Abstract
Alcohol abuse increases vulnerability to infections and infection-related mortality. In previous studies, we found that acute alcohol abuse in a binge-drinking model in mice decreased resistance to bacterial sepsis when alcohol was administered near the time of bacterial challenge. In the present study, we investigated the effects of alcohol administered later in the course of sepsis (18 hr after injection of Escherichia coli). Our working hypothesis was that decreased production of cytokines caused by alcohol at this time would actually improve survival, because overproduction of pro-inflammatory mediators is thought to be the proximate cause of mortality in sepsis. Unexpectedly, administration of alcohol late in the course of sepsis led to a rapid increase in the number of viable bacteria in the peritoneal cavity. Significant increases in the concentrations of several cytokines and chemokines coincided with the increased number of bacteria in alcohol-treated mice and decreased survival time. These results demonstrated our working hypothesis to be incorrect, and reiterated the complexity of sepsis. Hypothermia is a consistent feature in this model of sepsis, and in control mice (E. coli only), body temperature was near normal by 18 hr or 21 hr after administration of E. coli, but in mice treated with alcohol 18 hr after E. coli, hypothermia was significant 3 hr later and ultimately mortality was significantly increased. However, counteracting the hypothermic effect of alcohol by external warming of mice led to earlier mortality, demonstrating that hypothermia was not the major cause of mortality. These results along with previous results from studies in which alcohol was given before initiation of sepsis suggest that decreased cytokine and chemokine production may not be the key effect of alcohol that decreases resistance to sepsis. It seems more likely that suppression of mechanisms by which macrophages and neutrophils kill bacteria is critical, and this can occur even in the presence of high levels of cytokines and chemokines.
Introduction
Sepsis is a systemic microbial infection that induces a severe and sometimes life threatening systemic inflammatory response. Pathogens activate innate immunity and inflammation, which generally function to clear the host of infectious agents. However, under some circumstances, a disseminated infection can produce an exaggerated inflammatory response that can lead to multiple organ failure, shock, and death (Vincent and De Backer, 2013). Recent results indicate that the mortality rates in patients with septic shock are still nearly 50% (Chawla and DeMuro, 2014). Sepsis is the 10th leading cause of death in the U.S. (Melamed and Sorvillo, 2009), and mortality rates have not improved substantially, in spite of many years of research and numerous clinical trials (Iskander et al., 2013).
Binge drinking and a measurable blood alcohol concentration at the time of burn injury are associated with an increased risk of a poor outcome from sepsis, which often occurs after such injuries (Choudhry et al., 2004). Several studies have shown that both acute and chronic alcohol consumption suppress innate immune system functions and increase susceptibility to infection (Bagby et al., 2006, Brown et al., 2006, Gluckman and MacGregor, 1978, Szabo et al., 1999). Acute alcohol consumption has been reported by several research groups to contribute to sepsis-associated mortality in patients and animal models, with suppression of innate immunity and protective inflammatory responses (Huttunen et al., 2007, McGill et al., 1995, Nelson and Kolls, 2002). Suppressed activation of the innate immune system (Goral et al., 2008) via inhibition of toll-like receptor (TLR) function (Dai et al., 2005, Dolganiuc et al., 2006, Goral and Kovacs, 2005, Pruett et al., 2004, Szabo et al., 2007) as well as suppressed chemokine (Happel et al., 2007) and cytokine production (Mason et al., 2000, Nelson et al., 1989, Pruett et al., 2003, Szabo et al., 1999) have been reported following acute alcohol consumption in rodent models. However, the intricacy and redundancy of pro-inflammatory cytokine functions has not allowed complete elucidation of the mechanism of increased susceptibility to sepsis and increased mortality in sepsis associated with excessive alcohol consumption.
In rodent models (Bhatty et al., 2011, Pruett et al., 2010a, Pruett et al., 2003, Pruett et al., 2004, Wu and Pruett, 1996) and in humans (Gluckman et al., 1977), acute alcohol exposure decreases early inflammatory responses and bacterial clearance, presumably allowing sepsis to develop. However, mortality in sepsis is generally associated with overproduction of inflammatory mediators leading to shock, blood coagulation, organ failure, and death. Thus, in our previous studies, administration of alcohol shortly before induction of sepsis by intraperitoneal administration of E. coli led to an initial suppression of inflammatory mediators followed by decreased bacterial clearance at 18-24 hr and then by overproduction of pro-inflammatory cytokines and chemokines (Pruett et al., 2010a). The hypothesis tested in the present study was that administration of alcohol 18 hr after E. coli challenge would suppress the excessive (and presumably harmful) production of pro-inflammatory mediators and potentially enhance survival. This was suggested in part by results in which isopropanol decreased mortality in response to a Staphylococcal enterotoxin B (Desy et al., 2008). The results presented here disproved this hypothesis with regard to alcohol and demonstrated that administration of alcohol 18 hr after bacterial challenge leads to rapid regrowth of bacteria and overproduction of pro-inflammatory mediators associated with increased mortality.
In a subpopulation of humans with sepsis and in most mouse models of sepsis, hypothermia is a prominent feature. Humans who exhibit hypothermia, rather than the more common hyperthermia, tend to have worse outcomes (Laupland et al., 2004, Wang et al., 1989). Thus, it was of interest to determine if hypothermia in our mouse model contributed to poor outcome, particularly when alcohol was administered at 18 hr, a time when body temperature normally begins to return toward normal in mice that have only been treated with E. coli and were apparently recovering from sepsis (Tan and Pruett, 2015). In the present study, increased hypothermia was observed in alcohol-treated mice, and it was associated with decreased survival. However, intervening by increasing the ambient temperature and thereby increasing body temperature did not improve, but slightly decreased, survival. Therefore, the results reported here suggest that hypothermia may actually be protective at some stages of the infection, though it remains possible that prolonged hypothermia eventually contributes to mortality.
Methods and Materials
Bacterial growth and culture conditions
A non-pathogenic isolate of E. coli (characterized by the Clinical Microbiology Laboratory, College of Veterinary Medicine, Mississippi State University) obtained from the colon of mice from a specific pathogen-free colony was employed in this study (Pruett et al., 2010a). This bacterial culture was grown in Luria Bertani medium at 37°C and was used in experiments during log growth phase. Bacterial numbers were estimated using a growth curve established by plotting colony forming units (determined by plate counts using serially diluted bacterial samples) vs. optical density at 650 nm.
Animal Model
The mice used for this study were female C57Bl/6 x C3H F1 mice at 8 - 12 weeks of age, obtained from Charles River Labs (Wilmington, MA) through the animal program of National Cancer Institute. After receipt, the mice were allowed to acclimate and recover from shipping stress for two weeks in our laboratory animal facility, which is accredited by the American Association for Accreditation of Laboratory Animal Care. Animal care and use was conducted in accord to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health) and the policies and procedure of Mississippi State University. Mice were housed in filter-top shoebox cages, at five mice per cage. Food (Purina rodent chow) and water was provided ad libitum. The mice were housed on autoclaved bedding and maintained in the animal facility at 70-78°F and with relative humidity of 40-60%. Sentinel mice were housed along with the other mice employed in this study. These sentinel mice were negative for common mouse pathogens during the period of this study.
The sepsis model used in this study was described by Pruett et al (Pruett et al., 2010a). Briefly, viable E. coli were grown as described above and a suspension of 2 x 108 log-phase bacteria in 0.1 ml of phosphate buffered saline was prepared. This suspension was employed for intraperitoneal administration to mice. This model is relevant with regard to sepsis in persons who abuse alcohol. In more than half of cases in alcohol abusers treated for sepsis of abdominal origin, Escherichia coli is the only microbe isolated, even if the initial infection was polymicrobial (Blot et al., 2003, De Waele et al., 2008). In addition, for the purposes described here, our model is preferable to the widely used cecal ligation and puncture method, because the variation within groups with regard to all parameters is much less using the former as compared to the latter. This is especially the case relatively early in the infection (0-21 hr).
The binge drinking model employed in this study was developed by Carson and Pruett (Carson and Pruett, 1996). Briefly, a 32% (v/v) solution of alcohol prepared in tissue culture grade water (Sigma Chemical Co., St. Louis, MO) was administered orally to the mice via an 18-guage stainless steel gavage needle. Each mouse received a dose of 6 g/kg of alcohol which yields a peak blood alcohol concentration of ~0.4% (~87 mM) (Carson and Pruett, 1996). This blood alcohol concentration represents the higher end of the range observed in humans, but it is not particularly rare and has been reported in a number of studies (Jones and Holmgren, 2009, Urso et al., 1981). It should also be noted that mice clear alcohol much more rapidly than humans and that the lethal blood concentration of alcohol in mice is approximately twice that in humans (Baker et al., 1987), so producing biologically equivalent effects of alcohol in mice as in human binge drinkers requires a higher dosage in mice. This is consistent with the effects of many toxicants for which equivalent biological effects are obtained when the compound is administered at equivalent dosages per body surface area rather than per body weight (Travis and White, 1988). The conversion of a body weight to a body surface area-based dosage requires a 10-12 times higher dosage in mice than in humans (based on body weight) to yield biologically equivalent effects for many toxicants in the two species based on body surface area, due to the higher body surface area to weight ratio of mice compared to humans (Travis and White, 1988). Finally, it should be noted that the effects of binge alcohol exposure in this mouse model are typically dose dependent and statistically significant at dosages as low as 3-4 g/kg (which yields a peak blood alcohol concentration of 25-43 mM, which is a common range in human binge drinkers). In fact, cytokine concentrations induced by toll-like receptor ligands and in the E. coli model used here are significantly decreased by these dosages (Pruett and Pruett, 2006, Pruett et al., 2010a, Pruett et al., 2003). However, a higher dosage was used in this study to maximize effects so that treatments that alter these effects could be distinguished statistically from control (E. coli only) and alcohol treated groups. Our binge drinking model has been employed in other studies in our lab (Collier and Pruett, 2000, Collier et al., 2000, Han et al., 1993, Wu and Pruett, 1996, 1997, 1999), and it has been cited more than 100 times by other investigators (Scopus). The control mice in this study were administered water (vehicle control) instead of alcohol along with the E. coli infection dosage.
Survival of the mice was assessed using three different treatment times (relative to the time of E. coli challenge). Accordingly, the mice were divided into four groups, each group consisting of ten mice. One group received vehicle (water) prior to E. coli challenge, the second received alcohol (by gavage) 30 min prior to intraperitoneal E. coli challenge (early treatment group). The other two groups received alcohol either 2 h or 18 h after the E. coli challenge. The animals were observed at least every 6 hr after challenge. Animals found to be moribund were euthanized, and the time of euthanasia was recorded as the time of death. However, most animals with a lethal outcome were found dead, and the time at which death was observed was recorded as the time of death.
Temperature Monitoring
The body temperature of the mice in survival studies was monitored employing an electric thermometer with a rectal probe designed specifically for mice (Physiotemp BAT-12 microprobe thermometer purchased from Physiotemp Instruments Inc., Clifton, NJ). Mice were restrained manually with care. The probe of the thermometer was lubricated with sterile glycerol and inserted into the rectum of the mouse to record the temperature periodically. To study the effect of loss of temperature on the outcome of infection, some cages containing 5 mice each were placed on heating pads (Wetproof model number 4570-200) after alcohol treatment at 18 hr and the heating pads were maintained at ~37°C.
Collection of blood and peritoneal lavage fluid and preparation of cytospin slides
Experiments were designed with a group size of 5 mice. Mice were treated intraperitoneally with E. coli (2 x 108 per mouse). Mice then were given vehicle (water) or alcohol by gavage at 18 hr after E. coli infection. Samples from the mice were collected 3 hr after alcohol administration (21 hr after E. coli challenge). Blood was collected by retro-orbital bleeding under anesthesia induced by inhalation of isoflurane. Prior to peritoneal lavage, mice were anesthetized using isoflurane and euthanized by inhalation of carbon dioxide. One ml of sterile PBS with 10% fetal bovine serum was injected into the peritoneal cavity of the mice and the abdominal area was massaged to distribute the PBS within the peritoneal cavity. Then, the skin was dissected to expose the peritoneal membrane and the peritoneal lavage fluid was withdrawn using a 25-guage needle. In most cases, at least 0.7 ml of the original 1 ml injected was recovered. This initial sample was used to obtain supernatants for cytokine analysis and the cell pellet was saved. Each mouse was given a second injection of 7 ml of PBS with 10% fetal bovine serum in the peritoneal cavity. After massage, the liquid was withdrawn, centrifuged, and the supernatant was discarded. The cell pellet from the 1 ml and 7 ml injections for each mouse were pooled and resuspended in 1 ml of PBS with 10% fetal bovine serum and 20 μl of this cell suspension was used to make cytospin preparations (using a Thermo Cytospin 4 cytocentrifuge). The slides were stained with Wright-Giemsa stain and differential cell counts were done manually at 600 x magnification.
Bacterial number
The bacterial viability was assessed employing the peritoneal fluid samples. Briefly, peritoneal fluid was obtained as noted above (the initial 1 ml sample), serially diluted and plated on LB agar. These plates were incubated at 37°C overnight and colony forming units (CFU) were enumerated the following day to estimate the number of viable E. coli. Plates with 30-300 colonies were used for determination of bacterial concentration.
Estimation of Phagocytosis Efficiency
Quantitative analysis was conducted by differential counts of cytospin preparations of the phagocyte population of the peritoneal fluid. Cytospin slides were prepared as described above. Phagocytic cells associated with 3 or more bacteria were regarded as positive and cells with 2 or less bacteria were regarded as negative, as described in our previous study (Pruett et al., 2010a). In that study, virtually all phagocytic cells contained numerous bacteria at 2 hr after E. coli challenge, with or without prior alcohol administration. However, by 18 or 24 hr after challenge, most phagocytic cells from mice treated with E. coli only were negative for bacteria, indicating effective killing and digestion of bacteria. In contrast, a significant percentage of phagocytic cells from alcohol-treated mice contained numerous bacteria at these time points.
Cytokine, Chemokine, and Growth Factor Assays
The concentrations of a panel of the cytokines, chemokines, and growth factors were determined in serum and peritoneal fluid obtained as already described and centrifuged to obtain the supernatant. Chemokines, cytokines, and growth factors in these samples were analyzed using Milliplex AP Assay Kits (Millipore corporation, Billierica, MA). The protocol and appropriate standards were provided with Milliplex kits and analysis was done using a Luminex analyzer (Luminex, Austin, TX). All values shown except 0 (undetectable) values were within the range of the standards included in the Milliplex kit.
Statistical Analysis
Data analysis was conducted with Prism 6.0 software (Graph Pad, San Diego, CA). Phagocytosis data were analyzed by analysis of variance (ANOVA), followed by Newman-Keuls post-hoc test to identify differences in group means. The Mantel-Cox log rank test was used for analyzing the survival data. Student paired or unpaired t-tests were employed to compare treatment and control groups as appropriate. Welch’s correction was applied, because the variances in the data for each group were not always equal. A p-value of 0.05 or less was considered significant.
Results
Alcohol increases the rate of mortality by a mechanism that is not reversible by mitigating hypothermia.
When assessing the survival of the infected binge-drinking mice, we observed a significant decrease in survival in the E. coli infected mice that were subjected to treatment with alcohol 30 min before (−30), 2 hr after (+2), or 18 hr after (+18) challenge with bacteria (Figure 1A). Most of the mice in the control group receiving water 18 hr post E. coli challenge survived more than 72 hr. However, the mice in the treatment group receiving alcohol 18 hr after bacterial challenge were all dead (or moribund) by 40 hr. This group exhibited no significant difference in time to death (or time to onset of moribund status) compared to the groups treated with alcohol at −30 min and +2 hr after E. coli. However, the mice treated with alcohol at +18 hr all died by 40 ± 6 hr, whereas mice in the other treatment groups began to die earlier and the percent of survivors decreased gradually over time (Figure 1).
Figure 1.

Percent survival of mice infected with E. coli (E. coli only) and mice subjected to alcohol (6 g/kg) treatment 30 min before (−30 min), 2 hr after (+2 hr), or 18 hr after (+18 hr) E. coli challenge under ambient temperature conditions (panel A) and with heating to prevent severe hypothermia (panels B and C). The control mice and treatment groups received 2 x 108 cells of mid-log viable E. coli. Mice were monitored every 6 hr for 72 hr. The group size for survival experiments was 10 mice per group (panels A and B), and the group size for body temperature determination was 5 (panel C). Significant differences in time to death were determined using the Mantel-Cox log rank test. In panel B, increasing the ambient temperature by placing the mouse cage on a heating pad significantly accelerated mortality. Results in panel C confirm that the heating protocol prevented the profound hypothermia caused by E. coli infection and alcohol. The body temperature of untreated, naive control mice was 36.4 ± 0.2 °C (results not shown).
In the model for sepsis used in this study, mice develop profound hypothermia shortly after E. coli administration, and those that recover gradually return to normal body temperature as bacteria are cleared (Pruett et al., 2010a). Although this is probably secondary to elevated bacterial counts and cytokine concentrations, it is important to note that hypothermia of this extent is almost always fatal in humans (Thomas and Cahill, 2000), so we thought it reasonable to assess whether hypothermia per se contributed to lethal outcome in our mouse model. To determine if hypothermia was a prerequisite for mortality in this model, hypothermia was mitigated in a group of mice treated with alcohol 18 hr after E. coli challenge by placing cages on heating pads at the time of alcohol administration to maintain near normal body temperature (Figs. 1B and 1C). The results indicate that warming the mice actually accelerated mortality (Figure 1B). This was not because the warming was ineffective in mitigating hypothermia, because mice that were warmed had body temperatures that were near normal (~33°C) compared to significantly lower temperatures in mice treated with E. coli and alcohol but not warmed (~28°C) (Figure 1C). Results shown in F igure 2 indicate that mice treated with E. coli but not alcohol had near normal body temperature at 18 and 21 hr after challenge (Figure 2), but administration of alcohol at 18 hr followed 3 hr later by measurement of body temperature indicated a substantial decrease in body temperature similar to that noted in the alcohol-treated but unwarmed mice in Figure 1C. It should also be noted that in our previous study, mice treated with alcohol 30 minutes before challenge with E. coli exhibited decreased survival during a 72 hour period, and increased number of bacterial in the peritoneal cavity at 8 and 21 hr, and increased concentrations of many pro-inflammatory cytokines at 21 hr. However, the concentrations noted in the present report 3 hr after administration of alcohol 18 hr after challenge with E. coli were greater in almost every case, indicating a higher level of inflammation. In control, mice the number of bacteria in the peritoneal cavity decreased to almost undetectable levels by 8 hr and diminished further by 21 hr in control (non-alcohol treated) mice (Pruett et al., 2010a).
Figure 2.
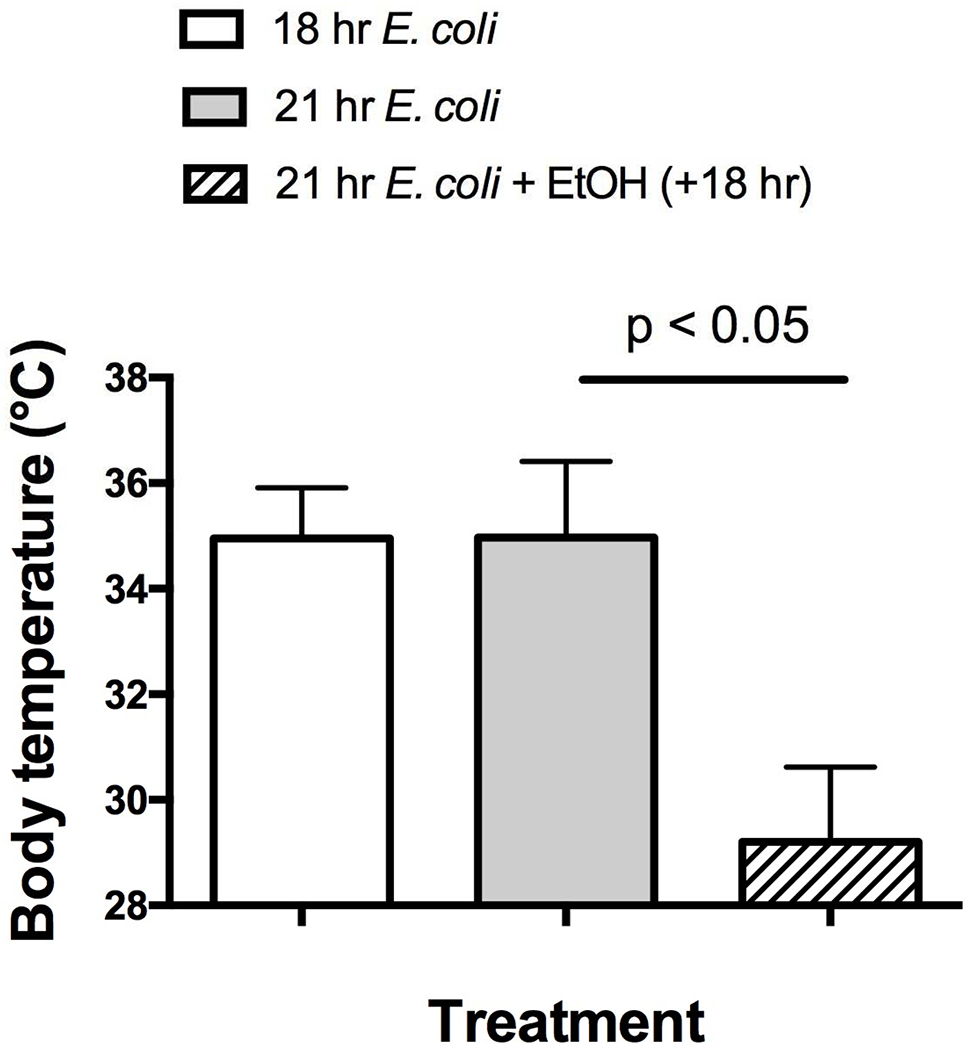
Alcohol administered at 18 hr caused late hypothermia (21 hr) in mice treated with E. coli, whereas mice treated with E. coli only had almost completely recovered by 18 or 21 hr from the hypothermia we have previously reported shortly after alcohol treatment. Results shown are means ± SEM and statistically significant differences as indicated by a t-test with Welch’s correction are shown (p < 0.05). The group size was 5 mice per group.
Alcohol treatment influences the number of bacteria in the peritoneal cavity
We assessed the change in the number of viable E. coli in mice treated with vehicle or alcohol 18 hr post infection (Figure 3). Samples taken 3 hr after alcohol treatment (21 hr after E. coli) contained significantly more bacteria than samples from control mice, which had mostly cleared the bacteria by 21 hr. This is consistent with our previous studies indicating that E. coli is almost entirely cleared by 18 hr in our sepsis model in mice treated only with E. coli or E. coli plus the vehicle for alcohol (water) (Pruett et al., 2010a). However, at 21 hr, mice treated with alcohol at 18 hr had a significantly larger number of E. coli in the peritoneal cavity, more consistent with mice treated with alcohol before E. coli challenge, most of which did not survive (Pruett et al., 2010a).
Figure 3.

Viability assessment of E. coli in peritoneal lavage fluid of alcohol-treated mice. Qualitative assessment of E. coli in peritoneal lavage fluid from control mice (A) and mice treated with alcohol at 6 g/kg 18 hr after E. coli challenge and 3 hr before sampling for bacterial quantitation (B) show an increase in the number of viable E. coli in the peritoneal cavity. Colony counts with 5 mice per group (C) also indicated an increase in E. coli counts in alcohol-treated mice. Significance was determined by the Student’s t-test (* = p < 0.05).
Effect of alcohol on chemokine, cytokine, and growth factor responses
The cytokine and chemokine concentrations were measured at the site of bacterial challenge (the peritoneal cavity) and in serum, which predominantly represents cytokine production in other anatomical locations with substantial macrophage populations (e.g., spleen and liver). Most cytokines and chemokines were elevated in mice receiving alcohol 18 hr after the E. coli challenge, as compared to the control group (Figures 4 and 5). The cytokines and chemokines measured in this study are mostly considered pro-inflammatory, but as has typically been noted in sepsis (Wu et al., 2009), the anti-inflammatory cytokine, IL-10, was elevated as well. Cytokines were measured at 21 hr in these experiments (3 hr after alcohol administration). Although alcohol at the doses used here is not a direct stimulus for cytokine or chemokine production (Pruett et al., 2004), the observation that bacterial numbers were substantially increased at 21 hr in mice treated with alcohol suggested that growth of bacteria was rapid, and this could have stimulated cytokine production. The results presented here show no suppression of pro-inflammatory cytokine and chemokine production by alcohol administered 18 hr after E. coli challenge such as we reported previously when alcohol was administered 30 min prior to bacterial challenge (Pruett et al., 2010a). It remains possible that alcohol 18 hr after bacterial challenge decreases the pro-inflammatory cytokine response to some degree, but that the overgrowth of bacteria induced by alcohol administration has an even greater effect on induction of pro-inflammatory cytokines and chemokines. In any case, most cytokines and chemokines were significantly increased in the peritoneal fluid and the serum of mice treated with alcohol 18 hr after bacterial challenge. There were a few differences in the relative increase in cytokines and chemokines in serum as compared to peritoneal fluid. For example, IL-9 was significantly elevated by alcohol in the peritoneal fluid, but it was not in serum (Figures 4 and 5). However, there was an overall similarity in the pattern of change induced by alcohol treatment for most cytokines, chemokines, and growth factors, even if the change was significant in some cases but not others.
Figure 4.

Peritoneal cytokines and chemokines (A) <1000 pg/ml and (B) > 1000 pg/ml increased due to the late alcohol treatment. Values shown are means ± SEM (n = 5). Significance was assessed by unpaired t-test using Welch’s correction and differences between control and alcohol-treated groups (6 g/kg given 18 hr after E. coli challenge with sampling 3 hr after alcohol treatment) are indicate by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001).
Figure 5.

Serum cytokines and chemokines (A) <1000 pg/ml and (B) > 1000 pg/ml increased due to the late alcohol treatment. Values shown are means ± SEM (n = 5). Significance was assessed by unpaired t-test using Welch’s correction and differences between control and alcohol-treated groups (6 g/kg given 18 hr after E. coli challenge with sampling 3 hr after alcohol treatment) are indicate by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001).
Alcohol administration at 18 hr decreases bacterial clearance and allows bacterial growth in phagocytic cells.
Phagocytic clearance of E. coli from the host was examined by collecting cells from the peritoneal fluid and determining the percentage of macrophages and neutrophils associated with three or more bacteria. Our previous study indicated that this measure reflects bacterial clearance by phagocytic cells (Pruett et al., 2010a). Indeed, the increased number of phagocytic cells associated with bacteria obtained from alcohol treated mice (Figure 6B) corresponds to the increased number of viable bacteria in these mice (Figure 3). Figure 6A illustrates that although neutrophils were attracted to the site of the original infection (the peritoneal cavity) by 21 hr and most of them cleared the E. coli they had ingested, E. coli were abundantly present in both neutrophils and macrophages in the alcohol-treated mice but not in the control mice (Figure 6). Results of quantitative assessment of the percentage of macrophages and neutrophils with three or more E. coli bacteria are shown in Figure 7. These results clearly indicate that alcohol treatment at 18 hr post E. coli challenge significantly increases the percentage of phagocytic cells that are associated with three or more E. coli, indicating that bacterial clearance is adversely affected by alcohol treatment. These results suggest that one or more anti-microbial mechanisms of phagocytic cells is inhibited by alcohol administered at 18 hr after challenge, a time when control mice have largely cleared the bacteria. However, these mechanisms cannot be strictly dependent on concurrent pro-inflammatory cytokine and chemokine production, because these were increased by alcohol treatment.
Figure 6.

Giemsa staining of peritoneal phagocytes revealing a larger number of E. coli in neutrophils and macrophages in peritoneal fluid collected from mice 3 hr following alcohol treatment 18 hr after E. coli challenge (B) as compared to control mice that did not receive the alcohol treatment (A). E. coli are visible as the small dark rods within or near the phagocytes. Phagocytes from E. coli-challenged control mice have prominent phagocytic vacuoles with very few visible bacteria.
Figure 7.
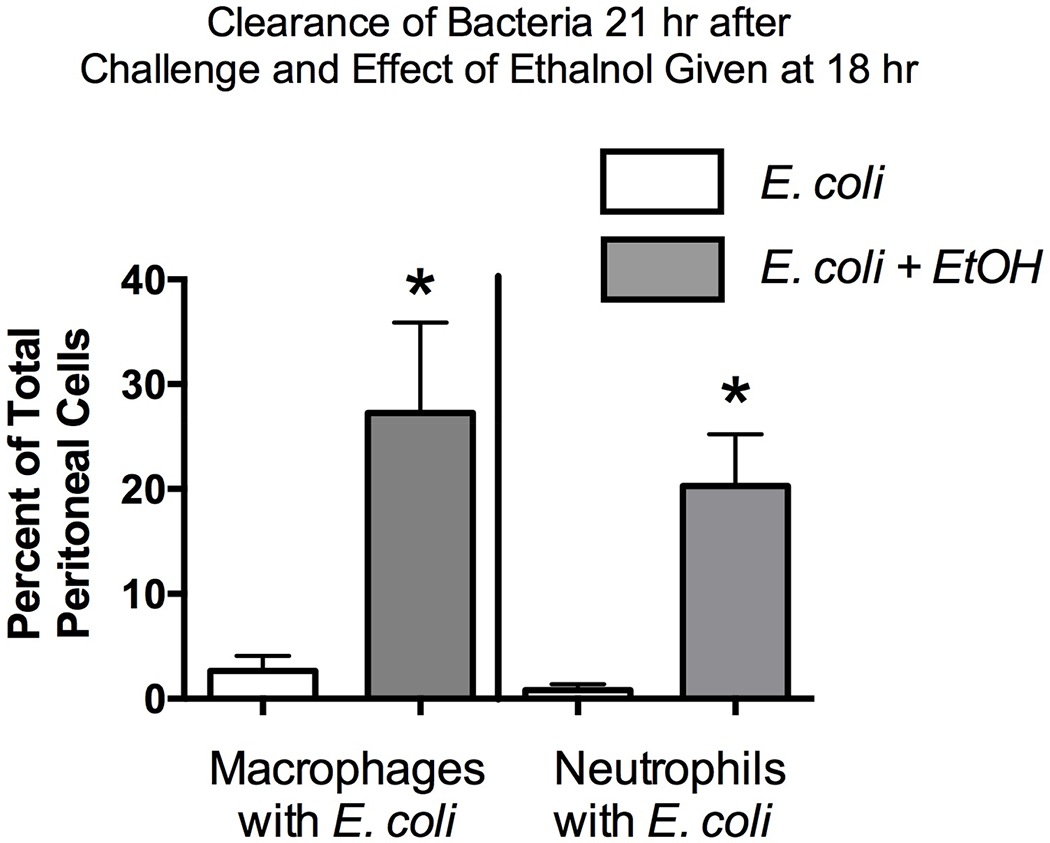
Percentage difference in phagocytes with and without E. coli engulfed. Control mice receiving E. coli only and mice treated with E. coli then treated with alcohol (EtOH) 18 hr later are shown. Samples were collected 3 hr after the 18 hr alcohol treatment. Cytospin preparations were stained and enumerated. The group size was 5, and the results for E. coli compared to E. coli + Alcohol were compared by a t-test with Welch’s correction. Statistical significance is indicated by * (P < 0.05).
Discussion
Previous studies in our lab have indicated that exposure of mice to alcohol shortly before or after infection decreases resistance to infection, at least in part by decreasing cellular signaling and consequently production of inflammatory mediators that are important in innate resistance to infection (Bhatty et al., 2011, Pruett and Pruett, 2006, Pruett et al., 2010a, Pruett et al., 2004). However, others have reported that administration of isopropanol, which is thought to act on the immune system by a mechanism similar to that of alcohol, increases resistance to mortality when administered late in the course of Staphylococcal enterotoxin B (SEB) toxicity. In the SEB model, elevated cytokine and chemokine concentrations contribute to pathology and lethality (Desy et al., 2008). This was the basis for our working hypothesis that the same phenomenon would occur in our sepsis model and that survival would be enhanced by decreased cytokine and chemokine responses caused by administering alcohol at a time when cytokines are known to contribute to mortality (late in the course of sepsis). The results conclusively refuted our working hypothesis and indicated that, in contrast with effects of isopropanol on mortality due to a non-replicating bacterial toxin, alcohol’s effects on an active bacterial infection (sepsis) were harmful due to a late loss of control of bacterial replication and an associated (presumably detrimental) substantial elevation of pro-inflammatory cytokines and chemokines. It has been well documented that in mice, a number of pro-inflammatory mediators, when produced in excessive amounts contribute to mortality in sepsis (Frazier et al., 2009). Such a “cytokine storm” can also clearly cause adverse effects in humans (Suntharalingam et al., 2006), but the precise relationship between the effects of elevated pro-inflammatory mediators in humans and in mice is not entirely clear and remains the subject of active investigation and debate (Seok et al., 2013).
It was remarkable that we observed an increase in E. coli number within 3 hours after alcohol treatment 18 hr after E. coli administration. This suggests the possibility that binge drinking both before and after the initiation of infection can be detrimental with regard to patient outcome. However, in humans this could be complicated by adverse effects of alcohol withdrawal in patients who are physically dependent. In this case, administration of the minimum amount of alcohol needed to ameliorate the withdrawal symptoms might not significantly affect resistance to infection. In our mouse model, we have previously shown that the anti-inflammatory effects of alcohol are dose dependent and are minimal or non-existent at doses less than 3 g/kg, a dosage that produces peak blood levels of about 0.18% (v/v) (Pruett and Pruett, 2006). However, it is difficult to equate human and mouse blood levels of alcohol without measuring the area under the concentration vs. time curve, because mice clear alcohol much more quickly than do humans. Thus, a peak concentration that would be considered high in humans would probably be less biologically effective in mice (Deltour et al., 1999).
Hypothermia is one of the thermoregulatory mechanisms employed by the host when under systemic inflammation; the other being fever (Romanovsky and Szekely, 1998). In rodent models of sepsis, hypothermia is observed and seems to be more common than a febrile response. Hypothermia is also observed in some human sepsis patients, and these patients typically have a poorer outcome than patients who exhibit a fever response (Laupland et al., 2004, Wang et al., 1989). Some of the effects of hypothermia on inflammation have been reported to be reversible (Johnson et al., 1995, Myers, 1981). However, it was not clear whether alcohol-induced hypothermia was one of the major factors that induced the suppression of the innate immune response to bacterial infection in our previous studies of the effects of alcohol on resistance to sepsis (Bhatty et al., 2011, Pruett et al., 2010a, Pruett et al., 2004). Interestingly, in this study we observed that alcohol administered 18 hr post-infection rapidly induced hypothermia. However, there were two new and unexpected observations: (1) alcohol treatment at 18 hr did not decrease cytokine concentrations as it does at 2-3 hr after infection (Pruett et al., 2010a) and (2) preventing the hypothermia induced by alcohol treatment at 18 hr did not save the treatment group from lethal sepsis. On receiving alcohol 18 hrs after the E. coli challenge, the temperature of the mice in the treatment group was observed to be reduced (hypothermia) compared to the control group receiving water (Figure 2). External warming of the hypothermic mice in the treatment group increased the body temperature (Figure 1C). However, the survival plots comparing the survival of the treatment groups with and without warming showed the increase in temperature to cause the majority of the mice to succumb to an earlier death. Thus, the results indicate that alcohol induced-hypothermia of brief duration may be a protective mechanism in mice, and it may enhance survival in cases in which animals would otherwise die from septic shock.
The observed increase in production of pro-inflammatory cytokines and chemokines when alcohol was administered 18 hr after bacterial challenge was probably mediated indirectly by the large increase in the number of E. coli caused by alcohol treatment. The sudden loss of control of microbial replication in macrophages and neutrophils while chemokine and cytokine concentrations were highly elevated demonstrate that there are mechanisms by which alcohol decreases microbial clearance by phagocytic cells that do not involve decreased production of cytokines and chemokines.
Consistent with the measured high levels of neutrophil chemoattractant chemokines, we noted increased percentages of neutrophils over time in the peritoneal cavity (figure 6). However, increased chemokine production induced by alcohol administered 18 hr after challenge did not lead to a further increase in neutrophils in the peritoneal cavity. It is possible that unknown inhibitory actions of alcohol counteracted the effects of the increased chemokine concentrations with regard to this particular function. In our study, alcohol treatment 18 hr after E. coli challenge led to highly elevated levels of CXCL9 and CXCL10 in the peritoneal cavity and CXCL9 in the serum as compared to the control group (Figures 4 and 5). These chemokines have the potential to attract T lymphocytes. However, in the present model it is unlikely that acquired immunity plays a significant role in decreased survival of infected hosts. Acquired responses require 7-10 days of induction, and the effects of alcohol in this model were evident within 3 hr after dosing, strongly suggesting that the effects on survival in this model represent adverse actions on innate, not acquired, immunological mechanisms. However, attraction of T regulatory or other classes of regulatory innate lymphoid cells might contribute to immunosuppression often reported in survivors of sepsis.
In any case, the 18 hr alcohol treatment was observed to enhance the proinflammatory response in the host without leading to bacterial clearance by 3 hr after alcohol administration. It is interesting that alcohol decreases cytokine and chemokine production 2-8 hr after E. coli challenge when the alcohol is given 30 min before E. coli challenge, but alcohol also only slightly decreases bacterial clearance at this time (Bhatty et al., 2011, Pruett et al., 2010a). These findings demonstrate that suppression of the production of cytokines and chemokines by alcohol is not required for inhibition of bacterial clearance when alcohol is administered 18 hr after E. coli. This calls into question the general assumption that early production of cytokines and chemokines is critical for the initial clearance of bacteria early in the infection. We found that mice with a mutated TLR4 gene produce substantially lower concentrations of pro-inflammatory cytokines and chemokines, but these mice ultimately survive better whether treated with alcohol or not than their wild-type counterparts (Bhatty et al., 2011). Thus, it seems that an emphasis on early events in innate immunity to sepsis should focus more on mechanisms by which phagocytic cells kill and clear bacteria than on production of cytokines and chemokines generally thought to be important in enhancing bacterial clearance.
We propose that the exaggerated cytokine response induced by the alcohol treatment at the later stages of infection contributed to lethality in the mice of our treatment group. In congruence with our postulate, Kellum and colleagues found that increases in both anti-inflammatory and pro-inflammatory cytokines are associated with a higher rate of mortality (Kellum et al., 2007). Thus, we propose that late alcohol treatment along with preventing the final clearance of bacteria and allowing their growth induced an exaggerated pro-inflammatory response that may have contributed to the rapid death in our binge drinking-infection model. Pro-inflammatory cytokines and chemokines, when produced in excessive amounts are known to be associated with coagulopathies leading to multi-organ failure and death (Pruett et al., 2010b). In future studies we plan to investigate these mechanisms of mortality.
Although hypothermia is a striking characteristic of E. coli-treated mice which receive alcohol at 18 hr after challenge, ameliorating the hypothermia by increasing the ambient temperature did not improve survival. This suggests that hypothermia is not a major contributor to mortality under these experimental conditions. However, we have also observed that in mice treated with a dithiocarbamate pesticide before E. coli challenge prolonged hypothermia is strongly correlated with mortality (Tan and Pruett, 2015). Therefore, it is possible that prolonged hypothermia contributes to mortality, whereas hypothermia of shorter duration after alcohol treatment at 18 hr does not contribute to mortality. In fact, hypothermia for a short period of time might be expected to decrease damage associated with hypoxia and to improve survival.
The results reported here are consistent with some aspects of the generally accepted narrative that lethal outcome in sepsis is associated with overgrowth of bacteria, excessive production of cytokines, chemokines, and growth factors (causing shock), and dysregulation of body temperature. However, the results reported here also suggest the novel conclusions that a key mechanism of survival in sepsis involves activation of critical phagocytic anti-microbial mechanisms that are important both early and late in the course of sepsis. This mechanism can be lost even after induction by cytokines and chemokines early in the course of infection, as illustrated by the rapid overgrowth of bacteria within 3 hr after administration of alcohol at 18 hr after initiation of infection. These conclusions suggest that loss of control of microbial growth in sepsis and lethal outcome is not strictly dependent on events early in the course of infection, but it can be influenced by environmental factors (such as alcohol) many hours after the initiation of infection. Understanding the mechanism of these effects and the mechanism responsible for maintenance of late resistance to infection could lead to new therapeutic approaches including regulation of environmental influences that might adversely affect resistance. In addition, although hypothermia is often considered to be associated with poor outcome in sepsis, it is possible that short-term hypothermia could be a protective mechanism. If so, it will be important to understand the duration as well as the degree of hypothermia that is associated with adverse effects, so intervention can be conducted to alleviate hypothermia of prolonged duration but not to alleviate hypothermia during brief periods during which it might be protective.
Highlights.
Alcohol administered (by gavage) 18 hr after initiation of sepsis.
Rapid bacterial overgrowth, hypothermia, and high levels of cytokines noted 3 hr after alcohol.
Interference with bacterial clearance rather than early suppression of cytokine production seems to be the key effect of alcohol that causes mortality in sepsis.
Acknowledgments
Funding: This work was supported by Grant R01AA009505 from the National Institute on Alcohol and Alcoholism and SBP, WT, and BN were supported by Center of Biomedical Research Excellence Grant #P20GM103646 from the National Institute of General Medical Sciences, Center for Research Capacity Building.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcoholism, clinical and experimental research. 2006;30:1781–90. [DOI] [PubMed] [Google Scholar]
- Baker RC, Smolen A, Smolen TN, Deitrich RA. Relationship between acute ethanol-related responses in long-sleep and short-sleep mice. Alcoholism, clinical and experimental research. 1987;11:574–8. [DOI] [PubMed] [Google Scholar]
- Bhatty M, Jan BL, Tan W, Pruett SB, Nanduri B. Role of acute ethanol exposure and TLR4 in early events of sepsis in a mouse model. Alcohol (Fayetteville, NY. 2011;45:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot S, Vandewoude K, Hoste E, De Waele J, Kint K, Rosiers F, Vogelaers D, Colardyn F. Absence of excess mortality in critically ill patients with nosocomial Escherichia coli bacteremia. Infect Control Hosp Epidemiol. 2003;24:912–5. [DOI] [PubMed] [Google Scholar]
- Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcoholism, clinical and experimental research. 2006;30:1624–31. [DOI] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcoholism, clinical and experimental research. 1996;20:132–8. [DOI] [PubMed] [Google Scholar]
- Chawla S, DeMuro JP. Current controversies in the support of sepsis. Curr Opin Crit Care. 2014;20:681–4. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol (Fayetteville, NY. 2004;33:199–208. [DOI] [PubMed] [Google Scholar]
- Collier SD, Pruett SB. Mechanisms of suppression of poly I:C-induced activation of NK cells by ethanol. Alcohol (Fayetteville, NY. 2000;21:87–95. [DOI] [PubMed] [Google Scholar]
- Collier SD, Wu WJ, Pruett SB. Ethanol suppresses NK cell activation by polyinosinic-polycytidylic acid (poly I:C) in female B6C3F1 mice: role of endogenous corticosterone. Alcoholism, clinical and experimental research. 2000;24:291–9. [PubMed] [Google Scholar]
- Dai Q, Zhang J, Pruett SB. Ethanol alters cellular activation and CD14 partitioning in lipid rafts. Biochemical and biophysical research communications. 2005;332:37–42. [DOI] [PubMed] [Google Scholar]
- De Waele JJ, Hoste EA, Blot SI. Blood stream infections of abdominal origin in the intensive care unit: characteristics and determinants of death. Surg Infect (Larchmt). 2008;9:171–7. [DOI] [PubMed] [Google Scholar]
- Deltour L, Foglio MH, Duester G. Metabolic deficiencies in alcohol dehydrogenase Adh1, Adh3, and Adh4 null mutant mice. Overlapping roles of Adh1 and Adh4 in ethanol clearance and metabolism of retinol to retinoic acid. The Journal of biological chemistry. 1999;274:16796–801. [DOI] [PubMed] [Google Scholar]
- Desy O, Carignan D, Caruso M, de Campos-Lima PO. Immunosuppressive effect of isopropanol: down-regulation of cytokine production results from the alteration of discrete transcriptional pathways in activated lymphocytes. J Immunol. 2008;181:2348–55. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcoholism, clinical and experimental research. 2006;30:76–85. [DOI] [PubMed] [Google Scholar]
- Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, Cato AC, Liu Y. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J Immunol. 2009;183:7411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman SJ, Dvorak VC, MacGregor RR. Host defenses during prolonged alcohol consumption in a controlled environment. Arch Intern Med. 1977;137:1539–43. [PubMed] [Google Scholar]
- Gluckman SJ, MacGregor RR. Effect of acute alcohol intoxication on granulocyte mobilization and kinetics. Blood. 1978;52:551–9. [PubMed] [Google Scholar]
- Goral J, Karavitis J, Kovacs EJ. Exposure-dependent effects of ethanol on the innate immune system. Alcohol (Fayetteville, NY. 2008;42:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456–63. [DOI] [PubMed] [Google Scholar]
- Han YC, Lin T-L, Pruett SB. Thymic atrophy caused by ethanol in a mouse model for binge drinking: involvement of endogenous glucocorticoids. Toxicol Appl Pharmacol. 1993;123:16–25. [DOI] [PubMed] [Google Scholar]
- Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby Gj, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol (Fayetteville, NY. 2007;41:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen R, Laine J, Lumio J, Vuento R, Syrjanen J. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC infectious diseases. 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiological reviews. 2013;93:1247–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Haddix T, Pohlman T, Verrier ED. Hypothemia Reversibly Inhibits Endothelial Cell Expression of E-Selectin and Tissue Factor. Journal of Cardiac Surgery. 1995;10:428–35. [DOI] [PubMed] [Google Scholar]
- Jones AW, Holmgren A. Age and gender differences in blood-alcohol concentration in apprehended drivers in relation to the amounts of alcohol consumed. Forensic Sci Int. 2009;188:40–5. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland KB, Davies HD, Church DL, Louie TJ, Dool JS, Zygun DA, Doig CJ. Bloodstream infection-associated sepsis and septic shock in critically ill adults: a population-based study. Infection. 2004;32:59–64. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Kolls JK, Nelson S. Ethanol and murine interleukin (IL)-12 production. Alcoholism, clinical and experimental research. 2000;24:553–9. [PubMed] [Google Scholar]
- McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–4. [DOI] [PubMed] [Google Scholar]
- Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Critical care (London, England). 2009;13:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R Alcohol’s effect on body temperature: hypothermia, hyperthermia or poikilothermia? Brain research bulletin. 1981;7:209–20. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WG. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–9. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–9. [DOI] [PubMed] [Google Scholar]
- Pruett BS, Pruett SB. An explanation for the paradoxical induction and suppression of an acute phase response by ethanol. Alcohol (Fayetteville, NY. 2006;39:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Cheng B, Glover M, Tan W, Deng X. Innate immunity and inflammation in sepsis: mechanisms of suppressed host resistance in mice treated with ethanol in a binge-drinking model. Toxicol Sci. 2010a;117:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q. Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life Sci. 2003;72:1825–39. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Tan W, Sebastian T, Liu D. Innate Immunity and Inflammation in Organ Failure. In: McQueen CA, editor. Comprehensive Toxicology, 2nd Edition. Kidlington OX5 1GB, United Kingdom: Elsevier Ltd.; 2010b. p. 299–322. [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol (Fayetteville, NY. 2004;33:147–55. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Szekely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses. 1998;50:219–26. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran Ns, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation, Host Response to Injury LSCRP. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England journal of medicine. 2006;355:1018–28. [DOI] [PubMed] [Google Scholar]
- Szabo G, Chavan S, Mandrekar P, Catalano D. Acute alcohol consumption attenuates interleukin-8 (IL-8) and monocyte chemoattractant peptide-1 (MCP-1) induction in response to ex vivo stimulation. J Clin Immunol. 1999;19:67–76. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–9. [DOI] [PubMed] [Google Scholar]
- Tan W, Pruett SB. Effects of sodium methyldithiocarbamate on selected parameters of innate immunity and clearance of bacteria in a mouse model of sepsis. Life Sci. 2015;139:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Cahill CJ. Successful defibrillation in profound hypothermia (core body temperature 25.6 degrees C). Resuscitation. 2000;47:317–20. [DOI] [PubMed] [Google Scholar]
- Travis CC, White RK. Interspecific scaling of toxicity data. Risk Anal. 1988;8:119–25. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–6. [DOI] [PubMed] [Google Scholar]
- Vincent JL, De Backer D. Circulatory shock. The New England journal of medicine. 2013;369:1726–34. [DOI] [PubMed] [Google Scholar]
- Wang FD, Wang LS, Liu CY, Cheng DL, Duh RW, Tsai IK. Septicemia in adults: II. Factors in prognosis. Zhonghua Yi Xue Za Zhi (Taipei). 1989;44:89–94. [PubMed] [Google Scholar]
- Wu HP, Chen CK, Chung K, Tseng JC, Hua CC, Liu YC, Chuang DY, Yang CH. Serial cytokine levels in patients with severe sepsis. Inflamm Res. 2009. [DOI] [PubMed] [Google Scholar]
- Wu W-J, Pruett SB. Suppression of splenic natural killer cell activity in a mouse model for binge drinking. I. Direct effects of ethanol and its major metabolites are not primarily responsible for decreased natural killer cell activity. J Pharmacol Exp Ther. 1996;278:1325–30. [PubMed] [Google Scholar]
- Wu W-J, Pruett SB. Involvement of catecholamines and glucocorticoids in ethanol-induced suppression of splenic natural killer cell activity in a mouse model for binge drinking. Alcohol Clin Exp Res. 1997;21:1030–6. [PubMed] [Google Scholar]
- Wu W-J, Pruett SB. Ethanol decreases host resistance to pulmonary metastases in a mouse model: role of natural killer cells and the ethanol-induced stress response. Int J Cancer. 1999;82:886–92. [DOI] [PubMed] [Google Scholar]


