Abstract
STUDY QUESTION
To what extent does fecundability vary across seasons?
SUMMARY ANSWER
After accounting for seasonal patterns in pregnancy planning, we observed higher fecundability in the fall and lower fecundability in the spring, particularly at lower latitudes.
WHAT IS KNOWN ALREADY
In human populations, there are strong seasonal patterns of births that vary across geographic regions and time periods. However, previous studies of seasonality and fecundity are limited because they examine season of birth rather than season of conception and therefore neglect to account for seasonal variation in initiating attempts to conceive or pregnancy loss or differences in gestational length.
STUDY DESIGN, SIZE, DURATION
We conducted a preconception cohort study of 14 331 women residing in North America (June 2013–May 2018: n = 5827) and Denmark (June 2007–May 2018: n = 8504). Participants were attempting to conceive without fertility treatment and had been attempting pregnancy for ≤6 menstrual cycles at enrolment.
PARTICIPANTS/MATERIAL, SETTING, METHODS
We collected information on season of each pregnancy attempt using last menstrual period dates over the study period. Pregnancy was reported on female bi-monthly follow-up questionnaires. We fit log-binomial models with trigonometric regression to examine periodic variation in fecundability. We accounted for seasonal variation in initiation of pregnancy attempts by including indicator variables for menstrual cycle of attempt in the regression models.
MAIN RESULTS AND THE ROLE OF CHANCE
Initiation of pregnancy attempts peaked in September, with stronger seasonality in North America than in Denmark (48 vs. 16% higher probability initiating attempts in September compared with March). After accounting for seasonal variation in initiation of pregnancy attempts, we observed modest seasonal variation in fecundability, with a peak in the late fall and early winter in both cohorts, but stronger peak/low ratios in North America (1.16; 95% confidence interval [CI]: 1.05, 1.28) than in Denmark (1.08; 95% CI: 1.00, 1.16). When we stratified the North American data by latitude, we observed the strongest seasonal variation in the southern USA (peak/low ratio of 1.45 [95% CI: 1.14, 1.84]), with peak fecundability in late November.
LIMITATIONS, REASONS FOR CAUTION
We estimated menstrual cycle dates between follow-up questionnaires, which may have introduced exposure misclassification, particularly when women skipped follow-up questionnaires. We were unable to measure seasonally varying factors that may have influenced fecundability, including ambient temperature, vitamin D levels or infectious disease.
WIDER IMPLICATIONS OF THE FINDINGS
An understanding of how fecundability varies across seasons could help identify factors that can impair reproductive function. Neglecting to account for seasonal variation in initiation of pregnancy attempts could bias estimates of seasonal patterns in fecundability. This is the first preconception cohort study to examine seasonal variation in fecundability after accounting for seasonality in initiation of pregnancy attempts. Fecundability was highest in the fall and lowest in the spring, with stronger effects in southern latitudes of North America, suggesting that seasonal exposures may affect fecundity.
STUDY FUNDING/COMPETING INTEREST(S)
This research was funded by the Eunice K. Shriver National Institute of Child Health and Human Development (R21-050264, R01-HD060680, R21-HD072326 and R01-HD086742) and the Danish Medical Research Council (271-07-0338). The authors declare no conflicts of interest.
Keywords: fecundability, fertility, internet-based study, preconception cohort, season, seasonality
Introduction
In human populations, there are well-documented seasonal patterns of birth (Becker 1991; Roenneberg and Aschoff, 1990a; 1990b) that vary by geographic location and time period. In the USA, births tend to peak during August and September and reach a trough during April and May, with stronger spring declines in the southern regions (Centers for Disease Control and Prevention, 2018a; Lam and Miron, 1994, 1996; Seiver 1985). A recent study provided evidence that birth seasonality in the USA follows a gradient by latitude, with births peaking in the spring and summer in northern states, and in the autumn in southern states (Martinez-Bakker et al., 2014). In Northern Europe, there tends to be a peak in births during the late spring and a trough in the late fall and early winter (Lam and Miron, 1994; Russell et al. 1993). This finding was confirmed in a Danish cohort study: women had longer time to pregnancy before conceiving in February, March and April, and shorter time to pregnancy before conceiving in August, September and October (Stolwijk et al., 1996a). Seasonal variation in births may be caused by environmental, behavioural or cultural factors that vary across seasons, such as sunlight-related variation in vitamin D levels, air pollution, daylight length, infectious diseases and intercourse frequency.
Seasonal birth patterns may also reflect seasonal variation in when couples choose to initiate pregnancy attempts (Basso et al. 1995; Stolwijk et al., 1996b). Couples may time their pregnancy attempts for a variety of reasons including employment schedules (e.g. more flexible summer hours), comfort (e.g. to avoid being pregnant during hot months) or personal choice (e.g. desiring a fall baby). Fecundability, the average per-cycle probability of conception amongst couples engaged in regular unprotected intercourse declines over pregnancy attempt time. Thus, seasonal variation in initiation of pregnancy attempts could cause seasonal variation in fecundability.
Prior studies examining seasonal patterns in human reproduction have primarily used data on births, rather than conceptions. These data are problematic because they fail to account for seasonal variation in initiation of pregnancy attempts or pregnancy loss, and they ignore differences in gestational length, which can cause inaccuracies in estimating conception dates. In the present analysis, we use prospective data from two preconception cohorts in North America and Denmark to measure seasonal variations in initiation of pregnancy attempts and fecundability and the extent to which these patterns differ across geographic regions. Seasonal variation in timing of pregnancy initiation attempts results from cultural and behavioural factors, whereas seasonal variation in fecundability stems from seasonal factors affecting the underlying biological mechanisms of fecundity.
Materials and Methods
Study design and population
Pregnancy Study Online (PRESTO) is a web-based preconception cohort study of pregnancy planners from the USA and Canada (Wise et al., 2015). Eligible women are 21–45 years old and trying to conceive without fertility treatments. Participants complete a baseline questionnaire on demographic, behavioural, medical and reproductive data and follow-up questionnaires every 8 weeks for up to 12 months. Women are randomised with 50% probability to receive a subscription to FertilityFriend.com, a menstrual charting and fertility tracking app. From June 2013 through May 2018, 7443 women completed the baseline questionnaire. We excluded 91 women who reported at baseline that the first day of their most recent menstrual cycle was at least 6 months before baseline, 11 women who reported implausible baseline menstrual cycle dates and 24 women with no prospectively reported menstrual cycle dates (i.e. women not menstruating over follow-up). We additionally excluded 1490 women who had been attempting conception for more than 6 cycles at baseline, because women attempting conception for longer than 6 cycles are more likely to have changed their behaviours in response to subfertility. Our final sample included 5827 women.
Snart Gravid (SG; 2007–2011) and Snart Foraeldre (SF; 2011–present) are web-based preconception cohort studies of the Danish pregnancy planners. Eligibility criteria include women age 18–45 years who are attempting to conceive without fertility treatment. Participants complete a baseline questionnaire and follow-up questionnaires every 8 weeks for up to 12 months. From June 2007 through May 2018, 11 655 women completed the baseline questionnaire. We excluded 138 women whose first day of the most recent menstrual cycle dates reported at baseline was at least 6 months before baseline, 202 with implausible baseline menstrual cycle dates and 222 women with no prospectively reported menstrual cycle dates. We additionally excluded 2589 women who had been attempting conception for more than 6 cycles at baseline, for a final sample of 8504 women.
Assessment of season
We collected prospective information on the first day of menstrual cycles that occurred during the follow-up/preconception period. On the baseline and follow-up questionnaires, women reported the date of the first day of their most recent menstrual cycle. To estimate menstrual cycle dates between questionnaires, which were ≥ 8 weeks apart, we used data from several sources (Fig. 1). In PRESTO, we used data from FertilityFriend on the first day of each menstrual cycle, when available (n = 551). For those without FertilityFriend data, we subtracted the length of the most recent cycle from the most recent menstrual cycle date, ascertained on each follow-up questionnaire, or, if missing, the typical cycle length, ascertained at baseline. If there were more than one period between questionnaires, we subtracted typical cycle length from the first estimated menstrual cycle date to calculate a second estimated menstrual cycle date. We repeated this process several times for women who skipped questionnaires. In SG/SF, 80.9% of women completed questionnaires consecutively and 16.0, 2.3, 0.5, 0.2 and 0.1% of women skipped 1, 2, 3, 4 and 5 consecutive follow-up questionnaires, respectively. In PRESTO, 80.8% completed consecutive questionnaires and 13.5, 3.2, 1.2, 0.8 and 0.5%, skipped 1, 2, 3, 4 and 5 consecutive questionnaires, respectively.
Figure 1.
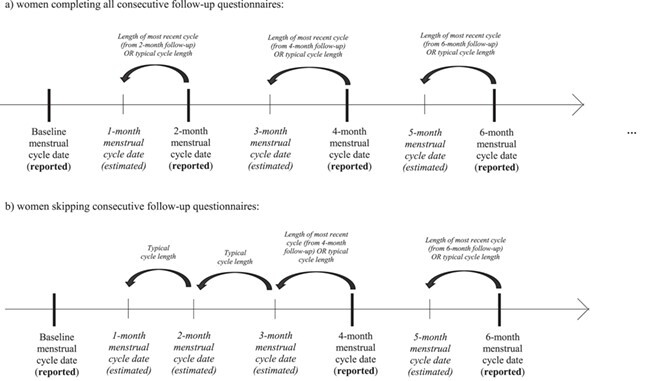
Schematic diagram depicting the information used to estimate menstrual cycle dates in between follow-up questionnaires.
At baseline, we asked women how many months they had been trying to conceive. We subtracted this number from the date they completed the baseline questionnaire to identify the month in which they started trying to conceive.
Assessment of fecundability
We used self-reported and estimated menstrual cycle dates to calculate total observed menstrual cycles at risk. On follow-up questionnaires, women reported whether they were currently pregnant or whether they had initiated fertility treatment. They also reported whether they had experienced any intervening pregnancy losses since their last questionnaire. Women who were not currently pregnant were asked if they were still trying to conceive.
Assessment of covariates
We collected information on demographic (e.g. age, education, income), behavioural (e.g. physical activity, cigarette smoking, alcohol use), medical (e.g. height, weight, medication use) and reproductive (e.g. parity, intercourse frequency) variables potentially associated with fecundability on the baseline and follow-up questionnaires.
Statistical analysis
We used life-table methods to estimate the cumulative rate of conception amongst participants over follow-up. We generated one observation per menstrual cycle to account for left truncation and allow for the update of season and covariates over time (Therneau and Grambsch 2000). Women contributed menstrual cycles to the analysis from study entry until pregnancy (regardless of the outcome), initiation of fertility treatment, cessation of pregnancy attempt, loss to follow-up or 12 cycles of attempt time, whichever came first.
To describe seasonal patterns in initiation of pregnancy attempts, we used Edwards’ method (Brookhart and Rothman 2008), which is a simplified modification of periodic regression that fits monthly frequencies to a sine curve. The model assumes a single annual peak that is exactly 6 months from the low point.
To examine seasonal variation in fecundability, we fit periodic regression models (Stolwijk et al. 1999) by converting the date of each menstrual cycle (ranging from 1 [January 1] to 365/366 [December 31]) to an angle (x). We then fit the following log-binomial regression model:
Log(probability of pregnancy given time x and j failed cycles) = αj + β 1*cos(x) + β 2*sin(x) + …
We included indicator variables for cycle of attempt (1 through 12) to account for seasonal variation in initiation of pregnancy attempt and the decline in mean fecundability with increasing number of failed cycles. From both the Edwards’ analysis and the periodic regression, we estimated the peak/low ratio, which is a measure of the intensity of seasonality occurrence. It estimates the ratio of the probability of becoming pregnant in the peak month to the probability in the low month. From the periodic regression, we obtain the ratio as follows:
Peak/low ratio = [1 + sqrt(β 1 2 + β 2 2)]/[1 − sqrt(β 1 2 + β 2 2)]
We estimated the timing of the peak as arctan (β 2/β 1) and converted the estimated angle to the corresponding day of the year. We conducted separate analyses in each of the two cohorts, followed by an analysis that pooled the data from the two cohorts.
We controlled for factors associated with fecundability that may vary with season, including BMI (calculated from self-reported height and weight), current smoking, intercourse frequency, doing something to improve chances of conception (e.g. charting menstrual cycles), sugar-sweetened beverage intake, multivitamin/folate supplementation, Perceived Stress Scale (PSS)-10 score (PRESTO only), physical activity, medication use in the past 4 weeks (antidepressants or anxiolytics; migraine medication; antibiotics [PRESTO only]; asthma medication; hay fever medication) and influenza vaccination in the past 2 months.
We stratified final models by current smoking, parity and latitude (PRESTO only) to examine whether seasonal variation in fecundability differed across these variables.
We imputed missing outcome and covariate data using a Markov chain Monte Carlo method. We generated five imputation data sets and combined point estimates and standard errors across data sets. We assigned 1 cycle of follow-up to women with no follow-up data (13% in PRESTO and 11% in SG/SF) and imputed their pregnancy status; women who were lost to follow-up after completing at least one follow-up questionnaire were censored at that time. Covariate missingness ranged from 0 (age) to 4% (household income).
The peak/low ratio is an unusual measure, in that the null value for this measure, 1.0, is also the smallest possible value, because for any sine curve, by definition, the peak must exceed the trough. Therefore, even without any seasonal pattern, random variation will produce a peak/low ratio above 1, thereby creating a positive bias in the measure. To assess the size of this bias for these data, we used ‘plasmode simulation’ (Gadbury et al. 2008) similar to that conducted by Skajaa et al. (2018). We randomly assigned a day of the year to be the start of each woman’s first observed cycle and then the calculated dates of subsequent cycles from the woman’s actual cycle length data. From these simulated data, we fit a periodic regression model and estimated the peak/low ratio. We repeated this procedure 1000 times. The mean peak/low ratio over these iterations is the expected value of the estimate when there is no seasonal pattern. We ran this simulation separately for PRESTO and SG/SF data.
Results
We followed 14 331 women for 48 795 menstrual cycles. During follow-up, 77.7% of women became pregnant (69.8% in PRESTO and 82.3% in SG/SF). In PRESTO, 17% of the participants were lost to follow-up, compared with 18% in SG/SF. The mean age of female participants across cohorts was 29.2 years (standard deviation (SD) = 4.2) (Table I). Participants were geographically dispersed across all states (USA) and provinces (Canada) and almost all municipalities (Denmark). Median pregnancy attempt time at study entry in both cohorts was 2 cycles. SG/SF participants were more likely to have <16 years of education than PRESTO participants but were less likely to have an annual household income <$50 000/300000 DKK. Approximately 30% of PRESTO women and 35% of SG/SF women were parous. Mean BMI was higher, and physical activity was lower in PRESTO relative to SG/SF participants. SG/SF participants were more likely to be current regular cigarette smokers but less likely to be current marijuana users. Multivitamin and folate supplementation, cycle irregularity and infrequent intercourse yet doing something to improve chances of conception were more prevalent amongst PRESTO participants, whereas hormonal being the last method of contraception was more common amongst SG/SF participants.
Table I.
Selected baseline characteristics of participants in the PRESTO (2013–2018), Snart Gravid (2007–2011) and Snart Foraeldre (2011–2018) cohorts.
| Characteristic | PRESTO (n = 5827) | SG/SF (n = 8504) |
|---|---|---|
| Age (years), mean | 29.9 | 28.7 |
| Male partner’s age (years), mean | 31.9 | 31.0 |
| Cycles of pregnancy attempt at study entry, mean | 2.1 | 2.0 |
| White, non-Hispanic, % | 83.5 | —a |
| <College degree, % | 26.6 | 33.4 |
| Annual household income <$50 000/300 000 DDK | 20.6 | 14.3 |
| Parous, % | 29.6 | 35.4 |
| Unemployed, % | 4.1 | 2.8 |
| BMI (kg/m2), mean | 27.7 | 24.1 |
| Physical activity (MET-hours/week), mean | 34.8 | 45.6 |
| Current regular smoker, % | 6.9 | 10.2 |
| Alcohol intake (drinks/week), mean | 3.2 | 2.8 |
| Caffeine intake (mg/day), mean | 124.0 | 133.6 |
| Sugar-sweetened beverage (drinks/week), mean | 2.9 | 1.0 |
| Current marijuana use, % | 12.4 | 0.6 |
| Daily multivitamin or folic acid intake, % | 79.0 | 55.5 |
| Medication use in past 4 weeks, % | ||
| Hay fever medication | 4.7 | 3.0 |
| Antibiotics | 8.4 | 6.7 |
| Antidepressants or anxiolytics | 11.4 | 1.9 |
| Asthma medication | 5.9 | 1.8 |
| Migraine medication | 9.6 | 2.1 |
| Influenza vaccine in past 2 months, % | 11.2 | 0.9 |
| Irregular menstrual cycles, % | 17.1 | 7.6 |
| Menstrual cycle length (days), mean | 30.0 | 30.3 |
| Intercourse frequency < 1 time/week, % | 21.9 | 17.7 |
| Doing something to improve conception chances, % | 75.3 | 59.4 |
| Hormonal last method of contraception, % | 39.6 | 60.9 |
aWe did not collect data on race/ethnicity in Denmark.
In PRESTO, the highest proportion of women started trying to conceive in September (10.1%), followed by October (9.4%) and December (9.4%). The fewest women started trying to conceive in February (6.1%), followed by March (6.7%) and April (6.9%). In SG/SF, we observed a higher likelihood of initiating pregnancy attempts in the summer (9.1 and 9.2% for July and August, respectively) and a lower likelihood in the late winter/early spring (6.5 and 7.2% for February and March, respectively), although the patterns were inconsistent and weaker than in PRESTO. When we fit these data using the Edwards method (Fig. 2), for PRESTO, initiating pregnancy attempts peaked in late September and was estimated to be 1.48 times as likely at this time compared with late March (95% CI: 1.37, 1.59) (Fig. 2a). In SG/SF, initiating pregnancy attempts peaked in early September, but the peak/low ratio was not as strong (1.16, 95% CI: 1.09, 1.23) (Fig. 2b).
Figure 2.
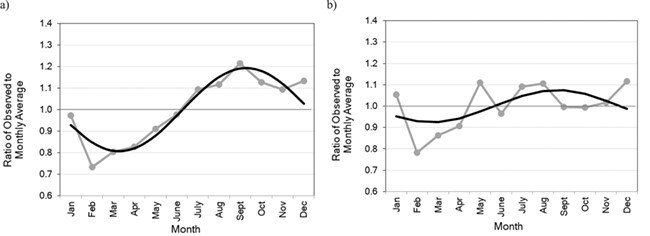
Crude seasonal patterns in initiation of pregnancy attempts fitted using sine curves in (a) PRESTO and (b) Snart Gravid and Snart Foraeldre. Gray lines show the ratio of observed to expected initiation of pregnancy attempts by month and black smoothed lines show the fitted sine curve.
In Table II, we present two models for each cohort: adjusted for timing of initiation of pregnancy attempts (by inclusion of indicator variables for cycle number at risk) or adjusted for both timing of initiation of pregnancy attempts and seasonally varying factors. The timing of the peak was similar in PRESTO (25 November–2 December 2) and SG/SF (25 November 25–1 December). However, the peak/low ratio was larger in PRESTO than in SG/SF. Adjustment for seasonally varying covariates did not meaningfully change either the size or the timing of the peak in either cohort.
Table II.
Results of periodic regression estimating seasonal variation in fecundability in the PRESTO, Snart Gravid and Snart Foraeldre cohorts.
| Cohort | Model | β cos | β sin | Peak/low ratio (95% CI) | Timing of peak |
|---|---|---|---|---|---|
| PRESTO | Adjusteda | 0.0605 | −0.0425 | 1.16 (1.05, 1.28) | November 25 |
| Fully adjustedb | 0.0718 | −0.0394 | 1.18 (1.07, 1.30) | December 2 | |
| SG/SF | Adjusteda | 0.0333 | −0.0186 | 1.08 (1.00, 1.16) | December 1 |
| Fully adjustedb | 0.0277 | −0.0198 | 1.07 (1.00, 1.15) | November 25 | |
| Pooled | Adjusteda | 0.0478 | −0.0331 | 1.12 (1.06, 1.19) | November 26 |
| Fully adjustedb | 0.0501 | −0.0304 | 1.12 (1.06, 1.19) | November 29 |
aAdjusted for timing of pregnancy attempt initiation (using indicator variables for cycle of attempts 1–12).
bAdjusted for timing of pregnancy attempt initiation and seasonally varying factors (BMI, current smoking, intercourse frequency, doing something to improve chances of conception, sugar-sweetened beverage intake, multivitamin or folic acid intake, PSS-10 score, physical activity, medication use in the past 4 weeks (antidepressants/anxiolytics, antibiotics, medications for migraines, asthma, hay fever) and influenza vaccination in the past 2 months.
In PRESTO models stratified by latitude, we found the strongest seasonal pattern in fecundability amongst women from latitudes <35° north (peak/low ratio of 1.45 [95% CI: 1.14, 1.84], with peak fecundability in late November; Table III). There was some evidence of a dose-response trend, with peak/low ratios of 1.45, 1.21, 1.12 and 1.14 for latitudes of <35, 35–39, 40–44 and ≥45°. Peak fecundability progressed later into the winter with increasing latitude. In both cohorts, associations were stronger amongst parous women. There were no substantial differences in associations between smokers and non-smokers.
Table III.
Results of periodic regression estimating seasonal variation in fecundability in the PRESTO, Snart Gravid and Snart Foraeldre cohorts, stratified by select factors.
| Cohort | Modela | β cos | β sin | Peak/low ratio (95% CI) | Timing of peak |
|---|---|---|---|---|---|
| PRESTO | Non-smokers | 0.0722 | −0.0410 | 1.18 (1.07, 1.30) | December 1 |
| Smokers | −0.0686 | −0.0671 | 1.21 (1.00, 1.69) | August 15 | |
| Nulliparous | 0.0741 | 0.0023 | 1.16 (1.03, 1.30) | January 2 | |
| Parous | 0.0607 | −0.1484 | 1.38 (1.16, 1.64) | October 23 | |
| Latitude <35° | 0.1441 | −0.1161 | 1.45 (1.14, 1.84) | November 22 | |
| Latitude 35–39° | 0.0587 | −0.0732 | 1.21 (1.00, 1.48) | November 9 | |
| Latitude 40–44° | 0.0533 | −0.0244 | 1.12 (1.00, 1.29) | December 6 | |
| Latitude ≥45° | 0.0298 | 0.0594 | 1.14 (1.00, 1.46) | February 5 | |
| SG/SF | Non-smokers | 0.0138 | −0.0216 | 1.05 (1.00, 1.14) | November 3 |
| Smokers | 0.1020 | −0.0120 | 1.23 (1.01, 1.49) | December 24 | |
| Nulliparous | 0.0212 | 0.0118 | 1.05 (1.00, 1.16) | January 30 | |
| Parous | 0.0366 | −0.0665 | 1.16 (1.03, 1.31) | October 30 | |
| Pooled | Non-smokers | 0.0471 | −0.0307 | 1.12 (1.05, 1.19) | November 27 |
| Smokers | 0.0741 | −0.0304 | 1.17 (1.00, 1.39) | December 8 | |
| Nulliparous | 0.0473 | 0.0032 | 1.10 (1.02, 1.18) | January 4 | |
| Parous | 0.0540 | −0.0948 | 1.24 (1.13, 1.37) | October 31 |
aAdjusted for timing of pregnancy attempt initiation and seasonally varying factors (BMI, current smoking, intercourse frequency, doing something to improve chances of conception, sugar-sweetened beverage intake, multivitamin or folic acid intake, PSS-10 score, physical activity, medication use in the past 4 weeks (antidepressants/anxiolytics, antibiotics, medications for migraines, asthma, hay fever) and influenza vaccination in the past 2 months.
The mean peak/low ratio in the simulation study, where we specified no systematic seasonal variation in fecundability, was 1.06 (range: 1.00–1.18) in PRESTO and 1.04 (range: 1.00–1.14) in SG/SF. These values estimate the peak/low ratio estimate under the null hypothesis of no seasonal variation.
Discussion
After accounting for noticeable seasonal patterns in initiation of pregnancy attempts, we observed peak fecundability in late November and early December in both Denmark and North America. The magnitude of seasonality was approximately twice as strong in North America as in Denmark. Simulation results indicate that random error could easily account for the magnitude of the Danish results, but not the North American cohort. Furthermore, the magnitude of the peak/low ratio in Denmark was similar to that seen in the northern latitudes of North America. Consistent with the observation of weaker seasonal variation in Denmark, the effect of seasonality was the strongest at the lowest latitudes in the North American cohort. There was little attenuation of our results when controlling for seasonally varying factors including intercourse frequency, sugar-sweetened beverage intake, smoking and medication use. This is one of the first studies of seasonal variation in human reproduction to enrol couples preconceptionally and to account for seasonal patterns in the time when couples start trying to conceive.
There is a broad literature examining seasonal patterns of births across time periods and geographic regions. The most consistent findings show a peak in births in August and September in the southern USA (Lam and Miron, 1994; Lam and Miron, 1996; Seiver 1985) and in March and April in Northern Europe (Lam and Miron, 1994; Russell et al. 1993). Assuming a 9-month gestation and assuming no seasonality in pregnancy loss, this pattern corresponds to a peak in conceptions in November and December in the southern USA, and in July and August in Northern Europe. We observed peak fecundability in late November and early December in both cohorts. However, there are several analytic and interpretative problems that arise when examining births, rather than conceptions, to assess seasonal patterns in human fertility. Analyses of births do not capture information on pregnancy losses, which may also exhibit seasonal patterns (Weinberg et al., 1994). Estimation of conception month based on birth month assumes that all pregnancies last 9 months; however, ~10% of infants are born preterm (Centers for Disease Control and Prevention, 2018b). Finally, seasonal patterns of births may reflect seasonal patterns in initiation of pregnancy attempts, rather than an underlying environmental or behavioural cause. The most fecund women conceive the most rapidly and will therefore conceive in a month closer to their desired month of conception than less fecund women. In the presence of strong seasonal patterns of initiation of pregnancy attempts, this can lead to bias when births are studied. The use of conception month, rather than month in which the pregnancy attempt began, amplifies this bias (Stolwijk et al., 1996b). In addition, researchers have demonstrated that seasonal variation in initiation of pregnancy attempts can lead to bias in studies of seasonal patterns of adverse reproductive outcomes other than fecundity, including pregnancy loss (Basso et al., 1995) and preterm birth (Weinberg et al., 2015).
Seasonal variation in other reproductive variables and events has been observed. Semen concentrations and counts decline in the summer, with suggested etiology related to heat, day length or an endogenous biological clock (Levine 1994; Levine 1999). In a study of bovine embryos, fall was associated with improved embryo development and higher cleavage rates (Chrenek et al. 2015). Studies have generally found no association between season and assisted reproductive technology (ART) success (Fleming et al. 1994; Gindes et al. 2003; Revelli et al. 2005; Wunder et al. 2005; Kirshenbaum et al. 2018; Xiao et al. 2018), which may reflect that fertility treatments are conducted in a controlled environment with few outside influences. The handful of studies supporting seasonal variation in ART outcomes have reported improved embryo quality (Rojansky et al. 2000) and/or a higher implantation rate (Wood et al. 2006) during the spring and summer and higher live birth rates following conception cycles immediately after months with more sunshine and less rain (Vandekerckhove et al. 2016). Several hypotheses have been proposed to explain these observations, including infectious disease, temperature, air pollution levels, length of daylight and vitamin D levels.
Distinct seasonal patterns of reproduction exist in non-human mammals, primarily dictated by food availability and ambient temperature, with reproduction timed so that births occur during the most energetically favourable season (Bronson 2009). While food availability is an unlikely driver behind seasonal birth patterns in contemporary human populations in developed countries, other factors related to timing of births, rather than timing of conceptions, may favour conception during specific seasons. For example, in northern climates, infants born in the winter relative to summer have higher levels of immunoglobulin E antibodies and greater risk of allergic disease (Susanto et al. 2017), higher risk of acute lymphoblastic leukaemia and Hodgkin’s lymphoma in childhood (Basta et al. 2010) and higher risk of schizophrenia (Castrogiovanni et al. 1998). Our finding of lower fecundability in the spring (with subsequent births in the winter) may reflect natural selection against having births in the winter. However, our results stratified by latitude do not support this hypothesis, as fecundability was lower in the spring compared with the fall in the southern USA.
One possible explanation for our results is that extreme heat or higher air pollution during the summer in the southern USA is related to higher risk of early pregnancy loss, which can manifest as longer time to pregnancy. Women who conceive in late May would experience pregnancy losses in July and August (~6–12 weeks after last menstrual period date). If heat or air pollution causes more unrecognised early losses in summer months, particularly in the southern USA, fecundability may appear lower in the spring.
Our study has several limitations. First, we had to estimate menstrual cycle dates between follow-up questionnaires, which may have introduced some exposure misclassification, particularly when women skipped follow-up questionnaires. Second, we lacked data on temperature, vitamin D levels or infectious disease, although we collected data on several seasonally varying factors that may influence fecundability. Third, we were unable to examine seasonal patterns within different geographic regions of North America due to small numbers.
Overall, our analysis indicates that there is at most a modest seasonal pattern to fecundability, after accounting for seasonal patterns in when couples start trying to conceive. The seasonality was stronger in North America than in Denmark, which may reflect differences in climate or other seasonally varying factors. The factors that account for the seasonal pattern in fecundability are yet to be identified. Our finding of stronger seasonal variation in the South may help generate hypotheses related to seasonally varying causes of temporal variation in human fecundity.
Acknowledgements
We are grateful to Mr Michael Bairos for developing and maintaining the web-based infrastructure of the PRESTO study.
Authors’ roles
A.K.W. took the lead on data analysis and drafting of the manuscript. All authors made substantial contributions to the conception or design of the work or to the acquisition, analysis or interpretation of data for the work; critically revised the work for important intellectual content; approved the final submitted version; and agreed to be accountable for all aspects of the work.
Funding
This research was funded by the Eunice K. Shriver National Institute of Child Health and Human Development (R21-050264, R01-HD060680, R21-HD072326 and R01-HD086742) and the Danish Medical Research Council (271-07-0338). The funding agencies played no role in the conduct of the research or the decision to publish the research.
Conflict of interest
The authors declare no conflicts of interest.
References
- Basso O, Olsen J, Bisanti L, Juul S, Boldsen J. Are seasonal preferences in pregnancy planning a source of bias in studies of seasonal variation in reproductive outcomes? The European Study Group on Infertility and Subfecundity. Epidemiol 1995;6:520–524. [DOI] [PubMed] [Google Scholar]
- Basta NO, James PW, Craft AW, McNally RJ. Season of birth and diagnosis for childhood cancer in Northern England, 1968-2005. Paediatr Perinat Epidemiol 2010;24:309–318. [DOI] [PubMed] [Google Scholar]
- Becker S. Seasonal patterns of birth and conception throughout the world. In: Zorgniotti AW (ed). Temperature and Environmental Effects on the Testis. Boston: Springer-Verlag, 1991,59–72 [Google Scholar]
- Bronson FH. Climate change and seasonal reproduction in mammals. Philos Trans R Soc Lond B Biol Sci 2009;364:3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookhart MA, Rothman KJ. Simple estimators of the intensity of seasonal occurrence. BMC Med Res Methodol 2008;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrogiovanni P, Iapichino S, Pacchierotti C, Pieraccini F. Season of birth in psychiatry. A review. Neuropsychobiol 1998;37:175–181. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Births and deaths: provisional data for 2010-December 2015: United States. National Vital Statistics Monthly Provisional Reports. National Center for Health Statistics, 2018a. [Google Scholar]
- Centers for Disease Control and Prevention . Preterm birth. Centers for Disease Control and Prevention, 2018b [Google Scholar]
- Chrenek P, Kubovicova E, Olexikova L, Makarevich AV, Toporcerova S, Ostro A. Effect of body condition and season on yield and quality of in vitro produced bovine embryos. Zygote 2015;23:893–899. [DOI] [PubMed] [Google Scholar]
- Fleming C, Nice L, Hughes AO, Hull MG. Apparent lack of seasonal variation in implantation rates after in-vitro fertilization. Hum Reprod 1994;9:2164–2166. [DOI] [PubMed] [Google Scholar]
- Gadbury GL, Xiang Q, Yang L, Barnes S, Page GP, Allison DB. Evaluating statistical methods using plasmode data sets in the age of massive public datasets: an illustration using false discovery rates. PLoS 2008;4:e1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindes L, Yoeli R, Orvieto R, Shelef M, Ben-Rafael Z, Bar-Hava I. Pregnancy rate fluctuations during routine work in an assisted reproduction technology unit. Hum Reprod 2003;18:2485–2488. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum M, Ben-David A, Zilberberg E, Elkan-Miller T, Haas J, Orvieto R. Influence of seasonal variation on in vitro fertilization success. PLoS One 2018;13:e0199210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DA, Miron JA. Global patterns of seasonal variation in human fertility. Ann N Y Acad Sci 1994;709:9–28. [DOI] [PubMed] [Google Scholar]
- Lam DA, Miron JA. The effects of temperature on human fertility. Demography 1996;33:291–305. [PubMed] [Google Scholar]
- Levine RJ. Male factors contributing to the seasonality of human reproduction. Ann N Y Acad Sci 1994;709:29–45. [DOI] [PubMed] [Google Scholar]
- Levine RJ. Seasonal variation of semen quality and fertility. Scand J Work Environ Health 1999;25:34–37. [PubMed] [Google Scholar]
- Martinez-Bakker M, Bakker KM, King AA, Rohani P. Human birth seasonality: latitudinal gradient and interplay with childhood disease dynamics. Proc Biol Sci 2014;281: 20132438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelli A, La Sala GB, Gennarelli G, Scatigna L, Racca C, Massobrio M. Seasonality and human in vitro fertilization outcome. Gynecol Endocrinol 2005;21:12–17. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Aschoff J. Annual rhythm of human reproduction: I. biology, sociology, or both? J Biol Rhythms 1990a;5:195–216. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Aschoff J. Annual rhythm of human reproduction: II. Environmental correlations. J Biol Rhythms 1990b;5:217–239. [DOI] [PubMed] [Google Scholar]
- Rojansky N, Benshushan A, Meirsdorf S, Lewin A, Laufer N, Safran A. Seasonal variability in fertilization and embryo quality rates in women undergoing IVF. Fertil Steril 2000;74:476–481. [DOI] [PubMed] [Google Scholar]
- Russell D, Douglas AS, Allan TM. Changing seasonality of birth—a possible environmental effect. J Epidemiol Community Health 1993;47:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiver DA. Trend and variation in the seasonality of U.S. fertility, 1947-1976. Demography 1985;22:89–100. [PubMed] [Google Scholar]
- Skajaa N, Horvath-Puho E, Sundboll J, Adelborg K, Rothman KJ, Sorensen HT. Forty-year seasonality trends in occurrence of myocardial infarction, ischemic stroke, and hemorrhagic stroke. Epidemiol 2018;29:777–783. [DOI] [PubMed] [Google Scholar]
- Stolwijk AM, Olsen J, Schaumburg I, Jongbloet PH, Zielhuis GA. Seasonal variation in the time to pregnancy: a secondary analysis of three Danish databases. Eur J Epidemiol 1996a;12:437–441. [DOI] [PubMed] [Google Scholar]
- Stolwijk AM, Straatman H, Zielhuis GA. Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Community Health 1999;53:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolwijk AM, Straatman H, Zielhuis GA, Jongbloet PH. Seasonal variation in the time to pregnancy: avoiding bias by using the date of onset. Epidemiology 1996b;7:156–160. [DOI] [PubMed] [Google Scholar]
- Susanto NH, Vicendese D, Salim A, Lowe AJ, Dharmage SC, Tham R, Lodge C, Garden F, Allen K, Svanes C et al. Effect of season of birth on cord blood IgE and IgE at birth: a systematic review and meta-analysis. Environ Res 2017;157:198–205. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer, 2000 [Google Scholar]
- Vandekerckhove F, Van der Veken H, Tilleman K, De Croo I, Van den Abbeel E, Gerris J, De Sutter P. Seasons in the sun: the impact on IVF results one month later. Facts Views Vis Obgyn 2016;8:75–83. [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Moledor E, Baird DD, Wilcox AJ. Is there a seasonal pattern in risk of early pregnancy loss? Epidemiol 1994;5:484–489. [PubMed] [Google Scholar]
- Weinberg CR, Shi M, DeRoo LA, Basso O, Skjaerven R. Season and preterm birth in Norway: a cautionary tale. Int J Epidemiol 2015;44:1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA et al. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol 2015;29:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Quinn A, Troupe S, Kingsland C, Lewis-Jones I. Seasonal variation in assisted conception cycles and the influence of photoperiodism on outcome in in vitro fertilization cycles. Hum Fertil (Camb) 2006;9:223–229. [DOI] [PubMed] [Google Scholar]
- Wunder DM, Limoni C, Birkhauser MH, Swiss F-G. Lack of seasonal variations in fertilization, pregnancy and implantation rates in women undergoing IVF. Hum Reprod 2005;20:3122–3129. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Wang M, Liu K. The influence of seasonal variations on in vitro fertilization and fresh/frozen embryo transfer: a retrospective study. Arch Gynecol Obstet 2018;298:649–654. [DOI] [PubMed] [Google Scholar]


