Abstract
Aims
Left ventricular pressure overload is associated with activation of the cardiac renin–angiotensin system, which may contribute to myocardial fibrosis and worse clinical outcomes. We sought to assess the association between treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) at baseline and clinical outcomes in patients with symptomatic, severe aortic stenosis (AS) undergoing transcatheter aortic valve replacement (TAVR) in the PARTNER 2 trial and registries.
Methods and results
A total of 3979 intermediate, high, or prohibitive risk patients who underwent TAVR in the PARTNER 2 trial and registries (excluding the valve in valve registry) were included in the study. Clinical outcomes at 2 years were compared according to baseline ACEI/ARB treatment status using Kaplan–Meier event rates and study-stratified multivariable Cox proportional hazards regression models. Sensitivity analysis was conducted using propensity score matching. Of 3979 patients who were included in the current analysis, 1736 (43.6%) were treated and 2243 (56.4%) were not treated with ACEI/ARB at baseline. Treatment with ACEI/ARB was associated with lower 2-year all-cause mortality (18.6% vs. 27.5%, P < 0.0001), cardiovascular mortality (12.3% vs. 17.9%, P < 0.0001), and non-cardiovascular mortality (7.2% vs. 11.7%, P < 0.0001). Angiotensin-converting enzyme inhibitor/ARB treatment at baseline remained independently associated with a lower hazard of 2-year all-cause and cardiovascular mortality after multivariable adjustment, and propensity score matching.
Conclusion
In a large cohort of patients with severe symptomatic AS from the PARTNER 2 trial and registries, ACEI/ARB treatment at baseline was independently associated with a lower risk of 2-year all-cause and cardiovascular mortality.
Keywords: Transcatheter aortic valve replacement, TAVR, TAVI, ACEI/ARB, Balloon-expandable valve, PARTNER
See page 955 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz819)
Introduction
Excessive left ventricular (LV) hypertrophy and fibrosis are associated with worse prognosis in patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve replacement (TAVR), especially if they persist after the valvular obstruction was treated.1 , 2 Both LV hypertrophy and fibrosis occur in response to the global haemodynamic load imposed on the left ventricle, which is the sum of the load imposed by the valvular obstruction (AS) and the arterial load (blood pressure).3–5 LV pressure overload is associated with activation of the renin–angiotensin system (RAS), which plays a major role in the progression of myocardial hypertrophy and enhances the increase in collagen I and III mRNA expression leading to myocardial fibrosis.6 , 7 Given the direct deleterious effects of RAS activation on the myocardium in AS, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) may moderate myocardial hypertrophy and fibrosis and may have a beneficial effect on LV remodelling in patients with severe AS. Angiotensin-converting enzyme inhibitors and ARBs also decrease blood pressure, thereby reducing the global haemodynamic load on the left ventricle.8 Via these mechanisms, ACEIs and ARBs may influence clinical outcomes for patients with severe AS. In the past, drugs like ACEIs/ARBs were considered relatively unsafe and even contraindicated in patients with significant AS,9 , 10 as they can theoretically lead to a significant drop in blood pressure. Nevertheless, this risk has never been demonstrated and in fact, a recent meta-analysis which included observational and small randomized trials found that these drugs are safe, and might be even beneficial, in patients with AS.11
The purpose of the current study was to examine whether baseline treatment with ACEI/ARB is an independent predictor of clinical outcomes in patients with severe symptomatic AS by analysing a large population of intermediate to high surgical risk patients undergoing TAVR in the PARTNER 2 trial and registries.
Methods
Study design and population
The analysis cohort is comprised of patients undergoing TAVR in the PARTNER 2 studies (ClinicalTrials.gov Identifier: NCT01314313), which included the PARTNER 2A and PARTNER 2B randomized cohorts and the PARTNER 2 S3 high-risk (S3HR) and intermediate risk (S3i) registries, details of which have been previously published.12 , 13 In brief, in the PARTNER 2A cohort, intermediate risk patients (predicted risk of 30-day mortality 4–8%, based on either the Society of Thoracic Surgeons (STS) score or clinical assessment by a multidisciplinary heart team) were randomly assigned to receive either surgical aortic valve replacement or TAVR using the SAPIEN XT THV. In the PARTNER 2B cohort, patients at prohibitive risk for surgery (heart team estimation of ≥50% risk of death or serious, irreversible morbidity with surgery) were randomly assigned to receive TAVR with either the original SAPIEN transcatheter heart valve (THV) or the 2nd generation SAPIEN XT THV. In the S3HR (high-risk patients, STS >8%) and S3i (intermediate-risk patients, STS 4–8%) registries, TAVR was performed using the newest generation S3 THV. In all cohorts, patients had severe AS, defined as a mean gradient >40 mmHg or jet velocity greater than 4.0 m/s and aortic valve area ≤0.8 cm2 or <0.5 cm2/m2, in addition to New York Heart Association (NYHA) functional Class II or greater symptoms. Key exclusion criteria for all cohorts included baseline creatinine >3 mg/dL or renal replacement therapy, a congenitally bicuspid aortic valve, severe aortic regurgitation, LV ejection fraction (LVEF) lower than 20%, and estimated life expectancy of less than 2 years. The protocols were approved by the institutional review boards of each participating site, and all patients provided written informed consent.
Both the randomized trial and the registries utilized electrocardiogram (ECG) and echocardiography core laboratories. Clinical events were adjudicated by an independent clinical events committee throughout 2 years of follow-up. The current analysis utilized an as treated population of patients who underwent TAVR in the PARTNER 2 randomized trial and registries, except the valve in valve registry, and excluded those with missing information regarding treatment with ACEI/ARB at baseline. The remaining patients were categorized based on whether they were treated with ACEI/ARB at baseline, and clinical outcomes were compared between groups by univariate and multivariable analysis.
Endpoints
Clinical data, ECGs, and transthoracic echocardiograms were obtained at baseline, hospital discharge, 30 days, 1 year, and 2 years. All ECGs and echocardiograms were interpreted by independent core laboratories using methodology previously described.14 The primary endpoint for the current analysis was all-cause death.
Statistical analysis
Continuous variables are presented as mean ± standard deviation and compared by Student’s t-test. Categorical variables are reported as percentages and frequencies, and compared by χ2 test or Fisher’s exact test, as appropriate. Time-to-event variables are presented as Kaplan–Meier event rates and compared by the log-rank test.
The primary analysis was performed using multivariable Cox proportional hazards regression. Competing risk analyses were also performed for the subtype of death (cardiovascular and non-cardiovascular) as a sensitivity analysis. Hazard ratios (HRs) were estimated using Fine and Gray’s proportional hazard regression model. Covariates included in the adjusted models were age, sex, body mass index (BMI), dyslipidaemia, diabetes mellitus, hypertension, chronic obstructive lung disease (COPD), COPD treated with oxygen, chronic kidney disease (serum creatinine ≥2 mg/dL), current smoker, previous or current cancer, peripheral vascular disease, history of atrial fibrillation or flutter, coronary artery disease (CAD), permanent pacemaker or automated implantable cardioverter-defibrillator, prior myocardial infarction, prior percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), prior stroke or transient ischaemic attack, STS risk score, access (transfemoral vs. transthoracic), prior hospitalization for symptoms of AS (within 6 months), frailty, treatment with beta-blockers at baseline, NYHA class, LVEF, aortic regurgitation (moderate or severe) at baseline, mitral regurgitation (moderate or severe) at baseline, tricuspid regurgitation (moderate or severe) at baseline, anaemia, thrombocytopenia, and serum albumin at baseline. All models (adjusted or non-adjusted) were stratified by study.
The propensity score model was developed using logistic regression, which modelled the likelihood of treatment with ACEI/ARB at baseline. This model included the aforementioned covariates with the following additional variables: hypothyroidism, cirrhosis, congestive heart failure, alcohol consumption, recreational drug usage, cerebrovascular disease, dementia, and treatment at baseline with dual antiplatelets, alpha 1 blockers, antiarrhythmics, calcium channel blockers, digoxin, diuretics, or statins. A greedy nearest neighbour algorithm was used to match those with and without ACEI/ARB treatment at baseline in a 1:1 ratio. The propensity-score matched cohort included a total of 2520 patients—1260 who were treated with ACEI/ARB at baseline and 1260 who were not. Baseline comparisons are reported using standardized differences. Survival analyses are performed using study-stratified marginal Cox proportional hazards regression to account for the matched nature of the data.
Results
Patient population and baseline characteristics
A total of 3990 patients underwent TAVR in the PARTNER II randomized trial and registries, excluding the valve in valve registry. Of these, 11 patients (0.3%) were excluded due to missing information regarding treatment with ACEI/ARB at baseline, resulting in a final study population of 3979 patients (Supplementary material online, Figure S1). Among these patients, 1736 (43.6%) were treated (1057 with ACEI and 636 with ARB) and 2243 (56.4%) were not treated with ACEI/ARB. Table 1 presents the baseline clinical characteristics of the patients according to baseline ACEI/ARB treatment status. Treatment with ACEI/ARB was associated with younger age, higher BMI, lower rates of chronic renal disease, and lower STS score. Coronary artery disease, prior myocardial infarction, PCI, or CABG were all more prevalent among patients who were treated with ACEI/ARB. Likewise, hypertension, dyslipidaemia, and diabetes were more common in patients who were treated with ACEI/ARB. New York Heart Association Class III or IV rates were similar in both groups. Systolic blood pressure was higher in patients treated with ACEI/ARB, while diastolic blood pressure did not differ significantly between the two groups. At discharge, 92.2% of patients (1600/1735) who were treated at baseline, and 11.9% of patients (267/2240) who were not treated at baseline, were treated with ACEI/ARB (Supplementary material online, Table S1). During 2-year follow-up, these percentages remained relatively steady in both groups. Patients treated with ACEI/ARB were more likely to be treated with beta-blockers and calcium channel blockers (Supplementary material online, Table S2). Treatment with beta-blockers slightly increased at discharge in both groups and remained stable during the 2-year follow-up. Treatment with calcium channel blockers, digoxin, and antiarrhythmic remained relatively steady during the 2-year follow-up.
Table 1.
Baseline characteristics
| ACEI/ARB treatment (n = 1736) | No ACEI/ARB treatment (n = 2243) | P-value | |
|---|---|---|---|
| Age (years) | 81.7 (7.2) | 82.9 (7.7) | <0.0001 |
| Male sex | 60.8 (1055/1736) | 58.4 (1311/2243) | 0.14 |
| Body mass index (kg/m2) | 28.8 (6.5) | 27.8 (6.2) | <0.0001 |
| STS score | 7.2 (3.8) | 7.7 (4.2) | <0.0001 |
| <4 | 7.9 (138/1736) | 7.9 (177/2243) | |
| 4–8 | 63.5 (1102/1736) | 56.6 (1269/2243) | |
| >8 | 28.6 (496/1736) | 35.5 (797/2243) | |
| Logistic EuroSCORE | 8.8 (9.1) | 9.6 (10.5) | 0.02 |
| NYHA functional class | 0.06 | ||
| III | 60.0 (1041/1736) | 58.0 (1301/2243) | |
| IV | 22.8 (395/1736) | 26.1 (585/2243) | |
| III or IV | 82.7 (1436/1736) | 84.1 (1886/2243) | 0.25 |
| CAD | 81.0 (1407/1736) | 76.0 (1705/2243) | 0.0001 |
| Prior MI | 21.0 (365/1736) | 17.1 (383/2243) | 0.002 |
| Prior PCI | 33.8 (587/1736) | 30.8 (690/2243) | 0.04 |
| Prior CABG | 36.2 (628/1736) | 26.6 (596/2243) | <0.0001 |
| Frailty | 19.3 (335/1735) | 26.0 (582/2239) | <0.0001 |
| PVD | 34.7 (602/1736) | 32.4 (727/2243) | 0.13 |
| Prior stroke or TIA | 18.7 (325/1736) | 18.9 (424/2243) | 0.88 |
| Hypertension | 96.3 (1672/1736) | 89.7 (2012/2243) | <0.0001 |
| Systolic blood pressure | 134.4 (21.6) | 131.2 (20.4) | <0.0001 |
| Diastolic blood pressure | 67.8 (11.5) | 67.5 (11.0) | 0.43 |
| Dyslipidaemia | 84.4 (1466/1736) | 79.4 (1780/2243) | <0.0001 |
| Diabetes mellitus | 40.6 (704/1736) | 32.5 (729/2243) | <0.0001 |
| Renal disease (Cr ≥2 mg/dL) | 7.2 (125/1736) | 9.8 (219/2243) | 0.004 |
| Liver disease | 1.7 (30/1736) | 2.5 (55/2243) | 0.12 |
| COPD | 34.7 (600/1730) | 31.8 (712/2239) | 0.056 |
| Oxygen dependent COPD | 7.4 (127/1724) | 9.3 (207/2234) | 0.03 |
| History of cancer | 31.8 (552/1736) | 33.0 (741/2243) | 0.41 |
| Haemoglobin (g/dL) | 12.2 (1.6) | 12.1 (1.8) | 0.04 |
| Electrocardiographic characteristics | |||
| Atrial fibrillation | 21.1 (360/1707) | 21.6 (476/2202) | 0.69 |
| First-degree AVB | 21.9 (377/1722) | 22.2 (491/2213) | 0.83 |
| LBBB | 6.8 (117/1722) | 5.6 (124/2213) | 0.12 |
| RBBB | 8.2 (141/1722) | 8.5 (188/2213) | 0.73 |
Values are mean (standard deviation) or % (n/N).
AVB, atrioventricular block; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; Cr, creatinine; EuroSCORE, European System for Cardiac Operative Risk Evaluation; LBBB, left bundle branch block; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; RBBB, right bundle branch block; STS, Society of Thoracic Surgeons; TIA, transient ischaemic attack.
Echocardiographic characteristics
Baseline echocardiographic characteristics are shown for patients with vs. without baseline ACEI/ARB treatment in Table 2. Treatment with ACEI/ARB was associated with slightly larger aortic valve area, LV mass, and LV stroke volume; however, after indexation to body surface area, these differences were no longer statistically significant. On the other hand, moderate or severe tricuspid regurgitation was more common among patients who were not treated with ACEI/ARB. Valvuloarterial Impedance (Zva) index was similar in patients treated vs. not treated with ACEI/ARB.
Table 2.
Baseline echocardiographic characteristics
| ACEI/ARB treatment (n = 1736) | No ACEI/ARB treatment (n = 2243) | P-value | |
|---|---|---|---|
| AV mean gradient (mmHg) | 44.7 (13.0) | 44.5 (13.5) | 0.64 |
| AV peak gradient (mmHg) | 76.3 (21.3) | 75.8 (21.7) | 0.47 |
| Aortic valve area (cm2) | 0.69 (0.18) | 0.68 (0.18) | 0.05 |
| Aortic valve area index (cm2/m2) | 0.36 (0.09) | 0.37 (0.09) | 0.34 |
| LV mass (g) | 236.2 (73.7) | 227.2 (70.7) | 0.0002 |
| LV mass index (g/m2) | 123.4 (33.5) | 122.1 (34.4) | 0.25 |
| Hypertrophya | 66.1 (1039/1571) | 64.0 (1277/1997) | 0.17 |
| LVEFb | 54.5 (13.7) | 54.9 (13.3) | 0.34 |
| LV stroke volumec (mL) | 70.9 (18.3) | 69.2 (18.1) | 0.004 |
| LV stroke volume index (mL/m2) | 37.3 (9.4) | 37.3 (9.7) | 0.97 |
| Valvuloarterial impedance (Zva) index | 5.05 (1.36) | 4.97 (1.32) | 0.08 |
| E/A ratio | 1.2 (0.8) | 1.2 (0.8) | 0.61 |
| E/E′ (lateral) | 11.5 (7.7) | 12.2 (8.3) | 0.03 |
| LA volume index (mL/m2) | 44.5 (15.5) | 44.1 (15.9) | 0.44 |
| RV function: moderately/severely decreased | 7.4 (60/806) | 7.3 (84/1152) | 0.90 |
| AR: moderate/severe | 9.6 (161/1674) | 10.3 (221/2153) | 0.51 |
| MR: moderate/severe | 15.7 (252/1603) | 18.0 (373/2072) | 0.07 |
| TR: moderate/severe | 12.4 (184/1489) | 16.4 (310/1893) | 0.001 |
| RV systolic pressure (mmHg) | 36.9 (13.5) | 37.5 (13.6) | 0.21 |
Values are mean (standard deviation) or % (n/N).
AR, aortic regurgitation; AV, aortic valve; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; TR, tricuspid regurgitation.
Defined as LV mass index >115 g/m2 for males and >95 g/m2 for female.
Visual or Simpson.
Assessed by Doppler.
Echocardiographic data 30 days after TAVR are shown in Supplementary material online, Table S3. The pressure gradient across the implanted aortic valve was slightly but significantly higher in patients treated with ACEI/ARB, whereas the rate of moderate or severe paravalvular aortic regurgitation after TAVR was similar in the two groups.
Procedural characteristics
Treatment with ACEI/ARB was more common among the cohort of patients who received the SAPIEN 3 valve than among the earlier cohorts. There were no significant differences in access route, or prosthesis size between the groups (Supplementary material online, Table S4). Time to discharge after TAVR was shorter for patients who were treated with ACEI/ARB.
Clinical outcomes
At 30 days after TAVR, the rate of NYHA III or IV class was significantly lower in patients treated with ACEI/ARB (10.8% vs. 14.1%, P = 0.003). The rates of 30-day adverse clinical outcomes did not differ significantly between the two groups (Table 3).
Table 3.
30-Day and 2-year clinical outcomes after TAVR in patients treated vs. not treated with ACEI/ARB
| ACEI/ARB treatment (n = 1736) | No ACEI/ARB treatment (n = 2243) | P-value | |
|---|---|---|---|
| 30-Day | |||
| All-cause death | 2.3 (40) | 3.2 (71) | 0.10 |
| Cardiovascular | 1.8 (32) | 2.5 (56) | 0.16 |
| Non-cardiovascular | 0.5 (8) | 0.7 (15) | 0.38 |
| Disabling stroke | 2.0 (34) | 2.1 (46) | 0.83 |
| Death or disabling stroke | 3.9 (67) | 4.8 (107) | 0.16 |
| Myocardial infarction | 1.3 (23) | 1.2 (27) | 0.74 |
| Periprocedural | 0.8 (14) | 0.9 (20) | 0.77 |
| Major vascular complicationsa | 7.1 (123) | 7.5 (167) | 0.66 |
| Life threatening or disabling bleedinga | 5.8 (101) | 7.1 (160) | 0.09 |
| Acute kidney injurya | 18.8 (265) | 17.8 (326) | 0.36 |
| New permanent pacemaker | 10.8 (187) | 10.1 (224) | 0.45 |
| 2-Year | |||
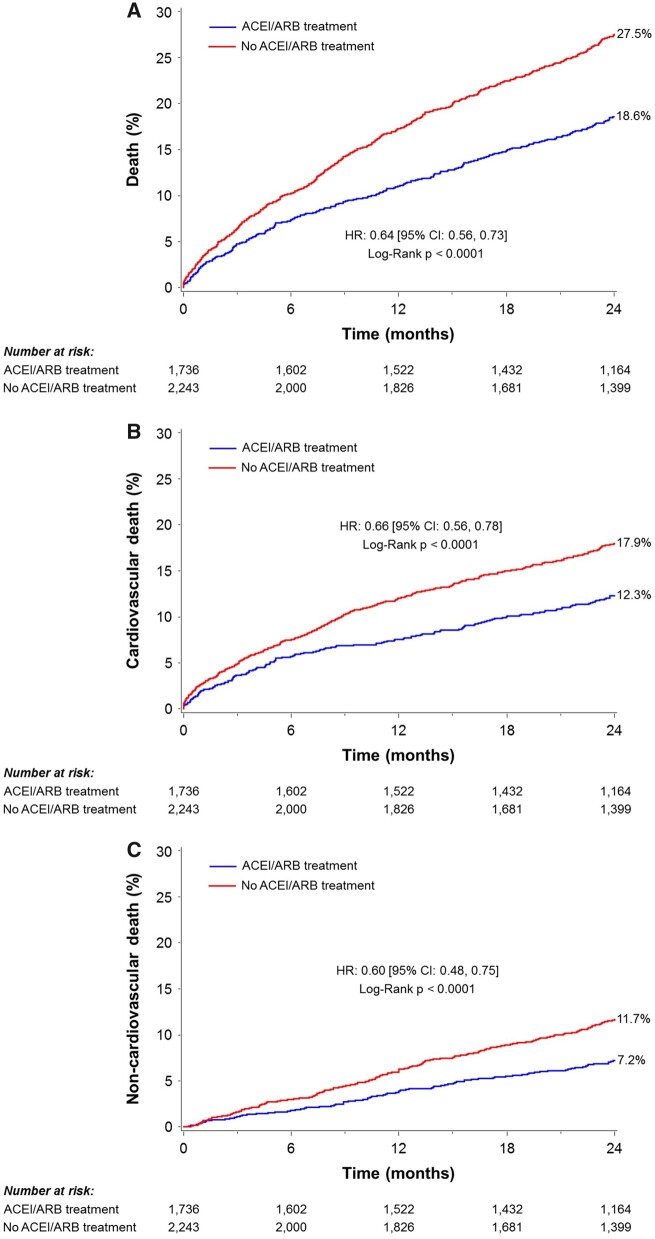
| All-cause death | 18.6 (316) | 27.5 (606) | <0.0001 |
| Cardiovascular | 12.3 (203) | 17.9 (378) | <0.0001 |
| Non-cardiovascular | 7.2 (113) | 11.7 (228) | <0.0001 |
Values are % (n).
According to VARC 2 definition.
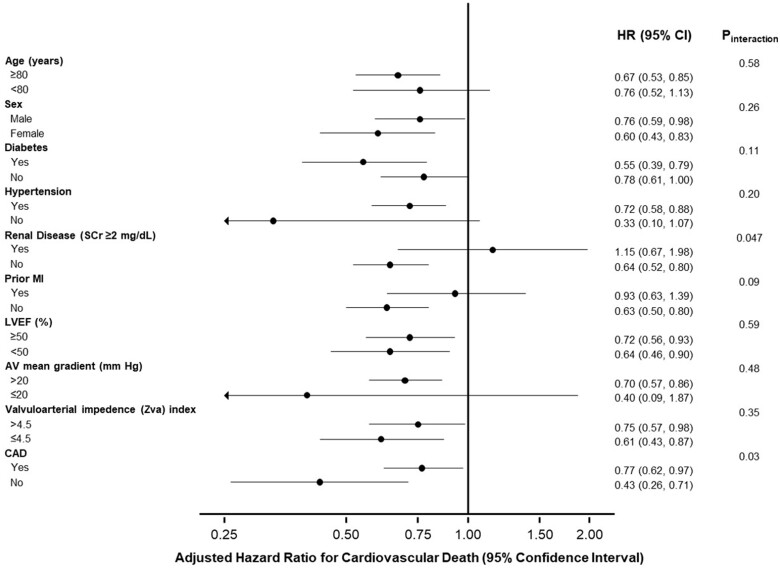
Compared with patients who were not treated with ACEI/ARB, the rate of the 2-year all-cause death was significantly lower for patients who were treated with ACEI/ARB at baseline (Table 3 and Figure 1). The reduction in all-cause mortality for patients with vs. without ACEI/ARB was driven by higher rates of both cardiovascular and non-cardiovascular death. These results remained consistent after multivariable adjustment (Table 4). Similarly, these results remained consistent after adjustment for competing risks (cardiovascular and non-cardiovascular death) (Supplementary material online, Table S5). The association between ACEI/ARB treatment at baseline and lower risk of cardiovascular death was consistent in most subgroups, with the exception of patients with chronic renal failure (P interaction = 0.047) and patients with CAD (P interaction = 0.03) (Table 5 and Figure 2).
Figure 1.
Two-year unadjusted clinical outcomes. Crude Kaplan–Meier failure rates according to angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment at baseline in patients undergoing transcatheter aortic valve replacement. (A) All-cause death, (B) cardiovascular death, and (C) non-cardiovascular death. CI, confidence interval; HR, hazard ratio.
Table 4.
Multivariable adjusted 2-year outcomes in patients treated vs. not treated with ACEI/ARB
| Adjusted hazard ratioa (95% confidence interval) | P-value | |
|---|---|---|
| All-cause death | 0.70 (0.60–0.82) | <0.0001 |
| Cardiovascular | 0.69 (0.56–0.84) | 0.0003 |
| Non-cardiovascular | 0.72 (0.56–0.93) | 0.01 |
Covariates included in the adjusted models were age, sex, BMI, dyslipidaemia, diabetes mellitus, hypertension, chronic obstructive lung disease (COPD), COPD treated with oxygen, chronic kidney disease (serum creatinine ≥2 mg/dL), current smoker, previous or current cancer, peripheral vascular disease, history of atrial fibrillation or flutter, coronary artery disease, permanent pacemaker or automated implantable cardioverter-defibrillator, prior myocardial infarction, prior percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), prior stroke or transient ischaemic attack (TIA), Society of Thoracic Surgeons (STS) risk score, access (transfemoral vs. transthoracic), prior hospitalization for symptoms of AS (within 6 months), frailty, treatment with beta-blockers at baseline, NYHA class, left ventricular ejection fraction (LVEF), aortic regurgitation (moderate or severe) at baseline, mitral regurgitation (moderate or severe) at baseline, tricuspid regurgitation (moderate or severe) at baseline, anaemia, thrombocytopenia, and albumin at baseline.
Adjusted hazard ratio and 95% confidence interval for patients treated vs. not treated with ACEI/ARB.
Table 5.
Multivariable adjusted 2-year outcomes in patients treated vs. not treated with ACEI/ARB in various subgroups
| Variables | Adjusted hazard ratioa for cardiovascular death (95% confidence interval) | P interaction |
|---|---|---|
| Age | ||
| ≥80 | 0.67 (0.53–0.85) | 0.58 |
| <80 | 0.76 (0.52–1.13) | |
| Sex | ||
| Male | 0.76 (0.59–0.98) | 0.26 |
| Female | 0.60 (0.43–0.83) | |
| Diabetes | ||
| Yes | 0.55 (0.39–0.79) | 0.11 |
| No | 0.78 (0.61–1.00) | |
| Hypertension | ||
| Yes | 0.72 (0.58–0.88) | 0.20 |
| No | 0.33 (0.10–1.07) | |
| Renal disease (Cr ≥2 mg/dL) | ||
| Yes | 1.15 (0.67–1.98) | 0.047 |
| No | 0.64 (0.52–0.80) | |
| Prior MI | ||
| Yes | 0.93 (0.63–1.39) | 0.09 |
| No | 0.63 (0.50–0.80) | |
| LVEF | ||
| ≥50% | 0.72 (0.56–0.93) | 0.59 |
| <50% | 0.64 (0.46–0.90) | |
| AV mean gradient (baseline) | ||
| >20 mmHg | 0.70 (0.57–0.86) | 0.48 |
| ≤20 mmHg | 0.40 (0.09–1.87) | |
| Valvuloarterial impedance (Zva) index | ||
| >4.5 | 0.75 (0.57–0.98) | 0.35 |
| ≤4.5 | 0.61 (0.43–0.87) | |
| CAD | ||
| Yes | 0.77 (0.62–0.97) | 0.03 |
| No | 0.43 (0.26–0.71) | |
AV, aortic valve; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Adjusted hazard ratio and 95% confidence interval for patients treated vs. not treated with ACEI/ARB.
Figure 2.
Adjusted 2-year outcomes in patients treated vs. not treated with angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker in various subgroups. Adjusted hazard ratio for cardiovascular death at 2 years after transcatheter aortic valve replacement, in patients treated vs. not-treated with angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker at baseline, in various subgroups.
When compared individually to no treatment with either drug, both ACEI and ARB treatment were independently associated with lower all-cause death and cardiovascular death, and no significant difference was seen when the two drugs were compared to each other regarding the effect on all-cause (P = 0.39) or cardiovascular mortality (P = 0.65) (Supplementary material online, Table S6).
Propensity-score matched cohort analysis
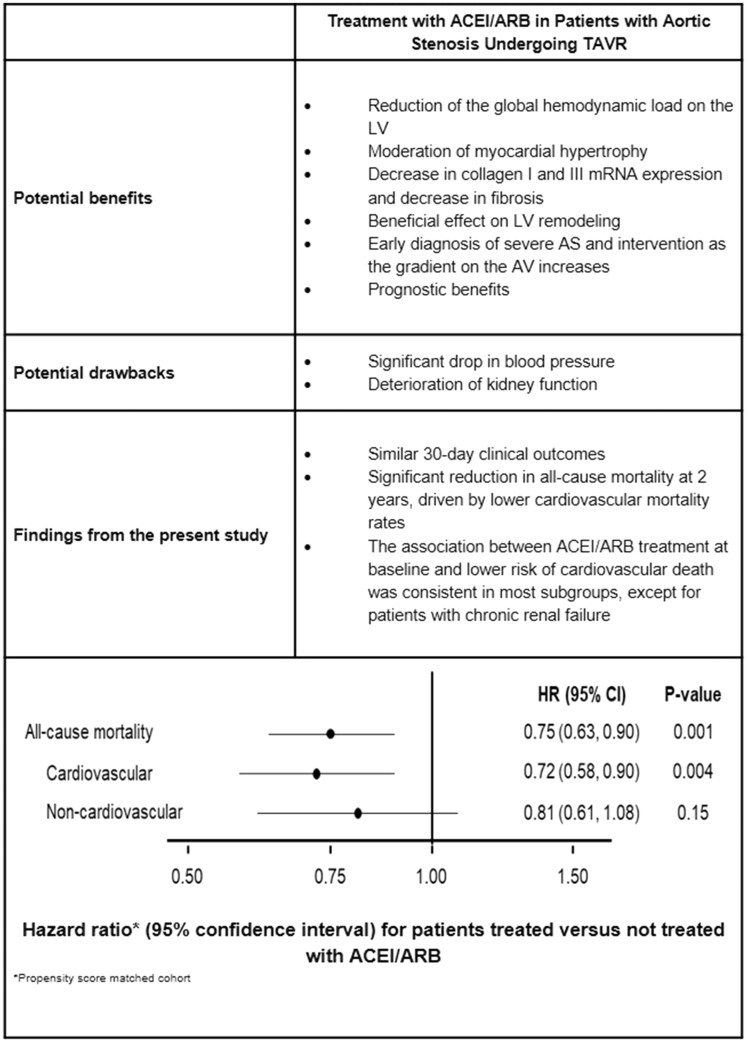
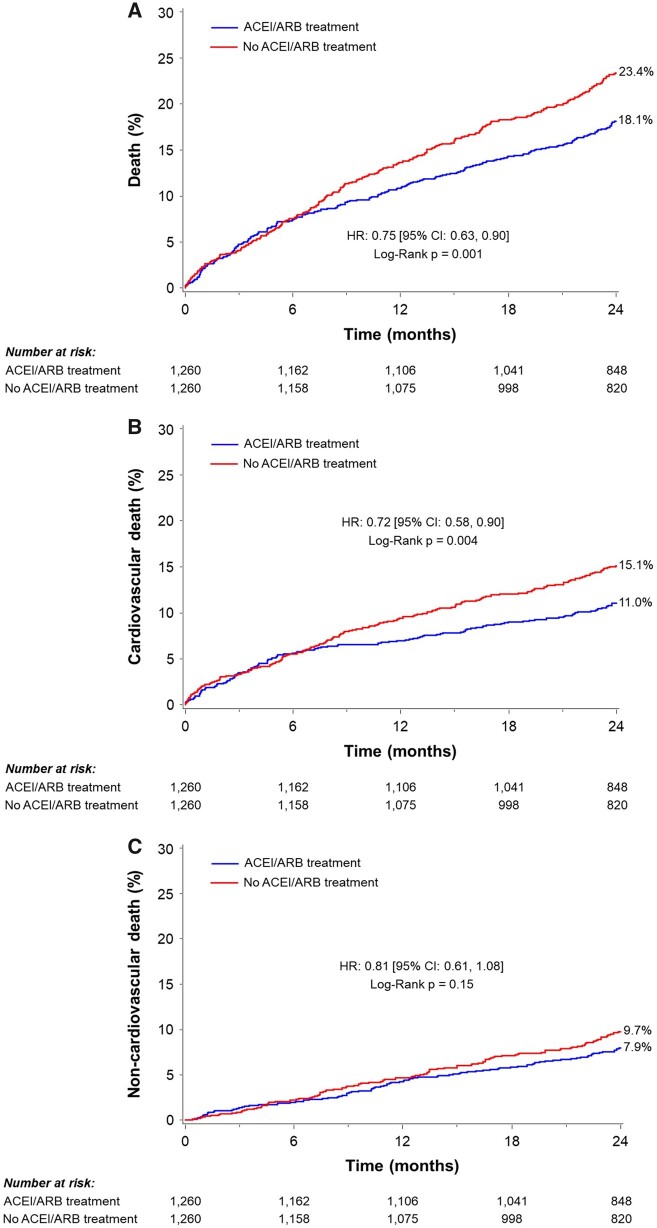
To minimize the potential selection bias inherent with the baseline treatment with ACEI/ARB, we performed a propensity-score matched cohort analysis. The propensity-score matched cohort included 2520 patients (1260 patients who were treated vs. 1260 patients who were not treated with ACEI/ARB at baseline). Baseline clinical, echocardiographic, and procedural characteristics were similar between the matched groups (Supplementary material online, Tables S7–S9). The HR for ACEI/ARB vs. no ACEI/ARB was 0.75 [95% confidence interval (CI) 0.63–0.90, P = 0.001] for all-cause death, 0.72 (95% CI 0.58–0.90, P = 0.004) for cardiovascular death, and 0.81 (95% CI 0.61–1.08, P = 0.15) for non-cardiovascular death (Figure 3). These results are summarized in the Take home figure.
Figure 3.
Two-year clinical outcomes in a propensity-score matched population. The Kaplan–Meier failure rates according to angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment at baseline in patients undergoing transcatheter aortic valve replacement. (A) All-cause death, (B) cardiovascular death, and (C) non-cardiovascular death. CI, confidence interval; HR, hazard ratio.
Take home figure.
The potential benefits and drawbacks of treatment with angiotensin-converting enzyme inhibitor/angiotensin II receptor at baseline in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement.
Discussion
The current study from the PARTNER 2 trial and registries examined the clinical implications of treatment with ACEI/ARB of patients with severe symptomatic AS at baseline before TAVR, on post-TAVR clinical outcomes. The major finding from this analysis is that among patients with severe symptomatic AS, treatment with ACEI/ARB at baseline was independently associated with decreased 2-year risks of all-cause and cardiovascular mortality after TAVR. To the best of our knowledge, the current study is the largest retrospective analysis examining the effect of pre-TAVR treatment with ACEI/ARB in patients with symptomatic severe AS. The data used in the current study was collected prospectively and comprehensively, echocardiographic and electrocardiographic data was analysed by a central core laboratory, allowing adequate adjustment to minimize the inherent risk of confounding. In addition, all events were adjudicated, including type of death (Cardiovascular vs. non-cardiovascular), allowing examination of the effect on specific outcome (cardiovascular death) and reducing the risk of confounding.
In the past, treatment of patients with severe AS with ACEI/ARB was considered unsafe and even contraindicated,9 , 10 as these drugs might induce severe hypotension resulting from vasodilatation in the presence of fixed LV outflow obstruction. However, this notion was based on theoretical risk and was not supported by clinical evidence. In fact, studies which examined ACEI/ARB treatment found that these drugs were well tolerated in patients with mild to moderate AS15 and even with symptomatic severe AS,16 although patients with congestive heart failure, LV dysfunction and low normal blood pressure were prone to have hypotension if treated with ACEI.16 , 17 More recent studies did not find any increase in mortality in patients with moderate to severe AS treated with ACEI/ARB,18 , 19 and some studies even demonstrated a favourable effect of RAS inhibitors on mortality,20 LV mass,18 , 19 and AS progression.21 A single retrospective study found a significant increase in mortality, at least with ACEI but not with ARB.21 A recent meta-analysis which included the above studies concluded that the use of RAS inhibitors in patients with AS was not associated with higher mortality rate, and was associated with lower rate of aortic valve replacement.11 Our results are consistent with a retrospective analysis from the STS/American College of Cardiology Transcatheter Valve Therapies (TVT) registry that reported that prescription with RAS inhibitors at hospital discharge in patients who underwent TAVR was associated with a lower risk of mortality and heart failure readmission at 1 year.22 In this analysis from the TVT registry, almost all patients who were treated with RAS inhibitors at discharge were already treated with these drugs before having TAVR. Thus, the beneficial effect could be attributed to treatment at baseline. Alternatively, given the fact that the majority of patients who were treated with RAS inhibitors at baseline in our study remained on RAS inhibition at discharge, the beneficial effect of baseline RAS inhibition observed in our study could be attributed at least in part to continued RAS inhibition post-discharge. In another recently published study, post-TAVR RAS blockade therapy has been found to be associated with greater LV mass index regression and reduced all-cause mortality.23 Nevertheless, data regarding treatment at baseline is lacking and categorization was performed according to post-TAVR RAS blockade therapy, which may introduce an additional selection bias, as patients who underwent a successful TAVR are more likely to be treated with ACEI/ARB.
In the current study, treatment of patients with severe symptomatic AS, with ACEI/ARB was not only safe but also associated with lower mortality rates after TAVR. One can speculate that these results are confounded due to a selection bias, i.e. that more stable, healthier patients can be expected to be more likely to be treated with ACEI/ARB than sicker, less stable patients for whom these drugs may be withheld. However, although patients who were treated with ACEI/ARB were slightly younger, had slightly lower STS score and were less likely to have renal disease than patients without ACEI/ARB, they had similar rates of NYHA class III or IV symptoms, and higher rates of CAD and cardiovascular risk factors including diabetes. More importantly, the association between ACEI/ARB treatment and lower risk of all-cause and cardiovascular mortality persisted after multivariable adjustment, and propensity score matching. Moreover, we included treatment with beta-blockers in the multivariable models. Beta-blockers are also usually given to relatively stable patients and avoided in decompensated and unstable patients, and would therefore be expected to be associated with the same residual confounding as ACEI/ARB treatment. In contrast to ACEI/ARB treatment, which remained significantly associated with all-cause and cardiovascular mortality after inclusion of beta-blocker treatment in the multivariable model, treatment with beta-blocker was not significantly associated with either all-cause or cardiovascular mortality.
One possible explanation for the observed association between ACEI/ARB treatment and favourable outcomes for patients with AS who undergo TAVR is the reduction of the global pressure overload of the left ventricle, with subsequent attenuation of irreversible damage to the LV induced by chronic stress and inappropriate, pathological hypertrophy. Although no difference was observed in hypertrophy rates between patients treated vs. not treated with ACEI/ARB, the extent of inappropriate, pathological hypertrophy is not known. Nevertheless, Zva which reflects the global load imposed on the left ventricle,24 did not differ significantly between the two groups, and thus, could not support this explanation. Indeed, systolic blood pressure was higher in patients treated with ACEI/ARB, implying that the effect of these drugs was not exerted via reduction of the global pressure overload. Furthermore, beta-blockers, which reduce haemodynamic and metabolic overload in patients with AS,25 were not associated with decreased mortality post-TAVR.
Another possible explanation for the observed association between ACEI/ARB treatment and favourable outcomes in the present study relates to the inhibitory effect of ACEI/ARB on hypertrophy and fibrosis.26 Physiologically, the LV hypertrophies in response to pressure overload, in order to maintain a relatively constant wall stress (Laplace law). Unlike physiological hypertrophy, which occurs in response to a relatively shorter stress, prolonged AS-related hypertrophic stress may cause non-compensatory pathological hypertrophy that reactivates the foetal gene expression of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP).27 Furthermore, chronic, prolonged pressure overload leads to myocardial hypertrophy that consists of not only hypertrophied myofibrils but also collagen and fibronectin depositions.28 The hormones of the RAS, angiotensin II and aldosterone, primarily involved in promoting the adverse structural remodelling of the myocardial collagen matrix.26 These maladaptive changes are only partially reversible after aortic valve replacement and likely to be responsible for persistent abnormalities in diastolic function and increased morbidity and mortality after valve replacement.28 Hypertrophy has been shown to be associated with increased mortality in patients with AS29 even after valve replacement.30 , 31 Attenuation of hypertrophy and fibrosis by ACEI/ARB,26 might favourably influence prognosis in severe AS patients undergoing TAVR.
Interestingly, the beneficial effect of ACEI/ARB was smaller in patients with CAD and absent in patients with chronic kidney disease. In patients with CAD and severe AS the decrease in coronary perfusion pressure induced by ACEI/ARB via the decrease in blood pressure, might be significant. In patients with chronic renal disease and severe AS, ACEI/ARB might decrease renal perfusion and worsen renal function, which may negate the beneficial cardiac effects.
As the current study is based on retrospective analysis and needs to be corroborated by prospective well-designed studies, randomized trials are warranted to address whether ACEI/ARB treatment in these patients is safe and improve prognosis, and whether the treatment pre- or post-TAVR (or both) is more influential. In fact, one such trial, the RASTAVI trial, which will randomize patients who have received TAVR to ramipril vs. placebo, is already underway.32
Limitations
The present study is a post hoc analysis and should be considered hypothesis generating. The PARTNER 2 trial was not designed or powered to examine outcomes according to treatment with ACEI/ARB at baseline. Although our findings regarding lower all-cause and cardiovascular mortality among patients treated with ACEI/ARB at baseline remained statistically significant after multivariable adjustment and after propensity score matching, we cannot rule out the possibility that the analysis is confounded by other unmeasured factors that are correlated with treatment with ACEI/ARB treatment at baseline. As the majority of patients who were treated pre-TAVR continued to be treated after the procedure we cannot differentiate between the treatment before and after the procedure, and whether the observed beneficial effect is attributed to ACEI/ARB treatment before TAVR, after TAVR, or both. In addition, we did not collect data regarding the dosage and duration of treatment pre-TAVR.
In conclusion, in a large cohort of patients from the PARTNER 2 trial and registries, treatment with ACEI/ARB at baseline was independently associated with lower 2-year mortality. This association was observed regardless of the baseline LVEF or presence of hypertension but was not observed in patients with chronic renal disease and was less pronounced in patients with CAD. Further studies should be conducted investigating the effects of ACEI/ARB treatment on clinical outcomes in patients undergoing TAVR.
Perspectives
Competency in patient care and procedural skills
In patients with severe symptomatic AS treated with TAVR, treatment with ACEI/ARB at baseline is independently associated with better 2-year prognosis, including lower risk of both all-cause and cardiovascular mortality.
Translational outlook
Treatment of patients with severe AS with ACEI/ARB could improve long-term outcomes after TAVR. Randomized studies should assess whether the association between treatment with ACEI/ARB and better outcomes are causative, and whether ACEI/ARB treatment pre- or post-TAVR (or both) is important to improve prognosis.
Funding
The PARTNER 2 Trial was funded by Edwards Lifesciences.
Conflict of interest: T.N. reports personal fees from Edwards Lifesciences, Boston Scientific, Medtronic, and BioTrace, outside the submitted work. A.K. reports grants from Medtronic, Abbott Vascular, Boston Scientific, Abiomed, CathWorks, Siemens, Philips, ReCor Medical, and Spectranetics, outside the submitted work. V.H.T. reports grants and personal fees from Edwards Lifesciences, outside the submitted work. T.P.V. reports institutional grants from Edwards Lifesciences and Medtronic, personal fees from JenaValve and Abbott, outside the submitted work. P.S.D. reports grants from Edwards Lifesciences, during the conduct of the study. S.K.K. reports grants from Edwards Lifesciences, during the conduct of the study; grants from Medtronic and Boston Scientific, grants and personal fees from Abbott Vascular, non-financial support from Dura Biotech and Thubrikar Aortic Valve, Inc.; personal fees from Claret Medical, Admedus, and Meril Lifesciences, and other from Biotrace Medical, outside the submitted work. M.B.L. reports institutional research grants from Edwards Lifesciences, Medtronic, and Boston Scientific, outside the submitted work. All other authors report that they have nothing to disclose.
Supplementary Material
References
- 1. Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271–1279. [DOI] [PubMed] [Google Scholar]
- 2. Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone G. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011;97:301–307. [DOI] [PubMed] [Google Scholar]
- 3. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pibarot P, Dumesnil JG. Improving assessment of aortic stenosis. J Am Coll Cardiol 2012;60:169–180. [DOI] [PubMed] [Google Scholar]
- 5. Antonini-Canterin F, Huang G, Cervesato E, Faggiano P, Pavan D, Piazza R, Nicolosi GL. Symptomatic aortic stenosis: does systemic hypertension play an additional role? Hypertension 2003;41:1268–1272. [DOI] [PubMed] [Google Scholar]
- 6. Shimizu M, Tanaka R, Fukuyama T, Aoki R, Orito K, Yamane Y. Cardiac remodeling and angiotensin II-forming enzyme activity of the left ventricle in hamsters with chronic pressure overload induced by ascending aortic stenosis. J Vet Med Sci 2006;68:271–276. [DOI] [PubMed] [Google Scholar]
- 7. Fielitz J, Hein S, Mitrovic V, Pregla R, Zurbrugg HR, Warnecke C, Schaper J, Fleck E, Regitz-Zagrosek V. Activation of the cardiac renin-angiotensin system and increased myocardial collagen expression in human aortic valve disease. J Am Coll Cardiol 2001;37:1443–1449. [DOI] [PubMed] [Google Scholar]
- 8. Routledge HC, Townend JN. ACE inhibition in aortic stenosis: dangerous medicine or golden opportunity? J Hum Hypertens 2001;15:659–667. [DOI] [PubMed] [Google Scholar]
- 9. Poullis M. Aortic stenosis and ACE inhibitors. Lancet 1998;352:821.. [DOI] [PubMed] [Google Scholar]
- 10. Rimington H, Takeda S, Chambers J. Aortic stenosis and ACE inhibitors. Lancet 1998;352:820–821. [DOI] [PubMed] [Google Scholar]
- 11. Andersson C, Abdulla J. Is the use of renin-angiotensin system inhibitors in patients with aortic valve stenosis safe and of prognostic benefit? A systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother 2017;3:21–27. [DOI] [PubMed] [Google Scholar]
- 12. Kodali S, Thourani VH, White J, Malaisrie SC, Lim S, Greason KL, Williams M, Guerrero M, Eisenhauer AC, Kapadia S, Kereiakes DJ, Herrmann HC, Babaliaros V, Szeto WY, Hahn RT, Pibarot P, Weissman NJ, Leipsic J, Blanke P, Whisenant BK, Suri RM, Makkar RR, Ayele GM, Svensson LG, Webb JG, Mack MJ, Smith CR, Leon MB. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J 2016;37:2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, Investigators P. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 14. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA Jr, Nakatani S, Quinones MA, Rakowski H, Rodriguez LL, Swaminathan M, Waggoner AD, Weissman NJ, Zabalgoitia M; American Society of Echocardiography's Guidelines and Standards Committee; Task Force on Prosthetic Valves; American College of Cardiology Cardiovascular Imaging Committee; Cardiac Imaging Committee of the American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography; American College of Cardiology Foundation ; American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975–1014, quiz 1082–4. [DOI] [PubMed] [Google Scholar]
- 15. O'Brien KD, Zhao XQ, Shavelle DM, Caulfield MT, Letterer RA, Kapadia SR, Probstfield JL, Otto CM. Hemodynamic effects of the angiotensin-converting enzyme inhibitor, ramipril, in patients with mild to moderate aortic stenosis and preserved left ventricular function. J Investig Med 2004;52:185–191. [DOI] [PubMed] [Google Scholar]
- 16. Chockalingam A, Venkatesan S, Subramaniam T, Jagannathan V, Elangovan S, Alagesan R, Gnanavelu G, Dorairajan S, Krishna BP, Chockalingam V; Symptomatic Cardiac Obstruction-Pilot Study of Enalapril in Aortic Stenosis. Safety and efficacy of angiotensin-converting enzyme inhibitors in symptomatic severe aortic stenosis: symptomatic Cardiac Obstruction-Pilot Study of Enalapril in Aortic Stenosis (SCOPE-AS). Am Heart J 2004;147:E19. [DOI] [PubMed] [Google Scholar]
- 17. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 18. Bull S, Loudon M, Francis JM, Joseph J, Gerry S, Karamitsos TD, Prendergast BD, Banning AP, Neubauer S, Myerson SG. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging 2015;16:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bang CN, Greve AM, Kober L, Rossebo AB, Ray S, Boman K, Nienaber CA, Devereux RB, Wachtell K. Renin-angiotensin system inhibition is not associated with increased sudden cardiac death, cardiovascular mortality or all-cause mortality in patients with aortic stenosis. Int J Cardiol 2014;175:492–498. [DOI] [PubMed] [Google Scholar]
- 20. Nadir MA, Wei L, Elder DH, Libianto R, Lim TK, Pauriah M, Pringle SD, Doney AD, Choy AM, Struthers AD, Lang CC. Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol 2011;58:570–576. [DOI] [PubMed] [Google Scholar]
- 21. Capoulade R, Clavel MA, Mathieu P, Cote N, Dumesnil JG, Arsenault M, Bedard E, Pibarot P. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur J Clin Invest 2013;43:1262–1272. [DOI] [PubMed] [Google Scholar]
- 22. Inohara T, Manandhar P, Kosinski AS, Matsouaka RA, Kohsaka S, Mentz RJ, Thourani VH, Carroll JD, Kirtane AJ, Bavaria JE, Cohen DJ, Kiefer TL, Gaca JG, Kapadia SR, Peterson ED, Vemulapalli S. Association of renin-angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter aortic valve replacement. JAMA 2018;320:2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ochiai T, Saito S, Yamanaka F, Shishido K, Tanaka Y, Yamabe T, Shirai S, Tada N, Araki M, Naganuma T, Watanabe Y, Yamamoto M, Hayashida K. Renin-angiotensin system blockade therapy after transcatheter aortic valve implantation. Heart 2018;104:644–651. [DOI] [PubMed] [Google Scholar]
- 24. Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol 2005;46:291–298. [DOI] [PubMed] [Google Scholar]
- 25. Hansson NH, Sörensen J, Harms HJ, Kim WY, Nielsen R, Tolbod LP, Frøkiær J, Bouchelouche K, Dodt KK, Sihm I, Poulsen SH, Wiggers H. Metoprolol reduces hemodynamic and metabolic overload in asymptomatic aortic valve stenosis patients: a randomized trial. Circ Cardiovasc Imaging 2017;10: [DOI] [PubMed] [Google Scholar]
- 26. Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J 1995;16(Suppl O):107–109. [DOI] [PubMed] [Google Scholar]
- 27. Dietz R, Haass M, Kubler W. Atrial natriuretic factor. Its possible role in hypertension and congestive heart failure. Am J Hypertens 1989;2:29S–33S. [DOI] [PubMed] [Google Scholar]
- 28. Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG. Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications. J Thorac Cardiovasc Surg 2012;143:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capoulade R, Clavel M-A, Le Ven F, Dahou A, Thébault C, Tastet L, Shen M, Arsenault M, Bédard É, Beaudoin J, O’Connor K, Bernier M, Dumesnil JG, Pibarot P. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging 2017;18:1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Une D, Mesana L, Chan V, Maklin M, Chan R, Masters RG, Mesana TG, Ruel M. Clinical impact of changes in left ventricular function after aortic valve replacement: analysis from 3112 patients. Circulation 2015;132:741–747. [DOI] [PubMed] [Google Scholar]
- 31. Beach JM, Mihaljevic T, Rajeswaran J, Marwick T, Edwards ST, Nowicki ER, Thomas J, Svensson LG, Griffin B, Gillinov AM, Blackstone EH. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014;147:362–369.e8. [DOI] [PubMed] [Google Scholar]
- 32. Amat-Santos IJ, Catalá P, Diez del Hoyo F, Fernandez-Diaz JA, Alonso-Briales JH, Del Trigo M, Regueiro A, Juan-Salvadores P, Serra V, Gutierrez-Ibanes E, Muñoz-García AJ, Nombela-Franco L, Sabate M, Jimenez-Diaz VA, García del Blanco B, López J, Varela-Falcón LH, Sevilla T, Arnold R, Revilla A, San Roman JA. Impact of renin-angiotensin system inhibitors on clinical outcomes and ventricular remodelling after transcatheter aortic valve implantation: rationale and design of the RASTAVI randomised multicentre study. BMJ Open 2018;8:e020255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.