Abstract
Background:
An NIH clinical coagulopathy score has been devised for trauma patients, but no such clinical score exists in transplantation surgery. We hypothesize that that this coagulopathy score can effectively identify laboratory defined coagulopathy during liver transplantation and correlates to blood product utilization.
Methods:
TEGs were performed and coagulopathy scores (1, normal bleeding – 5, diffuse coagulopathic bleeding) were assigned by the surgeons at 5 intra-operative time points. Blood products used during the case were recorded between time points. Statistical analyses were performed to identify correlations between coagulopathy scores, TEG-detected abnormalities, and blood product utilization.
Result:
Transfusions rarely correlated with the appropriate TEG measurements of coagulation dysfunction. Coagulopathy score had significant correlation to various transfusions and TEG-detected coagulopathies at multiple points during the case. High aggregate coagulopathy scores identified patients receiving more transfusions, re-operations, and longer hospital stays
Conclusion:
The combination of viscoelastic testing and a standardized clinical coagulopathy score has the potential to optimize transfusions if used in tandem as well as standardize communication between surgery and anesthesia teams about clinically evident coagulopathy.
Keywords: Liver transplantation, Hemostatic resuscitation, Thrombelastography, Clinical coagulopathy, Coagulopathy score
Introduction
The dynamic changes of coagulopathy during liver transplantation make achieving hemostasis challenging. Empirically transfusing patients when there is clinical evidence of bleeding with intermittent laboratory testing is one strategy to control bleeding, while scheduled laboratory testing to prevent progression of coagulopathy provides another.1 Routine whole blood viscoelastic testing during liver transplantation originated from early reports of liver transplantation in the 1960s.2 Since this time, numerous observational studies have supported the use of thrombelastography in liver transplantation.3 Several randomized controlled trials have supported that viscoelastic testing during liver transplantation reduces blood product administration.4,5
Despite decades of research on optimizing laboratory assessment of coagulation in liver transplantation,3 strategies to quantify coagulopathic bleeding have received less attention. A standardized clinical assessment of bleeding to determine the need for coagulation could provide a valuable clinical tool to reduce blood transfusions. However, clinical scoring of coagulopathy in liver transplantation is lacking. A coagulopathy score was recently generated from a consortium group at the National Institute of Health for bleeding related to trauma.6 This Likert scale of bleeding breaks down the clinical assessment of bleeding into a clinical score distinguishing hemostatic needs ranging from expected bleeding to the need for empiric transfusion. Concurrent categorization of clinically determined coagulopathy paired with viscoelastic assessment would aid in validating this trauma coagulopathy score in the setting of liver transplantation, in which there are several timepoints of anticipated changes in coagulation.7 We hypothesize that this coagulopathy score can effectively identify laboratory defined coagulopathy during liver transplantation and correlates to blood product utilization.
Methods
Patient population
Liver transplant patients were enrolled in a Colorado Multi-Institutional Review Board study to prospectively collect blood samples through the first 24 h following surgery. Enrollment criteria were adult (>18 years) and cadaveric liver donor recipient.
Blood samples for viscoelastic testing
Blood was collected and stored in a 3.5-mL tubes containing 3.2% citrate, and immediately transferred for analysis via a trained professional research assistant. All viscoelastic assays were completed within 2 h of blood draw. Serial blood samples were obtained before the surgical incision (pre-op), during the native hepatectomy (after hepatic artery ligation), during the anhepatic phase of surgery (15 min after removal of native liver from recipient), 30 min after reperfusion (determined as 30 min after unclamping the portal vein; reperfusion 30), 120 min after reperfusion (reperfusion 120), and on postoperative day 1 (POD1).
Thrombelastography
Blood samples were assayed with the TEG 5000 Hemostatic Analyzer (Haemonetics, Braintree, MA) according to manufacturer’s recommendations. The following measurements were recorded: R time (minutes), angle (a, degrees), maximum amplitude (MA, mm), and lysis 30 min after MA (LY30, %). Samples were run native, without any activator (n-TEG).
Coagulopathy score
The coagulopathy score ranged from 1 to 5. These scores were determined by the attending transplant surgeon or fellow during the operation. This Likert based scale was generated from the National Heart Lung and Blood Institute collaborative definition for defining clinical coagulopathy in trauma.6 In brief, a score of 1 represented normal bleeding and clotting during surgery, a score of 3 represented more than expected bleeding with concerns for the need for potential hemostatic blood product resuscitation warranting coagulation assessment, and 5 represented diffuse coagulopathy requiring packing an empiric hemostatic blood product transfusion. A score of 2 and 4 were in between the other measurements. The first coagulopathy score was recorded at the start of the operation (placement of surgical retractors), and the following coagulation scores were done concurrently with TEG blood draws. Neither the coagulopathy score nor the TEG results were used for clinical decision-making; the anesthesiologists were blinded to both and provided usual and customary care.
Blood product utilization
The amount of red blood cell units (RBC), plasma, cryoprecipitate, and platelets were recorded between each blood draw/coagulation score. For example, the first interval blood product utilization was recorded from skin incision until hepatectomy score, when blood was drawn for TEG analysis. The final interval blood product utilization at reperfusion 120 included the remaining time in the operating room and PACU prior to transfer to the intensive care unit or surgical ward. Cell saver blood was included in total red blood cell transfusions with a conversion of 300 mls equating to 1 RBC. Transfusions were also recorded from the time out of the operating until post-operative day 1 (24 h from incision). A massive transfusion was considered >10 RBC in the operating room.
Thrombelastography indications for transfusions
Based on the first randomized control trial demonstrating a reduction in blood product in liver transplant using TEG transfusion triggers were assigned to indices5; R time of >10 min was an indication for a plasma transfusion, Angle <45° was an indication for cryoprecipitate, and MA < 55 mm was an indication for platelets. These were recorded for each research TEG drawn during surgery. Based on the coagulopathy score definitions, a patient with a score from 1 to 2 had a low probability of TEG abnormality and was defined as low risk, a score of 3 was intermediate risk of having a TEG abnormality, and a score of 4 or greater was indicative of the patient being high risk of having a TEG abnormality.
Outcomes
The primary outcome of interest was the correlation of coagulopathy score to interval blood product utilization. Secondary outcomes of interest included the correlation between coagulation score and TEG, accuracy of coagulopathy score to predict if the patient had an indication for obtaining a TEG, its accuracy in predicting the need for hemostatic blood products based on TEG indices, and the ability of the score to predict who would need to return to the operating room the following day. Additional variables and outcomes were contrasted between the average coagulopathy score of patients during the duration of surgery stratified by low, intermediate, and high risk. These outcomes included hospital length of stay, 90 day mortality, return to the operating room, reason for return to operating room, complications; bleeding excluding return to operating room (e.g. gastro-intestinal, hemorrhagic stroke), thrombotic (thrombotic stroke, myocardial infarct, pulmonary embolism, deep vein thrombosis), infectious (cellulitis, cholangitis, deep space infection),cardiac (arrest, arrhythmia requiring medication), acute kidney injury (requirement of dialysis post-operative)
Statistical analysis
Statistical analysis was performed using SPSS 23 software (Microsoft, Armonk, NY). Normally distributed data were described as mean and standard deviation and non-normally distributed data were described as the median value with the 25th to 75th percentile values. A receive operating characteristic curve (ROC) was used to assess the performance of the score in predicting a massive transfusion during surgery. This included the individual scores and a composite score including all points added to one large number. The coagulopathy score was corelated to interval blood product utilization and TEG indices using a Spearman’s Rho. Indications for transfusions were contrasted between low, intermediate, and high-risk scores using a chi square during the different intervals. Other outcomes were contrasted between the 5 individual scores using a chi square analysis. We powered the study to greater than 90% to detect a moderate correlation8 between coagulation score interval blood product utilization setting alpha to 0.05.
Results
Patient population
40 Patients were enrolled in this study from July 2019 to February 2020. The median age of the population was 54 (40–60) with a median lab MELD-Na of 24 (16–29). The most common cause for end stage liver disease was alcohol (48%) followed by viral hepatitis (23%). A massive transfusion occurred in 75% of patients and 33% of patients returned to the operating room on post-operative day 1.
Coagulopathy score
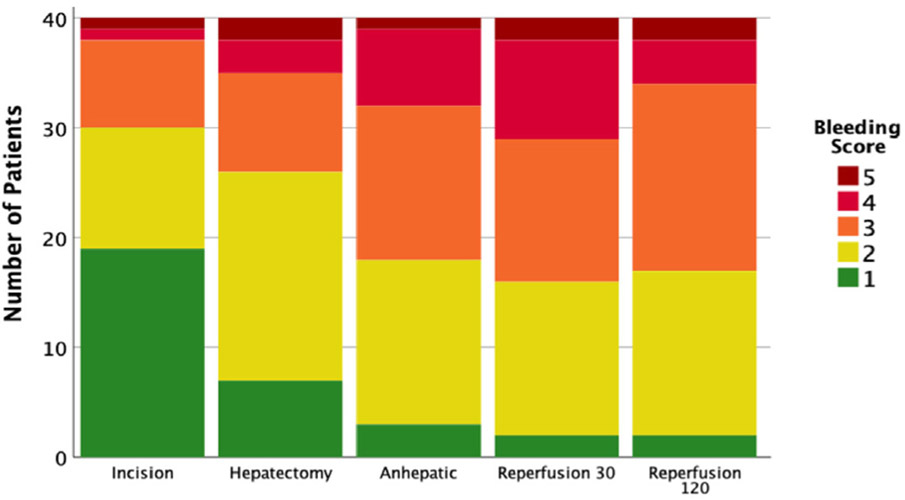
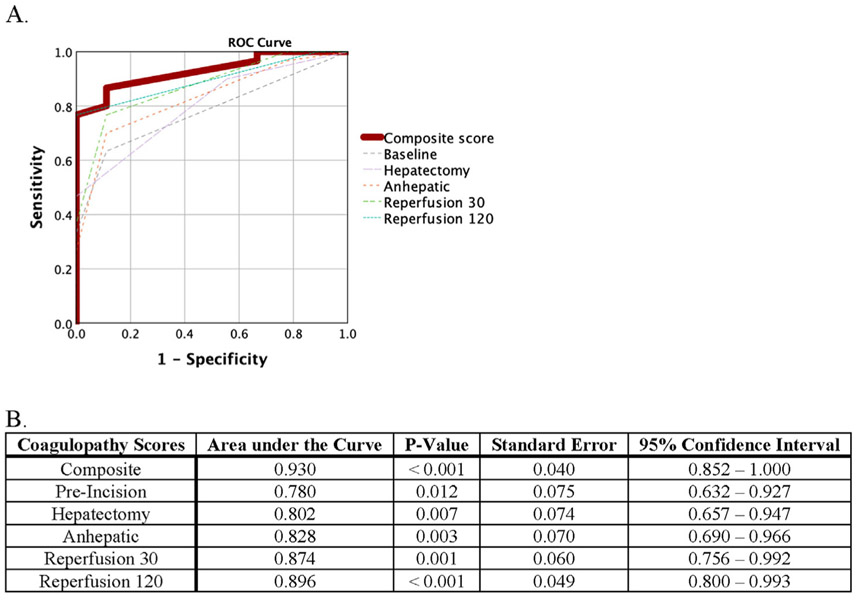
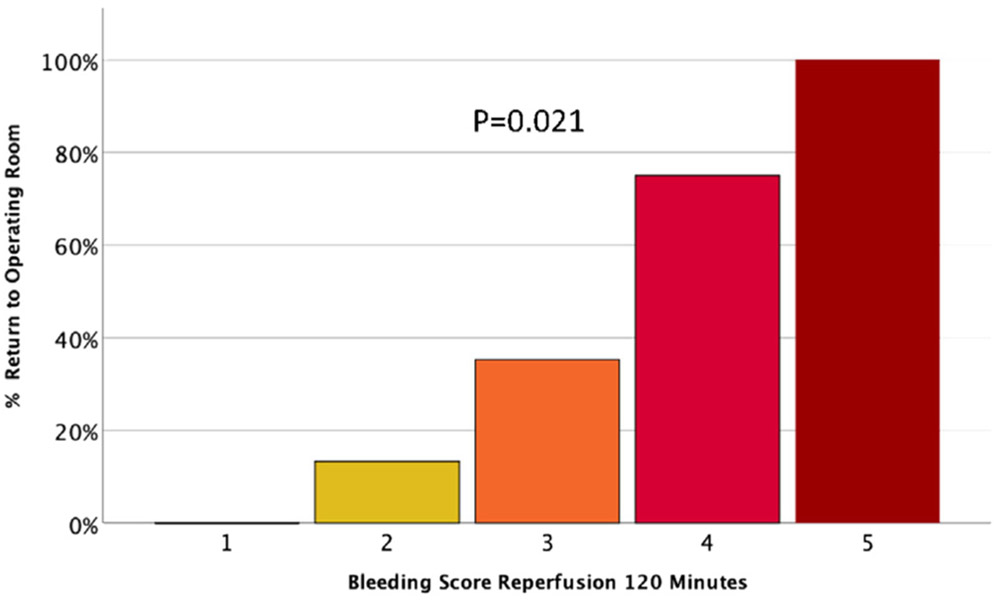
The coagulopathy score progressively increased over the duration of surgery (p = 0.001) peaking at reperfusion 30 (Fig. 1) with a reduction at reperfusion 120. Each interval score had a high performance for predicting massive transfusion, with the total composite score having an area under the curve of 0.930 (P < 0.001 Fig. 2). The reperfusion 120 coagulopathy score also significantly predicted which patients would require return to the operating room in a dose-like fashion (p = 0.021 Fig. 3).
Fig. 1.
Coagulopathy scores stratified by timepoint.
Fig. 2. Receiver Operant Characteristic (ROC) Curve For Coagulopathy Score Predicting Massive Transfusion.
Graphical (A) and Tabular (B) Representations are included. Massive transfusion was defined as more than 10 Red Blood Cell transfusions during the operation. ROC curves were constructed for the composite score as well as each timepoint
Fig. 3.
Patients requiring Re-operation following transplantation stratified by coagulopathy score at reperfusion 120.
Stratifying patients by the average coagulopathy score throughout the case identified that pre-operative coagulation assessment differentiated groups (Table 3). Patients with an average coagulopathy score stratified as persistently high-risk during surgery had lower baseline (prior to incision) angle, MA and higher INR compared to patients with low and moderate coagulopathy scores. This high-risk group were transfused significantly more blood products during surgery compared to the other patients with a median total RBC transfusion of 41 units; almost 4-fold compared to the other groups. This high-risk group also received more transfusions after the operating room, had higher drain outputs, and spent longer in the hospital after surgery with a median of 26 days.
Table 3.
Patient characteristics, transfusions, TEG indices, and post-operative outcomes stratified by average coagulopathy score.
| Average Low n = 15 | Average Intermediate N = 13 |
Average High N = 12 |
P Value | |
|---|---|---|---|---|
| Recipient | ||||
| Age (years) | 53 (44–64) | 53 (40–57) | 58 (47–62) | 0.496 |
| Female | 67% | 50% | 27% | 0.168 |
| Lab MELD-Na | 18 (10–26) | 23 (15–28) | 29 (19–44) | 0.084 |
| Pre-Incision | ||||
| R time (minutes) | 8 (7–11) | 9 (7–10) | 11 (9–14) | 0.148 |
| Angle (degrees) | 59 (54–68) | 55 (35–63) | 37 (23–43) | 0.003 |
| Maximum Amplitude (mm) | 53 (47–68) | 46 (33–59) | 35 (25–40) | 0.003 |
| LY30 (percent) | 0 (0–0.0) | 0 (0–0) | 0 (0–0) | 0.415 |
| INR | 1.7 (1.2–2.2) | 2.0 (1.5–2.5) | 2.5 (2.0–3.6) | 0.025 |
| Platelet Count (/mm3) | 83 (53–176) | 77 (49–100) | 40 (29–70) | 0.069 |
| Donor | ||||
| Age (years) | 34 (20–59) | 39 (34–58) | 38 (33–47) | 0.8585 |
| Female | 33% | 50% | 27% | 0.485 |
| BMI (kg/m2) | 26 (21–28) | 27 (24–31) | 28 (24–37) | 0.511 |
| Downtime | 0 (0–0) | 1 (0–27) | 1 (0–23) | 0.107 |
| Post Op TEG | ||||
| R time (minutes) | 8 (7–11) | 10 (9–11) | 10 (7–15) | 0.258 |
| Angle (degrees) | 56 (45–62) | 55 (44–62) | 44 (37–57) | 0.209 |
| Maximum Amplitude (mm) | 54 (50–60) | 52 (46–56) | 48 (42–51) | 0.253 |
| LY30 (percent) | 0 (0–0) | 0 (0–0.4) | 0 (0–.2) | 0.775 |
| INR | 1.5 (1.4–1.6) | 1.6 (1.4–1.7) | 1.5 (1.3–1.7) | 0.740 |
| Platelet Count (/mm3) | 60 (43–91) | 51 (45–64) | 57 (40–69) | 0.640 |
| Total OR | ||||
| Red Blood Cells (units) | 8 (6–12) | 14 (11–42) | 41 (24–69) | <0.001 |
| Plasma (units) | 10 (4–12) | 15 (9–30) | 31 (19–38) | <0.001 |
| Cryoprecipitate (pooled units) | 0 (0–1) | 1 (0–3) | 2 (1–5) | 0.004 |
| Platelets (units) | 1 (0–2) | 3 (1–4) | 3 (3–5) | <0.001 |
| Post Op Transfusions | ||||
| Red Blood Cells (units) | 0 (0–0) | 0 (0–4) | 8 (0–18) | <0.001 |
| Plasma (units) | 0 (0–1) | 0 (0–2) | 3 (0–10) | <0.001 |
| Cryoprecipitate (pooled units) | 0 (0–0) | 0 (0–1) | 1 (0–1) | <0.001 |
| Platelets (units) | 0 (0–0) | 0 (0–1) | 1 (0–2) | <0.001 |
| Massive Transfusion | 40% | 92% | 100% | <0.001 |
| Drain Output (liters) | 0.8 (0.5–1.1) | 1.3 (1.0–1.7) | 2.2 (0.5–3.0) | 0.034 |
| Return to OR | 20% | 35% | 45% | 0.366 |
| Reason | ||||
| Bleeding | 20% | 18% | 36% | 0.504 |
| Anastomosis | 0% | 10% | 9% | |
| Thrombosis | 0% | 7% | 0% | |
| Total ICU Days | 1 (0–4) | 0 (0–3) | 3 (1–13) | 0.055 |
| Post-operative Complications | 20% | 27% | 46% | 0.431 |
| Type | ||||
| Biliary | 0% | 0% | 8% | 0.739 |
| Thrombotic | 10% | 0% | 0% | |
| Infectious | 0% | 7% | 15% | |
| Bleeding | 0% | 10% | 0% | |
| Cardiac | 0% | 0% | 8% | |
| Acute Kidney Injury Requiring Dialysis | 10% | 10% | 15% | |
| Total Hospital Days Post-Transplantation | 10 (7–16) | 8 (7–14) | 26 (10–60) | 0.022 |
| 30-Day Mortality | 0% | 0% | 0% | 0.999 |
| 90-Day Mortality | 0% | 0% | 17% | 0.999 |
Abbreviations: MELD, Model for End Stage Liver Disease; LY30, lysis at 30 min; INR, international normalized ratio; BMI, body mass index; OR, operating room; ICU, intensive care unit.
Correlation between coagulopathy scores, blood product administration, and research thrombelastography
Table 1 demonstrates the correlation between coagulopathy score and individual blood product transfusions. The coagulopathy score had no correlation to the blood products transfused between incision and ligation of the hepatic artery. However, each subsequent coagulation score had a significant correlation to RBC transfusion requirements. Plasma transfusions correlated with coagulopathy score during the hepatectomy, anhepatic, and reperfusion 120. Cryoprecipitate only correlated with coagulation score at reperfusion 120, while platelet transfusions correlated with the coagulopathy score during reperfusion 30 and 120.
Table 1. Coagulopathy Score Correlations with Blood Product Transfusions During Surgery.
Highlighted boxes indicate significant correlations between a coagulopathy score at a certain timepoint and a blood product transfusion.
| Incision | Hepatectomy | Anhepatic | Reperfusion 30 | Reperfusion 120 | |
|---|---|---|---|---|---|
| Red Blood Cells | .128 p=0.430 | .592 p<0.001 | .542 p=0.001 | .466 p=0.002 | .674 p<0.001 |
| Plasma | .134 p=0.409 | .534 p<0.001 | .407 p=0.009 | .296 p=0.064 | .713 p<0.001 |
| Cryoprecipitate | na | .027 p=0.871 | .130 p=0.422 | .249 p=0.121 | .540 p<0.001 |
| Platelets | na | −.245 p=0.128 | .029 p=0.862 | .465 p=0.002 | .623 p<0.001 |
The correlation between TEG measurements and interval blood product requirements are depicted in Table 2. Similar to coagulopathy score, TEG indices had no correlation to blood product utilization during the initial phase of surgery. During hepatectomy, angle had an inverse correlation to RBC and plasma transfusion, but did not correlate to cryoprecipitate, which is the targeted hemostatic blood product. TEG angle during the anhepatic phase timepoint had an inverse correlation with RBC transfusions and cryoprecipitate, which would be an on-target blood product transfusion. MA had an inverse correlation to RBC units during the anhepatic phase, but an off-target inverse association with plasma; the on-target blood product should be platelet administration which lacked a correlation to MA at this timepoint. During reperfusion 30, there were no associations between TEG abnormalities and RBC transfusions, but there was an inverse correlation with MA and platelet transfusion supporting an on target hemostatic transfusion. Cryoprecipitate transfusions also had an off-target inverse correlation to MA. During reperfusion 120 none of the TEG indices had a correlation with interval blood product before the patient arrived to the floor or ICU following surgery, despite the coagulopathy score having a high correlation to all blood products transfused (Table 1).
Table 2. TEG Indices Correlations with Blood Product Transfusion During Surgery.
Boxes highlighted in yellow represent correlations between TEG indices and red blood cell transfusions. Boxes highlighted in light blue are correlations between TEG indices and on-target blood product transfusions. Boxes highlighted in purple represent correlations between TEG indices and off-target blood product transfusions. In the event of an off-target transfusion, boxes outlined in blue represent what would have been the on-target blood product for that specific TEG abnormality.2
| Incision | R time | Angle | Maximum Amplitude |
|---|---|---|---|
| Red Blood Cells | −0.185, p=0.252 | −0.095, p=0.558 | −0.145, p=.371 |
| Plasma | −0.061, p=0.719 | −0.170, p=0.294 | −0.179, p=0.270 |
| Cryoprecipitate | N/A | N/A | N/A |
| Platelets | N/A | N/A | N/A |
| Hepatectomy | R time | Angle | Maximum Amplitude |
| Red Blood Cells | 0.222, p=0.173 | −0.360, p=0.024 | −0.256, p=0.115 |
| Plasma | 0.125, p=0.449 | −0.363, p=0.023 | −0.258, p=0.113 |
| Cryoprecipitate | 0.186, p=0.257 | −0.134, p=0.415 | −0.134, p=0.415 |
| Platelets | 0.087, p=0.600 | 0.029, p=0.862 | 0.029, p=0.862 |
| Anhepatic | R time | Angle | Maximum Amplitude |
| Red Blood Cells | 0.299, p=0.075 | −0.496, p=0.001 | −0.592, p<0.001 |
| Plasma | 0.007, p=0.968 | −0.307, p=0.057 | −0.456, p=0.004 |
| Cryoprecipitate | −0.256, p=0.116 | −0.317, p=0.049 | −0.251, p=0.123 |
| Platelets | 0.092, p=0.581 | −0.295, p=0.072 | −0.252, p=0.126 |
| Reperfusion 30 | R time | Angle | Maximum Amplitude |
| Red Blood Cells | −0.041, p=0.805 | −0.029, p=0.861 | −0.188, p=0.252 |
| Plasma | −0.066, p=0.961 | −0.014, p=0.934 | −0.065, p=0.696 |
| Cryoprecipitate | 0.022, p=0.896 | −0.233, p=0.154 | −0.525, p=0.001 |
| Platelets | 0.084, p=0.612 | −0.246, p=.132 | −0.473, p=0.002 |
| Reperfusion 120 | R time | Angle | Maximum Amplitude |
| Red Blood Cells | 0.110, p=0.507 | −0.208, p=.203 | −0.180, p=.273 |
| Plasma | 0.027, p=0.872 | −0.102, p=0.539 | −0.064, p=0.697 |
| Cryoprecipitate | −0.190, p=0.247 | 0.077, p=0.643 | 0.076, p=0.646 |
| Platelets | 0.010, p=0.951 | −0.038, p=0.818 | 0.053, p=0.749 |
TEG transfusion triggers compared to products transfused stratified by coagulopathy score
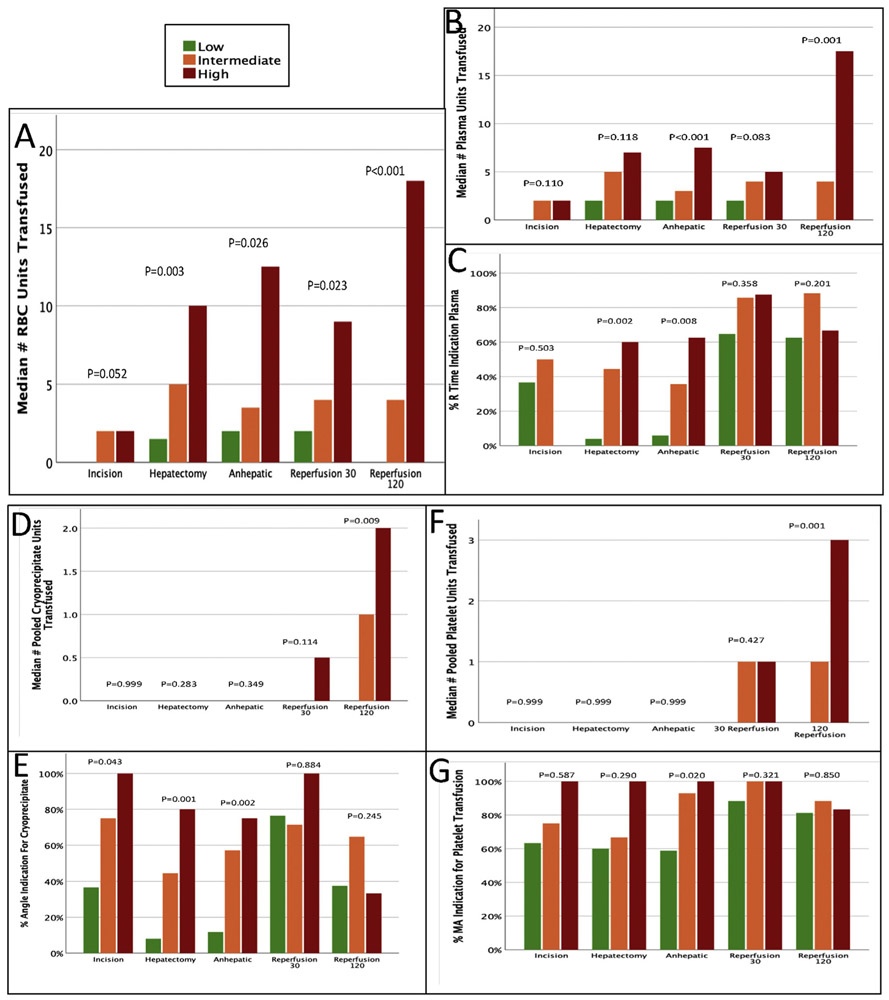
After stratifying patients into low, intermediate, and high risk of coagulopathy based on average coagulopathy score throughout the case, there was a significant difference in RBC transfusions between the different groups at all timepoints except at incision (<2, low; 2–4, intermediate; >4, high, Fig. 4A). The transfusion triggers used for hemostatic blood products (based on the randomized controlled trial) demonstrated a large number of patients throughout surgery had TEG-detected abnormalities indicating a coagulopathy. The coagulopathy score could appropriately differentiate patients for TEG abnormality warranting plasma transfusion during the hepatectomy and anhepatic phase of surgery (Fig. 4C). This matched plasma transfusion based on coagulation risk only during the anhepatic phase of surgery (Fig. 4B). The coagulopathy score also stratified patients with TEG-detected abnormalities indicative of cryoprecipitate transfusion during incision, hepatectomy, and anhepatic phase of surgery. The coagulopathy score did not significantly stratify patients for differences in platelet transfusion as the most patients did not receive platelet transfusions despite the majority of all patients from incision on had an TEG-derived indication for transfusion that persisted through the entire case (Fig. 4F and G). During reperfusion 120 the coagulopathy score risk significantly stratified patients by the number of RBC, plasma, cryoprecipitate, and platelet transfusions (Fig. 4A, B, D, and F) despite the coagulopathy score risk groups having no association with any TEG inflection points for transfusion (Fig. 4 panels C, E, G).
Fig. 4. Blood Products Transfused and TEG-indicated Transfusion Triggers Stratified by Timepoint and Coagulopathy Risk.
TEG indicated transfusion triggers were determined based on prior literature. Coagulopathy risk was determined by average bleeding score (<2, low; 2–4, intermediate; >4, high).
Discussion
This prospective observational study evaluating a trauma derived coagulopathy score in liver transplantation identified that the score had a high correlation with certain transfusions at different timepoints, as well as with TEG-derived coagulopathies. This suggests the potential for a complementary system consisting of clinically observed coagulopathy scores and viscoelastic testing for guiding transfusions during liver transplantation. When stratifying the data by average coagulopathy score during the case as in Fig. 4, this trend remained evident; plasma was extensively transfused throughout the case, while cryoprecipitate and fibrinogen were not given until the end of the case. TEG-detected coagulopathies warranting transfusion of cryoprecipitate and platelets were apparent in >80% of high-risk coagulopathy patients for the duration of surgery without transfusion until reperfusion 120. By the end of the case, no TEG indices correlated with any blood product administration or the coagulopathy score, which brings up concerns that patients were being transfused well after the development of coagulopathy in a “catch-up” fashion.
To our knowledge, this is the first study to investigate the correlation between a viscoelastic assay and a coagulopathy score in the setting of liver transplantation. The coagulopathy score used in this study was previously developed in the setting of trauma surgery.6 Studies that have prospectively evaluated this score have shown that it is associated with TEG-detected abnormalities including hyperfibrinolysis and blood product utilization.9-11 While those studies only scored patients at one timepoint, this study had multiple timepoints thus we were able to assess correlations to TEG indices more adequately. Coagulopathy scores have shown utility in a variety of other research and clinical settings. A coagulopathy score has been used in investigations of controlled hypotension in various surgical settings.12,13 A 6-point scoring system similar to that of this study including severity of bleeding was used, but also incorporated the amount of suctioning occurring in the surgical field. The International Society on Thrombosis and Haemostasis Bleeding Assessment Tool grades bleeding based on site of hemorrhage as well as the necessary interventions (ie packing vs transfusing requirement), which is utilized in the nontrauma setting. This coagulopathy score is currently used in clinical practice and correlates with bleeding severity as well as presence of disease in Factor 13 and Von Willebrand deficiencies.14,15
The coagulopathy score may aid in the clinical decision for laboratory testing for specific coagulation dysfunction during surgery. The utility of viscoelastic assays during liver transplantation has been established.5,16-20 Wang et al. first published a randomized controlled trial showing significantly less fresh-frozen plasma was used in patients monitored via TEG versus those monitored with conventional assays without changing outcomes.5 More recently in 2019, a randomized controlled trial showed that the median amount of intra-operative transfusions was reduced in the viscoelastic group compared with the standard group without a difference in post-operative outcomes.4 In that study, FFP and tranexamic acid were administered less often in the viscoelastic group, and fibrinogen infused more often. Our results complement these findings as we observed liberal FFP transfusion without TEG-derived indications, and lack of cryoprecipitate and platelet transfusions despite TEG indications throughout the surgery.
The decision to screen for then treat coagulopathy remains an area of debate in liver transplantation.3 Reluctance to treat coagulopathy early in liver transplant is multifactorial. There are general risks associated with blood product administration including anaphylaxis, infections, lung injury, and circulatory overload.21 There are also adverse events associated with transfusions in liver transplant. Platelet transfusions are associated with adverse outcomes in transplant22 and there is concern that transfused platelets are getting sequestered in the spleen.23 This concern for platelet administration is apparent in our study as the majority of liver transplant recipient have a TEG indication for platelet transfusions for the duration of surgery (Fig. 4G) but did not get transfused until after graft reperfusion (4F). We also appreciated a similar finding with TEG angle indicating a need for fibrinogen/cryoprecipitate transfusions (Fig. 4E), particularly for patients with high coagulopathy scores. Unlike platelet transfusions in liver transplant there is data lacking that cryoprecipitate use is associated with adverse outcomes. In fact, several studies concluded that fibrinogen replacement did not increase occurrence of thrombotic complications,24,25 and fibrinogen concentrate was associated with improved outcomes in liver transplantation when compared to fresh frozen plasma.26
While most blood products were used sparingly for treatment of coagulopathy during surgery, FFP was used more liberally through the duration of surgery (Fig. 4B). High volumes of FFP transfusions during liver transplant have recently been associated with an increased risk of early mortality.27 Plasma does not treat fibrinogen and platelet dysfunction, which was frequently transfused when a research TEG indicated one of these abnormalities (Table 2). Large volumes of FFP in attempt to correct these other coagulopathies will result in increased central venous pressure that can exacerbate surgical bleeding.28 Therefore, targeted correction of coagulopathy is an appealing strategy in liver transplantation. Optimal hemostasis is essential during transplantation as the number of red blood cell transfusions is associated with increased morbidity and mortality.29-31
There also appear to be times during surgery when TEG detected coagulopathy does not warrant treatment. Fig. 4A demonstrates that patients with coagulopathy scores of 2 at 120 min or reperfusion despite a TEG indication for transfusion in more than 30% of patients. These data support the classical philosophy indoctrinated by Dr. Starzl that “moderate bleeding such as experienced in [liver transplant] patients should not necessarily be regarded with alarm nor treated pharmacologically since spontaneous improvement can be expected…” and if “treatment should become necessary for a hemorrhagic diathesis, it should be guided by frequent measures of clotting parameters…“.2 We believe that the coagulopathy score is a starting point to standardize language in transplant surgery for when a patient has transitioned to from “moderate” to high rate of bleeding than tolerated.
The data from this study was collected at a single institution, thus limiting the generalizability of the results. Additionally, it is difficult to account for variability of scoring between individual transplant surgeons. To mitigate this variability, each transplant surgeon was trained on how to use the scoring system properly, and each score was determined by consensus, as often there were multiple attendings and fellows in the case. The surgeons were not blinded to the ongoing transfusions requirements of the patient during the case. None of the operating surgeons asked anesthesia input before making their decision on the coagulation score, which was based on the operative field. However, there is a potential for bias if the patient was actively being transfused. Research TEGs were performed at routine times regardless of the patient clinical bleeding status, and the results were blinded to the surgeon. These results also finalized 10–30 min after the clinical score was given. A clinical TEGs performed in a separate clinical laboratory were also ordered by some of the anesthesia providers at their own clinical discretion. These clinical TEGs ordered by the anesthesia providers used a kaolin activator, while research TEGs did not use an activator (native). While interpretation of the results between the two assays is comparable,32 only the kaolin TEG is FDA approved for clinical use. A clinical trial implementing this score would require the use of an activated TEG assay.
Conclusion
A standardized clinical coagulopathy score serves as a tool to improve communication between surgery and anesthesia teams. Implementation of the clinical coagulopathy score as an indicator for obtaining laboratory assessment and/or empiric transfusion of hemostatic blood products is warranted to assess if this strategy can result in reduction in blood transfusions while improving patient outcomes.
Acknowledgments
This study was supported in part by National Heart Lung and Blood Institute: K99HL151887, American Society of Transplant Surgeons Veloxis Fellowship Award, and University of Colorado Academic Enrichment Fund.
Footnotes
Declaration of competing interest
The authors of this paper have no conflicts of interest to declare.
References
- 1.Bezinover D, Dirkmann D, Findlay J, et al. Perioperative coagulation management in liver transplant recipients. Transplantation. 2018;102(4):578–592. [DOI] [PubMed] [Google Scholar]
- 2.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg. 1969;98(1):31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins RB, Raymond SL, Hartjes T, et al. Review: the perioperative use of thromboelastography for liver transplant patients. Transplant Proc. 2018;50(10):3552–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet A, Gilquin N, Steer N, et al. The use of a thromboelastometry-based algorithm reduces the need for blood product transfusion during orthotopic liver transplantation: a randomised controlled study. Eur J Anaesthesiol. 2019;36(11):825–833. [DOI] [PubMed] [Google Scholar]
- 5.Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42(7):2590–2593. [DOI] [PubMed] [Google Scholar]
- 6.Neal MD, Moore HB, Moore EE, et al. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: a TACTIC proposal. J Trauma Acute Care Surg. 2015;79(3):490–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bababekov YJ, Nydam TL, Pomposelli JJ, Moore HB. Goal-directed management of coagulation: the right treatment, the right patient, the right time. Transplantation. 2018;102(6):e303–e304. [DOI] [PubMed] [Google Scholar]
- 8.Akoglu H User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang R, Fox EE, Greene TJ, et al. Abnormalities of laboratory coagulation tests versus clinically evident coagulopathic bleeding: results from the prehospital resuscitation on helicopters study (PROHS). Surgery. 2018;163(4):819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore HB, Moore EE, Chapman MP, et al. Does tranexamic acid improve clot strength in severely injured patients who have elevated fibrin degradation products and low fibrinolytic activity, measured by thrombelastography? J Am Coll Surg. 2019;229(1):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore HB, Moore EE, Huebner BR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg. 2017;83(6):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Sun H, Jin H, Tan H. The effect of low central venous pressure on hepatic surgical field bleeding and serum lactate in patients undergoing partial hepatectomy: a prospective randomized controlled trial. BMC Surg. 2020;20(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang B, Zhang L, Lin Y, Zhang W, Li FS, Chen S. Comparison of effects and safety in providing controlled hypotension during surgery between dexmedetomidine and magnesium sulphate: a meta-analysis of randomized controlled trials. PloS One. 2020;15(1), e0227410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathare A, Al Omrani S, Al Hajri F, Al Obaidani N, Al Balushi B, Al Falahi K. Bleeding score in Type 1 von Willebrand disease patients using the ISTH-BAT questionnaire. Int J Lab Hematol. 2018;40(2):175–180. [DOI] [PubMed] [Google Scholar]
- 15.Naderi M, Cohan N, Haghpanah S, et al. Correlation of bleeding score with frequency and severity of bleeding symptoms in FXIII deficiency assessing by the ISTH Bleeding Assessment Tool. Transfus Apher Sci. 2019;58(4):495–497. [DOI] [PubMed] [Google Scholar]
- 16.Clevenger B, Mallett SV. Transfusion and coagulation management in liver transplantation. World J Gastroenterol. 2014;20(20):6146–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011;(12), CD009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson PJ, Moore HB, Moore EE, et al. Preoperative thrombelastography maximum amplitude predicts massive transfusion in liver transplantation. J Surg Res. 2017;220:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donohue CI, Mallett SV. Reducing transfusion requirements in liver transplantation. World J Transplant. 2015;5(4):165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamper RPC, Amorim TC, Costa L, Takaoka F, Serpa AN. The role of thromboelastometry in the assessment and treatment of coagulopathy in liver transplant patients. Einstein (Sao Paulo). 2017;15(2):243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazier SK, Higgins J, Bugajski A, Jones AR, Brown MR. Adverse reactions to transfusion of blood products and best practices for prevention. Crit Care Nurs Clin. 2017;29(3):271–290. [DOI] [PubMed] [Google Scholar]
- 22.Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108(4):1083–1091. [DOI] [PubMed] [Google Scholar]
- 23.Saab S, Brown RS Jr. Management of thrombocytopenia in patients with chronic liver disease. Dig Dis Sci. 2019;64(10):2757–2768. [DOI] [PubMed] [Google Scholar]
- 24.Danes AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JB. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang. 2008;94(3):221–226. [DOI] [PubMed] [Google Scholar]
- 25.Kirchner C, Dirkmann D, Treckmann JW, et al. Coagulation management with factor concentrates in liver transplantation: a single-center experience. Transfusion. 2014;54(10 Pt 2):2760–2768. [DOI] [PubMed] [Google Scholar]
- 26.Kozek-Langenecker S, Sorensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15(5):R239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertacco A, Barbieri S, Guastalla G, et al. Risk factors for early mortality in liver transplant patients. Transplant Proc. 2019;51(1):179–183. [DOI] [PubMed] [Google Scholar]
- 28.Saracoglu A, Saracoglu KT. Coagulopathy during liver transplantation. J Anaesthesiol Clin Pharmacol. 2018;34(3):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massicotte L, Denault AY, Beaulieu D, et al. Transfusion rate for 500 consecutive liver transplantations: experience of one liver transplantation center. Transplantation. 2012;93(12):1276–1281. [DOI] [PubMed] [Google Scholar]
- 30.Rana A, Petrowsky H, Hong JC, et al. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg. 2013;216(5):902–907. [DOI] [PubMed] [Google Scholar]
- 31.Xia VW, Du B, Braunfeld M, et al. Preoperative characteristics and intra-operative transfusion and vasopressor requirements in patients with low vs. high MELD scores. Liver Transpl. 2006;12(4):614–620. [DOI] [PubMed] [Google Scholar]
- 32.Coleman JR, Moore EE, Chapman MP, et al. Rapid TEG efficiently guides hemostatic resuscitation in trauma patients. Surgery. 2018;164(3):489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]