Abstract
The Reversion Inducing Cysteine Rich Protein With Kazal Motifs (RECK) is a glycosylphosphatidylinositol (GPI) anchored membrane-bound regulator of matrix metalloproteinases (MMPs). It is expressed throughout the body and plays a role in extracellular matrix (ECM) homeostasis and inflammation. In initial studies, RECK expression was found to be downregulated in various invasive cancers and associated with poor prognostic outcome. Restoring RECK, however, has been shown to reverse the metastatic phenotype. Downregulation of RECK expression is also reported in non-malignant diseases, such as periodontal disease, renal fibrosis, and myocardial fibrosis. As such, RECK induction has therapeutic potential in several chronic diseases. Mechanistically, RECK negatively regulates various matrixins involved in cell migration, proliferation, and adverse remodeling by targeting the expression and/or activation of multiple MMPs, A Disintegrin And Metalloproteinase Domain-Containing Proteins (ADAMs), and A Disintegrin And Metalloproteinase With Thrombospondin Motifs (ADAMTS). Outside of its role in remodeling, RECK has also been reported to exert anti-inflammatory effects. In cardiac diseases, for example, it has been shown to counteract several downstream effectors of Angiotensin II (Ang-II) that play a role in adverse cardiac and vascular remodeling, such as Interleukin-6 (IL-6)/IL-6 receptor (IL-6R)/glycoprotein 130 (IL-6 signal transducer) signaling and Epidermal Growth Factor Receptor (EGFR) transactivation. This review article focuses on the current understanding of the multifunctional effects of RECK and how its downregulation may contribute to adverse cardiovascular remodeling.
Keywords: Adverse Remodeling, Fibrosis, Metallopeptidases, RECK, EGFR, Inflammation
1. Introduction
The processes governing extracellular matrix (ECM) structure and composition are tightly regulated, involving a variety of cell types and signaling intermediates. Under physiological conditions, the matrix adapts to allow proper organ development and function, cellular migration, vascularization, and tissue remodeling in response to injury. However, persistent remodeling disrupts matrix homeostasis and leads to excess deposition of ECM proteins, leading to fibrosis and adverse remodeling [1]. Fibroblasts, a major contributor to fibrosis, undergo a process of activation characterized by differentiation and α-smooth muscle actin expression followed by proliferation. The differentiated fibroblasts (often referred to as myofibroblasts) then express and deposit ECM proteins in excess, leading to adverse remodeling and disease. For example, adverse remodeling occurs prior to hypertension-associated end-organ damage and atherosclerosis [2]. Furthermore, increased load on the heart resulting from hypertension, aortic stiffening, or valvular stenosis initially promotes compensatory remodeling to handle the increased wall stress, characterized by myocardial hypertrophy and fibrosis [3]. However, sustained remodeling eventually leads to myocardial dysfunction and heart failure development [3]. In the case of vascular remodeling, vascular smooth muscle cells de-differentiate from a contractile to a synthetic phenotype and undergo hypertrophy, hyperplasia, and migration [4].
Cardiovascular remodeling occurs in response to a wide range of stimuli, such as inflammation, growth hormone release, activation of the Renin-Angiotensin-Aldosterone System (RAAS), hypoxia, and increased wall stress. Diverse enzymes and signaling molecules regulate this remodeling process, including matrix metalloproteinases (MMPs), A Disintegrin And Metalloproteinase Domain-Containing Proteins (ADAMs), A Disintegrin And Metalloproteinase With Thrombospondin Motifs (ADAMTS), inflammatory mediators, growth factors and the Reversion Inducing Cysteine Rich Protein With Kazal Motifs (RECK). During the remodeling process, the extracellular matrix is degraded by increased release and activation of various MMPs, such as MMPs-1,2,3,7,8,9,13,14, which contribute to cell migration and growth hormone release [5]. A more detailed overview of MMPs, ADAMs, ADAMTS in cardiovascular diseases has been previously reviewed [6–9]. This article focuses on RECK and its potential mechanistic contribution to fibrosis and pathologic cardiovascular remodeling.
2. RECK Structure
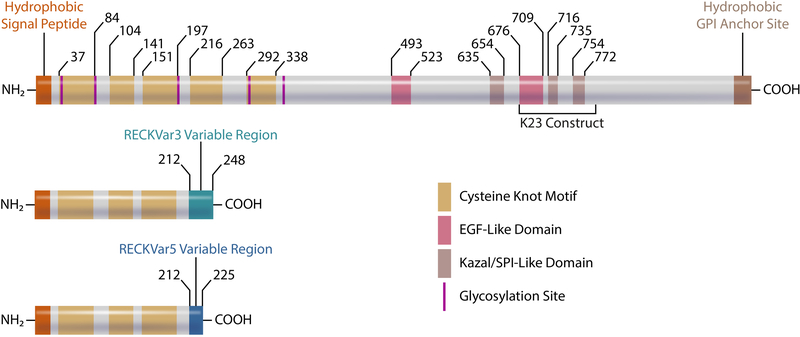
RECK was first cloned and characterized in 1998 by Noda and colleagues [10]. The primary structure of the protein is shown in Fig. 1. The human RECK gene spans 87 kb located on chromosome region 9p13 [10]. The gene encodes a 971 amino acid protein with cysteine accounting for 9% of residues. At the nucleotide level, human RECK shows ~93% homology with mouse, ~86% with rat, ~94% with bovine, and ~98% with monkey. RECK expression was found to be critical for proper development as constitutive RECK deletion leads to lethality in mice due to defective blood vessel maturation at approximately embryonic day 10.5 [11]. The NH2- and COOH-terminals of RECK contain hydrophobic regions. The hydrophobic portion of the NH2-terminal serves as a secretory signal peptide while the COOH-terminal contributes to RECK’s membrane anchoring via a Glycosylphosphatidylinositol (GPI). This GPI anchor contributes to RECK’s ability to regulate various membrane bound proteases such as MT1-MMP, ADAM10, and ADAM17 [8, 12, 13]. It has been shown that Glycerophosphodiester Phosphodiesterase 2 (GDE2), an enzyme that cleaves GPI anchors, releases RECK from the cell surface [14]. Upon release, RECK appears to lose its ability to inhibit protease activity [14]. Interestingly, RECK also contains two epidermal growth factor (EGF)-like domains similar to that described in ADAMs, TGF-β, fibrillin, and EGFR ligands [10, 15]. EGF-like domains have been implicated in ligand shedding, calcium binding, and blood coagulation [15, 16]. Currently, the function of EGF-like domains in RECK is not known, and warrants further investigation. In addition, RECK contains three Kazal motifs. Kazal motifs belong to the I1 family of serine protease inhibitors and typically contain three disulfide bonds between 6 cysteine residues [10, 17]. In the canonical RECK protein, residues 635–654 match a Kazal motif, while the other two domains (716–735 and 754–772) resemble incomplete Kazal motifs [10].
Fig. 1:
Domains/motifs of canonical RECK (top) and RECK isoforms, including corresponding amino acid residues. RECKVar3 and RECKVar5 share the first 212 amino acids with canonical RECK but differ in the COOH-terminal. Illustration made in Adobe Illustrator.
The NH2-terminal of RECK contains five cysteine knot motifs and five asparagine (Asn) residues [10]. The cysteine knot motifs are shown to promote proper development of the blood-brain barrier by facilitating Wingless-Type MMTV Integration Site Family Member 7a/b (Wnt7A/B) signaling in endothelial cells [18, 19]. The first cysteine knot motif (CC-1) has been shown to interact with Adhesion G Protein-Coupled Receptor A2 (Gpr124, an endothelial cell receptor involved in angiogenesis), whereas the fourth cysteine knot motif (CC-4) interacts directly with Wnt7a/Wnt7b [19–21]. Together, these interactions help facilitate the formation of a protein complex that increases Wnt7a/Wnt7b signaling and central nervous system angiogenesis [19]. RECK also has asparagine residues that act as glycosylation sites to regulate RECK’s function. It has been shown that glycosylation at Asn297 contributes to suppression of MMP-9 secretion, and glycosylation at Asn352 is required for RECK’s ability to impair MMP-2 activation [10, 22]. Importantly, blocking glycosylation at Asn86, Asn297, and Asn352 prevents RECK’s ability to suppress tumor cell invasion [22].
The role of RECK in tumor suppression has been widely described, and while a wide range of human tissues express RECK, including the heart, it’s expression is low or undetectable in transformed cancer cell lines [10]. Restoring RECK expression in these transformed cancer cells suppressed their invasive ability by reducing MMP-9 activation. Taken together, the protein was named RECK for its ability to reverse the malignant phenotype, the high percentage of cysteine residues, and the presence of Kazal motifs [10].
Similar to many other genes, several isoforms of RECK have been described [23]. Specifically, two shorter isoforms of RECK have been shown to counteract some of the anti-migratory and anti-growth effects of the canonical isoform [24, 25]. The first 212 amino acid residues at the NH2-terminal are identical between the isoforms, and they all possess the first three cysteine knot motifs (Fig. 1). However, the shorter isoforms lack several domains of canonical RECK, including the three Kazal motifs involved in protease regulation, the two EGF-like domains and the GPI anchor. The lack of a GPI anchor on the shorter isoforms may indicate that these are secreted proteins, and may interact with and regulate canonical RECK and other extracellular proteins away from the cell surface. In fact, it was demonstrated that RECKVar5 can interact with canonical RECK’s Kazal motif, preventing the 110 kDa isoform from inhibiting MMP-9, but not MMP-2 [24]. Interestingly, the expression of the 25 kDa isoform (RECKVar5) is upregulated during proliferation and after Transforming Growth Factor- β1 (TGF-β1) treatment [24]. The ratio of RECKVar5 to canonical RECK has also been shown elevated in more aggressive breast cancers. RECKVar3, another RECK isoform, has been shown to promote glioma cell growth by increasing anchorage-independent growth [23, 25]. Furthermore, a higher ratio of RECKVar3 to canonical RECK has been shown to correlate with lower survival rates in melanoma patients [25]. Interestingly, increased expression of RECKVar3 leads to elevated MMP-14 and MMP-9 mRNA expression, but decreased canonical RECK induction [25]. Expression and regulation of these alternative RECK isoforms in the heart and vasculature during health and disease, however, is currently not known, and warrants investigation.
3. RECK Regulation
RECK is a highly regulated gene. Its expression is regulated at both transcriptional and post-transcriptional levels, including regulation by histone acetylation, DNA methylation, and modulation by microRNAs [24–27]. There are two Specificity Protein 1 (SP1)-binding sites in its proximal promoter region, and activation of SP1 by extracellular signal-regulated kinase (ERK) downregulates RECK in cancer cells [26, 28]. We have previously demonstrated that Ang-II suppresses RECK expression through an ERK/SP1-dependent pathway, and that forced expression of canonical RECK inhibits Ang-II-induced cardiac fibroblast migration [29]. Proinflammatory cytokines negatively regulate RECK expression; we previously reported that IL-18, whose increased expression contributes to adverse cardiac remodeling, suppresses RECK expression in cardiac fibroblasts [30]. Estrogen has been shown to reduce RECK expression in mouse uterine epithelial cells [31] via mechanisms not fully known. Whether RECK expression is differently regulated in the heart and vessels in females is not known, and warrants further investigation. While many pathways have been shown to downregulate RECK, some of which are described above, it can be upregulated by activation of the farnesoid X receptor (FXR) response element in the first intron of the gene [32].
Promoter methylation also regulates RECK expression. Specifically, hypermethylation of the RECK promoter in oral and hepatic cancer cells is associated with reduced RECK mRNA and protein expression [33, 34]. In addition, RECK transcription is regulated by histone acetylation, and increased histone deacetylase (HDAC) activity is associated with reduced RECK expression [33]. In fact, the HDAC inhibitor apicifin is shown to upregulate RECK expression by blocking HDAC4 interaction with SP1 binding elements in the RECK promoter [35]. We have previously demonstrated that the HDAC inhibitors Trichostatin A and mocetinostat reverse Ang-II-induced RECK suppression in cardiac fibroblasts by blocking SP1 binding to its promoter [36].
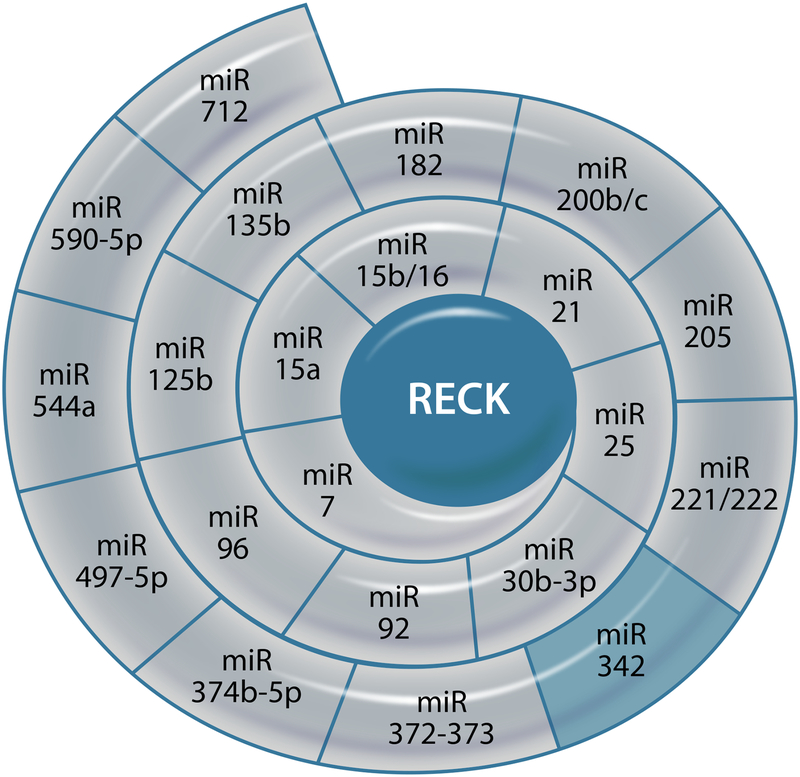
As shown in Fig. 2, multiple miRNAs have been experimentally demonstrated to affect the post-transcriptional regulation of RECK. Of note, while many of these non-coding RNAs suppress RECK expression, some are shown to promote its induction [37–59]. For example, miR-342 has been shown to restore RECK expression in colorectal cancer cells by reducing RECK promoter methylation by inhibiting DNA methyltransferase 1 [55].
Fig. 2.
Post-transcriptional regulation of RECK by microRNAs. Those in light grey suppress RECK expression, while the one in light blue promotes its expression. Illustration made in Adobe Illustrator.
In addition to microRNAs, a long non-coding RNA (lncRNA) known as Growth Arrest Specific 5 (GAS5) has been shown to increase RECK protein expression by binding to miR-21, and reducing miR-21-mediated RECK degradation [60]. Recently, transfection of cells with double-stranded RNA complementary to the RECK promoter region was shown to induce its protein expression, while suppressing MMP expression and activity [61]. However, the translational significance of double stranded RNA in a clinical setting is currently not known.
4. RECK and Tissue Inhibitors of Metalloproteinases
Even though RECK shares structural and functional characteristics with other MMP inhibitors, such as the tissue inhibitors of metalloproteinases (TIMPs), it possesses unique characteristics and features in regulating tissue remodeling as summarized in Table 1. Whereas Kazal motifs contribute to RECK’s regulation of proteases, TIMPs contain a conserved netrin (NTR) module involved in chelating the Zn2+ cofactor from the MMP active site, leading to MMP inhibition [62, 63]. While both Kazal motifs and NTR modules possess three disulfide bonds between six cysteine residues, only the NTR module chelates Zn2+ and inhibits MMP activation with high potency [62–64]. Furthermore, TIMPs are smaller proteins, with less than 200 residues [65, 66]. Interestingly, RECK and TIMP-3 are not soluble, whereas TIMPs 1, 2, and 4 can be secreted to act distally [67]. RECK is bound to the cell surface through a GPI anchor, whereas TIMP-3 is bound to the ECM [68].
Table 1:
Similarities and differences between RECK and TIMPs 1–4, focused on their structure, substrates and functions, as well as their knockout phenotype
| Enzyme | RECK | TIMP1 | TIMP2 | TIMP3 | TIMP4 |
|---|---|---|---|---|---|
| Substrates | MMP-(2,7,9,14,17), ADAMTS, ADAM10/17, EGFR, IL-6R, uPA | MMP1, MMP2, MMP3, MMP7, MMP8, MMP9, MMP10, MMP11, MMP12, MMP13 and MMP16 Low affinity for membrane type MMPs |
pro-MMP2, MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-13, MMP-14, MMP-15, MMP-16 and MMP-19. | MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-13, MMP-14 and MMP-15. Broadest inhibition spectrum, inhibits several members of the ADAM and ADAMTS families |
MMP-1, MMP-2, MMP-3, MMP-7 and MMP-9. |
| KO phenotype | • Embryonic lethality due to improper blood vessel maturation | • Increased ventricular remodeling • Altered ventricularstructure and function • Accelerated hepatocyte cell cycle • Increased resistance to corneal and pulmonary infection • Decreased adiposetissue weight on HFD • Impaired learning and memory |
• Motor defects and deficiency in prepulse inhibitor of the startle reflex | • Enlargement of airspace in lungs • Enhanced apoptosis during mammary gland involution • Excessive cardiac fibrosis • Increased TGFβ1and TNFα. • Increased risk of endotoxin shock • Unaltered tumorigenesis and angiogenesis |
• Reduced adipose tissue hypertrophy and fibrosis on a high fat diet. • Lower metabolic rate and energy expenditure. |
| Role in Angiogene sis | • Negatively Regulates | • Negatively Regulates | • Negatively Regulates • Enhances expression of RECK by interacting with α3β1 integrin switching signaling from Rac 1 to Rap 1 |
• NegativelyRegulates • Binds VEGFR2 to block VEGF signaling on endothelial cells. • Binds AT2R |
• Negatively Regulates |
| Role in Cell Migration | • Inhibitsendothelial cell migration • SuppressesSMC proliferation and migration |
• Increases lungcancer cell migration • Increases hepatoma cell migration • Increases cancer associated fibroblasts migration • Decreases microvascular endothelial cell migration |
• Inhibits cancercell migration • Inhibitsmacrophage migration to atherosclerotic plaques |
• Suppresses SMC proliferation and migration | • Inhibits SMCs migration |
| Role in Proliferation | • Anti-proliferative | • Promotes growth ofkeratinocytes and fibroblasts • Increases Ras-GTP • Inhibits caspase mediated apoptosis |
• Potentiates erythroid activity and cell growth in metanephric mesenchyme cells • Increases amount of Ras-GTP • Reduces apoptosis |
• Promotes apoptosis in a number of cancer cell lines and rat vascular smooth muscle cells | • Induces aortic SMC apoptosis |
| Structure | • Rich incysteine residues • 971 residues • 110kDa • 6 disulfidebonds (Kazal motif) • 5 asparagine glycosylation sites |
• Conserved cysteineresidues • 207 residues • 25kDa • 6 disulfide bonds (NTR module) • 2 asparagine glycosylation sites |
• Conserved cysteine residues • 220 residues • 25kDa • 6 disulfide bonds (NTR module) |
• Conserved cysteineresidues • 211 residues • 25kDa • 6 disulfide bonds (NTR module) |
• Conserved cysteine residues • 224 residues • 25kDa • 6 disulfidebonds (NTR module) |
While RECK and all four TIMPS work to limit excess angiogenesis, they target different substrates [67]. TIMP-1 possesses fewer substrates compared to other TIMPs and RECK, weakly targeting membrane-type MMPs 14, 16, 19 and 24. Both TIMP-2 and TIMP-3 can target a majority of MMPs, but TIMP-3 can also inhibit various members of the ADAM/ADAMTS family. RECK most closely resembles TIMP-3, regulating a broad spectrum of MMPs, ADAM10/17, and ADAMTS. Further, RECK and TIMP-3 are both able to suppress proliferation and migration of vascular smooth muscle cells and promote apoptosis [67, 69–71]. Constitutive knockout of TIMP-1 leads to structural changes in the heart, including exacerbated remodeling following infarction, whereas TIMP-2 KO leads to motor defects [67]. TIMP-3 KO leads to increased cardiac fibrosis and elevated cardiac TGF-β1 and Tumor Necrosis Factor-α (TNF-α) expression in aged mice, and increased myocardial hypertrophy and fibrosis in aortic banded mice [72, 73]. On the other hand, RECK deletion is embryonically lethal, implicating an irreplaceable role for RECK in vascular development [11]. Despite several similarities with TIMPs, RECK’s membrane localization and requirement for proper development suggest a unique role in tissue homeostasis that requires further characterization.
5. RECK and Matrix Metalloproteinases
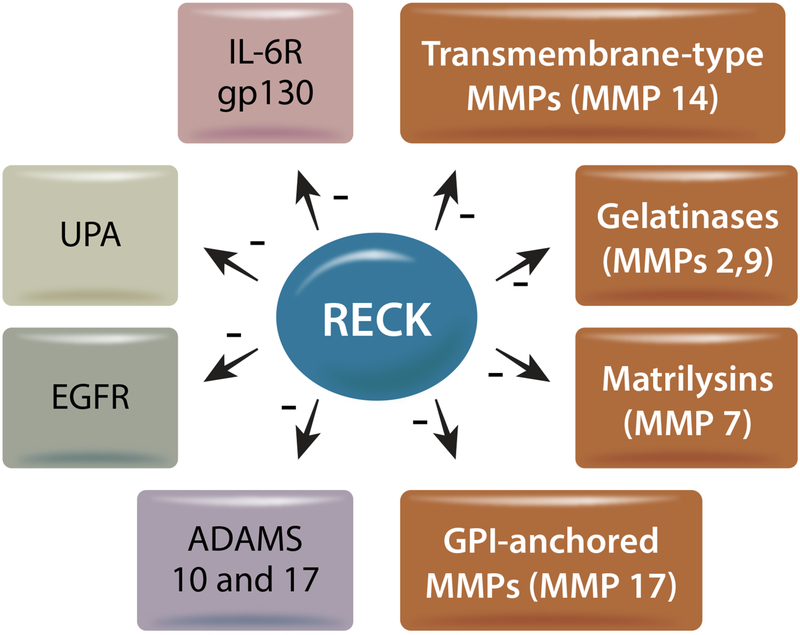
The anchorage of RECK to the membrane allows it to interact with and regulate the activity of other membrane associated proteins, as well as secreted proteins, including those responsible for activating matrixins and various pathophysiological signaling pathways [11]. RECK’s extracellular portion contains protease inhibitor-like domains that sequester pro-MMP-9 and prevent its activation. Elevated expression and persistent activation of the gelatinase MMP-9 contributes to adverse cardiac remodeling, in part through MMP-9-mediated degradation of the ECM and activation of latent growth factors. RECK has also been shown to inhibit MMP9 transcription in cultured cells [11, 74] by blocking binding of the Activator Protein (AP)-1 subunits Fra-1 and c-Jun to the TRE (12-O-tetradecanoylphorbol-13-acetate-responsive element) site in its promoter region [74]. However, these studies did not find RECK in the nucleus, suggesting that RECK-mediated suppression of MMP9 transcription is indirect. Outside of MMP-9, RECK has also been shown to inhibit the catalytic activity of MMPs 2, 7, and 14 [11, 75]. Chang et al. tested full length RECK and several shorter constructs spanning the cysteine knot motif (residues 285–368), all three Kazal motifs (K123, residues 605–799), and a third construct containing the last two Kazal motifs (K23, residues 676–799) to identify the critical region responsible for RECK’s MMP inhibitory activity. Their results suggest that the K23 domain of RECK impaired MMP-9 secretion and activity in lung cancer cells. In fact, immunoprecipitation assays demonstrated that the K23 domain binds and inhibits active MMP-9 [76].
Interestingly, a recent study proposed that earlier results demonstrating the direct inhibition of MMP-9 catalytic activity by RECK stemmed from contamination with a serine protease during purification of RECK protein [77]. By adding a serine inhibitor, the study found that neither RECK nor the two constructs containing the Kazal motifs (residues 621–797, 697–797) are able to significantly influence MMP activity [77]. However, these authors indicated that RECK could still influence MMP activity in vivo outside of direct inhibition, potentially via downregulation of MMP transcription, reducing MMP secretion, or by binding/sequestering MMPs at the cell surface [77]. It is also of note that the constructs in this recent study spanned slightly different residues compared to those from the study by Chang et al. While RECK may not have direct inhibitory action on the catalytic domain of MMP-9, it still appears to negatively regulate MMP-9 activity. This suggests that part of RECK’s role is to slow down MMP-9-mediated remodeling.
In addition to MMP-9, RECK has also been shown to inhibit pro-MMP-2 secretion and activation in human fibrosarcoma-derived HT1080 cells [11] by physically interacting with MT1-MMP (also known as MMP-14) [12]. Recently, Noda et al. reported that RECK also promotes pro-MT1-MMP processing to mature MT1-MMP, and that RECK combined with ADAMTS10 could influence the gelatinolytic and collagenolytic activity of both pro-MT1-MMP and mature MT1-MMP [64]. Interestingly, RECK and ADAMTS10 interaction led to increased proteolytic activity of pro-MT1-MMP, but decreased proteolytic activity of mature MT1-MMP. Furthermore, they also found that RECK, in association with MT1-MMP, enhanced fibrillin and fibronectin deposition [64]. Taken together, these data indicate that RECK may influence MT1-MMP function in a context-dependent manner, it inhibits the proteolytic activity of mature MT1-MMP and MMP-2, but promotes fibrillin and fibronectin deposition in association with MT1-MMP [11, 64].
More recently, RECK has been shown to inhibit the activation of MMP-7, a matrilysin involved in adverse cardiac remodeling [75]. It also inhibits activation of MMP-17, a GPI-anchored MMP and reduces MMP-17-dependent neural crest cell migration [78]. It is however not known whether RECK regulates the activation of MMP3, a stromelysin, whose increased expression contributes to fibrosis [79]. Overall, RECK appears to negatively regulate multiple matrixins involved in migration and remodeling. Therefore, inducing or sustaining RECK expression has therapeutic potential in inhibiting adverse cardiac and vascular remodeling.
Besides targeting multiple MMPs, RECK also targets ADAMs 10 and 17 [80]. In fact, RECK has been shown to inhibit Notch ligand shedding and signaling by targeting ADAM10 [13, 80]. ADAM17, which is also known as TNF-α Converting Enzyme (TACE), acts as a sheddase and releases TNF-α and other transmembrane proteins that play a role in inflammation, cardiac hypertrophy and fibrosis [81]. Together, these reports indicate that RECK is a membrane anchored multi-functional protein that can target activation and/or expression of MMPs and ADAMs.
6. RECK and TGF-β1
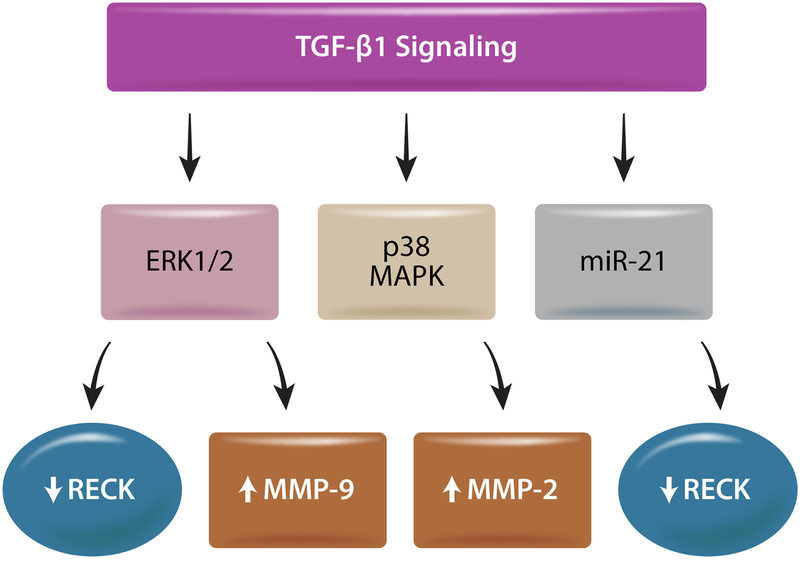
TGF-β1, which plays a role in cardiac hypertrophy and fibrosis, induces the expression of MMPs 2 and 9 and TIMP2 in breast cancer cells, but suppresses RECK [82], indicating that TGF-β1 is a negative regulator of RECK in these cells. Further, inhibition of ERK½ prevented TGF-β1-induced increases in MMP-9 and TIMP-2, but not MMP-2, and reversed RECK suppression. Moreover, targeting p38 MAPK inhibited TGF-β1-induced MMP-2 expression, but not MMP-9 or RECK [82] (Fig. 3). In endothelial cells, exposure to TGF-β1 increases miR-21 expression and promotes endothelial to mesenchymal transition (EndMT) [83]. The process of EndMT increases the number of fibroblasts and accounts for a third of fibroblasts in fibrotic regions of the diseased heart [84]. TGF-β1 also suppresses RECK protein expression in hepatic oval cells, while upregulating miR-21 (Fig. 3), and targeting miR-21 prevents TGF-β1-induced EndMT and renal fibrosis in diabetic nephropathy [85].
Fig. 3:
Downstream effects of TGF-β1 signaling. TGF-β1 increases MMP-9 expression but suppresses RECK through ERK½ activation in cancer cells. It also upregulates MMP-2 through p38 MAPK activation. TGF-β1 has also been shown to suppress RECK expression in part via miR-21 in hepatic oval cells. Illustration made in Adobe Illustrator.
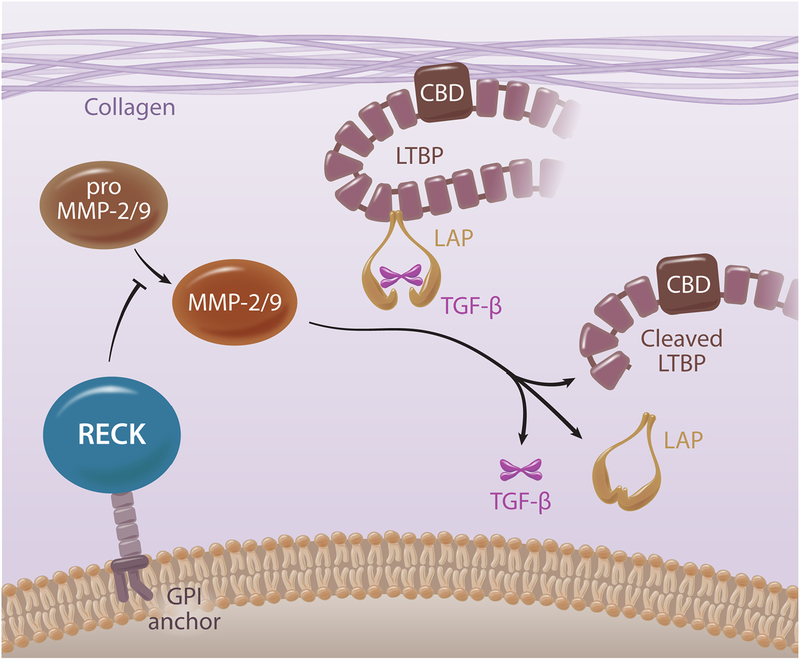
Activation of TGF-β1 is a multistep process, including removal of the Latency TGF- β1 Binding Protein (LTBP) [86, 87]. The LTBP contains a Collagen Binding Domain (CBD) that sequesters latent TGF-β1 to collagen fibers, allowing it to be activated by changes in protease activity or physical strain on the ECM [88]. Since both MMPs 2 and 9 play a role in TGF-β1 release, suggesting a potential role for RECK in excess TGF-β1 activation [89, 90] (Fig. 4). Though anti-TGF-β1 therapies have shown promise, questions remain regarding their efficacy and safety. Therefore, identifying RECK inducers may provide beneficial effects in tissue fibrosis and adverse remodeling by inhibiting excess TGF-β1 activation and signaling. However, unlike in cancer cells, it is not known whether TGF-β1 suppresses RECK expression in cardiac fibroblasts or vascular cells, and needs further investigation.
Fig. 4:
Schematic for protease mediated activation of latent TGF-β1, and the potential role RECK plays in regulating this process. Collagen Binding Domain (CBD), Latency Associated Peptide (LAP), Latent TGF-β1 Binding Protein (LTBP). Illustration made in Adobe Illustrator.
7. RECK and Urokinase-Type Plasminogen Activator
Another target of RECK involved in extracellular matrix degradation is the plasminogen activator (PA) system [91]. The Urokinase-Type Plasminogen Activator (UPA) is a key serine protease involved in transforming plasminogen into active plasmin. This allows active plasmin to initiate a proteolytic cascade to degrade components of the ECM and promote cell migration (288). Both human and animal studies report that macrophage accumulation and increased PA activity contribute to the pathogenesis of cardiac fibrosis. In fact, elevated PA activity was detected in failing human hearts [92, 93]. Consistent with this observation, UPA knockout mice are resistant to developing cardiac fibrosis [94]. The UPA receptor (UPAR) is bound to the cell membrane by a GPI anchor, and UPA ligand binding increases the ability of UPA to convert plasminogen to plasmin [95, 96]. It has been shown that RECK can physically associate with the UPAR and interfere with UPA activity. RECK knockdown increases UPA secretion and invasion of breast cancer cells, and is rescued by concomitant knockdown of UPA [91] or RECK induction [91]. Therefore, further investigations are required to determine whether RECK induction inhibits UPA secretion, ECM remodeling, cell migration, macrophage accumulation, and fibrosis in a diseased or injured heart.
8. Potential Targets of RECK: IL-6R and EGFR
Chronic inflammation, mild or severe, contributes to adverse cardiac and vascular remodeling. It has been previously reported that monocyte recruitment following myocardial infarction promotes myocardial wound healing by recruiting myofibroblasts to the injured area [97], and that these myofibroblasts express pattern recognition receptors that can respond to pathogen-associated molecules to induce the secretion of various proinflammatory cytokines and chemokines [98, 99]. Upon stimulation with lipopolysaccharide (LPS), human cardiac fibroblasts have been shown to secrete IL-1β, TNF-α, IL-6, and macrophage chemoattractant protein-1 (MCP-1) [98]. Cardiac fibroblasts are also shown to express increased levels of IL-1β and IL-18 following myocardial infarction [99]. Inflammatory mediators contribute to remodeling and changes in cell behavior. For instance, IL-17A promotes the migration and proliferation of vascular smooth muscle cells (VSMC), whereas IL-18 induces cardiac fibroblast migration [30, 100]. In both instances, these proinflammatory cytokines suppressed RECK expression, and restoring RECK reversed their migratory and proliferative responses.
Increased circulating IL-6 is a known risk factor in chronic cardiac diseases, including hypertension, cardiac hypertrophy, fibrosis, congestive heart failure, and atherosclerosis [101–109]. Circulating IL-6 levels serve as a marker of vascular inflammation and are released by vessels in response to vascular injury, Ang-II, and inflammatory mediators such as TNF-α and IL-1 [107, 110–114]. In addition, circulating IL-6 levels positively corelate with blood pressure, plasma Ang-II, and vascular hypertrophy [115]. IL-6 signals by binding to IL-6 receptor α (IL-6Rα) on the cell surface [116]. This association leads to oligomerization of the IL-6/IL-6Rα complex with gp130, the signal transducing subunit of the heterodimer receptor, resulting in IL-6/IL-6Rα/gp130 complex [117], and activation of the JAK/STAT pathway that contribute adversely to cardiac and vascular remodeling. Interestingly, using immunoprecipitation and immunoblotting, RECK has been shown to physical associate with IL-6R and gp130 in breast cancer cells [91]. However, in that study, the biological consequence of their interaction has not been investigated.
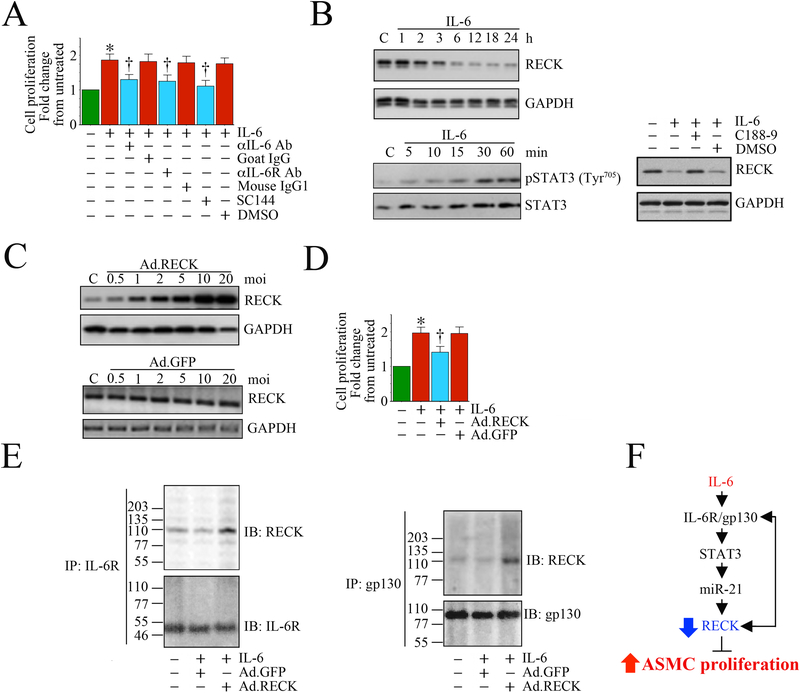
To investigate the role of RECK in IL-6 signaling, we performed a preliminary set of experiments to examine whether RECK inhibits IL-6-mediated VSMC proliferation and determined whether RECK physically interacts with IL-6R and gp130. We found that treatment with IL-6 induced SMC proliferation, and pretreatment with IL-6R neutralizing antibody and exposure to gp130 inhibitor SC144 each inhibited IL-6-mediated STAT3 phosphorylation and SMC proliferation (Fig. 5). For the first time, we also show that IL-6 suppresses RECK expression in a STAT3-dependent manner. Moreover, forced expression of RECK by adenoviral transduction suppressed IL-6-induced SMC proliferation. Confirming earlier results, immunoprecipitation and immunoblotting showed that RECK physically associates with IL-6R and gp130, suggesting that RECK induction blunts IL-6’s pro-mitogenic effects by binding to IL-6R and gp130. Taken together, these results suggest that RECK may inhibit migration and proliferation of VSMCs in response to IL-6 and may help protect against vascular inflammation and remodeling.
Fig. 5: Ectopic expression of RECK suppresses IL-6 mediated aortic smooth muscle cell (ASMC) proliferation. RECK physically associates with IL-6R and gp130.
A, Recombinant human IL-6 induces human ASMC (SMC) proliferation. SMC were grown in SmGM-2 medium, and at 70% confluency, the culture medium was replaced with basal medium containing 0.5% BSA (conditioning medium). After 48 h incubation, the quiescent SMC were incubated with IL-6 (10 ng/ml) for 48 h and analyzed for proliferation by CyQuant assay (n=6). Specificity of IL-6 was verified by incubating quiescent SMC with neutralizing IL-6 or IL-6R antibody (10 mg/ml for 1 h) or the gp130-specific inhibitor SC144 (5 μM in DMSO for 24 h) prior to IL-6 addition (n=6). B, IL-6 suppresses RECK via STAT3 activation. Quiescent SMC were incubated with IL-6 (10 ng/ml) for the indicated time periods. In a subset of experiments, SMC were incubated with the STAT3 inhibitor C188–9 (10 mM in DMSO for 15 min) prior to IL-6 addition (10 ng/ml for 6 h). RECK expression (upper panel) and STAT3 phosphorylation (lower panel) were analyzed in cleared whole cell homogenates (20 μg) by Western blotting. GAPDH and total STAT3 served as loading controls. C, Adenoviral transduction of RECK (upper panel), but not control GFP (lower panel), increases RECK expression in a dose-dependent manner. Quiescent SMC were transduced with Ad.RECK (upper panel) or Ad.GFP (lower panel) at the indicated multiplicity of infection (moi) for 24 h. RECK protein expression was analyzed by Western blotting. D, Forced expression of RECK inhibits IL-6-induced SMC proliferation. SMC were transduced with Ad.RECK or Ad.GFP (moi10 for 24 h) were treated with IL-6 (10 ng/ml for 48h) and analyzed for proliferation as in A (n=6). E, RECK physically associates with IL-6R and gp130. SMC transduced with Ad.RECK or Ad.GFP and then treated with IL-6 were analyzed for IL-6R/RECK and gp30/RECK association by immunoprecipitation (IP) and immunnoblotting (IB) using soluble membrane fractions. F, Schematic showing the signaling pathway involved in IL-6/IL-6R/gp130-mediated STAT3 activation, RECK suppression and SMC proliferation. Importantly, forced expression of RECK suppressed IL-6-mediated SMC proliferation. Double head arrow: Physical association of RECK with IL-6R or gp130. *P<0.05 vs. untreated, †P<0.05 vs. IL-6.
9. RECK and EGFR Transactivation
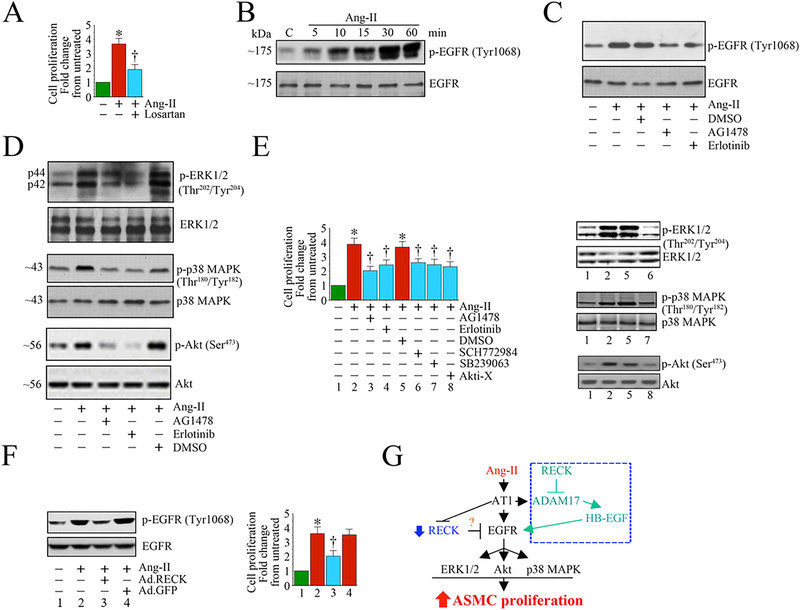
Another potential target of RECK that warrants further investigation is the transactivation of the Epidermal Growth Factor Receptor (EGFR). Increased activation of EGFR has been linked to vascular remodeling, and suppression of its activity could serve as a therapeutic target in adverse remodeling [118]. Transactivation of EGFR also contributes to hypertrophy, migration and proliferation of VSMCs [119–122]. Knockdown of ADAM10/17 in VSMCs reduces Ang-II-mediated EGFR transactivation and hypertrophy, suggesting that ADAM10/17 play a critical intermediate step in Ang-II/AT1R-mediated EGFR transactivation [123, 124]. Blocking EGFR also reduces Ang-II-induced cardiomyocyte hypertrophy and upregulation of fibronectin in cardiac fibroblasts [125, 126]. Of note, VSMC and endothelial cells are known to express some of the EGFR ligands, including EGF, transforming growth factor-α (TGF-α), heparin-binding EGF-like growth factor (HBEGF), betacellulin (BTC), and epiregulin (EREG) [127]. These ligands are produced as transmembrane precursors that must be cleaved by sheddases, such as ADAMs10/17 or MMPs 3 and 7, to become solubilized and bind to EGFR. ADAM10/17 are responsible for the shedding of TGF-α, while ADAM17 facilitates the release of soluble/active EGF [128–130]. Importantly, RECK is a negative regulator of ADAM10/17. Therefore, inducing RECK may indirectly suppress EGFR transactivation by targeting ADAM10/17-mediated ligand release, thereby providing an avenue to put the brakes on vascular and cardiac adverse remodeling. In Fig. 6, we show that Ang-II induces aortic SMC proliferation through transactivation of EGFR, and that adenoviral RECK transduction attenuated Ang-II-induced EGFR transactivation and aortic SMC proliferation.
Fig. 6:
Ectopic expression of RECK suppresses Angiotensin (Ang)-II-induced human aortic smooth muscle cell (SMC) proliferation by inhibiting EGFR activation. A, Angiotensin (Ang)-II stimulates SMC proliferation via AT1. Quiescent SMC were incubated with the AT1 antagonist Losartan potassium (10 μM) for 1 h prior to Ang-II addition (100 nM for 48 h). Cell proliferation was analyzed by CyQuant assay (n=6). B, C, Ang-II induces EGFR activation in a time-dependent manner and is inhibited by AG1478 and erlotinib. Quiescent SMC treated with Ang-II were analyzed for EGFR activation by Western blotting using cleared whole cell lysates and activation-specific antibodies. Total EGFR served as a loading control. In a subset of experiments (C), quiescent SMC were treated with the EGFR-specific inhibitors AG1478 (100 μM in DMSO for 30 min) or erlotinib (1 μM in DMSO for 1h) prior to Ang-II (100 nM for 30 min). D, E, Ang-II induces SMC proliferation via EGFR-dependent ERK½, p38 MAPK and Akt activation. Quiescent SMC were treated with AG1478 or erlotinib prior to Ang-II addition (100 nM for 1 h). Activation of ERK½, p38 MAPK, and Akt were analyzed by Western blotting using cleared whole cell lysates and activation-specific antibodies (D). In a subset of experiments, quiescent SMC incubated with EGFR inhibitors AG1478 or erlotinib, the ERK½ inhibitor SCH772984 (10 μM in DMSO for 1h), p38 MAPK inhibitor SB239063 (10 μM in DMSO for 1h) or the Akt inhibitor Akti-X (1 μM in DMSO for 1h) prior to Ang-II addition (100 nM for 48 h) were analyzed for proliferation as In A (E). The efficacy of inhibitors on respective target proteins was analyzed by Western blotting as shown on the right. F, Ectopic expression of RECK inhibits Ang-II-induced EGFR activation. SMC transduced with Ad.RECK or control GFP were incubated with Ang-II (100 nM for 30 min) were analyzed for EGFR activation by Western blotting (left hand panel). In a subset of experiments, SMC transduced with Ad.RECK or Ad.GFP were made quiescent, treated with Ang-II (100 nM for 48 h) and then analyzed for proliferation (right hand panel). G, Schema showing possible signaling pathways involved in Ang-II/AT1-mediated EGFR activation, RECK suppression and SMC proliferation. While Ang-II induced EGFR activation, it suppressed RECK expression. Further, targeting EGFR inhibits Ang-II-induced ERK½, Akt and p38 MAPK activation, and ASMC proliferation. Importantly, ectopic expression of RECK suppresses EGFR activation and inhibits Ang-II-induced SMC proliferation. RECK suppresses EGFR activation without physical association (data not shown), suggesting that RECK-mediated suppression of EGFR activation is indirect, and may involve (hypothesis) RECK inhibition of Ang-II/AT1/ADAM17-mediated cleavage and release of EGFR ligands such as HB-EGF from the cell surface, and binding to EGFR (blue box at the top right). *P<0.05 vs. untreated, †P<0.05
10. RECK Inducers
Since its discovery, decreased RECK expression has been associated with malignant transformation and progression of many types of cancer [131]. Previously, we reported that ectopic expression of RECK by adenoviral transduction inhibits Ang-II-induced cardiac fibroblast proliferation and migration by targeting MMPs 2, 9, and MMP-14 [29]. Adenoviral transduction of RECK also suppresses Ang-II- and inflammatory cytokine-induced cardiac fibroblast and SMC migration [29]. However, the use of viral vectors in clinical practice has been met with challenges. Therefore, it is important to identify RECK inducers from existing drugs (repurposing) or by developing newer small molecule inducers. Of note, Noda and colleagues used a reporter assay to test 880 bioactive compounds for their ability to induce activation of the RECK promoter, and identified 34 compounds that induced RECK expression [132]. Among these compounds, 12 were anticancer drugs, such as doxorubicin. However, several of these anticancer drugs are cardiotoxic thereby limiting their clinical utility. This screening process also identified minocycline as a RECK inducer, increasing RECK promoter activity by 2.6-fold. Minocycline, an FDA-approved second-generation semisynthetic tetracycline, exerts antioxidant, anti-apoptotic, and anti-inflammatory effects independent of its antimicrobial properties. We recently reported that minocycline inhibits Platelet Derived Growth Factor-BB (PDGF-BB)-induced human aortic smooth muscle cell proliferation and migration in vitro by reversing miR-221- and −222-mediated RECK suppression [133].
We have also previously reported that acetylsalicylic acid (aspirin) and docosahexanoic acid (DHA; an omega 3 lipid) induce RECK expression in cultured cells [30, 134]. In those studies, aspirin and DHA upregulated RECK expression by reducing the expression of oxidative stress-responsive miRNAs such as miR-21. These reports indicate that induction of RECK by minocycline, aspirin, and DHA are not direct. Though double-stranded RNA complimentary to the RECK promoter region induces RECK expression in vitro [61], their therapeutic potential in vivo needs investigation. Lastly, extracts from some natural products have shown to induce RECK in cancer cells [135, 136]. However, their effect on RECK induction also appears to be not specific or direct. Thus, identifying an effective inducer of RECK will have therapeutic benefit not only in cancer, but also in cardiovascular diseases.
11. RECK and Cardiovascular Diseases
While RECK has been shown to attenuate the fibrotic phenotype of cardiac fibroblasts and VSMC, the role of RECK in myocardial hypertrophy, ischemia, doxorubicin toxicity, myocardial cell death, and cardiac dysfunction has not been elucidated. Interestingly, Ang-II has been shown to induce cardiac hypertrophy through transactivation of the EGFR, which was attenuated by EGFR inhibition [137]. Furthermore, TGF-β1 deficient mice did not show increased left ventricular hypertrophy following Ang-II treatment [138]. This raises the possibility that RECK induction may help counteract myocardial hypertrophy by reducing EGFR transactivation and latent TGF-β1 activation. With respect to ischemia, it has been shown in human embryonic kidney epithelial cells that hypoxia reduces RECK expression via HDAC1 and Hypoxia Inducible Factor-1α (HIF-1α), and that in colorectal cancer cells hypoxia induced miR-590–5p expression, leading to RECK suppression [139, 140]. While the role and expression of RECK has not been investigated in a model of myocardial infarction/reperfusion, it is tempting to speculate that RECK expression would be downregulated allowing progression of compensatory remodeling to pathological remodeling and contractile dysfunction. As previously mentioned, doxorubicin increases RECK promoter activity in the HT-1080 fibrosarcoma cell line. Further studies are needed to determine if this induction also occurs in the heart or vasculature, and whether RECK plays a protective/detrimental role in doxorubicin cardiotoxicity.
12. Conclusions and future directions
Independent of underlying cause, the pathogenesis of cardiovascular diseases is associated with enhanced expression and/or activation of MMPs, ADAMs and UPA, proinflammatory mediators (e.g., gp130 ligands), growth factors (e.g., EGFR ligands: heparin-binding EGF [HBEGF], TGF-α), cytokines (e.g., TGF-β), cell surface receptors (e.g., β2-adrenergic receptors, vascular endothelial growth factor receptor-2), and altered ECM components. When overactivated, these diverse processes work in tandem to drive remodeling, and over time impair cardiovascular function. In this review article, we examined how RECK regulates critical steps of each pathway (summarized in Fig. 7), and how it may act as a brake to slow remodeling. As mentioned above, while RECK is expressed widely in various organs under physiological conditions, its expression is markedly suppressed in many diseases that promote remodeling, migration, and proliferation. In these RECKless conditions, the overactivation of proteases leads to the release of both membrane-bound and ECM-sequestered growth factors, as well as the breakdown of collagen, ultimately leading to adverse cardiovascular remodeling. Therefore, a RECK-centered strategy could inhibit the function of multiple pro-hypertrophic, pro-fibrotic and proinflammatory mediators to blunt adverse cardiac and vascular remodeling.
Fig. 7:
Regulation of RECK substrates involved in extracellular remodeling, inflammation, migration and proliferation. Illustration made in Adobe Illustrator.
Highlights.
RECK is a membrane-anchored matrix metalloproteinase regulator
RECK inhibits inflammation
RECK is antifibrotic
RECK reduces adverse cardiac remodeling
RECK induction is cardioprotective
Acknowledgements
We thank Christopher Baines for reviewing and critiquing this article. We appreciate Ms. Stacy Cheavins (Department of Orthopedic Surgery, University of Missouri) for illustrations.
Funding
This study was supported by grants from the Veterans Affairs Merit (VA-I01-BX004220) and Research Career Scientist (IK6BX004016) to BC. SBB is supported by NIH R01 HL136386. This work was supported by the use of facilities and resources at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, MO.
Abbreviation
- ADAM
Disintegrin And Metalloproteinase Domain-Containing Protein
- ADAMTS
A Disintegrin And Metalloproteinase with Thrombospodin Motifs
- Ang-II
Angiotensin II
- Asn
Asparagine
- AT1R
Angiotensin II Receptor Type 1
- BTC
Betacellulin
- CBD
Collagen Binding Domain
- CC
Cysteine Knot Motif
- CT-1
Cardiotrophin-1
- DHA
Docosahexanoic Acid
- ECM
Extracellular Matrix
- EGFR
Epidermal Growth Factor Receptor
- EndMT
Endothelial to Mesenchymal Transition
- ErbB
Epidermal Growth Factor Receptor Family
- EREG
Epiregulin
- ERK
Extracellular Signal-Regulated Kinase
- FXR
Farnesoid X Receptor
- GAS5
Growth Arrest Specific 5
- GDE2
Glycerophosphodiester Phosphodiesterase 2
- gp130
Interleukin 6 Signal Transducer
- GPCR
G-Protein Coupled Receptor
- GPR124
Adhesion G Protein-Coupled Receptor A2
- GPI
Glycosylphosphatidylinositol
- HB-EGF
Heparin Binding EGF-Like Growth Factor
- HDAC
Histone Deacetylase
- HF
Heart Failure
- HIF-1α
Hypoxia Inducible Factor 1 Subunit α
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- IL-6Rα
Interleukin-6 Receptor Subunit α
- JAK
Janus Kinase
- LAP
Latency Associated Peptide
- lncRNAs
Long non-coding RNAs
- LPS
Lipopolysaccharide
- LTBP
Latent Transforming Growth Factor β Binding Protein
- LV
Left Ventricle
- MCP-1
Monocyte Chemoattractant Protein-1
- MI
Myocardial Infarction
- miRNA
MicroRNA
- MMP
Matrix Metalloproteinase
- MT1-MMP
Membrane Type 1 Matrix Metalloproteinase
- NTR
Netrin
- PA
Plasminogen Activator
- PDGF-BB
Platelet Derived Growth Factor-BB
- RAAS
Renin-Angiotensin-Aldosterone System
- RECK
Reversion Inducing Cysteine Rich Protein With Kazal Motifs
- SC144
Small-molecule gp130 inhibitor
- sIL-6Rα
Soluble Interleukin-6 Receptor Subunit α
- SP1
Specificity Protein 1
- STAT
Signal Transducer And Activator Of Transcription
- TACE
TNF-α Converting Enzyme/ADAM17
- TGF-α
Transforming Growth Factor α
- TGF-β
Transforming Growth Factor β
- TIMP
Tissue Inhibitor of Metalloproteinase
- TNF-α
Tumor Necrosis Factor-α
- UPA
Urokinase-Type Plasminogen Activator
- UPAR
Urokinase-Type Plasminogen Activator Receptor
- VEGFR-2
Vascular Endothelial Growth Factor Receptor-2
- VSMCs
Vascular Smooth Muscle Cells
- Wnt7
Wingless-Type MMTV Integration Site Family Member 7
- α1-AR
α1-adrenoceptor
- β2-AR
β2-adrenoreceptor
Footnotes
Declaration of Competing Interest None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hinz B, The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship, Matrix Biology 47 (2015) 54–65. [DOI] [PubMed] [Google Scholar]
- [2].Briet M, Schiffrin EL, Treatment of Arterial Remodeling in Essential Hypertension, Current Hypertension Reports 15(1) (2013) 3–9. [DOI] [PubMed] [Google Scholar]
- [3].Burchfield JS, Xie M, Hill JA, Pathological Ventricular Remodeling, Circulation 128(4) (2013) 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Forrester SJ, Kawai T, O’Brien S, Thomas W, Harris RC, Eguchi S, Epidermal Growth Factor Receptor Transactivation: Mechanisms, Pathophysiology, and Potential Therapies in the Cardiovascular System, Annual Review of Pharmacology and Toxicology 56(1) (2016) 627–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li L, Zhao Q, Kong W, Extracellular matrix remodeling and cardiac fibrosis, Matrix Biology 68–69 (2018) 490–506. [DOI] [PubMed] [Google Scholar]
- [6].Liu P, Sun M, Sader S, Matrix metalloproteinases in cardiovascular disease, Canadian Journal of Cardiology 22 (2006) 25B–30B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spinale FG, Villarreal F, Targeting matrix metalloproteinases in heart disease: Lessons from endogenous inhibitors, Biochemical Pharmacology 90(1) (2014) 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhong S, Khalil RA, A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease, Biochemical Pharmacology 164 (2019) 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shirazi LF, Bissett J, Romeo F, Mehta JL, Role of Inflammation in Heart Failure, Current Atherosclerosis Reports 19(6) (2017). [DOI] [PubMed] [Google Scholar]
- [10].Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K, Kitaura Y, Takai S, Sasahara RM, Horimoto A, Ikawa Y, Ratzkin BJ, Arakawa T, Noda M, Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK, Proceedings of the National Academy of Sciences 95(22) (1998) 13221–13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S-I, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M, The Membrane-Anchored MMP Inhibitor RECK Is a Key Regulator of Extracellular Matrix Integrity and Angiogenesis, Cell 107(6) (2001) 789–800. [DOI] [PubMed] [Google Scholar]
- [12].Miki T, Takegami Y, Okawa K, Muraguchi T, Noda M, Takahashi C, The Reversion-inducing Cysteine-rich Protein with Kazal Motifs (RECK) Interacts with Membrane Type 1 Matrix Metalloproteinase and CD13/Aminopeptidase N and Modulates Their Endocytic Pathways, Journal of Biological Chemistry 282(16) (2007) 12341–12352. [DOI] [PubMed] [Google Scholar]
- [13].Muraguchi T, Takegami Y, Ohtsuka T, Kitajima S, Chandana EPS, Omura A, Miki T, Takahashi R, Matsumoto N, Ludwig A, Noda M, Takahashi C, RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity, Nature Neuroscience 10(7) (2007) 838–845. [DOI] [PubMed] [Google Scholar]
- [14].Park S, Lee C, Sabharwal P, Zhang M, Meyers CLF, Sockanathan S, GDE2 Promotes Neurogenesis by Glycosylphosphatidylinositol-Anchor Cleavage of RECK, Science 339(6117) (2013) 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wouters MA, Rigoutsos I, Chu CK, Feng LL, Sparrow DB, Dunwoodie SL, Evolution of distinct EGF domains with specific functions, Protein Sci 14(4) (2005) 1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Knott V, Downing AK, Cardy CM, Handford P, Calcium binding properties of an epidermal growth factor-like domain pair from human fibrillin-1, J Mol Biol 255(1) (1996) 22–7. [DOI] [PubMed] [Google Scholar]
- [17].Rawlings ND, Tolle DP, Barrett AJ, Evolutionary families of peptidase inhibitors, Biochemical Journal 378(3) (2004) 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY, Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis, Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cho C, Smallwood PM, Nathans J, Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation, Neuron 95(5) (2017) 1056–1073.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang T-H, Hsieh F-L, Smallwood PM, Gabelli SB, Nathans J, Structure of the RECK CC domain, an evolutionary anomaly, Proceedings of the National Academy of Sciences 117(26) (2020) 15104–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, Logsdon D, Hsiao E, Stein EV, Cuttitta F, Haines DC, Nagashima K, Tessarollo L, St B. Croix, GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood–brain barrier, Proceedings of the National Academy of Sciences 108(14) (2011) 5759–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Simizu S, Takagi S, Tamura Y, Osada H, RECK-Mediated Suppression of Tumor Cell Invasion Is Regulated by Glycosylation in Human Tumor Cell Lines, Cancer Research 65(16) (2005) 7455–7461. [DOI] [PubMed] [Google Scholar]
- [23].Trombetta-Lima M, Winnischofer SMB, Demasi MAA, Filho RA, Carreira ACO, Wei B, De Assis Ribas T, Konig MS, Bowman-Colin C, Oba-Shinjo SM, Marie SKN, Stetler-Stevenson W, Sogayar MC, Isolation and characterization of novel RECK tumor suppressor gene splice variants, Oncotarget 6(32) (2015) 33120–33133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee HN, Mitra M, Bosompra O, Corney DC, Johnson EL, Rashed N, Ho LD, Coller HA, RECK isoforms have opposing effects on cell migration, Molecular Biology of the Cell 29(15) (2018) 1825–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jacomasso T, Ribas HT, Trombetta-Lima M, Silberspitz Konig M, Trindade E.d.S., Martinez GR, Sogayar MC, Winnischofer SMB, The alternatively spliced RECK transcript variant 3 is a predictor of poor survival for melanoma patients being upregulated in aggressive cell lines and modulating MMP gene expression in vitro, Melanoma Research 30(3) (2020) 223–234. [DOI] [PubMed] [Google Scholar]
- [26].Sasahara RM, Takahashi C, Noda M, Involvement of the Sp1 site in ras-mediated downregulation of the RECK metastasis suppressor gene, Biochem Biophys Res Commun 264(3) (1999) 668–75. [DOI] [PubMed] [Google Scholar]
- [27].Han L, Yue X, Zhou X, Lan FM, You G, Zhang W, Zhang KL, Zhang CZ, Cheng JQ, Yu SZ, Pu PY, Jiang T, Kang CS, MicroRNA-21 Expression is regulated by β-catenin/STAT3 Pathway and Promotes Glioma Cell Invasion by Direct Targeting RECK, CNS Neuroscience & Therapeutics 18(7) (2012) 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hsu MC, Chang HC, Hung WC, HER-2/neu represses the metastasis suppressor RECK via ERK and Sp transcription factors to promote cell invasion, J Biol Chem 281(8) (2006) 4718–25. [DOI] [PubMed] [Google Scholar]
- [29].Siddesha JM, Valente AJ, Sakamuri SSVP, Yoshida T, Gardner JD, Somanna N, Takahashi C, Noda M, Chandrasekar B, Angiotensin II stimulates cardiac fibroblast migration via the differential regulation of matrixins and RECK, Journal of Molecular and Cellular Cardiology 65 (2013) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Siddesha JM, Valente AJ, Sakamuri SS, Gardner JD, Delafontaine P, Noda M, Chandrasekar B, Acetylsalicylic acid inhibits IL-18-induced cardiac fibroblast migration through the induction of RECK, J Cell Physiol 229(7) (2014) 845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang X, Healy C, Nothnick WB, Estrogen suppresses expression of the matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with Kazal motifs (RECK) within the mouse uterus, Endocrine 42(1) (2012) 97–106. [DOI] [PubMed] [Google Scholar]
- [32].Peng X, Wu W, Zhu B, Sun Z, Ji L, Ruan Y, Zhou M, Zhou L, Gu J, Activation of farnesoid X receptor induces RECK expression in mouse liver, Biochem Biophys Res Commun 443(1) (2014) 211–6. [DOI] [PubMed] [Google Scholar]
- [33].Pramanik KK, Singh AK, Alam M, Kashyap T, Mishra P, Panda AK, Dey RK, Rana A, Nagini S, Mishra R, Reversion-inducing cysteine-rich protein with Kazal motifs and its regulation by glycogen synthase kinase 3 signaling in oral cancer, Tumour Biol 37(11) (2016) 15253–15264. [DOI] [PubMed] [Google Scholar]
- [34].Zhang C, Ling Y, Zhang C, Xu Y, Gao L, Li R, Zhu J, Fan L, Wei L, The silencing of RECK gene is associated with promoter hypermethylation and poor survival in hepatocellular carcinoma, Int J Biol Sci 8(4) (2012) 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ahn MY, Kang DO, Na YJ, Yoon S, Choi WS, Kang KW, Chung HY, Jung JH, Min do S, Kim HS, Histone deacetylase inhibitor, apicidin, inhibits human ovarian cancer cell migration via class II histone deacetylase 4 silencing, Cancer Lett 325(2) (2012) 189–99. [DOI] [PubMed] [Google Scholar]
- [36].Somanna NK, Valente AJ, Krenz M, McDonald KS, Higashi Y, Noda M, Chandrasekar B, Histone deacetyltransferase inhibitors Trichostatin A and Mocetinostat differentially regulate MMP9, IL-18 and RECK expression, and attenuate Angiotensin II-induced cardiac fibroblast migration and proliferation, Hypertens Res 39(10) (2016) 709–716. [DOI] [PubMed] [Google Scholar]
- [37].Jung HM, Phillips BL, Patel RS, Cohen DM, Jakymiw A, Kong WW, Cheng JQ, Chan EK, Keratinization-associated miR-7 and miR-21 regulate tumor suppressor reversion-inducing cysteine-rich protein with kazal motifs (RECK) in oral cancer, J Biol Chem 287(35) (2012) 29261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xin C, Buhe B, Hongting L, Chuanmin Y, Xiwei H, Hong Z, Lulu H, Qian D, Renjie W, MicroRNA-15a promotes neuroblastoma migration by targeting reversion-inducing cysteine-rich protein with Kazal motifs (RECK) and regulating matrix metalloproteinase-9 expression, Febs j 280(3) (2013) 855–66. [DOI] [PubMed] [Google Scholar]
- [39].Guan Y, Guo L, Zukerberg L, Rueda BR, Styer AK, MicroRNA-15b regulates reversion-inducing cysteine-rich protein with Kazal motifs (RECK) expression in human uterine leiomyoma, Reproductive Biology and Endocrinology 14(1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Loayza-Puch F, Yoshida Y, Matsuzaki T, Takahashi C, Kitayama H, Noda M, Hypoxia and RAS-signaling pathways converge on, and cooperatively downregulate, the RECK tumor-suppressor protein through microRNAs, Oncogene 29(18) (2010) 2638–48. [DOI] [PubMed] [Google Scholar]
- [41].Fan X, Wang E, Wang X, Cong X, Chen X, MicroRNA-21 is a unique signature associated with coronary plaque instability in humans by regulating matrix metalloproteinase-9 via reversion-inducing cysteine-rich protein with Kazal motifs, Exp Mol Pathol 96(2) (2014) 242–9. [DOI] [PubMed] [Google Scholar]
- [42].Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, Zhao X, Lu C, Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21–5p delivery, J Exp Clin Cancer Res 38(1) (2019) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhao H, Wang Y, Yang L, Jiang R, Li W, MiR-25 promotes gastric cancer cells growth and motility by targeting RECK, Mol Cell Biochem 385(1–2) (2014) 207–13. [DOI] [PubMed] [Google Scholar]
- [44].Jian Y, Xu CH, Li YP, Tang B, Xie SH, Zeng EM, Down-regulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by up-regulating RECK, Biosci Rep 39(8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [45].Lin HY, Chiang CH, Hung WC, STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells, Br J Cancer 109(3) (2013) 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhou Z, Wang Z, Wei H, Wu S, Wang X, Xiao J, Promotion of tumour proliferation, migration and invasion by miR-92b in targeting RECK in osteosarcoma, Clin Sci (Lond) 130(11) (2016) 921–30. [DOI] [PubMed] [Google Scholar]
- [47].Guo H, Li Q, Li W, Zheng T, Zhao S, Liu Z, MiR-96 downregulates RECK to promote growth and motility of non-small cell lung cancer cells, Mol Cell Biochem 390(1–2) (2014) 155–60. [DOI] [PubMed] [Google Scholar]
- [48].Shi L, Wan Y, Sun G, Gu X, Qian C, Yan W, Zhang S, Pan T, Wang Z, You Y, Functional differences of miR-125b on the invasion of primary glioblastoma CD133-negative cells and CD133-positive cells, Neuromolecular Med 14(4) (2012) 303–16. [DOI] [PubMed] [Google Scholar]
- [49].Li Y, Xu D, Bao C, Zhang Y, Chen D, Zhao F, Ding J, Liang L, Wang Q, Liu L, Li J, Yao M, Huang S, He X, MicroRNA-135b, a HSF1 target, promotes tumor invasion and metastasis by regulating RECK and EVI5 in hepatocellular carcinoma, Oncotarget 6(4) (2015) 2421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ding J, Zhu X, Chen X, Guan J, Li H, MicroRNA-182 Suppresses Malignant Melanoma Proliferation by Targeting RECK, Clin Lab 66(4) (2020). [DOI] [PubMed] [Google Scholar]
- [51].Zheng H, Wang JJ, Zhao LJ, Yang XR, Yu YL, Exosomal miR-182 regulates the effect of RECK on gallbladder cancer, World J Gastroenterol 26(9) (2020) 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R, MicroRNA-182–5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer, PLoS One 8(1) (2013) e55502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pan Y, Liang H, Chen W, Zhang H, Wang N, Wang F, Zhang S, Liu Y, Zhao C, Yan X, Zhang J, Zhang CY, Gu H, Zen K, Chen X, microRNA-200b and microRNA-200c promote colorectal cancer cell proliferation via targeting the reversion-inducing cysteine-rich protein with Kazal motifs, RNA Biol 12(3) (2015) 276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim CW, Kumar S, Son DJ, Jang IH, Griendling KK, Jo H, Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice, Arterioscler Thromb Vasc Biol 34(7) (2014) 1412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang H, Wu J, Meng X, Ying X, Zuo Y, Liu R, Pan Z, Kang T, Huang W, MicroRNA-342 inhibits colorectal cancer cell proliferation and invasion by directly targeting DNA methyltransferase 1, Carcinogenesis 32(7) (2011) 1033–42. [DOI] [PubMed] [Google Scholar]
- [56].Xie J, Tan ZH, Tang X, Mo MS, Liu YP, Gan RL, Li Y, Zhang L, Li GQ, MiR-374b-5p suppresses RECK expression and promotes gastric cancer cell invasion and metastasis, World J Gastroenterol 20(46) (2014) 17439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen X, Shi C, Wang C, Liu W, Chu Y, Xiang Z, Hu K, Dong P, Han X, The role of miR-497–5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis, Scientific Reports 7(1) (2017) 40958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zheng WP, Meng FL, Wang LY, miR-544a Stimulates endometrial carcinoma growth via targeted inhibition of reversion-inducing cysteine-rich protein with Kazal motifs, Mol Cell Probes 53 (2020) 101572. [DOI] [PubMed] [Google Scholar]
- [59].Shen B, Yu S, Zhang Y, Yuan Y, Li X, Zhong J, Feng J, miR-590–5p regulates gastric cancer cell growth and chemosensitivity through RECK and the AKT/ERK pathway, Onco Targets Ther 9 (2016) 6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lin J, Liu Z, Liao S, Li E, Wu X, Zeng W, Elevation of long non-coding RNA GAS5 and knockdown of microRNA-21 up-regulate RECK expression to enhance esophageal squamous cell carcinoma cell radio-sensitivity after radiotherapy, Genomics 112(3) (2020) 2173–2185. [DOI] [PubMed] [Google Scholar]
- [61].Sakurai F, Nanjo Y, Okamoto S, Tachibana M, Mizuguchi H, Upregulation of RECK gene expression by small double-stranded RNA targeting the promoter region, Cancer Gene Ther 21(4) (2014) 164–70. [DOI] [PubMed] [Google Scholar]
- [62].Bányai L, Patthy L, The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases, Protein Sci 8(8) (1999) 1636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Agrawal A, Romero-Perez D, Jacobsen JA, Villarreal FJ, Cohen SM, Zinc-binding groups modulate selective inhibition of MMPs, ChemMedChem 3(5) (2008) 812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Matsuzaki T, Keene DR, Nishimoto E, Noda M, Reversion-inducing cysteine-rich protein with Kazal motifs and MT1-MMP promote the formation of robust fibrillin fibers, J Cell Physiol 236(3) (2021) 1980–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang Y, Rosen H, Madtes DK, Shao B, Martin TR, Heinecke JW, Fu X, Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: an oxidative mechanism for regulating proteolysis during inflammation, J Biol Chem 282(44) (2007) 31826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Caterina NC, Windsor LJ, Yermovsky AE, Bodden MK, Taylor KB, Birkedal-Hansen H, Engler JA, Replacement of conserved cysteines in human tissue inhibitor of metalloproteinases-1, J Biol Chem 272(51) (1997) 32141–9. [DOI] [PubMed] [Google Scholar]
- [67].Brew K, Nagase H, The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity, Biochim Biophys Acta 1803(1) (2010) 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pavloff N, Staskus PW, Kishnani NS, Hawkes SP, A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family, J Biol Chem 267(24) (1992) 17321–6. [PubMed] [Google Scholar]
- [69].Chen Y, Tsai YH, Tseng SH, HDAC Inhibitors and RECK Modulate Endoplasmic Reticulum Stress in Tumor Cells, Int J Mol Sci 18(2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Baker AH, Zaltsman AB, George SJ, Newby AC, Divergent effects of tissue inhibitor of metalloproteinase-1, −2, or −3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis, J Clin Invest 101(6) (1998) 1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Smith MR, Kung H, Durum SK, Colburn NH, Sun Y, TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells, Cytokine 9(10) (1997) 770–80. [DOI] [PubMed] [Google Scholar]
- [72].Fedak PW, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, Mickle DA, Watson KL, Hojilla CV, Cruz W, Weisel RD, Li RK, Khokha R, TIMP-3 deficiency leads to dilated cardiomyopathy, Circulation 110(16) (2004) 2401–9. [DOI] [PubMed] [Google Scholar]
- [73].Kassiri Z, Defamie V, Hariri M, Oudit GY, Anthwal S, Dawood F, Liu P, Khokha R, Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart, J Biol Chem 284(43) (2009) 29893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Takagi S, Simizu S, Osada H, RECK negatively regulates matrix metalloproteinase-9 transcription, Cancer Res 69(4) (2009) 1502–8. [DOI] [PubMed] [Google Scholar]
- [75].Omura A, Matsuzaki T, Mio K, Ogura T, Yamamoto M, Fujita A, Okawa K, Kitayama H, Takahashi C, Sato C, Noda M, RECK forms cowbell-shaped dimers and inhibits matrix metalloproteinase-catalyzed cleavage of fibronectin, J Biol Chem 284(6) (2009) 3461–9. [DOI] [PubMed] [Google Scholar]
- [76].Chang CK, Hung WC, Chang HC, The Kazal motifs of RECK protein inhibit MMP-9 secretion and activity and reduce metastasis of lung cancer cells in vitro and in vivo, J Cell Mol Med 12(6b) (2008) 2781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mendes SR, Amo-Maestro L.d., Marino-Puertas L, Diego I.d., Goulas T, Gomis-Rüth FX, Analysis of the inhibiting activity of reversion-inducing cysteine-rich protein with Kazal motifs (RECK) on matrix metalloproteinases, Scientific Reports 10(1) (2020) 6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Leigh NR, Schupp MO, Li K, Padmanabhan V, Gastonguay A, Wang L, Chun CZ, Wilkinson GA, Ramchandran R, Mmp17b is essential for proper neural crest cell migration in vivo, PLoS One 8(10) (2013) e76484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, Suzuki T, Campbell MN, Gauldie J, Radisky DC, Riches DW, Yu G, Kaminski N, McCulloch CA, Downey GP, Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis, Am J Pathol 179(4) (2011) 1733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hong K-J, Wu D-C, Cheng K-H, Chen L-T, Hung W-C, RECK Inhibits Stemness Gene Expression and Tumorigenicity of Gastric Cancer Cells by Suppressing ADAM-Mediated Notch1 Activation, Journal of Cellular Physiology 229(2) (2014) 191–201. [DOI] [PubMed] [Google Scholar]
- [81].Fan D, Takawale A, Shen M, Wang W, Wang X, Basu R, Oudit GY, Kassiri Z, Cardiomyocyte A Disintegrin And Metalloproteinase 17 (ADAM17) Is Essential in Post-Myocardial Infarction Repair by Regulating Angiogenesis, Circ Heart Fail 8(5) (2015) 970–9. [DOI] [PubMed] [Google Scholar]
- [82].Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC, TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK½ in highly invasive breast cancer cells, BMC Cancer 12 (2012) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T, Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21, Arterioscler Thromb Vasc Biol 32(2) (2012) 361–9. [DOI] [PubMed] [Google Scholar]
- [84].Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R, Endothelial-to-mesenchymal transition contributes to cardiac fibrosis, Nat Med 13(8) (2007) 952–61. [DOI] [PubMed] [Google Scholar]
- [85].Gao S, Chen D, Zou L, Haung L, Dai R, Zheng J, Mo Z, Hu W, Shan W, MiR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target RECK in hepatic oval cells, Int J Clin Exp Pathol 9(4) (2016) 4779–4785. [Google Scholar]
- [86].Sengle G, Ono RN, Sasaki T, Sakai LY, Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability, J Biol Chem 286(7) (2011) 5087–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].De Crescenzo G, Grothe S, Zwaagstra J, Tsang M, O’Connor-McCourt MD, Real-time monitoring of the interactions of transforming growth factor-beta (TGF-beta ) isoforms with latency-associated protein and the ectodomains of the TGF-beta type II and III receptors reveals different kinetic models and stoichiometries of binding, J Biol Chem 276(32) (2001) 29632–43. [DOI] [PubMed] [Google Scholar]
- [88].Saharinen J, Hyytiäinen M, Taipale J, Keski-Oja J, Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action, Cytokine Growth Factor Rev 10(2) (1999) 99–117. [DOI] [PubMed] [Google Scholar]
- [89].Horiguchi M, Ota M, Rifkin DB, Matrix control of transforming growth factor-β function, J Biochem 152(4) (2012) 321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yu Q, Stamenkovic I, Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis, Genes Dev 14(2) (2000) 163–76. [PMC free article] [PubMed] [Google Scholar]
- [91].Walsh LA, Roy DM, Reyngold M, Giri D, Snyder A, Turcan S, Badwe CR, Lyman J, Bromberg J, King TA, Chan TA, RECK controls breast cancer metastasis by modulating a convergent, STAT3-dependent neoangiogenic switch, Oncogene 34(17) (2015) 2189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pogwizd SM, McKenzie JP, Cain ME, Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy, Circulation 98(22) (1998) 2404–14. [DOI] [PubMed] [Google Scholar]
- [93].Tofler GH, Massaro J, O’Donnell CJ, Wilson PWF, Vasan RS, Sutherland PA, Meigs JB, Levy D, D’Agostino RB Sr., Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study, Thromb Res 140 (2016) 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Heymans S, Lupu F, Terclavers S, Vanwetswinkel B, Herbert JM, Baker A, Collen D, Carmeliet P, Moons L, Loss or inhibition of uPA or MMP-9 attenuates LV remodeling and dysfunction after acute pressure overload in mice, Am J Pathol 166(1) (2005) 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ellis V, Behrendt N, Danø K, Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor, J Biol Chem 266(19) (1991) 12752–8. [PubMed] [Google Scholar]
- [96].Ploug M, Rønne E, Behrendt N, Jensen AL, Blasi F, Danø K, Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol, J Biol Chem 266(3) (1991) 1926–33. [PubMed] [Google Scholar]
- [97].van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ, Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice, Am J Pathol 170(3) (2007) 818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sandstedt J, Sandstedt M, Lundqvist A, Jansson M, Sopasakis VR, Jeppsson A, Hultén LM, Human cardiac fibroblasts isolated from patients with severe heart failure are immune-competent cells mediating an inflammatory response, Cytokine 113 (2019) 319–325. [DOI] [PubMed] [Google Scholar]
- [99].Sandanger Ø, Ranheim T, Vinge LE, Bliksøen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A, The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury, Cardiovasc Res 99(1) (2013) 164–74. [DOI] [PubMed] [Google Scholar]
- [100].Mummidi S, Das NA, Carpenter AJ, Yoshida T, Yariswamy M, Mostany R, Izadpanah R, Higashi Y, Sukhanov S, Noda M, Siebenlist U, Rector RS, Chandrasekar B, RECK suppresses interleukin-17/TRAF3IP2-mediated MMP-13 activation and human aortic smooth muscle cell migration and proliferation, J Cell Physiol 234(12) (2019) 22242–22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A, Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events, Circulation 99(16) (1999) 2079–84. [DOI] [PubMed] [Google Scholar]
- [102].Chae CU, Lee RT, Rifai N, Ridker PM, Blood pressure and inflammation in apparently healthy men, Hypertension 38(3) (2001) 399–403. [DOI] [PubMed] [Google Scholar]
- [103].Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M, Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure, J Am Coll Cardiol 31(2) (1998) 391–8. [DOI] [PubMed] [Google Scholar]
- [104].Roig E, Orús J, Paré C, Azqueta M, Filella X, Perez-Villa F, Heras M, Sanz G, Serum interleukin-6 in congestive heart failure secondary to idiopathic dilated cardiomyopathy, Am J Cardiol 82(5) (1998) 688–90, a8. [DOI] [PubMed] [Google Scholar]
- [105].Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP, Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity, J Clin Endocrinol Metab 82(5) (1997) 1313–6. [DOI] [PubMed] [Google Scholar]
- [106].Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H, Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability, Circulation 101(12) (2000) 1372–8. [DOI] [PubMed] [Google Scholar]
- [107].Vázquez-Oliva G, Fernández-Real JM, Zamora A, Vilaseca M, Badimón L, Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects, Journal of Human Hypertension 19(6) (2005) 457–462. [DOI] [PubMed] [Google Scholar]
- [108].Chou CH, Hung CS, Liao CW, Wei LH, Chen CW, Shun CT, Wen WF, Wan CH, Wu XM, Chang YY, Wu VC, Wu KD, Lin YH, IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis, Cardiovasc Res 114(5) (2018) 690–702. [DOI] [PubMed] [Google Scholar]
- [109].Kanda T, Takahashi T, Interleukin-6 and cardiovascular diseases, Jpn Heart J 45(2) (2004) 183–93. [DOI] [PubMed] [Google Scholar]
- [110].Han Y, Runge MS, Brasier AR, Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors, Circ Res 84(6) (1999) 695–703. [DOI] [PubMed] [Google Scholar]
- [111].Cui R, Tieu B, Recinos A, Tilton RG, Brasier AR, RhoA mediates angiotensin II-induced phospho-Ser536 nuclear factor kappaB/RelA subunit exchange on the interleukin-6 promoter in VSMCs, Circ Res 99(7) (2006) 723–30. [DOI] [PubMed] [Google Scholar]
- [112].Akira S, Kishimoto T, IL-6 and NF-IL6 in acute-phase response and viral infection, Immunol Rev 127 (1992) 25–50. [DOI] [PubMed] [Google Scholar]
- [113].Recinos A 3rd, LeJeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG, Brasier AR, Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice, Atherosclerosis 194(1) (2007) 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Loppnow H, Libby P, Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6, J Clin Invest 85(3) (1990) 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gomolak JR, Didion SP, Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation, Front Physiol 5 (2014) 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hou T, Tieu BC, Ray S, Recinos Iii A, Cui R, Tilton RG, Brasier AR, Roles of IL-6-gp130 Signaling in Vascular Inflammation, Curr Cardiol Rev 4(3) (2008) 179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Boulanger MJ, Chow DC, Brevnova EE, Garcia KC, Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex, Science 300(5628) (2003) 2101–4. [DOI] [PubMed] [Google Scholar]
- [118].Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM, Angiotensin II and vascular injury, Curr Hypertens Rep 16(6) (2014) 431. [DOI] [PubMed] [Google Scholar]
- [119].Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK, Angiotensin II Stimulation of NAD(P)H Oxidase Activity, Circulation Research 91(5) (2002) 406–413. [DOI] [PubMed] [Google Scholar]
- [120].Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S, Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology, Clin Sci (Lond) 112(8) (2007) 417–28. [DOI] [PubMed] [Google Scholar]
- [121].Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T, Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells, J Biol Chem 273(15) (1998) 8890–6. [DOI] [PubMed] [Google Scholar]
- [122].Touyz RM, Wu XH, He G, Salomon S, Schiffrin EL, Increased angiotensin II-mediated Src signaling via epidermal growth factor receptor transactivation is associated with decreased C-terminal Src kinase activity in vascular smooth muscle cells from spontaneously hypertensive rats, Hypertension 39(2 Pt 2) (2002) 479–85. [DOI] [PubMed] [Google Scholar]
- [123].Elliott KJ, Bourne AM, Takayanagi T, Takaguri A, Kobayashi T, Eguchi K, Eguchi S, ADAM17 silencing by adenovirus encoding miRNA-embedded siRNA revealed essential signal transduction by angiotensin II in vascular smooth muscle cells, J Mol Cell Cardiol 62 (2013) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S, ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II, Arterioscler Thromb Vasc Biol 26(9) (2006) e133–7. [DOI] [PubMed] [Google Scholar]
- [125].Moriguchi Y, Matsubara H, Mori Y, Murasawa S, Masaki H, Maruyama K, Tsutsumi Y, Shibasaki Y, Tanaka Y, Nakajima T, Oda K, Iwasaka T, Angiotensin II-induced transactivation of epidermal growth factor receptor regulates fibronectin and transforming growth factor-beta synthesis via transcriptional and posttranscriptional mechanisms, Circ Res 84(9) (1999) 1073–84. [DOI] [PubMed] [Google Scholar]
- [126].Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J, An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II-mediated cardiac hypertrophy, Circ Res 99(5) (2006) 528–36. [DOI] [PubMed] [Google Scholar]
- [127].Holbro T, Hynes NE, ErbB receptors: directing key signaling networks throughout life, Annu Rev Pharmacol Toxicol 44 (2004) 195–217. [DOI] [PubMed] [Google Scholar]
- [128].Hinkle CL, Mohan MJ, Lin P, Yeung N, Rasmussen F, Milla ME, Moss ML, Multiple metalloproteinases process protransforming growth factor-alpha (proTGF-alpha), Biochemistry 42(7) (2003) 2127–36. [DOI] [PubMed] [Google Scholar]
- [129].Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M, Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site, J Biol Chem 272(50) (1997) 31730–7. [DOI] [PubMed] [Google Scholar]
- [130].Yu WH, Woessner JF Jr., McNeish JD, Stamenkovic I, CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling, Genes Dev 16(3) (2002) 307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Noda M, Takahashi C, Recklessness as a hallmark of aggressive cancer, Cancer Sci 98(11) (2007) 1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Murai R, Yoshida Y, Muraguchi T, Nishimoto E, Morioka Y, Kitayama H, Kondoh S, Kawazoe Y, Hiraoka M, Uesugi M, Noda M, A novel screen using the Reck tumor suppressor gene promoter detects both conventional and metastasis-suppressing anticancer drugs, Oncotarget 1(4) (2010) 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Higashi Y, Mummidi S, Sukhanov S, Yoshida T, Noda M, Delafontaine P, Chandrasekar B, Minocycline inhibits PDGF-BB-induced human aortic smooth muscle cell proliferation and migration by reversing miR-221- and −222-mediated RECK suppression, Cell Signal 57 (2019) 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Siddesha JM, Valente AJ, Yoshida T, Sakamuri SS, Delafontaine P, Iba H, Noda M, Chandrasekar B, Docosahexaenoic acid reverses angiotensin II-induced RECK suppression and cardiac fibroblast migration, Cell Signal 26(5) (2014) 933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kowshik J, Mishra R, Sophia J, Rautray S, Anbarasu K, Reddy GD, Dixit M, Mahalingam S, Nagini S, Nimbolide upregulates RECK by targeting miR-21 and HIF-1α in cell lines and in a hamster oral carcinogenesis model, Scientific Reports 7(1) (2017) 2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Murugan RS, Vinothini G, Hara Y, Nagini S, Black tea polyphenols target matrix metalloproteinases, RECK, proangiogenic molecules and histone deacetylase in a rat hepatocarcinogenesis model, Anticancer Res 29(6) (2009) 2301–5. [PubMed] [Google Scholar]
- [137].Peng K, Tian X, Qian Y, Skibba M, Zou C, Liu Z, Wang J, Xu Z, Li X, Liang G, Novel EGFR inhibitors attenuate cardiac hypertrophy induced by angiotensin II, J Cell Mol Med 20(3) (2016) 482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T, TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II, J Clin Invest 109(6) (2002) 787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lee KJ, Lee KY, Lee YM, Downregulation of a tumor suppressor RECK by hypoxia through recruitment of HDAC1 and HIF-1alpha to reverse HRE site in the promoter, Biochim Biophys Acta 1803(5) (2010) 608–16. [DOI] [PubMed] [Google Scholar]
- [140].Kim CW, Oh ET, Kim JM, Park JS, Lee DH, Lee JS, Kim KK, Park HJ, Hypoxia-induced microRNA-590–5p promotes colorectal cancer progression by modulating matrix metalloproteinase activity, Cancer Lett 416 (2018) 31–41. [DOI] [PubMed] [Google Scholar]