Abstract
Objectives
Tenofovir alafenamide produces lower plasma tenofovir and higher intracellular tenofovir diphosphate (DP) concentrations than tenofovir disoproxil fumarate but it is likely a victim of interactions with rifampicin. We aimed to investigate the pharmacokinetics of tenofovir alafenamide/emtricitabine with rifampicin.
Patients and methods
Healthy volunteers received tenofovir alafenamide/emtricitabine at 25/200 mg once daily, followed by tenofovir alafenamide/emtricitabine + rifampicin daily followed by tenofovir disoproxil fumarate. Plasma tenofovir alafenamide, tenofovir, emtricitabine and intracellular tenofovir-DP and emtricitabine triphosphate pharmacokinetics and genetic polymorphisms were assessed.
Results
Tenofovir alafenamide exposure decreased when tenofovir alafenamide/emtricitabine + rifampicin was used compared with tenofovir alafenamide/emtricitabine [geometric mean ratio (GMR) (90% CI): 0.45 (0.33–0.60)]. Plasma tenofovir and intracellular tenofovir-DP concentrations decreased with rifampicin [GMR (90% CI): 0.46 (0.40–0.52) and 0.64 (0.54–0.75), respectively]. GMR (90% CI) of intracellular tenofovir-DP AUC0–24 for tenofovir alafenamide/emtricitabine + rifampicin versus tenofovir disoproxil fumarate was 4.21 (2.98–5.95). Rifampicin did not affect emtricitabine pharmacokinetics. CYP3A4*22 rs35599367 was associated with higher plasma tenofovir alafenamide AUC0–24 at day 56.
Conclusions
Following tenofovir alafenamide/emtricitabine administration with rifampicin, intracellular tenofovir-DP concentrations were still 4.21-fold higher than those achieved by tenofovir disoproxil fumarate, supporting further study during HIV/TB co-infection.
Introduction
TB is a leading cause of death in people living with HIV (PLWH), accounting for 40% of all deaths globally in 2016. PLWH are 20–30 times more likely to present with active TB than HIV-negative individuals, and 1.4 million new cases of TB amongst PLWH were estimated in 2016.1 The first-line treatment modality for TB infection is a 2 month daily regimen of four compounds and 4 months of two compounds, including the antibiotic rifampicin.2 Initiation of ART in co-infected patients with TB is strongly advised for reducing mortality, and it is therefore important to evaluate the potential drug–drug interactions between anti-HIV and TB treatments.3–5
Tenofovir alafenamide is a prodrug of tenofovir with greater stability in plasma and increased penetration into PBMCs as compared with tenofovir disoproxil fumarate.6 In view of these characteristics, 80%–90% lower plasma concentrations of tenofovir are expected when tenofovir alafenamide is used as compared with tenofovir disoproxil fumarate. Tenofovir disoproxil fumarate is currently the primary form of tenofovir used in HIV treatment, and the only form approved for preventing HIV infection.3 The decrease in circulating tenofovir exposure results in an improved safety profile of tenofovir alafenamide compared with tenofovir disoproxil fumarate, mainly in terms of nephrotoxicity and bone health. Importantly, tenofovir alafenamide provides a high degree of efficacy and is as effective as tenofovir disoproxil fumarate in PLWH.7–11
Tenofovir alafenamide is a substrate of drug transporters such as P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), all of which are involved in the intestinal and hepatic uptake of tenofovir alafenamide.12 , 13 Once absorbed, tenofovir alafenamide is metabolized to tenofovir by cathepsin A and carboxylesterase 1 in PBMCs and hepatic cells, respectively, subsequently undergoing intracellular phosphorylation to the active metabolite tenofovir diphosphate (tenofovir-DP).14 , 15
Rifampicin is known to be a ligand of the pregnane X receptor (PXR), activation of which results in induction of target genes including ABCB1 (which encodes P-gp), ABCG2 (which encodes BCRP) and CYP3A4, leading to increased clearance of drugs that are substrates of these transporters.16 , 17
In view of the potential drug–drug interactions the co-administration of tenofovir alafenamide with rifampicin is not currently recommended.18 A recent randomized study investigated the co-administration of an additional 25 mg dose of tenofovir alafenamide administered with rifampicin in healthy volunteers receiving the fixed-dose combination tablet bictegravir/emtricitabine/tenofovir alafenamide 50/200/25 mg.19 Following co-administration of tenofovir alafenamide twice daily plus rifampicin, the total plasma tenofovir alafenamide, plasma tenofovir and intracellular tenofovir-DP concentrations over 24 h (AUC0–24) were reduced by approximately 15%, 20% and 24%, respectively, as compared with tenofovir alafenamide once daily. Interestingly, the mean steady-state concentration at 24 h post-dose (C 24) of tenofovir-DP was found to be above the historical steady-state tenofovir-DP concentrations achieved with 300 mg tenofovir disoproxil fumarate without rifampicin (359 versus 300 fmol/106 cells, respectively).
Tenofovir alafenamide is currently available on the US market as a single compound (Vemlidy) or co-formulated with other antiretrovirals (ARVs) in fixed-dose combinations: (i) with 200 mg of emtricitabine to generate a recommended dual-NRTI backbone, which is frequently preferred as initial HIV therapy; (ii) with emtricitabine and cobicistat plus either elvitegravir (Genvoya) or darunavir (Symtuza);20 , 21 or (ii) with emtricitabine and bictegravir (Biktarvy).
Data on tenofovir alafenamide/emtricitabine co-administered daily with rifampicin are not currently available. Therefore, the primary objective of this study was to evaluate the pharmacokinetics (PK) of plasma tenofovir alafenamide, plasma tenofovir, intracellular tenofovir-DP, plasma emtricitabine and its intracellular active metabolite emtricitabine triphosphate (TP) during co-administration of tenofovir alafenamide/emtricitabine and rifampicin in HIV-negative healthy volunteers to avoid the risk of achieving sub-therapeutic concentrations of ARVs in PLWH. We also aimed to compare tenofovir-DP concentrations following tenofovir alafenamide/emtricitabine given with rifampicin versus administration of tenofovir disoproxil fumarate alone.
Secondary objectives of the study were to assess the safety and tolerability of tenofovir alafenamide/emtricitabine in healthy volunteers receiving rifampicin and to describe the association between genetic polymorphisms in drug disposition genes and drug exposure in the studied individuals.
Materials and methods
Study population
Eligible participants for the study were HIV-negative males or non-pregnant and non-lactating females, aged between 18 and 60 years (inclusive), with a BMI between 18 and 35 kg/m2 (inclusive), using an adequate and effective double-barrier method of contraception and without any clinically significant acute or chronic medical illness. Subjects were required to have ALT, alkaline phosphatase and bilirubin ≤1.5 × upper limit of normal (unless bilirubin was fractionated and direct bilirubin was <35%). Other subject exclusion criteria were any significant organ dysfunction or clinically significant laboratory determination outside the normal range, and use of any prescription or over-the-counter medications or herbal preparations known to interfere with study drug metabolism within 2 weeks prior to commencing study drug dosing. Subjects with excessive alcohol or drug use (positive urine drug screen) considered by the investigator to potentially hinder compliance with treatment, evaluation or safety procedures were excluded. Patients were instructed to refrain from alcohol-containing beverages, caffeine-containing products, drinking or eating any St John’s wort, Seville oranges, grapefruit juice and Seville orange juice for 2 days prior to the intensive PK visits (days 28, 56 and 84).
Regulatory and ethics approval was obtained before initiating the study (London Hampstead Ethics Committee). Subjects signed written informed consent prior to being enrolled in the study. The study was registered at ClinicalTrials.gov (NCT03186482).
Study procedures
This was an open-label, single-centre, Phase I study that enrolled healthy volunteers. On day 1 subjects started 25 mg of tenofovir alafenamide co-formulated with emtricitabine daily with a standard meal (with 20 g fat content) until day 28 (PK1). Subsequently from day 29 to 56 (PK2) 600 mg rifampicin daily administration on an empty stomach was added, with the instruction to take it at least 30 min before a standard meal and followed by tenofovir alafenamide. Finally, participants discontinued both tenofovir alafenamide/emtricitabine and rifampicin and from day 57 to 84 they received 300 mg tenofovir disoproxil fumarate daily with a standard meal.
Steady-state plasma concentrations of tenofovir alafenamide, tenofovir and emtricitabine and intracellular tenofovir-DP and emtricitabine-TP were measured weekly from day 7 to day 56. Sampling for tenofovir-DP quantification was also performed weekly from day 63 to 84.
Safety procedures including review and assessment of adverse events (AEs) and concomitant medications, laboratory analysis and vital signs were conducted throughout the study. AEs were reported according to the Division of AIDS (DAIDS) grading scale (December 2004).
Intensive PK assessments
Intensive PK visits with serial blood sample collection over 24 h on the last day of each dosing sequence were collected after 28 days of tenofovir alafenamide/emtricitabine intake (day 28, PK1), 28 days into tenofovir alafenamide/emtricitabine and rifampicin co-administration (day 56, PK2), and 28 days after tenofovir disoproxil fumarate administration alone (day 84, PK3). Study drug plasma concentrations were evaluated from blood obtained from an intravenous catheter inserted into an arm vein pre-dose and 2, 4, 6, 8, 12 and 24 h post-dose. At PK1 and PK3 visits participants attended in a fasted state. Pre-dose PK blood samples for plasma and PBMCs were collected before they received a standard meal, followed by drug intake under direct observation by the clinical study staff. At PK2 visits subjects received rifampicin at least 30 min before a standard meal followed by tenofovir alafenamide/emtricitabine. Plasma tenofovir alafenamide, tenofovir and emtricitabine PK parameters were evaluated at PK1 and PK2; tenofovir plasma concentrations were also evaluated at PK3. Moreover, PK parameters of intracellular tenofovir-DP and emtricitabine-TP were calculated from PBMCs collected at 2, 8, 24 h post-dose at PK1 and PK2; tenofovir-DP intracellular concentrations were also evaluated at PK3.
Bioanalytical methods
All drug and metabolite concentrations were determined via LC–MS/MS analysis. All assays were validated in accordance with recommendations of the FDA, Guidance for Industry, Bioanalytical Method Validation guidelines. Tenofovir-DP and emtricitabine-TP were acquired from TriLink Biotechnologies (San Diego, CA, USA). 13C-labelled tenofovir and 13C,15N-labelled emtricitabine were acquired via Moravek Biochemicals, Inc. (Brea, CA, USA) and served as internal standards for the bioanalytical assay.
Quantification of plasma tenofovir alafenamide, tenofovir and emtricitabine were performed using previously described assays, in which lower limits of quantification were 0.03 ng/mL (tenofovir alafenamide) and 0.31 ng/mL (tenofovir and emtricitabine).22 , 23 Quantification of tenofovir-DP and emtricitabine-TP in PBMCs was performed using a modified form of a previously published method.24 Briefly, PBMC counts were determined using a quality control-checked automated cell counter (Adam-MC). PBMCs were lysed in 70% methanol, and 0.1 mL of lysate was used for downstream analysis. Tenofovir-DP and emtricitabine-TP were separated from parent compounds and intermediate metabolites via anion exchange chromatography with gradient KCl washes; metabolites of interest were eluted with 1 M KCl. Tenofovir-DP and emtricitabine-TP were enzymatically converted into parent compounds via sweet potato acid phosphatase (≥10.0 U/mg protein, Sigma, St Louis, MO, USA). Post-enzymatic conversion, compounds were isolated via solid-phase extraction on Oasis MCX 3 cc (60 mg) extraction cartridges (Waters Corporation, Milford, MA, USA). Compounds were eluted and evaporated to dryness. The indirect quantification of tenofovir-DP and emtricitabine-TP was conducted using a Shimadzu Nexera X2 LC system (Shimadzu Corporation, Kyoto, Japan) interfaced with an API 6500 QTRAP mass analyser (SCIEX, Framingham, MA, USA) operated in selective reaction monitoring and position ionization modes. Chromatographic separation was performed using an Acquity UPLC HSS T3 (1.8 μm, 2.1 × 50 mm) column (Waters Corporation, Milford, MA, USA). Assay lower limits of quantification were 5 fmol/sample (tenofovir-DP) and 50 fmol/sample (emtricitabine-TP). Results were normalized to number of cells evaluated and expressed as fmol/106 cells.
PK and statistical analysis
The primary endpoints were the PK parameters C 24, C max and AUC0–24 of plasma tenofovir alafenamide, plasma tenofovir, plasma emtricitabine and intracellular tenofovir-DP and emtricitabine-TP during administration of tenofovir alafenamide/emtricitabine and tenofovir disoproxil fumarate alone and during co-administration of tenofovir alafenamide/emtricitabine plus rifampicin. We also calculated T max at each PK visit. PK parameters were calculated using non-compartmental modelling techniques (WinNonlin® Phoenix, version 7.0; Pharsight Corp., Mountain View, CA, USA). PK parameters calculated both in plasma and in PBMCs were descriptive statistics, including geometric mean (GM), and 95% CIs were used to present all PK parameters. Within-participant changes in the assessed PK parameters (PK2 versus PK1, PK3 versus PK2, PK3 versus PK1) were assessed by calculating the geometric mean ratio (GMR) and 90% CI. Inter-individual variability in all PK parameters was expressed as a geometric coefficient of variation (GCV) calculated using the formula × 100%, where is the variance of the logarithmic transformation of the variable.25 Differences in drug concentrations through each study phase were assessed to establish when steady state was achieved (mixed effect test). StataIC.14 was used to conduct the intra-individual comparison of GMR and the 90% CI.
The sample size was determined based on the predicted effect of rifampicin on tenofovir-DP concentrations. For this sequential design, a sample size of 20 patients was calculated to provide at least 80% power to detect a decrease in tenofovir-DP C 24 of 40% during combined tenofovir alafenamide/rifampicin administration compared with tenofovir alafenamide administered without rifampicin.
The secondary endpoints were to assess the safety and tolerability of the co-administration of tenofovir alafenamide/emtricitabine + rifampicin. Descriptive statistics were used to summarize participants’ baseline characteristics and demographic data. The safety analysis included all participants who received at least one dose of study drug, and safety data were collected at least 28 days and up to 36 days after the last dose of study drug (between days 112 and 120). All safety data, including laboratory tests, vital signs, graded AEs and their incidence, were recorded.
Pharmacogenetic assays and analysis
Genomic DNA was extracted from whole blood with the manufacturers’ protocol (E.Z.N.A Blood DNA Mini Kit; Omega Bio-tek, Norcross, GA, USA). Extracted DNA was quantified using NanoDrop (ThermoFisher Scientific, Wilmington, DE, USA). Genotyping was completed using real-time allelic discrimination PCR assays on a DNA Engine Chromo4 system (Bio-Rad Laboratories, Hercules, CA, USA). All patients were genotyped for NR1I2 (encoding PXR) 63396C>T (rs2472677), NR1I3 [encoding the constitutive androstane receptor (CAR)] 540C>T (rs2307424), CYP3A4*22 522-191C>T (rs35599367) and ABCG2 421C>A (rs2231142). The PCR protocol followed denaturation at 95°C for 10 min, followed by 50 cycles of amplification at 92°C for 15 s and annealing at 60°C for 1 min 30 s. TaqMan Genotyping Mastermix and all assays were purchased from ThermoFisher Scientific (Wilmington, DE, USA). Opticon Monitor v.3.1 software (Bio-Rad Laboratories) was used to obtain allelic discrimination plots and identify genotypes.
Compliance with Hardy–Weinberg equilibrium was tested through previously outlined methods.26 Genotypes were coded for regression analyses as 0 = homozygous common allele, 1 = heterozygous and 2 = homozygous variant allele. Categorical variables were described using relative frequencies, and continuous variables were described using median and range. The Shapiro–Wilk test was used to test for normality with P ≤ 0.05 considered statistically significant. A univariate analysis through linear regression was carried out in order to identify independent variables associated with drug PK. Variables with P ≤ 0.2 for the univariate analysis were carried through to a linear backwards multivariate analysis where P ≤ 0.05 was classed as statistically significant. All statistical analyses were carried out using SPSS Statistics v.22 (IBM Armonk, NY, USA).
Results
Study population
A total of 25 subjects were screened for the study, and 2 were excluded as not meeting eligibility criteria. Twenty-three were enrolled in the study and included in the safety analysis after receiving at least one drug dose. A total of 21 subjects completed all three PK assessments and were included in the PK analysis. At baseline, the mean age was 33 years (range 22–58) and the mean BMI was 26 kg/m2 (range 19–35). The majority of participants were female (67%); ethnicity was white (53%) or of African ancestry (33%).
PK of tenofovir alafenamide/emtricitabine administered once daily with and without rifampicin and of tenofovir disoproxil fumarate administered alone
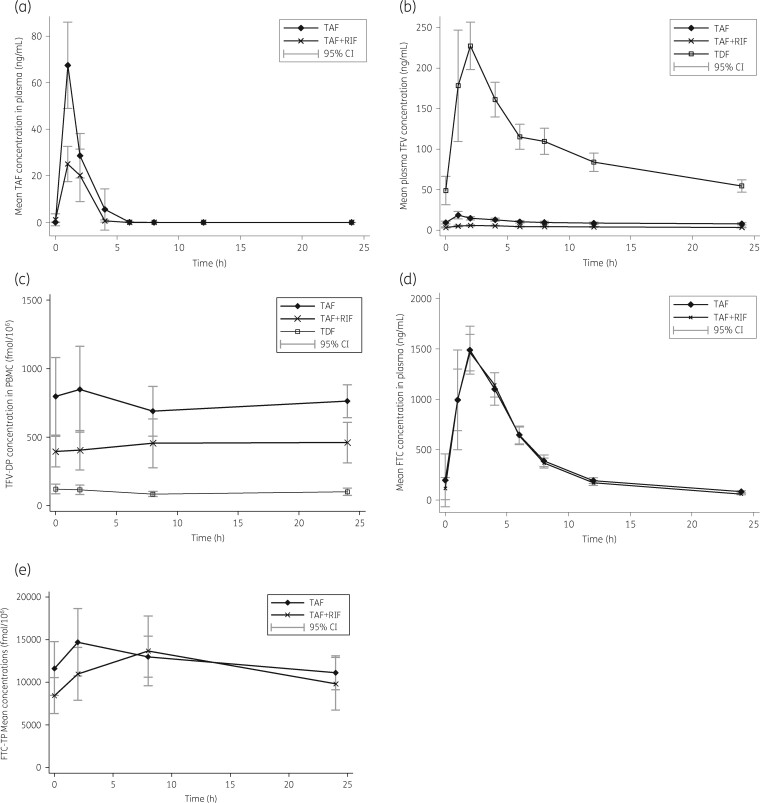
GM (95% CI) of plasma tenofovir alafenamide, plasma tenofovir, plasma emtricitabine, intracellular tenofovir-DP and emtricitabine-TP PK parameters measured at each PK visit are illustrated in Table 1. Plasma concentrations and intracellular GMR (90% CI) of the main PK parameters are presented in Table 2. Concentration–time profiles of plasma tenofovir alafenamide, tenofovir, emtricitabine and the NRTI metabolites tenofovir-DP and emtricitabine-TP (in the presence of absence of rifampicin) are shown in Figure 1.
Table 1.
Plasma tenofovir, plasma tenofovir alafenamide, plasma emtricitabine and intracellular tenofovir diphosphate and emtricitabine triphosphate PK parameters following 28 days of administration of 25/200 mg of tenofovir alafenamide/emtricitabine once daily, co-administration of 25/200 mg of tenofovir alafenamide/emtricitabine once daily and rifampicin for 28 days and following 28 days of 300 mg of tenofovir disoproxil fumarate in healthy volunteers
| PK parameter | TFV |
TAF |
FTC |
||||
|---|---|---|---|---|---|---|---|
| TAF/FTC | TAF/FTC+RIF | TDF | TAF/FTC | TAF/FTC+RIF | TAF/FTC | TAF/FTC+RIF | |
| C max (ng/mL) | 18 (16–22) | 6 (6–7) | 249 (214–289) | 64 (51–80) | 32 (27–39) | 1559 (1369–1774 ) | 1578 (1347–1849) |
| GCV (%) | 34 | 38 | 44 | 53 | |||
| C 24 (ng/mL) | 7 (6–9) | 3 (3–4) | 52 (45–60) | – | – | 89 (76–104) | 62 (53–72) |
| GCV (%) | 26 | 43 | |||||
| AUC0–24 (ng·h/mL) | 208 (172–251) | 95 (84–106) | 2307 (2040–2609) | 88 (69–112) | 39 (31–49) | 9299 (8262–10465) | 9160 (8418–9966) |
| GCV (%) | 30 | 36 | 53 | 59 | |||
| T max (h) | 1.3 (1.1–1.6) | 2 (1.5–2.7) | 1.7 (1.4–2.1) | 1.3 (1–1.7) | 1.4 (1.1–1.7) | 2.1 (1.7–2.4) | 1.9 (1.5–2.4) |
|
|
|||||||
|
TFV-DP |
FTC-TP |
||||||
| TAF/FTC | TAF/FTC+RIF | TDF | TAF/FTC | TAF/FTC+RIF | |||
|
|
|||||||
| C max (fmol/106 cells) | 808.2 (618.4–1056.4) | 499.4 (375.8–663.5) | 113.5 (81.9–157.2) | 14620.0 (11641.4–18360.6) | 14425.5 (11482.8–18122.4) | ||
| GCV (%) | 69 | 64 | |||||
| C 24 (fmol/106 cells) | 613.8 (481.1–783.1) | 352.9 (250.7–496.7) | 85.1 (63.7–113.7) | 9463.1 (7727.5–11588.6) | 8259.5 (6410.0–10642.7) | ||
| GCV (%) | 82 | 102 | |||||
| AUC0–24 (fmol·h/106 cells) | 13052.6 (8864.8–19218.8) | 8325.8 (6015.0–11524.3) | 1976.4 (1484.4–2631.6) | 230200.3 (161076.5–328990.8) | 237695.3 (185749.6–304167.8) | ||
| GCV (%) | 87 | 58 | |||||
| T max (h) | 4 (2.2–7.3) | 9.0 (3.8–11.8) | 3.2 (1.6–5.4) | 3.5 (2.3–5.3) | 7.0 (4.3–9.2) | ||
Data are GM (95% CI) except for T max, which is reported as median (IQR).
TFV, tenofovir; TAF, tenofovir alafenamide; FTC, emtricitabine; TFV-DP, tenofovir diphosphate; FTC-TP, emtricitabine triphosphate; RIF, rifampicin; TDF, tenofovir disoproxil fumarate.
Table 2.
Plasma tenofovir, tenofovir alafenamide, emtricitabine and intracellular tenofovir diphosphate and emtricitabine triphosphate GMRs of the main PK parameters following administration of 25/200 mg of tenofovir alafenamide/emtricitabine once daily with and without rifampicin and 300 mg of tenofovir disoproxil fumarate administered alone
| PK parameter | TFV GMR (90% CI) |
||
|---|---|---|---|
| TAF/FTC+RIF versus TAF/FTC | TDF versus TAF/FTC | TAF/FTC+RIF versus TDF | |
| C max | 0.35 (0.30–0.42) | 13.57 (11.98–15.36) | 0.03 (0.02–0.03) |
| C 24 | 0.45 (0.42–0.50) | 7.15 (6.56–7.79) | 0.06 (0.06–0.07) |
| AUC0–24 | 0.46 (0.40–0.52) | 11.10 (9.96–12.36) | 0.04 (0.04–0.04) |
|
| |||
| TAF GMR (90% CI) | FTC GMR (90% CI) | ||
| TAF/FTC/RIF versus TAF/FTC | TAF/FTC/RIF versus TAF/FTC | ||
|
| |||
| C max | 0.50 (0.42–0.61) | 1.01 (0.90–1.14) | |
| C 24 | – | 0.70 (0.61–0.79) | |
| AUC0–24 | 0.45 (0.33–0.60) | 0.99 (0.90–1.08) | |
|
| |||
| TFV-DP GMR (90% CI) | |||
|
| |||
| TAF/FTC+RIF versus TAF/FTC | TAF/FTC versus TDF | TAF/FTC+RIF versus TDF | |
|
| |||
| C max | 0.62 (0.52–0.74) | 7.14 (5.0–10) | 4.40 (3.09–6.27) |
| C 24 | 0.57 (0.47–0.71) | 7.14 (5.26–10) | 4.15 (2.89–5.94) |
| AUC0–24 | 0.64 (0.54–0.75) | 6.67 (4.35–10) | 4.21 (2.98–5.95) |
|
| |||
| FTC-TP GMR (90% CI) | |||
| TAF/FTC/RIF versus TAF/FTC | |||
|
| |||
| C max | 0.99 (0.85–1.15) | ||
| C 24 | 0.87 (0.71–1.07) | ||
| AUC0–24 | 1.03 (0.86–1.24) | ||
TFV, tenofovir; TAF, tenofovir alafenamide; FTC, emtricitabine; TFV-DP, tenofovir diphosphate; FTC-TP, emtricitabine triphosphate; RIF, rifampicin; TDF, tenofovir disoproxil fumarate.
Figure 1.
Mean (a) plasma tenofovir alafenamide (TAF), (b) plasma tenofovir (TFV), (c) intracellular tenofovir diphosphate (TVF-DP), (d) plasma emtricitabine (FTC) and (e) intracellular emtricitabine triphosphate (FTC-TP) concentration–time profiles and 95% CIs. RIF, rifampicin; TDF, tenofovir disoproxil fumarate.
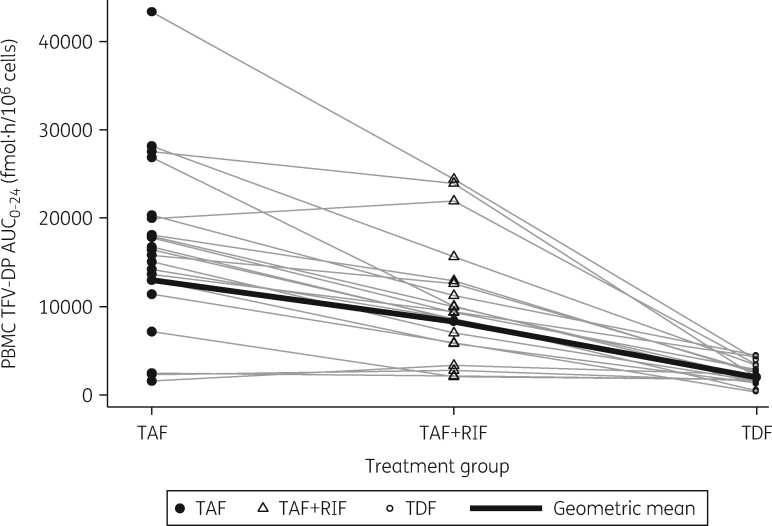
The co-administration of tenofovir alafenamide with rifampicin decreased plasma tenofovir alafenamide C max by 50% and plasma tenofovir alafenamide AUC0–24 by 55%. Plasma tenofovir C max, C 24 and AUC0–24 were reduced by 65%, 55% and 54%, respectively, in the presence of rifampicin. Intracellular tenofovir-DP C max, C 24 and AUC0–24 were decreased by 38%, 43% and 36%, respectively, when tenofovir alafenamide was co-administered with rifampicin. However, intracellular tenofovir-DP C max, C 24 and AUC0–24 GMRs were 4.40, 4.15 and 4.21, respectively, when tenofovir alafenamide was given with rifampicin versus those achieved by a standard dose of tenofovir disoproxil fumarate without rifampicin. Intra-individual changes in intracellular AUC0–24 for tenofovir-DP at each timepoint are shown in Figure 2.
Figure 2.
Intra-individual changes in tenofovir diphosphate (TFV-DP) intracellular AUC0–24 after 28 days of tenofovir alafenamide (TAF) intake without rifampicin (RIF), 28 days of tenofovir alafenamide administered with rifampicin and following 28 days of tenofovir disoproxil fumarate (TDF) administered alone. The black line represents TFV-DP AUC0–24 calculated GM.
Plasma emtricitabine and intracellular emtricitabine-TP parameters did not change significantly in the presence of co-administered rifampicin.
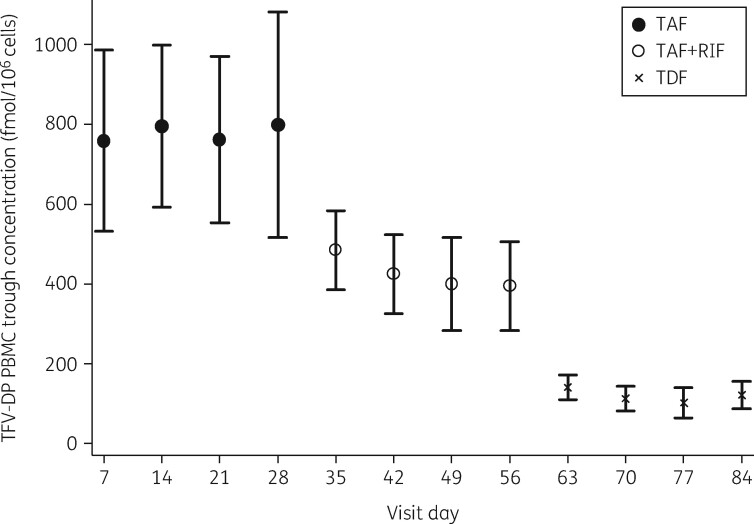
Plasma concentrations of tenofovir alafenamide, tenofovir and emtricitabine and intracellular concentrations of tenofovir-DP and emtricitabine-TP measured at days 7, 14, 21 and 28 were stable (Figure 3) and confirmed that all study subjects’ drug concentrations had achieved steady state by the time that full 24 h PK assessments were undertaken. GM tenofovir-DP C 24 measured was 589.0, 650.0, 558.6 and 653.4 fmol/106 cells at days 7, 14, 21 and 28, respectively, when subjects were administered tenofovir alafenamide/emtricitabine (P = 0.78). At days 35, 42, 49 and 56 during tenofovir alafenamide/emtricitabine plus rifampicin administration GM tenofovir-DP C 24 concentrations were 445.2, 370.6, 289.9 and 323.6 fmol/106 cells (P = 0.05), respectively. Finally, during tenofovir disoproxil fumarate intake, GM tenofovir-DP was 126.5, 91.8, 73.3 and 96.7 fmol/106 cells at days 63, 70, 77 and 84, respectively (P = 0.26)
Figure 3.
Steady-state intracellular tenofovir diphosphate (TFV-DP) C 24 at each PK visit. TAF, tenofovir alafenamide; RIF, rifampicin; TDF, tenofovir disoproxil fumarate.
Pharmacogenetics
All patients were included within the pharmacogenetic analysis (n = 21). All polymorphisms were in Hardy–Weinberg equilibrium. Through linear regression analysis CYP3A4*22 522-191C>T (rs35599367) was significantly associated with log10 tenofovir alafenamide plasma AUC at visit day 56 (P = 0.033, β = 0.25), with a 39% difference in tenofovir alafenamide AUC between homozygous CC (n = 17) and heterozygous CT (n = 4) individuals. Within our population no subjects possessed the homozygous variant genotype for CYP3A4*22 522-191C>T.
Safety and tolerability
Overall, tenofovir alafenamide/emtricitabine without and with rifampicin and tenofovir disoproxil fumarate were well tolerated by the study participants. Two subjects prematurely discontinued the study: one was withdrawn while on tenofovir alafenamide/emtricitabine alone owing to transient increase in ALT ≥8 × upper limit of normal, likely related to alcohol intake and unlikely to be related to the investigational products; another subject withdrew consent owing to the onset of grade 2 rifampicin-related gastrointestinal symptoms. No other grades 3–4 AEs, serious AEs or deaths were reported.
Discussion
We evaluated for the first time, to our knowledge, the administration of 25 mg of tenofovir alafenamide co-formulated with 200 mg of emtricitabine daily with rifampicin in healthy volunteers. We found that rifampicin co-administration with tenofovir alafenamide/emtricitabine resulted in significantly reduced plasma concentrations of tenofovir alafenamide and tenofovir, and the intracellular tenofovir-DP AUC0–24 was 36% lower than when tenofovir alafenamide/emtricitabine was co-administered without rifampicin. However, the intracellular concentrations of tenofovir-DP achieved when rifampicin was added to tenofovir alafenamide/emtricitabine were still >4-fold higher than those reached by tenofovir disoproxil fumarate administered alone, which has been the standard of care for many years.
Tenofovir alafenamide is a novel pro-drug of tenofovir that has proved to be an efficacious component of ARV combinations, is well tolerated, and provides some benefits to certain patients in terms of bone and renal toxicity. Owing to its improved access into cells and unique activation mechanism, tenofovir alafenamide produces higher concentrations of the pharmacologically active intracellular metabolite tenofovir-DP compared with tenofovir disoproxil fumarate.27 , 28 Once tenofovir alafenamide is absorbed and reaches the blood circulation, it enters HIV target cells and after diphosphorylation is converted into the active metabolite tenofovir-DP, which is responsible for tenofovir antiviral activity. Owing to its greater stability in plasma compared with tenofovir disoproxil fumarate, the intracellular concentrations of tenofovir-DP achieved by tenofovir alafenamide have been shown to be 7-fold higher than those achieved by tenofovir disoproxil fumarate.27 These observations were confirmed by our study, where we found intracellular concentrations of tenofovir-DP to be 6.67-fold higher when tenofovir alafenamide was given without rifampicin compared with tenofovir disoproxil fumarate. As a consequence of these beneficial characteristics as compared with tenofovir disoproxil fumarate, tenofovir alafenamide is likely to be used more widely in high-income countries, and eventually as part of first-line ARV therapy in low- and middle-income countries, where HIV/TB co-infection is common.29
Tenofovir alafenamide is a substrate of the drug efflux transporters P-gp and BCRP, but it is not an inhibitor or inducer of CYP3A4 in vivo.13 , 18 A dose adjustment of tenofovir alafenamide from 25 to 10 mg is required in many settings when co-administered with drugs that potently inhibit CYP3A4 or P-gp, such as cobicistat and ritonavir.18 , 20
Interestingly, in our study, we observed that the tenofovir alafenamide C max was the PK parameter that was most affected by rifampicin co-administration, as it was reduced by up to 65%. Tenofovir AUC0–24 and C 24 were decreased by rifampicin co-administration to a lesser extent, by 54% and 55%, respectively, both consistent with reduced tenofovir alafenamide bioavailability. These observations support our hypothesis that rifampicin acts mainly via reducing tenofovir alafenamide intestinal absorption rather than increasing tenofovir alafenamide clearance.
The mechanism behind the impaired absorption of tenofovir alafenamide is potentially explained by the rifampicin-mediated activation of xenobiotic nuclear receptors such as PXR and CAR that affect expression of drug transporters such as P-gp, known to be involved in tenofovir alafenamide disposition. PXR is mainly expressed in intestinal and hepatic cells, and only to a minor extent in the kidneys.30 The rifampicin-mediated P-gp induction via PXR would therefore mainly affect tenofovir alafenamide intestinal absorption without compromising renal clearance. Quantification of tenofovir excretion in the urine would confirm this hypothesis; however, this parameter was not assessed in our study. Another limitation is that this study was undertaken in HIV-negative individuals; this is because there are no existing data on tenofovir alafenamide administered daily with rifampicin and therefore it would not have been safe to enrol PLWH with TB before the existence of Phase I data confirming that it might be safe to co-administer tenofovir alafenamide daily and anti-TB treatment.
Importantly, both European and North American health authorities base their recommendation on whether a drug can be co-administered with tenofovir alafenamide on tenofovir alafenamide plasma concentrations only, without taking into account the intracellular metabolites that exert the ARV activity. Therefore, products that induce P-gp activity (e.g. rifampicin, rifabutin, carbamazepine, phenobarbital) that are expected to decrease the absorption of tenofovir alafenamide and to decrease plasma concentration of tenofovir alafenamide are contraindicated. However, our study showed that the intracellular concentrations of tenofovir-DP are definitely above those that have been demonstrated to be effective against HIV replication and our data will need confirmation in PLWH and those co-infected by TB to confirm the applicability of using 25 mg tenofovir alafenamide once a day with rifampicin.
Conversely, when tenofovir alafenamide is co-administered with the P-gp inhibitor cobicistat, its concentration increases and a dose reduction of tenofovir alafenamide from 25 to 10 mg once daily is warranted in many settings. No data are available, however, regarding the co-administration of rifampicin and tenofovir alafenamide with cobicistat, as the latter is contraindicated with rifampicin because it is a victim of rifampicin metabolic induction.18
We also found that rifampicin had no impact on plasma emtricitabine or intracellular concentrations of its active metabolite emtricitabine-TP, consistent with the fact that emtricitabine is not thought to be a substrate of P-gp or other intestinal transport proteins induced by rifampicin. Our data support the recommendation that no emtricitabine dose adjustment is needed when co-administered with rifampicin.31
Changes in the main PK parameters following introduction of rifampicin were not linked to genetic polymorphisms in drug disposition genes, with the exception of CYP3A4*22, which was linked to higher tenofovir alafenamide plasma AUC at visit day 56. As tenofovir alafenamide is minimally metabolized by CYP3A4 and CYP3A4*22 has been shown to result in reduced CYP protein levels and function,18 , 32 , 33 this relationship is most likely caused by reduced tenofovir alafenamide metabolism in patients heterozygous for CYP3A4*22, resulting in higher plasma concentrations. However, this is an extremely small study population for genetic analysis, correction for multiple comparison was not conducted, patient demographic data such as age and weight were not included and the association was only observed at day 56. Therefore, the link between tenofovir alafenamide plasma concentration and CYP3A4*22 should be confirmed in larger studies with appropriate sample size.
Conclusions
We studied for the first time the PK of tenofovir alafenamide following administration of 25/200 mg of tenofovir alafenamide/emtricitabine daily with rifampicin. Although rifampicin led to lower plasma concentrations of tenofovir alafenamide and tenofovir, the intracellular concentrations of the tenofovir active metabolite were still higher compared with those achieved by the approved dose of tenofovir disoproxil fumarate administered alone in the same individuals. Anti-HIV potency of both tenofovir alafenamide and tenofovir disoproxil fumarate is determined by the concentrations achieved in HIV-target cells of the active metabolite tenofovir-DP. Therefore, dose adjustment of tenofovir alafenamide when co-administered with rifampicin may not be necessary even if the plasma concentrations of tenofovir alafenamide and tenofovir are reduced. Our findings in healthy volunteers support further clinical evaluation of tenofovir alafenamide/emtricitabine with rifampicin in PLWH co-infected with TB.
Acknowledgements
We thank the St Stephen’s AIDS Trust research team for their hard work and the volunteers who took part in the study.
Some results of this study were presented at the Conference on Retroviruses and Opportunistic Infections (CROI), 3–7 March 2018, Boston, MA, USA (Abstract number 28LB).
Funding
The study was funded by Gilead Science (grant to Marta Boffito).
Transparency declarations
M. C. has received a travel grant from Gilead. A. O. has received research funding from Merck, AstraZeneca, Pfizer, ViiV Healthcare and Janssen and consultancy for Merck and ViiV Healthcare. He is also co-inventor of patents relating to nanotechnology-based drug delivery systems. A. P. has received of grants and research supports from ViiV, Merck, Gilead and Janssen. He has also received honoraria or consultation fees from ViiV, Merck, Gilead, Janssen. C. F. has served as a paid consultant to GlaxoSmithKline, Janssen, Merck, Mylan Pharmaceuticals, ViiV Healthcare and Cipla. M. B. has received travel and research grants from and has been advisor for Janssen, Roche, ViiV, Bristol-Myers Squibb, Merck Sharp & Dohme, Gilead, Mylan, Cipla and Teva. All other authors: none to declare.
References
- 1.World Health Organization. Fact sheets. Tuberculosis. http://www.who.int/mediacentre/factsheets/fs104/en/.
- 2.CDC. TB: Treatment of LTBI and TB for Persons with HIV. https://www.cdc.gov/tb/topic/treatment/tbhiv.htm.
- 3.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: WHO, 2016; 155. [PubMed] [Google Scholar]
- 4.World Health Organization. Treatment of Tuberculosis—Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care, 2017 Update. 2017. https://who.int/tb/publications/2017/dstb_guidance_2017/en/.
- 5.AIDSinfo. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 6. Ruane PJ, Dejesus E, Berger D et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndrom 2013; 63: 449–55. [DOI] [PubMed] [Google Scholar]
- 7. Sax PE, Zolopa A, Brar I et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndrom 2014; 67: 52–8. [DOI] [PubMed] [Google Scholar]
- 8. Mills A, Crofoot G, McDonald C et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndrom 2015; 69: 439–45. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy. Medicine (Baltimore) 2016; 95: e5146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Custodio JM, Fordyce M, Garner W et al. Pharmacokinetics and safety of tenofovir alafenamide in HIV-uninfected subjects with severe renal impairment. Antimicrob Agents Chemother 2016; 60: 5135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Post FA, Yazdanpanah Y, Schembri G et al. Efficacy and safety of emtricitabine/tenofovir alafenamide (FTC/TAF) vs. emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) as a backbone for treatment of HIV-1 infection in virologically suppressed adults: subgroup analysis by third agent of a randomized, double-blind, active controlled phase 3 trial. HIV Clin Trials 2017; 18: 135–40. [DOI] [PubMed] [Google Scholar]
- 12. Lepist EI, Phan TK, Roy A et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother 2012; 56: 5409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Begley R, Das M, Zhong L et al. Pharmacokinetics of tenofovir alafenamide when coadministered with other HIV antiretrovirals. 2018; 78: 465–72. J Acquir Immune Defic Syndr 2018; 78: 465–2. [DOI] [PubMed] [Google Scholar]
- 14. Babusis D, Phan TK, Lee WA et al. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm 2013; 10: 459–66. [DOI] [PubMed] [Google Scholar]
- 15. Birkus G, Kutty N, He G-X et al. Activation of 9-[(R)-2-[[(S)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases. Mol Pharmacol 2008; 74: 92–100. [DOI] [PubMed] [Google Scholar]
- 16. Greiner B, Eichelbaum M, Fritz P et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest 1999; 104: 147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niemi M, Backman JT, Fromm MF et al. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 2003; 42: 819–50. [DOI] [PubMed] [Google Scholar]
- 18.Gilead Sciences Ltd. Descovy 200 mg/10 mg Film Coated Tablets. https://www.medicines.org.uk/emc/medicine/31764.
- 19. Custodio JM, West SK, Lutz J et al. Twice daily administration of tenofovir alafenamide in combination with rifampin: potential for tenofovir alafenamide use in HIV-TB coinfection. Abstract PS13/14. In: Abstracts of the 16th European AIDS Conference (EACS), Milan, Italy, 2017. [Google Scholar]
- 20.Gilead Sciences Ltd. Genvoya 150mg/150mg/200mg/10mg Film Coated Tablets. https://www.medicines.org.uk/emc/product/5063/smpc.
- 21.Janssen-Cilag Ltd. Symtuza 800 mg/150 mg/200 mg/10 mg Film-Coated Tablets. https://www.medicines.org.uk/emc/product/8430/smpc.
- 22. Hendrix CW, Andrade A, Bumpus NN et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016; 32: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hummert P, Parsons TL, Ensign LM et al. Validation and implementation of liquid chromatographic-mass spectrometric (LC–MS) methods for the quantification of tenofovir prodrugs. J Pharm Biomed Anal 2018; 152: 248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King T, Bushman L, Kiser J et al. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Anal Technol Biomed Life Sci 2006; 843: 147–56. [DOI] [PubMed] [Google Scholar]
- 25. Kirkwood TBL. Geometric means and measures of dispersion. Source: Biometrics 1979; 35: 908–9. [Google Scholar]
- 26. Rodriguez S, Gaunt TR, Day INM. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 2009; 169: 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ray AS, Fordyce MW, Hitchcock MJM. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res 2016; 125: 63–70. [DOI] [PubMed] [Google Scholar]
- 28. Birkus G, Bam RA, Willkom M et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother 2016; 60: 316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maartens G, Boffito M, Flexner CW. Compatibility of next-generation first-line antiretrovirals with rifampicin-based antituberculosis therapy in resource limited settings. Curr Opin HIV AIDS 2017; 12: 355–8. [DOI] [PubMed] [Google Scholar]
- 30. Benson EA, Eadon MT, Desta Z et al. Rifampin regulation of drug transporters gene expression and the association of microRNAs in human hepatocytes. Front Pharmacol 2016; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilead Sciences Ltd. Emtriva 200 mg Hard Capsules. https://www.medicines.org.uk/emc/product/18/smpc.
- 32.Gilead Sciences Ltd. Vemlidy 25 mg Film Coated Tablets. https://www.medicines.org.uk/emc/product/2314/smpc.
- 33. Okubo M, Murayama N, Shimizu M et al. CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) is associated with reduced CYP3A4 protein level and function in human liver microsomes. J Toxicol Sci 2013; 38: 349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]