Abstract
Introduction
Ex-vivo normothermic limb perfusion (EVNLP) has been proven to preserve limb viability better than standard cold storage. Perfusates containing packed red blood cells (pRBC) improve outcomes when compared to acellular perfusates. Limitations of pRBC-based perfusion include limited availability, need for cross match, mechanical hemolysis, and activation of pro-inflammatory proteins. Hemoglobin-based oxygen carrier (HBOC)-201 (Hemopure) is a solution of polymerized bovine hemoglobin, characterized by low immunogenicity, no risk of hemolytic reaction, and enhanced convective and diffusive oxygen delivery. This is a preliminary study on the feasibility of EVNLP using HBOC-201 as an oxygen carrier.
Materials and Methods
Three porcine forelimb perfusions were performed using an established EVNLP model and an HBOC-201-based perfusate. The perfusion circuit included a roller pump, oxygenator, heat exchanger, and reservoir. Electrolytes, limb temperature, weight, compartment pressure, nerve conduction, and perfusion indicated by indocyanine green angiography and infra-red thermography were monitored. Histological evaluation was performed with hematoxylin and eosin and electron microscopy.
Results
Three limbs were perfused for 21.3 ± 2.1 hours. Muscle contractility was preserved for 10.6 ± 2.4 hours. Better preservation of the mitochondrial ultrastructure was evident at 12 hours in contrast to crystallization and destruction features in the cold-storage controls.
Conclusions
An HBOC-201-EVNLP produced outcomes similar to RBC-EVNLP with preservation of muscle contractility and mitochondrial structure.
1. Introduction
In the USA, limb loss secondary to trauma accounted for 45% of the prevalent cases in 2005 (704,000), and it is projected to reach an estimated 1,326,000 cases in 2050.1,2 As a result of the conflicts in Iraq, Syria, and Afghanistan, there are 1,645 military personnel with a major or partial limb amputation as of the 2015 Congressional Research Service report. 3 In the event of traumatic limb amputation, the best immediate option is replantation. The next best option is transplantation, if prosthetic rehabilitation fails. Hand transplantation is being performed in an increasing number of centers with encouraging mid- and long-term outcomes.4 Extremity segments containing large amounts of skeletal muscle can endure up to 6 hours of warm ischemia and 12 hours of cold ischemia following amputation. However, the best functional results are obtained when the cold ischemia is kept under 2 hours.5 Clinical reports of hand transplantation indicate that shorter ischemia results in superior graft function. Herzberg et al. accounted the increased ischemia time (9 hours) for modest functional recovery following bilateral hand transplantation.6 compared to the Austrian bilateral hand transplants where ischemia time was shorter (2.5–2.8 hours) and the acquisition of complex hand functions was achieved.7 Similarly, Cavadas et al. reported perioperative ischemic injury (3 hours of cold and 3.5 hours of warm ischemia) resulting in fibrotic contracture of forearm muscles following transplantation.8 Ischemic injury is also one of the several risk factors for acute and chronic rejection by activating immune responses.9 In a composite allograft model, relatively short ischemic times (1–3 hours) produced sufficient reperfusion injury to increase the risk of acute rejection.10
The current gold standard for limb preservation following amputation is cooling to decrease cell metabolism. During cold preservation, energy consumption due to metabolic activity is not halted, and ultimately, mitochondrial damage and subsequent adenosine triphosphate (ATP) depletion ensue. To extend the time to replantation or transplantation and to minimize ischemia-reperfusion injury, normothermic machine perfusion (NMP) systems have been implemented successfully in solid organ transplantations.11–17 NMP provides several advantages over cold preservation: (1) the ability to maintain oxygenation and temperature of the limb close to physiological values, (2) a perfusion solution providing all necessary nutrients at optimal concentrations, and (3) the ability to maintain and monitor physiological pH and electrolyte range to preserve function.
Perfusion solutions containing packed red blood cells (pRBC) as an oxygen carrier have shown to improve outcomes when compared to acellular perfusates.12–14,18 However, limitations of pRBC-based NMP include limited availability, need for cross match, mechanical hemolysis, activation of pro-inflammatory proteins, transmission of infectious diseases, and patient’s refusal to accept human blood products.18–20 Hemoglobin-based oxygen carrier (HBOC)-201 (Hemopure, HbO2 Therapeutics, Souderton, PA) is an oxygen carrier containing polymerized bovine hemoglobin characterized by low immunogenicity, no risk of hemolytic reactions, low viscosity, and enhanced convective and diffusive oxygen delivery.
In previous studies, HBOC-201 has been successfully used for liver NMP. When compared against pRBC-based perfusates, these studies found that HBOC-201 was as effective, with a higher oxygen extraction in HBOC-201-perfused groups and no significant difference in lactate clearance, reactive oxygen species, and histological findings.17,20
Replacement of pRBC with HBOC-201 as an oxygen carrier in limb NMP can help advance this technology to become highly available and avoid limitations associated with the use of pRBCs.
The authors undertook a preliminary study to assess the feasibility of using an HBOC-201-based perfusate in supporting limb viability during EVNLP.
2. Methods
This study was exempt from the Institutional Animal Care and Use Committee approval. Bilateral porcine forelimbs were procured from Yorkshire pigs, weighing 45 kg on average, immediately after euthanasia as previously published.11 Donor animals were used in Cleveland Clinic’s Simulation and Advanced Skills Center for the following procedures: laparoscopic colorectal surgery, gastrectomy, adrenalectomy, heminephrectomy, nephrectomy, and oophorectomies. Inclusion criteria for the recovery of the forelimbs were hemodynamic stability and no use of muscle relaxants.
After procurement, limbs were weighed, the subclavian artery was cannulated, and flushed with heparinized saline (100 U/mL). The right limbs were connected to the perfusion circuit. The left limbs were used as the control, wrapped with saline-soaked gauze, placed in a sterile polyurethane bag, and preserved on ice and water at 4°C.
2.1. Perfusion system
The ex-vivo normothermic limb perfusion (EVNLP) system (Fig. 1) consisted of a roller pump fitted with pulse module (Terumo Sarns 8000, Ann Arbor, MI, USA), oxygenator (QUADROX-i Adult, MAQUET GETINGE GROUP, Rastatt, Germany), gas regulator (ProStar Platinum, Praxair Inc., Danbury, CT, USA), heat exchanger (Biocal 370, Medtronic, Minneapolis, MN, USA), perfusate reservoir (Terumo Capiox Cardiotomy Reservoir 4000 mL, Ann Arbor, MI, USA), and support tray kept covered by a heated dome set at 39.8°C. The circuit was connected to the subclavian artery cannula and venous drainage occurred freely into the organ receptacle for recirculation.
Figure 1.

The components of EVNLP circuit are shown including a roller pump fitted with pulse module, oxygenator, gas regulator/mixer, heat exchanger, perfusate reservoir, and support tray kept covered by a heated dome set at 39.8°C. The circuit is connected to the subclavian artery cannula, and venous return drains freely into the organ receptacle for recirculation.
The perfusate consisted of a colloid solution with a physiological concentration of albumin, glucose, and electrolytes with the addition of HBOC-201 as an oxygen carrier. pH was adjusted using trometamol; tris-hydroxymethyl aminomethane (THAM) solution to reach a physiological level of 7.4 and correct base deficit. Vancomycin, methylprednisolone, heparin, and regular insulin were added to the perfusate. Glucose levels were maintained by adding 50% dextrose.
Twenty percent of the perfusate volume was exchanged every 3 hours, starting at 6 hours of perfusion. The perfusion was terminated when the systolic perfusion pressure exceeded 125 mmHg.
2.2. Perfusion monitoring
Arterial perfusate pressure and flow were recorded continuously (Philips Viridia 24C, Minuteman Rd. Andover, MA; Sams, Terumo CVS, Ann Arbor, MI, USA). Vascular resistance was calculated by the hydrostatic variation of the Ohms equation resistance = (mean arterial pressure-central venous pressure)/cardiac output (the venous pressure was set to zero given the negligible pressure from the open venous return and cardiac output replaced with the flow in the circuit).
Arterial and venous perfusate gases were measured hourly (i-STAT 1, Abaxis LLC, Union City, CA, USA).
Limb weight was recorded before the start and at the end of the perfusion (American Weight Scale AWR-SR-5, Ronald Reagan Blvd. Cumming, GA). Skin oxygen saturation (SaO2) was measured continuously by near-infrared spectroscopy (Vioptix Mission Falls Ct. Fremont, CA) and pulse oximetry. The surface temperature was assessed hourly by infrared thermography (IRT) (Fluke TiS Thermal Imager, Kuala Lumpur, Malaysia). Muscle temperature (Thermoworks Microtherma 2, Alpine, UT, USA) was recorded hourly.
Muscle and nerve functionality were assessed hourly by electrical stimulation of the motor nerves with a modified tens unit (TENS, Ultima 20; Largo, FL, USA) at 3 Hz and 250 μs ls, in both control and experimental limbs. Contractility was graded clinically using a 0 to 5 scale, with 0 representing no contraction and 5 representing full contraction. Additionally, the amplitude of compound muscle action potentials (CMAP), conduction velocity, and latency were measured with an electromyogram (EMG) unit (XLTEK, XCalibur LT, San Carlos, CA, USA) by direct stimulation of the median, ulnar, and radial nerves. Every 6 hours and at the end of perfusion, muscle, skin, and nerve biopsies were collected from control and perfused limbs. Peripheral perfusion was investigated with indocyanine green (ICG) angiography (SPY Elite System, Stryker, Kalamazoo, MI, USA) at the end of the perfusion. Compartment pressure was measured in the forearm flexor and extensor forearm compartments at the end of the perfusion (Stryker intracompartmental pressure monitor, Stryker, Kalamazoo, MI, USA).
2.3. Data analysis
The perfusion parameters were described as average and standard deviation (- ± SD). Comparison of the histological images of muscle samples was made between the perfused limbs and their control limbs after 12 hours of EVNLP or cold storage, respectively. Finally, perfusion parameters at 12 hours of EVNLP using the current HBOC-201-based perfusate were compared to our historical data of 12 hours of EVNLP using red blood cells (RBC)-based perfusate. 11 The Mann-Whitney U test was used for comparison, and a P value < 0.05 was considered significant.
3. Results
Three porcine forelimbs underwent EVNLP utilizing the HBOC-201-based perfusate. The primary procedure length prior to euthanasia and procurement of the limbs were 4 hours and 10 minutes (limb 1), 5 hours and 20 minutes (limb 2), and 5 hours and 55 minutes (limb 3). The average warm ischemia time from euthanasia to the start of the perfusion was 38 ± 14 minutes. At the beginning of the perfusion, all limbs were hypothermic with an average muscle temperature of 25.6 ± 4.6°C, which increased to 35.9 ± 1.2°C after 4 hours of perfusion (Fig. 2). Figure 3 shows the surface temperature of the limbs, which demonstrates evidence of satisfactory perfusion of the limb during the EVNLP. The limbs were perfused for an average of 21.3 ± 2.1 hours.
Figure 2.
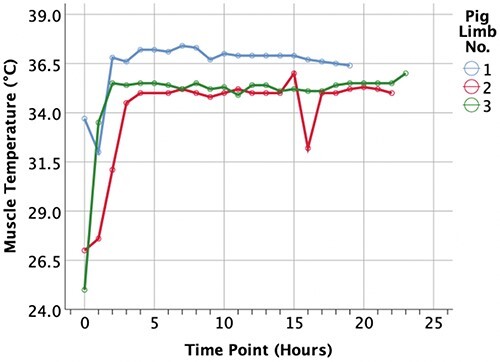
Readings of muscle temperature throughout the perfusion. At the start of the perfusion immediately after the limb procurement, the limbs were hypothermic with an average temperature of 25.6 ± 4.6°C, which increased to an average of 35.9 ± 1.2°C after 4 of perfusion. The limb muscle temperature remained normothermic until the end of the perfusion.
Figure 3.

The infrared thermography showed satisfactory limb perfusion till the endpoint. At the end of perfusion, areas of reduced perfusion with hypothermic periphery became apparent in limbs 2 and 3.
Muscle contractility was maximal at the beginning of the perfusion for a median of 6 hours (Fig. 4A). The amplitude of CMAP paralleled the strength of the muscle contraction (Fig. 4B). In the control limbs, no contraction could be elicited immediately after amputation and thereafter during the preservation period. At 12 hours of perfusion, CMAP was on average 62.5 ± 54.5% of the CMAP at the beginning of the perfusion.
Figure 4.

(A) Muscle contractility was graded visually from 0 to 5 with 0 indicating no contraction and 5 shows maximum contractility. All limbs showed adequate contraction for at least 6 hours of perfusion. (B) Amplitude of the CMAP. The difference in the amplitude between the limbs is affected by the difference of the exact location of the leads on each limb. However, the change in the amplitude over time parallels the visually assessed muscle contractility, showing adequate contractility for at least 6 hours of perfusion.
Vascular resistance showed a stable low resistance throughout EVNLP (167.2 ± 30.9 mmHg min/L) until a significant increase occurred at the end of the perfusion (257 ± 73 mmHg min/L).
The concentration of the electrolytes (sodium, potassium, and calcium) as well as perfusate pH, gases, and tissue SaO2 is shown in Figure 5. Table I shows the difference in these measurements at time point 12 and at the end of the perfusion for each limb. Oxidation of the HBOC-201 into methemoglobin (metHb) occurred at a constant rate of 5.7 ± 0.3% per hour and was only partially corrected with the perfusate exchanges (Fig. 6).
Figure 5.

Perfusate trends of electrolytes, pH, and blood gasses perfusion. There was a constant increase in potassium throughout EVNLP, which was not responsive to perfusate exchanges. Partial oxygen pressure (PaO2) and SaO2 were maintained for more than 12 hours for all of the three limbs.
TABLE I.
Perfusate Gases, Electrolytes Concentration, Lactate, Muscle Contraction and Temperature for Each Limb at 12 hours and at the End of the EVNLP are Shown A Breakdown of the Perfusate
| Variable | Limb 1 | Limb 2 | Limb 3 | |||
|---|---|---|---|---|---|---|
| 12 hours | End point | 12 hours | End point | 12 hours | End point | |
| Arterial PO2 | 423 mmHg | 554 mmHg | 561 mmHg | 121 mmHg | 509 mmHg | 50 mmHg |
| O2 saturation | 83.0% | 82% | 70% | 70% | 78% | 77% |
| pH | 7.535 | 7.445 | 7.351 | 7.3 | 7.389 | 7.222 |
| Sodium | 158 mmol/L | 165 mmol/L | 161 mmol/L | 179 mmol/L | 143 mmol/L | 145 mmol/L |
| Potassium | 7 mmol/L | 7.8 mmol/L | 9 mmol/L | >9 mmol/L | 9 mmol/L | >9 mmol/L |
| Calcium | 0.83 mmol/L | 1.00 mmol/L | 1.06 mmol/L | 0.62 mmol/L | 0.7 mmol/L | 0.64 mmol/L |
| Lactic acid | 15.75 mmol/L | 19.06 mmol/L | 23.0 mmol/L | 4.91 mmol/L | 28.0 mmol/L | 27.0 mmol/L |
| Contraction level (0–5) | 4 | 0 | 4 | 0 | 0 | 0 |
| Muscle temperature | 36.9°C | 36.4°C | 35.0°C | 35.0°C | 35.4°C | 36.0°C |
All the limbs displayed physiological or near physiological parameters at 12 hours of perfusion.
Figure 6.

Methemoglobin levels raised within the first 6 hours of perfusion at a rate of 5.7% per hour. This increase was partially stabilized with the continuous exchange of 20% of the perfusate every 3 hours after the 6th hour of perfusion.
ICG angiography performed at the end of the perfusion indicated adequate peripheral perfusion in limbs one and two, while limb three displayed patchy areas of hypoperfusion (Fig. 7).
Figure 7.

ICG angiography at the end of the perfusion. Pig limb (PL)1 showed uniform and strong contrast diffusion at the end of the perfusion at 19 hours. PL2 showed a uniform background distribution of the contrast with some areas of hypoperfusion, while PL3 had wider areas of hypoperfusion. Interestingly, all limbs displayed evidence of peripheral perfusion to the very distal part of the hoof.
The average limb weight at the beginning and the end of EVNLP were 2123 ± 852 g and 2670 ± 1009 g, respectively. The average weight change was an increase of 25.5 ± 11.7% from the initial weight.
Hematoxylin and eosin staining of muscle samples obtained at 12 hours of perfusion showed preservation of the myofibers, no signs of swelling, or loss of continuity, in contrast to those collected at 12 hours of cold storage that showed discoid and segmental disintegration of the myofibers (Fig. 8).
Figure 8.

Hematoxylin and eosin (H&E) staining of muscle samples obtained from EVNLP 1 and 2 in comparison with their contralateral control limbs in SCS. All samples obtained at 12 hours of preservation. (A) EVNLP 1 muscle sample showing preservation of the structure of the muscle fibers. In comparison, there is discoid and segmental disintegration of the myofibers in the contralateral limb after 12 hours of SCS (B). (C) EVNLP 2 muscle sample showing preservation of the structure of the muscle fibers. In comparison, there is discoid and segmental disintegration of the myofibers in the contralateral limb after 12 hours of SCS (D).
Electron microscopic images of muscle samples that were obtained at 12 hours perfusion showed preservation of the mitochondrial ultrastructure, the integrity of the outer membrane, and inner cristae. In contrast, muscle samples collected after 12 hours of cold storage showed signs of the fluid collection inside the mitochondria, swelling of the mitochondria, or the destruction of the organelle (Fig. 9).
Figure 9.

A side-by-side comparison of muscular mitochondrial morphology under the electronic microscope (13,000× magnification) after 12 hours of EVNLP versus the contralateral limbs after 12 hours of SCS. (A) Sample from EVNLP limb no. 1 after 12 hours, the mitochondria (black arrows) are compact with no signs of swelling or damage to their structural integrity. (B) In comparison, control limb no. 1 showed signs of swelling and separation of the mitochondrial cristae with loss of the integrity of the outer membrane after 12 hours of SCS (black arrows). (C) Sample from normothermic limb number 2: the mitochondria (black arrows) are compact with no signs of swelling or damage to their structural or membrane integrity after 12 hours of perfusion. (D) In comparison, control limb number 2 showed signs of swelling and separation of the mitochondrial crista at 12 hours of SCS (black arrows). (E) Sample from EVNLP limb number 3 after 12 hours of perfusion: the mitochondria (black arrows) showed signs of minor swelling with intact outer membrane and structural integrity. (F) In comparison, control limb number 3 showed signs of complete destruction and loss of structural integrity at 12 hours of SCS (black arrows).
Finally, perfusion parameters obtained at 12 hours of EVNLP using HBOC-201-based perfusate were compared to our historical data obtained at 12 hours of EVNLP using RBC-based perfusate. 11 There was difference in the arterial O2 content, which was higher in the HBOC-201 based-perfusate at 12 hours compared to RBC-based perfusate (497.7 ± 69.7 mmHg, 170.4 ± 140.3 mmHg, P = .036), and in the glucose concentration, which was higher in RBC-based perfusate compared to HBOC-201-based perfusate at 12 hours of perfusion (257 ± 199, 80 ± 20, P = .036). Other variables were comparable between the two types of perfusates at 12 hours of perfusion (Table II).
TABLE II.
Comparison of Perfusion Parameters Between RBC and HBOC-201-Based Perfusates at 12 hours of Perfusion
| Variable | RBC at TP12 (n = 5) |
HBOC-201 at time point 12 (n = 3) | P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Muscle temperature | 32.9 ± 2.3 | 35.8 ± 1.0 | 0.143 |
| Arterial pH | 7.292 ± 0.15 | 7.425 ± 0.097 | 0.250 |
| Arterial PCO2 (mmHg) | 27.8 ± 12.2 | 5.6 ± 0.7 | 0.095 |
| Arterial PO2 (mmHg) | 170.4 ± 140.3 | 497.7 ± 69.7 | 0.036 |
| Sodium (mmol/L) | 170.6 ± 9.1 | 154 ± 9.6 | 0.071 |
| Potassium (mmol/L) | 7.82 ± 1.6 | 8 ± 1.41 | 0.857 |
| Calcium (mmol/L) | 0.87 ± 0.13 | 0.86 ± 0.18 | 0.999 |
| Glucose (mmol/L) | 257 ± 199 | 80 ± 20 | 0.036 |
| Lactic Acid (mmol/L) | 11.4 ± 6.8 | 22.3 ± 6.2 | 0.143 |
| CK (U/L) | 53,344 ± 16,603 | 63,085 ± 19,896 | 0.571 |
| Myoglobin (ng/mL) | 875 ± 325 | 1,529 ± 185 | 0.095 |
Only the arterial PO2 content and glucose concentration were statistically different between the two perfusate types. P value < 0.05 was considered statistically significant.
4. Discussion
Significant advancements in upper extremity transplantation have been made since the first successful transplantation in 1998.21 The immunological and functional outcomes, as well as improved quality of life for upper extremity transplant recipients, have warranted the consistent increase in the number of procedures performed worldwide and a proposal to implement this procedure as the standard of care.21–23 Widespread adoption of hand transplantation in clinical practice is hindered by significant morbidity and mortality associated with the lifelong immunosuppression necessary to prevent and treat graft rejection. Extensive solid organ transplant research and emerging data on vascularized composite allograft models suggest that one of the most important determinants of rejection is represented by immune activation resulting from the ischemia-reperfusion injury.10,24 Moreover, longer ischemia is associated with worse functional outcomes.5–8
The current clinical practice of organ preservation is based on static cold storage (SCS). The two main principles are the reduction of metabolic activity (by cooling) and prevention of cellular swelling (by preservation solution). When the flow of oxygenated blood is stopped, the supply of oxygen, nutrients, and cofactors terminates concomitantly. However, cell metabolic activity is not halted, only slowed by 1.5- to 2-fold for every 10°C drop in temperature. Anaerobic metabolism continues, with resultant depletion of energy stores and accumulation of metabolic products.25 Depletion of ATP causes loss of transcellular electrolyte gradients, an influx of free calcium, and subsequent activation of phospholipases; this is the main contributor to cell swelling and lysis.26 In addition, accumulation of metabolic products during ischemia forms the basis for the production of toxic molecules after reperfusion, which promotes downstream pathways of ischemia-reperfusion injury.27
Normothermic machine preservation (NMP) represents a complete reversal of the current paradigm of organ preservation by maintaining the cellular metabolism at physiological temperatures. Ischemia is prevented by perfusing the organ with an oxygen-rich medium, continuous circulation of metabolic substrates, and removal of waste products. NMP has proven its effectiveness in clinical trials for heart, liver, kidney, and lung transplantation.28–31
Oxygen requirements during hypothermic or subnormothermic preservation are relatively low due to reduced metabolic activity and can be met by supplying a high fraction of oxygen dissolved in the perfusion fluid.32 At 37°C, adequate oxygen delivery with a dedicated oxygen carrier is required to enable and support full metabolic activities.33 To date, most clinical transplant series using organs preserved by NMP of the liver have used red blood cells as the oxygen carrier.34–39
We, initially, showed that EVNLP of porcine forelimbs using an RBC-based perfusate achieved preservation of limb viability for 12 hours. The structural integrity of the skeletal muscle was superior at 12 hours of perfusion compared to cold preservation.11 In a model of terminal perfusion (EVNLP until the perfusion pressure raised above 125 mmHg),40 we subsequently showed that perfused porcine forelimbs retain physiological parameters up to 44 hours with an average weight increase of 16.25 ± 17.86% and compartment pressure of 24.75 ± 7.79 mmHg. Thermography and ICG-angiography showed minimal variations of peripheral limb perfusion over time. Eventually, in a preclinical model, using human upper extremities donated for research, six arms underwent EVNLP with a perfusate containing pRBCs and plasma.41 The upper extremities retained physiological parameters and function up to 48 hours with a final weight increase of 8.33 ± 0.07%, mean muscle temperature of 35.5 ± 0.61°C, and tissue SaO2 of 90.44 ± 11.2%. Thermography and ICG angiography depicted uniform peripheral perfusion throughout the experiment. Electrical stimulation of median, ulnar, and radial nerves displayed no muscle contraction at the beginning; however, muscle contraction recovered gradually and was preserved until the end of perfusion.41
Whilst blood-based perfusion is physiological and effective, it has several potential disadvantages including immune-mediated phenomena, blood-borne infectious transmission, hemolysis, use of a precious resource, and logistical difficulties associated with using cross-matched blood.
HBOC-201 provides many logistical and physiological advantages over blood-based perfusates: low immunogenicity, no risk of hemolytic reactions, no risk of a donor-recipient mismatch, low viscosity, and enhanced convective and diffusive oxygen delivery.16,42 Oxygen delivery by HBOC-201 in the plasma is relatively insensitive to mechanisms regulating RBC distribution in the microcirculation, resulting in improved tissue oxygenation in more remote regions.17,43,44
Fontes et al. first explored the use of HBOC-201 during subnormothermic (21°C) machine perfusion as compared with SCS using porcine livers. The investigators noted significantly higher survival, superior graft function, and bile production after liver transplantation in the machine-perfused group compared with SCS livers. Laing et al. compared human liver NMP, using pRBCs, with HBOC-201 and reported similar perfusate flow, lactate clearance, and histological findings.18 They also reported significantly higher oxygen extraction in the HBOC-201-perfused group. Subsequently, Matton et al. perfused 12 discarded human livers with HBOC-201.15 Compared with pRBC, HBOC-201- perfused livers had significantly higher ATP content, bile production, and portal and arterial perfusate flows. 15 Later, five previously discarded livers were transplanted after dual hypothermic and NMP. 45
Hemodynamically, skeletal muscle perfusion is different from that of the liver. Mongan et al. measured regional blood flows in anesthetized swine that underwent a 50% isovolumic circulatory volume exchange with HBOC-201.46 Of the nine organs measured (liver, brain, kidney, heart, pancreas, small intestine, large intestine, gall bladder, and skeletal muscle), normal flow was maintained in all organs except skeletal muscle, which declined by up to 65.4% due to a 2.7-fold increase in vascular resistance. Other differences between perfusion conditions used in the liver studies described above and our perfused limbs are differences in perfusate compositions, perfusate HBOC-201 concentrations, %O2Hb saturation, perfusion pressures, the ratio of perfusate volume to organ volume, and “dual” liver perfusion via both the portal vein and hepatic artery.
There are also several notable differences in outcomes of perfused liver and limb perfusions. Livers generally sustained less weight gain (edema) per unit of perfusion time than limbs and are able to consume lactate by reducing it to pyruvate whereas limbs process little or no lactate during perfusion.15,47 The increases in metHb during liver perfusions were generally smaller per unit time than observed in our study,15 suggesting the possibility that livers may produce more reducing equivalents (eg, glutathione) than limbs and/or express higher levels of NADH-dependent cytochrome b5 Hb reductase [the enzyme in RBCs responsible for reducing metHb (Fe3+) to Hb (Fe2+)] than limbs.48,49 Despite these differences, our preliminary experience with HBOC-201-based EVNLP was broadly similar to the results of the liver-perfusion studies. In both organ types, NMP perfusion with HBOC-201 preserved tissue integrity and organ function better than SCS and comparably to perfusion with RBC-based perfusates. The venous oxygen concentrations and oxygen extraction ratios in our experiment and O2 extraction data from the liver experiment indicated that ample of oxygen was delivered to the tissue. 15 In neither of the experiments did metHb levels prevent oxygen delivery to the organ. MetHb itself appears to be well tolerated during liver perfusions. Human livers perfused with an HBOC-201-based perfusate containing up to 20% metHb, which were uniformly transplanted with full patient recoveries.44 However, none of the livers were perfused beyond 8 hours as opposed to 19 to 22 hours of limb EVNLP.15,18
Comparing the results of the three limbs presented here with our historical data from porcine limb perfusions using a pRBC-based perfusate, a substantial difference was not identified during the first 6 to 12 hours of perfusion. However, the HBOC-perfused limbs seemed to gain more weight with extended perfusion durations. While the HBOC-201-based perfusate avoids the risk of immunogenicity and progressive hemolysis, its 19 to 24 hours half-life and auto-oxidation to methemoglobin may require a more complete exchange of the perfusate to compensate for the decrease in the oxygen-carrying capacity during longer perfusions.
Transient systemic adverse reactions (elevation in blood pressure, oliguria, jaundice, GI symptoms, and elevated methemoglobin levels) have been reported when HBOC-201 was transfused to manage severe anemia. These effects have not been identified in liver transplant recipients following HBOC-201-based NMP. Following the completion of NMP, the donor livers were flushed to remove HBOC prior to transplantation. Hence, the amount of HBOC reaching the recipient following transplantation was negligible.19,44 HBOC-perfused donor limbs can be similarly flushed to eliminate HBOC-based perfusate prior to transplantation.
5. Study Limitations
This pilot study aimed at evaluating the feasibility of using an HBOC-201-based perfusate as a substitute to an RBC-based perfusate. A limitation of the study is represented by the small number of limbs used. Interestingly, HBOC-201-based perfusion parameters at 12 hours were comparable to those achieved using an RBC-based perfusate with higher oxygen content in the HBOC-201 group. Furthermore, ongoing experiments will confirm the findings of this study.
6. Conclusions
This preliminary study demonstrates the feasibility of EVNLP using an HBOC-201-based perfusate. Further investigation is planned to evaluate strategies involving perfusates and perfusion systems modified for optimal oncotic pressure and sustainable metHb reduction. These efforts may limit or prevent metHb accumulation and limb edema, allowing for longer ex-vivo preservations of limbs that retain high levels of structural integrity and function.
Presented as a poster at the 2018 Military Health System Research Symposium, August 2018, Kissimmee, FL; abstract # MHSRS-18-1264
HBOC-201 was donated by Hemoglobin Oxygen Therapeutics LLC., Home Souderton, PA, USA.
Funding
Internal funding from the department of Plastic Surgery, Cleveland Clinic, Cleveland, OH.
References
- 1. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R: Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008; 89(3): 422–9. [DOI] [PubMed] [Google Scholar]
- 2. Barmparas G, Inaba K, Dubose JJ, Criscuoli M, et al. : Epidemiology of post-traumatic limb amputation: a national trauma databank analysis. Am Surg 2010; 76(11): 1214–22. [DOI] [PubMed] [Google Scholar]
- 3. Fischer H. A guide to U.S. military casualty statistics: operation freedom’s sentinel, operation inherent resolve, operation new dawn, operation iraqi freedom, p 7. Federation of American Scientists, August 2015. [Google Scholar]
- 4. Shores J, Brandacher G, Lee WP: Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast Reconstr Surg 2015; 135(2): 360e. [DOI] [PubMed] [Google Scholar]
- 5. Stevanovic M, Sharpe F: Functional free muscle transfer for upper extremity reconstruction. Plast Reconstr Surg 2014; 134(2): 274e. [DOI] [PubMed] [Google Scholar]
- 6. Herzberg G, Weppe F, Masson N, Gueffier X, Erhard L: Clinical evaluation of two bilateral hand allotransplantations at six and three years follow-up. Chir Main 2008; 27(2): 109–17. [DOI] [PubMed] [Google Scholar]
- 7. Piza-Katzer H, Ninkovic M, Pechlaner S, Gabl M, Hussel H: Double hand transplantation: functional outcome after 18 months. J Hand Surgery 2002; 27(4): 385–90. [DOI] [PubMed] [Google Scholar]
- 8. Landin L, Cavadas PC, Garcia-Cosmes P, Thione A, Vera-Sempere F: Perioperative ischemic injury and fibrotic degeneration of muscle in a forearm allograft: functional follow-up at 32 months post transplantation. Ann Plast Surg 2011; 66(2): 202–9. [DOI] [PubMed] [Google Scholar]
- 9. Halloran PF, Homik J, Goes N, et al. : The “injury response”: a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc 1997; 29(1): 79–81. [DOI] [PubMed] [Google Scholar]
- 10. Pradka SP, Ong YS, Zhang Y, et al. : Increased signs of acute rejection with ischemic time in a rat musculocutaneous allotransplant model. Transplant Proc 2009; 41(2): 531–6. [DOI] [PubMed] [Google Scholar]
- 11. Duraes EFR, Madajka M, Frautschi R, et al. : Developing a protocol for normothermic ex-situ limb perfusion. Microsurgery 2018; 38(2): 185–94. [DOI] [PubMed] [Google Scholar]
- 12. Constantinescu MA, Knall MD, Erhard MD, et al. : Preservation of amputated extremities by extracorporeal blood perfusion; a feasibility study in a porcine model. J Surg Res 2011; 171(1): 291–9. [DOI] [PubMed] [Google Scholar]
- 13. Abdelhafez MM, Shaw J, Sutter D, et al. : Effect of C1-INH on ischemia/reperfusion injury in a porcine limb ex vivo perfusion model. Mol Immunol 2017; 88: 116–24. [DOI] [PubMed] [Google Scholar]
- 14. Ozer K, Rojas-Pena A, Mendias CL, et al. : The effect of ex situ perfusion in a swine limb vascularized composite tissue allograft on survival up to 24 hours. J Hand Surgery 2016; 41(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 15. Matton APM, Burlage LC, van Rijn R, et al. : Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl 2018; 24(4): 528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pearce LB, Gawryl MS: The pharmacology of tissue oxygenation by biopure's hemoglobin-based oxygen carrier, hemopure (HBOC-201). Adv Exp Med Biol 2003; 530: 261. [DOI] [PubMed] [Google Scholar]
- 17. Kocian R, Spahn DR: Haemoglobin, oxygen carriers and perioperative organ perfusion. Best Pract Res Clin Anaesthesiol 2007; 22(1): 63–80. [DOI] [PubMed] [Google Scholar]
- 18. Laing R, Bhogal R, Wallace L, et al. : The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation 2017; 101(11): 2746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Hemelrijck J, Levien L, Veeckman L, Pitman A, Zafirelis Z, Standl T: A safety and efficacy evaluation of hemoglobin-based oxygen carrier HBOC-201 in a randomized, multicenter red blood cell controlled trial in noncardiac surgery patients. Anesth Analg 2014; 119(4): 766–76. [DOI] [PubMed] [Google Scholar]
- 20. Mackenzie CF, Moon-Massat PF, Shander A, Javidroozi M, Greenburg AG: When blood is not an option: factors affecting survival after the use of a hemoglobin-based oxygen carrier in 54 patients with life-threatening anemia. Anesth Analg 2010; 110(3): 685–93. [DOI] [PubMed] [Google Scholar]
- 21. Dubernard J, Owen E, Herzberg G, et al. : Human hand allograft: report on first 6 months. Lancet 1999; 353(9161): 1315–20. [DOI] [PubMed] [Google Scholar]
- 22. Breidenbach W, Meister E, Becker G, et al. : A statistical comparative assessment of face and hand transplantation outcomes to determine whether either meets the standard of care threshold. Plast Reconstr Surg 2016; 137(1): 222e. [DOI] [PubMed] [Google Scholar]
- 23. Amaral S, Kessler SK, Levy TJ, et al. : 18-month outcomes of heterologous bilateral hand transplantation in a child: a case report. Lancet Child Adolesc Health 2017; 1(1): 35–44. [DOI] [PubMed] [Google Scholar]
- 24. Troppmann C, Gillingham KJ, Benedetti E, et al. : Delayed graft function, acute rejection and outcome after cadaver renal transplantation: the multivariate analysis. Transplantation 1995; 59(7): 962–8. [DOI] [PubMed] [Google Scholar]
- 25. Clavien P, Harvey P, Strasberg SM: Preservation and reperfusion injuries in liver allografts: overview and synthesis of current studies. Transplantation 1992; 53(5): 957–78. [DOI] [PubMed] [Google Scholar]
- 26. Carini R, Autelli R, Bellomo G, Albano E: Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res 1999; 248(1): 280–93. [DOI] [PubMed] [Google Scholar]
- 27. Petrosillo G, Ruggiero FM, Paradies G: Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J 2003; 17(15): 2202–8. [DOI] [PubMed] [Google Scholar]
- 28. Akateh C, Beal EW, Whitson BA, Black SM: Normothermic ex-vivo liver perfusion and the clinical implications for liver transplantation. J Clin Transl Hepatol 2018; 6(3): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamar M, Selzner M: Ex-vivo machine perfusion for kidney preservation. Curr Opin Organ Transplant 2018; 23(3): 369–74. [DOI] [PubMed] [Google Scholar]
- 30. Hosgood SA, Thompson E, Moore T, Wilson CH, Nicholson ML: Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. British J Surg 2018; 105(4): 388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koch A, Pizanis N, Olbertz C, et al. : One-year experience with ex vivo lung perfusion: preliminary results from a single center. Int J Artif Organs 2018; 41(8): 460–6. [DOI] [PubMed] [Google Scholar]
- 32. Dutkowski P, Polak WG, Muiesan P, et al. : First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg 2015; 262(5): 764–71. [DOI] [PubMed] [Google Scholar]
- 33. Liu Q, Nassar A, Farias K, Buccini L, et al. : Comparing normothermic machine perfusion preservation with different perfusates on porcine livers from donors after circulatory death. Am J Transplant 2016; 16(3): 794–807. [DOI] [PubMed] [Google Scholar]
- 34. Dhital KK, Iyer A, Connellan M, et al. : Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 2015; 385(9987): 2585–91. [DOI] [PubMed] [Google Scholar]
- 35. Boucek MM, Mashburn C, Dunn SM, et al. : Pediatric heart transplantation after declaration of cardiocirculatory death. NEJM 2008; 359(7): 709–14. [DOI] [PubMed] [Google Scholar]
- 36. Ardehali A, Esmailian F, Deng M, et al. : Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015; 385(9987): 2577–84. [DOI] [PubMed] [Google Scholar]
- 37. Ravikumar R, Jassem W, Mergental H, et al. : Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant 2016; 16(6): 1779–87. [DOI] [PubMed] [Google Scholar]
- 38. Watson CJE, Kosmoliaptsis V, Randle LV, et al. : Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant 2016; 16(1): 353–7. [DOI] [PubMed] [Google Scholar]
- 39. Mergental H, Perera MTPR, Laing RW, et al. : Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant 2016; 16(11): 3235–45. [DOI] [PubMed] [Google Scholar]
- 40. Dalla-Pozza E, Duraes E, Madajka M, et al. : Ex-situ extended limb preservation: a protocol for porcine limb perfusion. Plast and Reconstr Surg, 2019; 17(suppl 3). Available at https://atcmeetingabstracts.com/abstract/ex-situ-extended-limb-preservation-a-protocol-for-porcine-limb-perfusion/; accessed January 10, 2019. [Google Scholar]
- 41. Fahradyan V, Dalla Pozza E, Madajka M, et al. : Normothermic ex-situ perfusion of human upper extremity. Plast Reconstr Surg Glob Open 2018; 6(9S): 56–7. Available at https://journals.lww.com/prsgo/pages/articleviewer.aspx?year=2018&issue=08001&article=00080&type=Fulltext; accessed January 10, 2019. [Google Scholar]
- 42. Mackenzie CF, Dubé GP, Pitman A, Zafirelis M: Users guide to pitfalls and lessons learned about HBOC-201 during clinical trials, expanded access, and clinical use in 1,701 patients. Shock 2017. Available at https://www.ncbi.nlm.nih.gov/pubmed/29076972; accessed January 10, 2019. [DOI] [PubMed] [Google Scholar]
- 43. Keipert PE: Hemoglobin-based oxygen carrier (HBOC) development in trauma: previous regulatory challenges, lessons learned, and a path forward. Adv Exp Med Biol 2017; 977: 343. [DOI] [PubMed] [Google Scholar]
- 44. Standl T, Horn P, Wilhelm S, et al. : Bovine haemoglobin in more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolaemic haemodilution in dogs. Can J Anesth 1996; 43(7): 714–23. [DOI] [PubMed] [Google Scholar]
- 45. De Vries Y, Matton APM, Nijsten MWN, et al. : Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am J Transplant 2019. 19(4): 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mongan PD, Moon-Massat PF, Rentko V, Mihok S, Dragovich A, Sharma P: Regional blood flow after serial normovolemic exchange transfusion with HBOC-201 in anesthetized swine. J Trauma 2009; 67(1): 51–60. [DOI] [PubMed] [Google Scholar]
- 47. Fontes P, Lopez R, van der Plaats A, Vodovotz Y, et al. : Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant 2015; 15(2): 381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schantz PG, Källman M: NADH shuttle enzymes and cytochrome b5 reductase in human skeletal muscle: effect of strength training. J Appl Physiol 1989; 67(1): 123–7. [DOI] [PubMed] [Google Scholar]
- 49. Villalba JM, Navarro F, Gómez-Díaz C, Arroyo A, Bello RI, Navas P: Role of cytochrome b5 reductase on the antioxidant function of coenzyme Q in the plasma membrane. Mol Asp Med 1997; 18(Suppl): S7–13. [DOI] [PubMed] [Google Scholar]


