doi:10.1016/j.msec.2019.110100 [DOI] [PubMed] [Google Scholar]
- 30. Li Z, Hu Y, Howard KA, et al. Multifunctional bismuth selenide nanocomposites for antitumor thermo-chemotherapy and imaging. ACS Nano. 2016;10(1):984–997. doi:10.1021/acsnano.5b06259 [DOI] [PubMed] [Google Scholar]
- 31. Xia Y, Tang G, Wang C, et al. Functionalized selenium nanoparticles for targeted siRNA delivery silence Derlin1 and promote antitumor efficacy against cervical cancer. Drug Deliv. 2020;27(1):15–25. doi:10.1080/10717544.2019.1667452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hussain SP, Rao AR. Chemopreventive action of selenium on methylcholanthrene-induced carcinogenesis in the uterine cervix of mouse. Oncology. 1992;49(3):237–240. [DOI] [PubMed] [Google Scholar]
- 33. Ji J, Liu J, Liu H, Wang Y. Effects of fermented mushroom of cordyceps sinensis, rich in selenium, on uterine cervix cancer. Evid Based Complement Alternat Med. 2014;2014:173180. doi:10.1155/2014/173180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang C, Qi X, Shi Y, et al. Estimation of trace elements in mace (Myristica fragrans Houtt) and their effect on uterine cervix cancer induced by methylcholanthrene. Biol Trace Elem Res. 2012;149(3):431–434. doi:10.1007/s12011-012-9443-4 [DOI] [PubMed] [Google Scholar]
- 35. Misra S, Boylan M, Selvam A, Spallholz JE, Bjornstedt M. Redox-active selenium compounds-from toxicity and cell death to cancer treatment. Nutrients. 2015;7(5):3536–3556. doi:10.3390/nu7053536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karamali M, Nourgostar S, Zamani A, Vahedpoor Z, Asemi Z. The favourable effects of long-term selenium supplementation on regression of cervical tissues and metabolic profiles of patients with cervical intraepithelial neoplasia: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2015;114(12):2039–2045. doi:10.1017/S0007114515003852 [DOI] [PubMed] [Google Scholar]
- 37. Muecke R, Micke O, Schomburg L, et al. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology: follow-up analysis of the survival data 6 years after cessation of randomization. Integr Cancer Ther. 2014;13(6):463–467. doi:10.1177/1534735414541963 [DOI] [PubMed] [Google Scholar]
- 38. Gandin V, Khalkar P, Braude J, Fernandes AP. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radical Bio Med. 2018;127:80–97. doi:10.1016/j.freeradbiomed.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 39. Yang W, Diamond AM. Selenium-binding protein 1 as a tumor suppressor and a prognostic indicator of clinical outcome. Biomarker Res. 2013;1(1):15. doi:10.1186/2050-7771-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi:10.1016/j.biopha.2018.12.146 [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Wang X, Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci. 2008;101(1):22–31. doi:10.1093/toxsci/kfm221 [DOI] [PubMed] [Google Scholar]
- 42. Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 2007;42(10):1524–1533. doi:10.1016/j.freeradbiomed.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 43. Chen T, Wong YS, Zheng W, Bai Y, Huang L. Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf B Biointerfaces. 2008;67(1):26–31. doi:10.1016/j.colsurfb.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 44. Shi LG, Xun WJ, Yue WB, et al. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Ruminant Res. 2011;96(1):49–52. doi:10.1016/j.smallrumres.2010.11.005 [Google Scholar]
- 45. Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N. Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci. 2015;178:330–336. doi:10.1016/j.livsci.2015.05.004 [Google Scholar]
- 46. Liu CL, Ding L, Bai LX, et al. Folate receptor alpha is associated with cervical carcinogenesis and regulates cervical cancer cells growth by activating ERK1/2/c-Fos/c-Jun. Biochem Biophys Res Commun. 2017;491(4):1083–1091. doi:10.1016/j.bbrc.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 47. Mattheolabakis G, Milane L, Singh A, Amiji MM. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target. 2015;23(7-8):605–618. doi:10.3109/1061186x.2015.1052072 [DOI] [PubMed] [Google Scholar]
- 48. Mahmoodi Chalbatani G, Dana H, Gharagouzloo E, et al. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomedicine. 2019;14:3111–3128. doi:10.2147/IJN.S200253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qi W, Zhou X, Wang J, et al. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr Polym. 2020;237:116113. doi:10.1016/j.carbpol.2020.116113 [DOI] [PubMed] [Google Scholar]
- 50. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–2359. doi:10.1002/ijc.27485 [DOI] [PubMed] [Google Scholar]
- 51. Muecke R, Schomburg L, Glatzel M, et al. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int J Radiat Oncol Biol Phys. 2010;78(3):828–835. doi:10.1016/j.ijrobp.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 52. Muecke R, Micke O, Schomburg L, et al. Impact of treatment planning target volumen (PTV) size on radiation induced diarrhoea following selenium supplementation in gynecologic radiation oncology--a subgroup analysis of a multicenter, phase III trial. Radiat Oncol. 2013;8:72. doi:10.1186/1748-717X-8-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Olesen TB, Svahn MF, Faber MT, et al. Prevalence of human papillomavirus in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol. 2014;134(1):206–215. doi:10.1016/j.ygyno.2014.02.040 [DOI] [PubMed] [Google Scholar]
- 54. Hoefig CS, Renko K, Kohrle J, Birringer M, Schomburg L. Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J Nutr Biochem. 2011;22(10):945–955. doi:10.1016/j.jnutbio.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 55. Valdiglesias V, Pasaro E, Mendez J, Laffon B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: a review. Arch Toxicol. 2010;84(5):337–351. doi:10.1007/s00204-009-0505-0 [DOI] [PubMed] [Google Scholar]
- 56. Maiyo F, Singh M. Folate-targeted mRNA delivery using chitosan-functionalized selenium nanoparticles: potential in cancer immunotherapy. Pharmaceuticals (Basel). 2019;12(4):164. doi:10.3390/ph12040164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu T, Xu L, He L, et al. Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nano Today. 2020;35:100975. doi:10.1016/j.nantod.2020.100975 [Google Scholar]
- 58. Gao S, Li T, Guo Y, Sun C, Xianyu B, Xu H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv Mater. 2020;32(12):e1907568. doi:10.1002/adma.201907568 [DOI] [PubMed] [Google Scholar]
- 59. MacFarquhar JK, Broussard DL, Melstrom P, et al. Acute selenium toxicity associated with a dietary supplement. Arch Intern Med. 2010;170(3):256–261. doi:170/3/256 [pii]10.1001/archinternmed.2009.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vinceti M, Filippini T, Rothman KJ. Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Epidemiol. 2018;33(9):789–810. doi:10.1007/s10654-018-0422-8 [DOI] [PubMed] [Google Scholar]
- 61. Wang QQ, Yu SC, Xu CD, et al. Association between selenium in soil and diabetes in Chinese residents aged 35-74 years: results from the 2010 national survey of chronic diseases and behavioral risk factors surveillance. Biomed Environ Sci. 2020;33(4):260–268. doi:10.3967/bes2020.035 [DOI] [PubMed] [Google Scholar]
- 62. Kristal AR, Darke AK, Morris JS, et al. Baseline selenium status and effects of selenium and vitamin E supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106(3): djt456. doi:10.1093/jnci/djt456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wallenberg M, Misra S, Bjornstedt M. Selenium cytotoxicity in cancer. Basic Clin Pharmacol Toxicol. 2014;114(5):377–386. doi:10.1111/bcpt.12207 [DOI] [PubMed] [Google Scholar]
- 64. Gupta S, Kumar P, Das BC. HPV: molecular pathways and targets. Curr Probl Cancer. 2018;42(2):161–174. doi:10.1016/j.currproblcancer.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 65. Wang W, Yue Z, Tian Z, et al. Expression of Yin Yang 1 in cervical cancer and its correlation with E-cadherin expression and HPV16 E6. PLoS One. 2018;13(2):e0193340. doi:10.1371/journal.pone.0193340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kori M, Yalcin Arga K. Potential biomarkers and therapeutic targets in cervical cancer: Insights from the meta-analysis of transcriptomics data within network biomedicine perspective. PLoS One. 2018;13(7):e02007 17. doi:10.1371/journal.pone.0200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Steinbrenner H, Al-Quraishy S, Dkhil MA, Wunderlich F, Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv Nutr. 2015;6(1):73–82. doi:10.3945/an.114.007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang X, Zheng Z, Yingji S, et al. Downregulation of glutathione peroxidase 3 is associated with lymph node metastasis and prognosis in cervical cancer. Oncol Rep. 2014;31(6):2587–2592. doi:10.3892/or.2014.3152 [DOI] [PubMed] [Google Scholar]
- 69. Jablonska E, Vinceti M. Selenium and human health: witnessing a Copernican revolution? J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33(3):328–368. doi:10.1080/10590501.2015.1055163 [DOI] [PubMed] [Google Scholar]