Female humans appear to have an advantage in language, from early childhood through late adulthood, reported to include a larger vocabulary, more complex utterances, greater expressive language, and better verbal and pragmatic language comprehension [1]. Wakeful infants produce ‘protophones’ — precursors to speech that include vowel-like sounds, squeals, and growls — at a rate of four or five utterances per minute, more than five times the rate of crying, throughout the first year [2]. The massive number of protophones is in itself surprising, but equally surprising, given the presumed female language advantage, we found that, in the first year, boys produced 24% more protophones than girls. This sex bias was true of infants either at high risk (HR) or low risk (LR) for autism. Both genetic and cultural factors may be involved in this bias, and additional research is clearly called for to investigate the origins of the strong tendency of infants to produce protophones and the unexpected tendency for boys to do so to a greater extent.
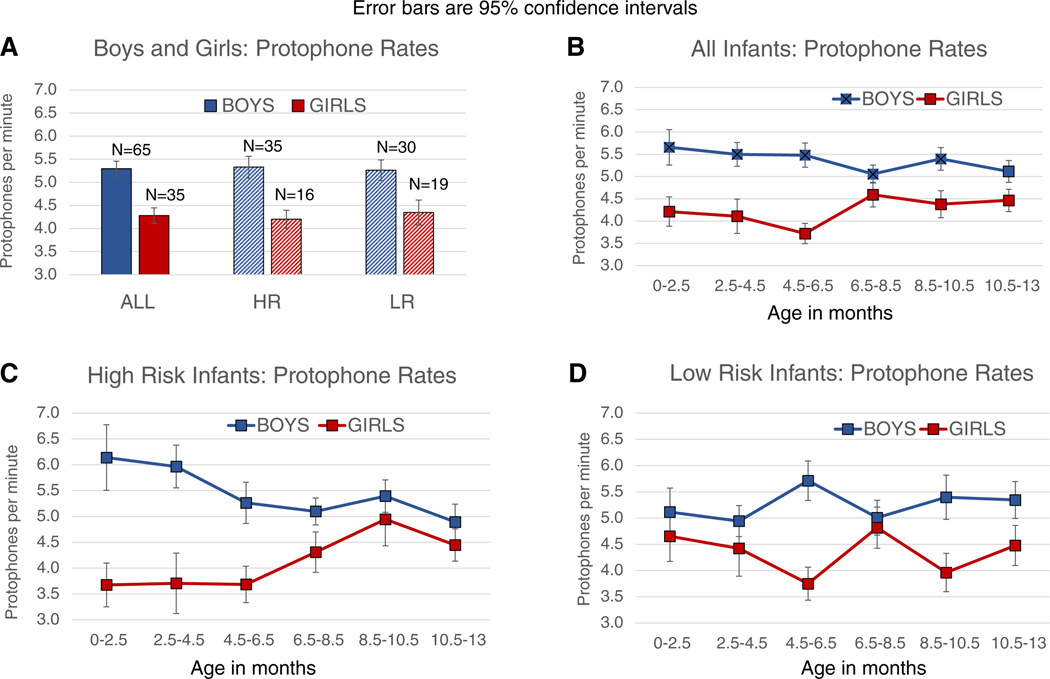
Figure 1A shows the highly significant result favoring boys (t-test, p < 0.0001) with an effect size (Cohen’s d = 0.89) more than four times larger than that typically reported for female language advantage [3]. Both HR and LR boys’ protophone rates were significantly higher than girls’ (HR, p < 0.005, boys 27% higher, d = 1.02; LR, p = 0.01, boys 21% higher rate, d = 0.78). Figure 1B displays rates for infants grouped by age, boys showing higher rates at all ages. Figure 1C,D shows results for HR and LR infants, with higher rates in boys at all ages. Generalized Estimating Equations (GEE) tested the Age, Sex, and Risk factors, revealing a Sex effect ( p< 0.0001) and an Age by Sex interaction (p < 0.05), corresponding to a decreasing difference between boys and girls across ages (Figure 1B), a pattern driven mostly by the diminishing difference across Age in the HR infants (Figure 1C). Thus, contrary to expectations, protophone rate was higher in boys than girls across the first year, with greatest difference at the earliest ages.
Figure 1. Protophone rates in boys and girls.
(A) 65 boys produced about one protophone per minute more (approximately a thousand more protophones per day) than 35 girls (p < 0.0001). The difference favoring boys applied significantly to both infants at high risk (HR) for autism and infants at low risk (LR). Error bars are 95% confidence intervals. Data pertain only to infants who were awake. (B,C,D) Age analysis revealed that both HR and LR boys produced more protophones at all ages across the first year.
We wondered if the higher protophone rate of the boys would correspond to more rapid development of advanced protophones, namely canonical babbling — baba, mama, and so on — which begins at approximately seven months and involves well-formed syllables that can be used in words [4]. The canonical babbling ratio (CBR) is the number of canonical syllables, such as [ba], divided by the total number of syllables an infant produces, including non-canonical syllables, usually vowellike sounds. Notably, whereas deaf infants show no reduction in protophone rate, they are sharply delayed in onset and rate of canonical babbling [5]. So protophone rate and canonical babbling may be somewhat independent.
Indeed, boys had no advantage over girls in CBR (Figure S1 in the Supplemental Information), which increased as expected significantly for both sexes across Age (p < 0.005) and Risk (LR higher, p < 0.05). Thus canonical babbling, a scaffold for first word acquisition, showed no sex bias, but did show the expected increase with age as well as a higher CBR in LR infants, a finding consistent with prior reports of disruption in canonical babbling of infants with or at risk for autism [6].
We did not set out to study sex effects in speech-precursors, but the longitudinal research reported here afforded us the opportunity to reliably evaluate sex effects through extensive human coding at considerable sample size both of intensive longitudinal home-recordings and of infants. The infants were recorded all day and approximately monthly across the first year (65 boys, M = 8.55 all-day recordings; 35 girls, M = 8.60) using a miniature audio recorder, yielding ~6800 hours of recording. Twenty-one randomly-sampled five-minute segments from each recording were coded in real-time by a trained team, yielding >330,000 protophones and >50,000 cries. Coders were blind regarding infant age, sex, and risk status. Coding reliability was high, and discrepancies among coders were small with regard to the effect, indicating boys produced more protophones than girls (see Supplemental Experimental Procedures for methods details and demographics, and Table S1).
Cultural factors could contribute to sex differences in protophone rates. But we know of no comparative cross-cultural research on vocal rates of infant boys and girls nor on possible differences in caregiver speech to boys and girls across cultures. A non-significant tendency for caregivers to speak more to boys was seen in our data (see Supplemental Results), and other possible cultural factors could also influence sex differences in infant vocal rates (see Supplemental Discussion).
It is possible that the sex difference is not closely related to language capability — the CBR did not show a sex difference — but rather to a difference in the tendency to vocalize, perhaps owing to sex differences in motoric activity level in infancy [7]. Boys might be said to show higher quantity but not quality in protophone production. Another hypothesis can be formulated in conjunction with a proposed explanation for the high rate (thousands per day throughout the first year) of human protophone production in both sexes (see Supplemental Discussion). The protophones appear to be produced largely endogenously — they are most commonly not directed toward other speakers, occurring at a rate of approximately four per minute even when infants are alone [8]. Even infants born more than two months prematurely and still in neonatal intensive care produce prodigious numbers of protophones [2]. Furthermore, as noted above, there is no sign that deafness reduces protophone rates [5].
This audible endogenous motoric activity, usually produced by infants in comfort, might be motivated by its value as a fitness signal for the altricial human infant, competing for parental investment [9]. One might then suggest that evolution has led to boys signaling their fitness more frequently than girls because they are more vulnerable to death in the first year [10]. This fitness signaling hypothesis could be explored, for example, by correlating parental investment with infant protophone rates. We are, however, seeking other possible explanations for this unexpected sex difference in infant vocal rates (see Supplemental Discussion).
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by grants DC011027 and DC015108 from the National Institute on Deafness and Other Communication Disorders, MH100029 from the National Institute on Mental Health, and by the Plough Foundation, the Holly Lane Foundation, the Marcus Foundation, the Woodruff-Whitehead Foundation, and the Georgia Research Alliance.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes one supplemental fi gure, one supplemental table, Supplemental Experimental Procedures, Supplemental Results, Supplemental Discussion, and Author Contributions and can be found with this article online at https://doi.org/10.1016/j.cub.2020.03.049.
REFERENCES
- 1.Bornstein MH, Hahn C-S, and Haynes OM (2004). Specific and general language performance across early childhood: stability and gender considerations. First Language 24, 267–304. [Google Scholar]
- 2.Oller DK, Caskey M, Yoo H, Bene ER, Jhang Y, Lee C-C, Bowman DD, Long HL, Buder EH, and Vohr B. (2019). Preterm and full term infant vocalization and the origin of language. Sci. Rep 9, 14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyde JS (2005). The gender similarity hypothesis. Am. Psychol 60, 581–592. [DOI] [PubMed] [Google Scholar]
- 4.Oller DK (2000). The Emergence of the Speech Capacity (Mahwah, NJ: Lawrence Erlbaum Associates; ). [Google Scholar]
- 5.Iyer SN and Oller DK (2008). Prelinguistic vocal development in infants with typical hearing and infants with severe-to-profound hearing loss. Volta Rev. 108, 115–138. [PMC free article] [PubMed] [Google Scholar]
- 6.Paul R, Feurst Y, Ramsay G, Chawarska K, and Klin A. (2010). Out of the mouths of babes: vocal production in infant siblings of children with ASD. J. Child Psychol. Psych 52, 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell DW, and Eaton WO (1999). Sex differences in the activity level of infants. Infant Child Dev. 8, 1–17. [Google Scholar]
- 8.Oller DK, Griebel U, Iyer SN, Jhang Y, Warlaumont AS, Dale R, and Call J. (2019). Language origin seen in spontaneous and interactive vocal rate of human and bonobo infants. Front. Psychol 10, 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locke JL (2006). Parental selection of vocal behavior: crying, cooing, babbling, and the evolution of language. Human Nature 17, 155–168. [DOI] [PubMed] [Google Scholar]
- 10.Pongou R. (2013). Why is infant mortality higher in boys than in girls? A new hypothesis based on preconception environment and evidence from a large sample of twins. Demography 50, 421–444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.