Abstract
Remdesivir is an antiviral drug initially designed against the Ebola virus. The results obtained with it both in biochemical studies in vitro and in cell line assays in vivo were very promising, but it proved ineffective in clinical trials. Remdesivir exhibited far better efficacy when repurposed against SARS-CoV-2. The chemistry that accounts for this difference is the subject of this study. Here, we examine the hypothesis that remdesivir monophosphate (RMP)-containing RNA functions as a template at the polymerase site for the second run of RNA synthesis, and as mRNA at the decoding center for protein synthesis. Our hypothesis is supported by the observation that RMP can be incorporated into RNA by the RNA-dependent RNA polymerases (RdRp) of both viruses, although some of the incorporated RMPs are subsequently removed by exoribonucleases. Furthermore, our hypothesis is consistent with the fact that RdRp of SARS-CoV-2 selects RMP for incorporation over AMP by 3-fold in vitro, and that RMP-added RNA can be rapidly extended, even though primer extension is often paused when the added RMP is translocated at the i+3 position (with i the nascent base pair at an initial insertion site of RMP), or when the subsequent nucleoside triphosphates (NTPs) are below their physiological concentrations. These observations have led to the hypothesis that remdesivir might be a delayed chain terminator. However, that hypothesis is challenged under physiological concentrations of NTPs by the observation that about three quarters of RNA products efficiently overrun the pause.
Keywords: Remdesivir, chain terminator, delayed chain termination, poly(A) tail, RMP-containing template, RMP-containing primer, translating ribosome, decoding center, codon-anticodon base pairing, Ebola virus, mouse hepatitis virus
Graphical Abstract
Topics of Content (TOC). Chemical structure and base pairing of remdesivir
Introduction
Remdesivir (R) is an analogue of adenosine (A), capable of forming Watson-Crick base pairs with uridine (U).1, 2 It has a cyano substitution at the H position of the C1’ center, a strong electron-withdrawing group that destabilizes the glycosidic C1’-N9 bond. To prevent hydrolysis of the glycosidic bond, C and N atoms within its aromatic base ring are repositioned for it to become 4-aza-7,9-dideazaadenosine such that a C-C bond occupies the equivalent glycosidic bond. This repositioning does not alter the hydrogen bonding capability of its base so it effectively mimics adenosine by hydrogen bonding to uridine mono-phosphate (UMP). Within the context provided by the active site of the polymerase (pol) within the pol replication complex, there is very little electronic difference of the base between remdesivir monophosphate (RMP) and adenosine monophosphate (AMP).
Remdesivir exhibited promising results both in vitro and in vivo studies as well as in animal models for treatment of Ebola virus; however, it exhibited very little efficacy in clinical trials.3, 4 Remarkably, it exhibited much better efficacy when repurposed for treatment of patients infected with SARS-CoV-2 although the mechanism of the inhibition remains uncertain.5, 6 Therefore, understanding how remdesivir inhibits the RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 remains an outstanding challenge that could provide valuable insights to development of new drugs with even better efficacy.
Base selectivity for RMP over AMP by RNA polymerases
Remdesivir exhibits very low toxicity in practice, as human RNA polymerases select AMP over RMP for nucleotide incorporation by as much as three orders of magnitude.4 RdRp from both SARS-CoV-2 and Ebola virus are able to efficiently incorporate RMP into RNAs, making it a potential inhibitor of viral multiplication.3, 7–9 In fact, RdRp of SARS-CoV-2 selects RMP for nucleotide incorporation over AMP by about 3-fold in vitro while RdRp of Ebola selects AMP over RMP, also by 3-fold.8 Thus, there is a 9-fold difference in RMP selectivity of the two pols.
An initial working hypothesis has been that remdesivir is a direct-acting nucleotide analog chain terminator that targets specifically viral RdRp. That proposal has been based on the observation that remdesivir can be selectively incorporated into viral RNAs and escaped from excision by exoribonuclease to some extent, but not into host RNAs. However, remdesivir has an extendable 3’-OH so that it differs from the classic chain terminators such as 3’-deoxy version of analogs or AZT that lack an extendable 3’-OH. Here, we propose that the 3’-deoxy, 3’-F, 3’-NH2, or 3’-N3 versions of remdesivir could be developed to be more effective inhibitors, assuming that they can be efficiently converted into the triphosphate form by the cellular machinery. These new true chain terminators could completely block, or significantly slow down, the synthesis of viral RNAs but should not affect the synthesis of host RNAs.
The chain termination hypothesis for remdesivir was supported by initial cell-based assays. An analysis of the level of the total RNAs of the SARS-CoV-2 infected cells showed that overall, 80% of the total RNAs were virally encoded RNA in the absence of treatment of remdesivir, whereas the percentage was reduced to ~ 10% when the cells were treated with remdesivir,10, 11 suggesting that remdesivir treatment significantly reduced the level of viral RNAs. In cell line assays in vivo, the amount of remdesivir needed to reduce 50% of viral RNA levels increased when the exoribonuclease-deficient SARS-CoV-1 or Ebola virus were studied, suggesting that RMP may act as a chain terminator and that the exoribonuclease was able to remove some of added RMP chain terminators so that it can restart primer extension. However, the level of viral RNA does not always correlate well with the level of viral replication and viral infection in the presence of antiviral drugs,12 because the level of viral infection is proportional to the number of cells being infected and because it is unclear whether all viral RNAs are functional for viral multiplication. Alternatively, viral RNAs synthesized in the presence of remdesivir may be unstable and degraded rapidly, which would be contradictory to the chain termination hypothesis.
A hypothesis of delayed chain termination
In vitro observations show that purified nsp12 or RdRp from SARS-CoV-2 incorporates RMP by ~3-fold more efficiently than AMP, and that the 3’-hydroxyl of added RMP can also serve as a new substrate for addition of the next Watson-Crick basepaired nucleotide,8, 9 ruling out the original hypothesis that remdesivir serves as a chain terminator. It was observed that primer extension for the next three nucleotides was very efficient even after the concentrations of the next NTPs were artificially decreased.8, 9, 13, 14 However, it has been observed that primer extension for the fourth nucleotide was stopped leading to a revised hypothesis, called a delayed chain termination.
Intracellular ATP concentration is the highest among four NTPs and varies from 3 to 10 mM, with the cellular concentrations of three other NTPs of about 0.5 mM.15–17 Therefore, the question was raised as to whether the primer extension remained stalled under the physiological concentrations of NTPs. A careful quantification showed that about 3/4 of RNA products rapidly extended primers as if RMP were never incorporated or as if RMP behaved exactly the same as AMP, and that only 1/4 of RNA products failed to be fully extended.13 If exoribonuclease were added, the stalled RNA products would likely have been hydrolyzed so that they could be further extended after removing RMP. Therefore, the paused primer extension observed in vitro at the i+3 site after RMP addition (with i the nascent base pair at an initial insertion site of RMP) is short lived and not likely to be biologically relevant to the reduced level of viral RNAs observed in the infected cells.
RNA synthesis during transcription-replication in SARS-CoV-2 and Ebola virus
SARS-CoV-2 is a positive-sense single-stranded (ss) RNA virus.18, 19 The ssRNA genome of SARS-CoV-2 is highly structured with a vast network of secondary structures, duplex stems, hairpins, and pseudoknots, stabilized by a large number of bound nucleocapsid proteins.20–22 The genome is properly positioned within the pre-packaged functional replication-transcription complex (RTC) inside the virus. After the virus enters permissive host cells where a pool of NTPs is available, RdRp rapidly transcribes a negative-strand fusion RNA product in a discontinuous manner, known as a transcription-intermediate (TI). Each TI serves as a template for repetitive synthesis of tens to hundreds copies of different positive-sense viral mRNAs for synthesis of viral proteins. All sub-genomic (sg) transcripts have identical 5’- and 3’-sequences whereas they differ in various deletions of middle sequences, flanked by a pair of transcription-regulatory sequences (TRS), through a strand-switching mechanism or jumping events. After accumulations of all necessary viral proteins, RdRp transcribes another specialized negative-strand replication-intermediate (RI) as a template for faithful synthesis of new genomic RNA in its entirety for viral packaging without any deletion. In the virally infected cells, the majority of viral RNAs are positive-sense mRNAs. The amount of negative-sense RNAs is very low (only a few percent) and difficult to study.18 Yet, they are essential as templates for making viral mRNAs and new genomic RNAs. Different from viral mRNAs which are often short-lived and rapidly turned over, both TI and RI are long lived, resistant to RNA degradation, likely due to some specific protections by the RTC to both of their 5’and 3’ ends. An unaddressed important question is whether RMP-containing RNA templates can be copied. If not, it may explain why the level of viral rRNAs is reduced in the infected cells after they were treated with remdesivir. The presence of RMP in the template could also result in high noise of random transcriptional jumping events or uncontrolled strand-switching in positive-sense RNAs and disrupt the normal functions of viral mRNAs.11
Ebola is a negative-sense ssRNA virus.23 The synthesis of its viral mRNAs does not involve a transcription-intermediate as a template for synthesis of viral mRNAs as in the case of SARS-CoV-2 because viral mRNAs are directly transcribed from the RNA genome. Therefore, the mechanism of transcription-replication cycle differs between SARS-CoV-2 and Ebola virus, as does the relative importance of the first and second runs of RNA synthesis. If remdesivir directly acts on the viral intermediate RNAs as templates, its effects on inhibition of viral multiplication will differ against these two viruses. In addition, the genome of SARS-CoV-2 is A and U enriched with A, U, G, and C contents of 30%, 32%, 20%, and 18%, respectively, whereas that of Ebola virus is enriched in G and C contents.24–26 Given the fact that RdRp of SARS-CoV-2 prefers RMP over AMP whereas RdRp of Ebola virus prefers AMP over RMP, these differences could be amplified to result in different efficacies of remdesivir for treatment of patients infected with one of these two viruses.
Structures of the replication complexes of RNA polymerases with RMP-containing primer/template RNA duplexes
The first structure of the replication complex of SARS-CoV-2 RNA polymerase with RMP-containing primer/template (P/T) was obtained upon incubation with remdesivir triphosphate (RTP) opposite to poly(U) template.27 It was found that the pol was able to add only single RMP with the pyrophosphate product remaining bound (Fig. 1a). The added RMP did not appear to be extended by a second RMP, a situation that differs from results of biochemical studies using a non-poly(U) template where primer extension after RMP is very efficient. An implication of this observation is that the genomically encoded poly(A) tails of viral mRNAs cannot be fully synthesized and are often shortened in the presence of remdesivir. This is because the synthesis of genomically encoded 33-nucleotide poly(A) tails of viral mRNAs is carried out by RdRp using 5’-poly(U) negative-sense (PUN) template of transcription intermediate (TI),28 whereas these of cellular RNAs are synthesized by cellular poly(A)-polymerase. Without the protection of poly(A) tails, viral RNAs will be degraded very rapidly, which would also explain the reduced level of viral RNAs in the SARS-CoV-2 infected cells after being treated with remdesivir. In fact, many viruses evolve an elaborate mechanism for protection of the poly(A) tails of their viral RNAs.29–32
Figure 1.
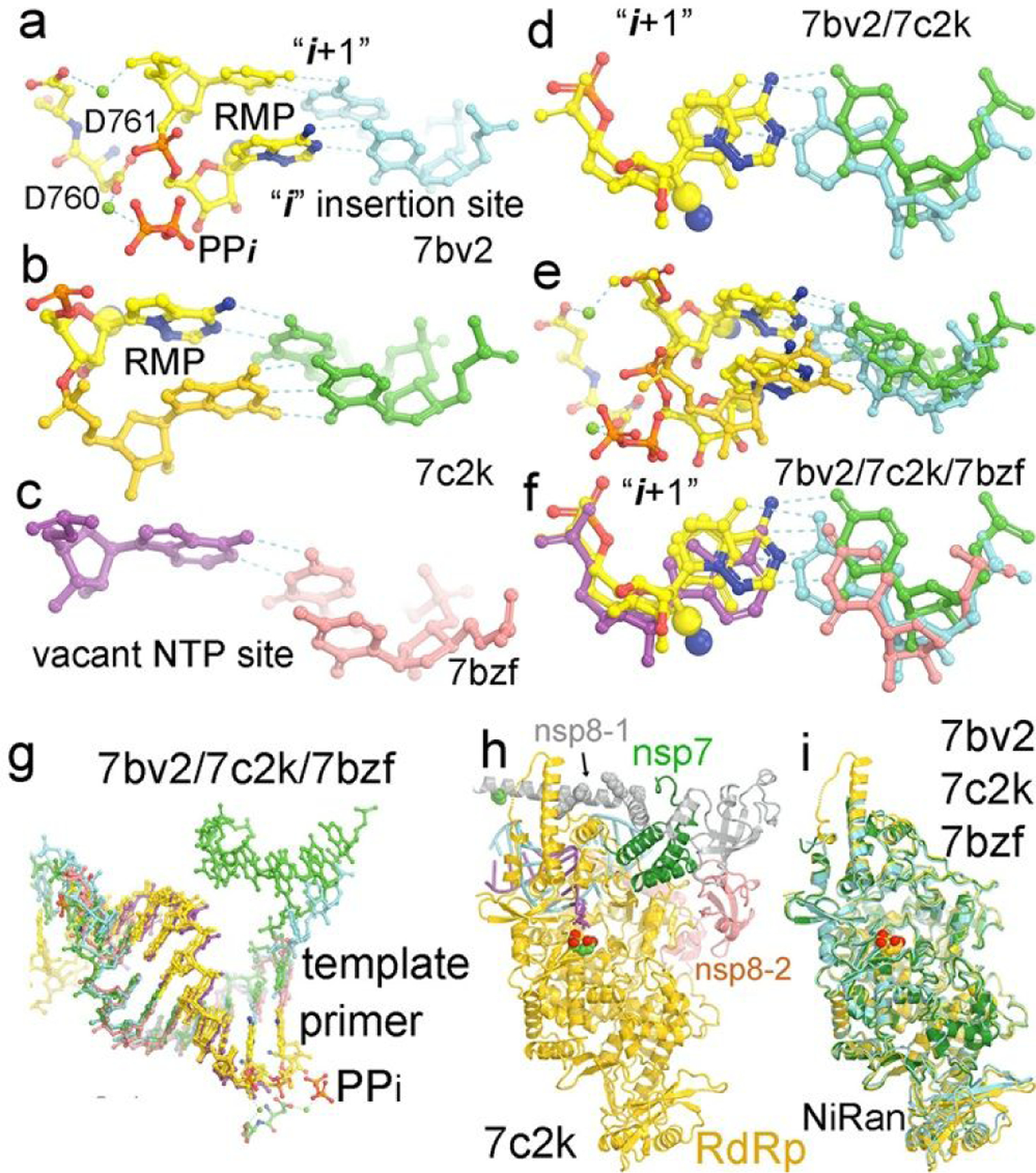
Conformations of RMP-containing primers in P/T replication complex of RdRp of SARS-CoV-2. (a) The 7bv2 structure with RMP at the “i” site and the pyrophosphate (PPi) bound. Two catalytic carboxylates D760 and D761 and two divalent metal ions are shown. (b) The 7c2k structure with RMP at the i+1 site in the pre-translocated product complex. (c) The 7bzf structure of a post-translocated product complex with a vacant NTP-binding pocket. (d) Comparison of the 7bv2 and 7c2k structures at the i+1 site. (e). Comparison of the 7bv2 and 7c2k structures at both the i and i+1 sites. (f) Comparison of the 7bv2, 7c2k and 7bzf structures at the i site. (g) Comparison of the 7bv2, 7c2k and 7bzf structures for the entire primer/template RNA duplexes. (h) The 7bv2 structure with one nsp8 in grey (nsp8–1) and the second nsp8 (nsp8–2) in salmon, nsp7 in green, and P/T in cyan, and the polymerase in gold and two catalytic carboxylates in large CPK models. (i) The superposition of the 7bv2, 7c2k, and 7bzf polymerase structures, which is a basis of comparison of corresponding P/T RNA duplexes.
Many additional structures of replication complexes with RMP added at different positions of the primer strands were obtained using chemically synthesized RMP-containing primers or enzymatically synthesized by the pols (Fig. 1).13, 14, 33–35 Similarly, primer extension assays with RMP added at different positions of the primer strands were studied using both chemically synthesized and enzymatically synthesized primers. Results are nearly identical regardless of the sources of RMP-containing primers. As expected, one-nucleotide extended RMP-containing primer forms a Watson-Crick base pair to the template as does exactly the AMP-containing primer (Fig. 1b).27 At this position, the extra cyano substitution is not in direct contact with any sidechain of the pol, which is why RMP-added primer can be rapidly translocated from the insertion “i” site to the i+1 site and be extended efficiently. Nevertheless, the substituted cyano group may interact with the pol through ordered water molecules, which remain unresolved due to relatively low resolution of cryo-electron microscopic (cryo-EM) structures. After incorporation of NMP and release of pyrophosphate, the synthesized RNA product is translocated to the i+1 position, which is known as the post-translocated product state with a vacant NTP binding site ready for accepting the next incoming NTP (Fig. 1c).27
RdRp selects incoming NTPs according to Watson-Crick base pairing principles opposite to the templating nucleotide as does DNA pols although the base selectivity for nucleotide incorporation by RNA pols is often much poorer than the base selectivity by replicative DNA pols. It is well known that many conformational changes of DNA pols occur upon initial binding of the Watson-Crick base paired incoming dNTP, formation of the closed replication complex, alignment of substrates, chemical step of nucleotide addition, and release of pyrophosphate, followed by the translocation of the P/T duplex product for the next nucleotide addition.36 When a non-Watson-Crick base paired dNTP binds, each of these events becomes slower so that the incorrect dNTP will be preferably rejected. Although it is likely that many equivalent conformational changes may also occur in RNA pols but with smaller amplitudes, they have not yet been fully characterized. After misincorporation of a non-Watson-Crick base paired NMP, primer extension is often stalled so that it can be removed either by pyrophosphorylysis or by exoribonuclease. When it is removed by exoribonuclease, mispaired NMP must first become unpaired and be transferred into the exoribonuclease active site for hydrolysis. If RMP remains Watson-Crick base paired and if RMP-added primers can be rapidly extended with the next Watson-Crick base paired NMPs, both of which are true for RdRp of SARS-CoV-1 and SARS-CoV-2, it is inevitable that the synthesized viral RNAs will contain a high level of RMPs.
A comparison of the i+1 base pair between two pol replication structures with RMP-containing primer at two different positions, one at the i position and the other at the i+1 position, shows that the RMP/UMP base pair is noticeably displaced away from the pol, relative to a normal Watson Crick base pair (> 0.5 Å) (Fig. 1d).27 As a result, the nascent base pair next to the i+1 positioned RMP-containing P/T base pair is also displaced from the pol (Fig. 1e).27 This conformational state likely differs from the catalytically active state. When comparing with the post-translocated state, resulting in a vacant NTP binding site, the i+1 base pair appears to be displaced towards the pol (Fig. 1f),27 likely making a strong interaction with the pol and thus driving the translocation process forward. After incorporation of RMP, the extra cyano substitution appears to prevent the RMP/UMP base pair at the i+1 position from being displaced towards the pol, as seen in the normal Watson-Crick base pair. This may explain why the translocation process after RMP incorporation is not spontaneous and the NTP-binding site is not yet vacant for accepting the next NTP. Therefore, it would require a higher concentration of the next NTP to drive the translocation forward after RMP incorporation.
The comparison above was based on superposition of the pol subunit between different complexes (Fig. 1).27 Within experimental errors, so far there is no large open-to-closed conformational change of the Fingers domain observed in these structures in contrast with those in DNA pols. Comparison with other structures of this pol further supports this conclusion.13, 14, 27, 33, 34 It is likely that some subtle conformational changes will occur for base selection of nucleotide incorporation, which would require much higher resolution to resolve than that observed in the current cryo-EM structures.
Models of RMP-containing RNA as a template strand at the polymerase site
A key feature of remdesivir is the cyano substitution at its C1’ position. This substitution can be easily modeled computationally according to the tetrahedral geometry of the C1’ center and known bond lengths to any position of nucleotides in any of known pol replication complex containing a P/T duplex. A systematic analysis of these structures of the RdRp from SARS-CoV-2 shows that there are two common features as exemplified in modeling of the 7bv2 structure (Fig. 2).27 As discussed elsewhere,8, 14 during the translocation of the RMP between the i+3 and i+4 site, the side chain of Ser861 of RdRp appears to become a roadblock, which explains a pause during primer extension. The closest interatomic distance between them is about 2.5 Å (Fig. 2e).27 Translocation could still occur if the P/T RNA duplex is slightly displaced away from Ser861 when there is enough driving energy, as in the presence of high concentration of the next incoming NTP.
Figure 2.
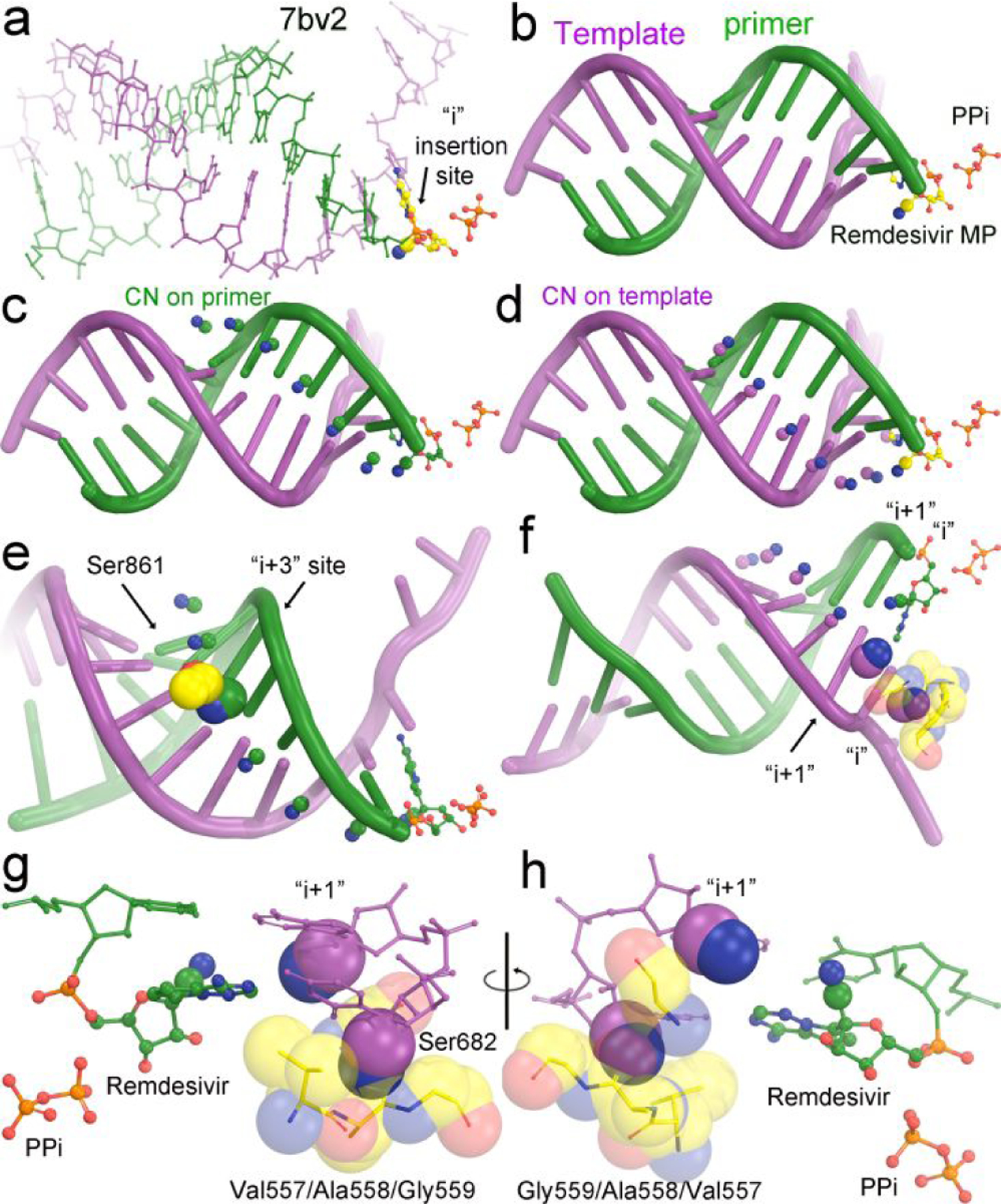
Modeling of the cyano substitutions at various positions of primer and template nucleotides. (a, b) Experimental structure of the 7bv2 complex with pyrophosphate and RMP in colored ball-and-stick model. The cyano group is shown in 30% of van der Waals radii. (c) Modeling of the cyano substitutions on the nucleotides of the primer strand based on (i) tetrahedral geometry of C1’ atom, (ii) the C-C single bond of 1.47 Å, and (iii) the C-N triple bond of 1.14 Å. (d) Modeling of the cyano substitutions on the nucleotides of the template strand. (e) Known clashes between the sidechain of Ser861 of RdRp and the cyano substitution during the translocation from the i+3 site to the i+4 site. (f) New severe clashes between the cyano substitution of the template nucleotide at the i site and backbone carbonyl group of Val557 of RdRp and a minor clash between the cyano substitution of the template nucleotide at the i+1 site and the backbone carbonyl of Ser682 of RdRp. (g,h) Close-up views of the first two base pairs in 180° orientation of (f).
When the cyano substitution is modeled onto the nucleotides of the template strand (Fig. 2),27 it is found that when it is in either the i or i+1 site there are stereochemical clashes. The interatomic distance between the backbone carbonyl group of Ala588 and the cyano substitution at the i site is 1.1 Å, which is a severe stereochemical clash, and it is 2.1 Å between the backbone carbonyl group of Ser682 and the cyano substitution at the i+1 site. Given the importance of substrate alignment of Watson-Crick base pairs at the i site, the severe clash of 1.1 Å at the templating RMP will cause severe misalignment of substrates. We predict that the RMP-containing template cannot be copied by RdRp of SARS-CoV-2. Therefore, the second run synthesis of viral mRNAs is reduced after incorporation of any RMP into the transient template strand during the first run of RNA synthesis.
Our modeling shows that the cyano substitution at the i site of the template strand has a stereochemical clash with the backbone carbonyl of Ala558 within the V557/A558/G559 stretch (Fig. 2).27 This clash would explain the inhibition of UMP incorporation opposite to RMP at the templating position. Interestingly, the mutation V557L reduces UMP incorporation opposite to AMP by 3-fold but improves it opposite to RMP by 5-fold. This mutation appears to counteract some inhibitory effects of the second run of RNA synthesis opposite the RMP-containing template. In vitro selection experiments resulted in special mutations that conferred a reduced susceptibility to remdesivir in two viral RNA polymerases.10, 37 These are F548S in Ebola viral lineage and V557L in the mouse hepatitis virus (MHV), each at the equivalent position of V557 of SARS-CoV-2 viral polymerase.
Models of RMP-containing RNA as a mRNA at the decoding center of protein synthesis
A small fraction of the transient RNA templates during the first run of RNA synthesis may be free of RMP incorporation even in the presence of remdesivir. These templates can be used for synthesis of a large quantity of viral mRNAs. However, it is inevitable that a large fraction of viral mRNA will also contain RMPs when in the presence of remdesivir. We carried out similar modeling of cyano substitutions at each of three codon sites of mRNA at the decoding center of the translating ribosome using the 7k00 structure (Fig. 3).38 At each codon position, the cyano group appears to overlap with the ribosomal nucleotides. Thus, we predict that translation will be stalled or slowed down when encountering the RMP-containing mRNAs.
Figure 3.
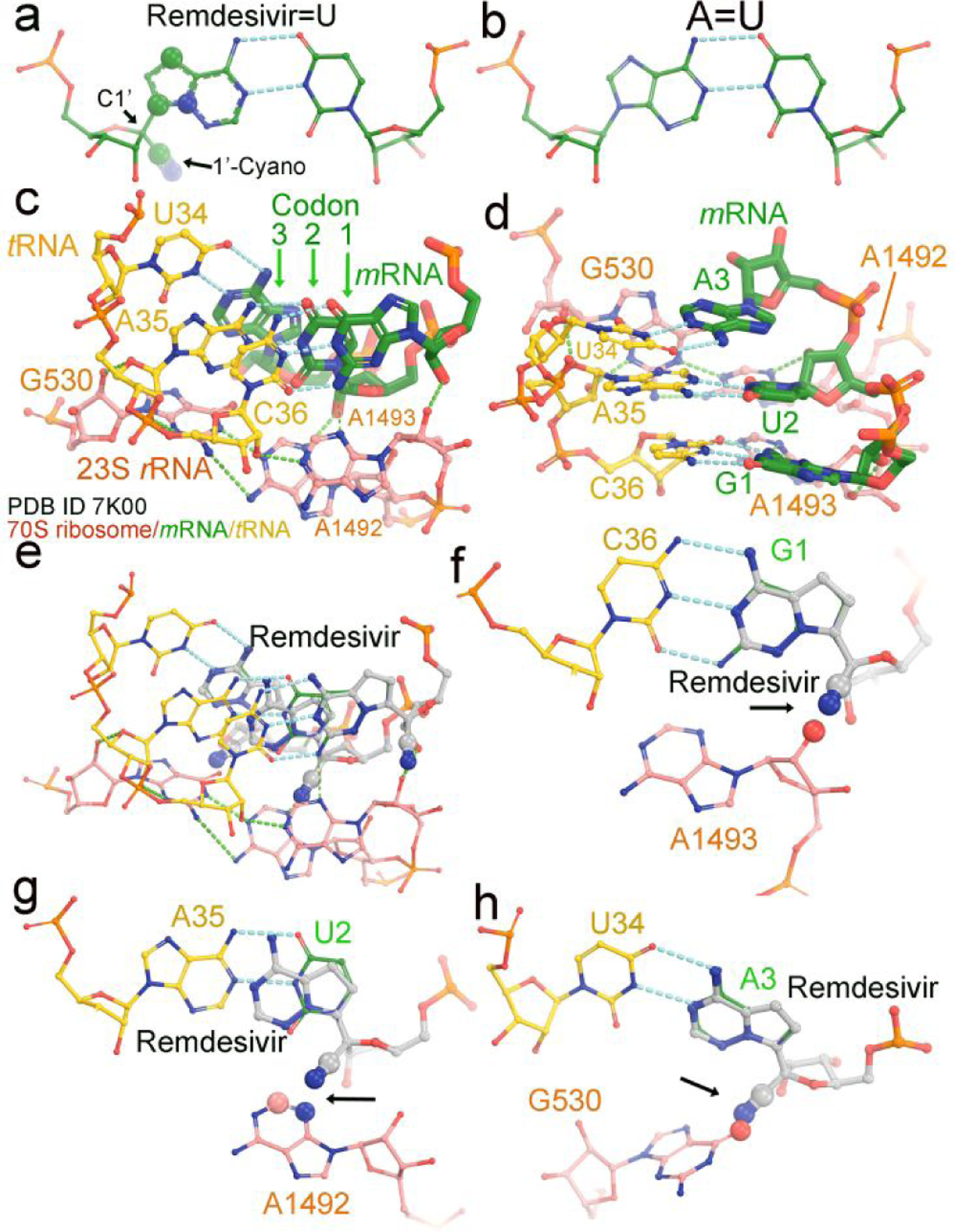
Modeling of remdesivir on a translating ribosome. (a) Base-pairing between RMP and UMP. The 1’-cyano substitution at C1’ and three other substitutions in the nucleobase are in large spheres (30% of van der Waals radii). (b) Watson-Crick AMP=UMP base pair. (c, d) Two views of the translating 70S E. coli ribosome cryo-EM structure (PDB ID 7k00) in complex with mRNA (green) and tRNA (yellow) at the decoding center. Three nucleotides of 23S rRNA, G530, A1492 and A1493 are shown in salmon. The three codon nucleotides in the structure are G1, U2 and A3 and in green. Three tRNA anticodon nucleotides are U34, A35 and G36 and in salmon. (e-h) Modeled remdesivir at the first, second, and third codon positions. Large spheres and arrows show where severe clashes occur.
Inside the cell, coronaviral mRNAs are loaded onto the ribosome by an apparatus that recognizes and interacts with both the 5’-caps and 3’-poly(A) tails.39, 40 Once loaded, translating mRNAs are scanned for the translation initiation codon “AUG”, which is sequestered by the secondary structure and surrounded by a poor Kozak context for coronavirus viral mRNAs,41 suggesting a possibly inefficient leaky scanning mechanism for translation initiation. This could be a reason why a large number of copies of coronaviral mRNAs are produced in the infected cells for synthesis of viral proteins. Once bound to the ribosome, viral mRNAs are also protected by the ribosome against RNA degradation. Evidence exists, as discussed above, that remdesivir may impair the synthesis of full-length 3’-poly(A) tail of viral mRNAs. Without a 3’-poly(A) tail or with shortened poly(A) tails, viral mRNAs cannot be properly loaded onto the ribosome for translation initiation, and thus they are rapidly degraded. A net consequence of remdesivir’s action appears to deplete the pool of NTPs through a futile RNA synthesis-RNA degradation cycle. This novel understanding of remdesivir’s action could provide a unique avenue for development of new antiviral drugs as a silver bullet to specifically find the SARS-CoV-2 infected cells, to deplete NTPs in these cells, and to kill them alongside with the virus.
Concluding remarks
Upon analysis of existing structures and examination of recent literature in this study, we have raised an issue with the commonly circulated mechanism that remdesivir is a chain terminator or a delayed chain terminator as a nucleotide analog inhibitor for RNA-dependent RNA polymerase of SARS-CoV-2. Evidence for that hypothesis appears relatively weak. Alternative experimentally testable hypotheses for the mechanism have been put forward, which are based on existing observations that remdesivir may affect the second run of RNA synthesis more than the first run and that it may also impair viral protein synthesis when viral mRNAs contain RMPs. In addition, nothing is known as to whether remdesivir will also inhibit the synthesis of RNA primer by the primase of SARS-CoV-2 or interfere with host tRNA synthetases or other proteins. These new hypotheses are inspired by results of our structural analysis and will stimulate many new experiments.
SYNOPSIS TOC:
Alternative mechanisms other than delayed chain termination seem to better explain existing data on remdesivir on inhibition of reproduction of SARS-CoV-2.
ACKNOWLEDGMENT
Authors thank Drs. P. B. Moore, J. A. Steitz, and W. V. Gilbert for discussion during course of this study.
Funding Sources
This work was supported by R01 GM106121 (V.S.B)
ABBREVIATIONS
- RdRp
RNA-dependent RNA polymerase
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- RMP
remdesivir mono phosphate
- RTP
remdesivir triphosphate
- P/T
primer/template
Footnotes
Competing interest statement
The authors declare that there is no competing interest in this study
REFERENCES
- [1].Seley-Radtke KL, and Yates MK (2018) The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold, Antiviral Res 154, 66–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yates MK, and Seley-Radtke KL (2019) The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold, Antiviral Res 162, 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, and Bavari S (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys, Nature 531, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tchesnokov EP, Feng JY, Porter DP, and Gotte M (2019) Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir, Viruses 11, E326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, and Hall MD (2020) Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19, ACS Cent Sci 6, 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Y, Zhang D, and Du G (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial (vol 395, 1569, 2020), Lancet 395, 1694–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tchesnokov EP, Gordon CJ, Woolner E, Kocinkova D, Perry JK, Feng JY, Porter DP, and Gotte M (2020) Template-dependent inhibition of coronavirus RNA-dependent RNA polymerase by remdesivir reveals a second mechanism of action, J Biol Chem 295, 16156–16165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, and Gotte M (2020) Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J Biol Chem 295, 6785–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, and Gotte M (2020) The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus, J Biol Chem 295, 4773–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, and Denison MR (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao Y, Sun J, Li Y, Li Z, Xie Y, Fen R, Zhao J, and Hu Y (2020) The strand-biased transcription of SARS-CoV-2 and unbalanced inhibitiion by remdesivir., BioRxiV preprint: doi: 10.1101/2020.10.15.325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alexandersen S, Chamings A, and Bhatta TR (2020) SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication, Nat Commun 11, 6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kokic G, Hillen HS, Tegunov D, Dienemann C, Seitz F, Schmitzova J, Farnung L, Siewert A, Hobartner C, and Cramer P (2021) Mechanism of SARS-CoV-2 polymerase stalling by remdesivir, Nat Commun 12, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A, Ji W, Yan L, Zhu Y, Zhu C, Fang X, Yang X, Huang Y, Gao H, Liu F, Ge J, Sun Q, Yang X, Xu W, Liu Z, Yang H, Lou Z, Jiang B, Guddat LW, Gong P, and Rao Z (2020) Structural basis for RNA replication by the SARS-CoV-2 polymerase, Cell 182, 417–428 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Traut TW (1994) Physiological concentrations of purines and pyrimidines, Mol Cell Biochem 140, 1–22. [DOI] [PubMed] [Google Scholar]
- [16].Stridh S (1983) Determination of ribonucleoside triphosphate pools in influenza A virus-infected MDCK cells, Arch Virol 77, 223–229. [DOI] [PubMed] [Google Scholar]
- [17].Kennedy EM, Gavegnano C, Nguyen L, Slater R, Lucas A, Fromentin E, Schinazi RF, and Kim B (2010) Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages, J Biol Chem 285, 39380–39391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sola I, Almazan F, Zuniga S, and Enjuanes L (2015) Continuous and discontinuous RNA synthesis in coronaviruses, Annu Rev Virol 2, 265–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].V’Kovski P, Kratzel A, Steiner S, Stalder H, and Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2, Nat Rev Microbiol 19, 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Simmonds P (2020) Pervasive RNA secondary structure in the genomes of SARS-CoV-2 and other coronaviruses, mBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miao Z, Tidu A, Eriani G, and Martin F (2021) Secondary structure of the SARS-CoV-2 5’-UTR, RNA Biol 18, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang D, and Leibowitz JL (2015) The structure and functions of coronavirus genomic 3’ and 5’ ends, Virus Res 206, 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jacob ST, Crozier I, Fischer WA 2nd, Hewlett A, Kraft CS, Vega MA, Soka MJ, Wahl V, Griffiths A, Bollinger L, and Kuhn JH (2020) Ebola virus disease, Nat Rev Dis Primers 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sanchez A, and Rollin PE (2005) Complete genome sequence of an Ebola virus (Sudan species) responsible for a 2000 outbreak of human disease in Uganda, Virus Res 113, 16–25. [DOI] [PubMed] [Google Scholar]
- [25].Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, and Zhang YZ (2020) A new coronavirus associated with human respiratory disease in China, Nature 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, and Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yin W, Mao C, Luan X, Shen DD, Shen Q, Su H, Wang X, Zhou F, Zhao W, Gao M, Chang S, Xie YC, Tian G, Jiang HW, Tao SC, Shen J, Jiang Y, Jiang H, Xu Y, Zhang S, Zhang Y, and Xu HE (2020) Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir, Science 368, 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hackbart M, Deng X, and Baker SC (2020) Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors, Proc Natl Acad Sci U S A 117, 8094–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mitton-Fry RM, DeGregorio SJ, Wang J, Steitz TA, and Steitz JA (2010) Poly(A) tail recognition by a viral RNA element through assembly of a triple helix, Science 330, 1244–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brown JA, Bulkley D, Wang J, Valenstein ML, Yario TA, Steitz TA, and Steitz JA (2014) Structural insights into the stabilization of MALAT1 noncoding RNA by a bipartite triple helix, Nat Struct Mol Biol 21, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Torabi SF, Chen YL, Zhang K, Wang J, DeGregorio SJ, Vaidya AT, Su Z, Pabit SA, Chiu W, Pollack L, and Steitz JA (2021) Structural analyses of an RNA stability element interacting with poly(A), Proc Natl Acad Sci U S A 118 (doi: 10.1073/pnas.2026656118). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Torabi SF, Vaidya AT, Tycowski KT, DeGregorio SJ, Wang J, Shu MD, Steitz TA, and Steitz JA (2021) RNA stabilization by a poly(A) tail 3’-end binding pocket and other modes of poly(A)-RNA interaction, Science 371 (doi: 10.1126/science.abe6523). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Naydenova K, Muir KW, Wu LF, Zhang Z, Coscia F, Peet MJ, Castro-Hartmann P, Qian P, Sader K, Dent K, Kimanius D, Sutherland JD, Lowe J, Barford D, and Russo CJ (2021) Structure of the SARS-CoV-2 RNA-dependent RNA polymerase in the presence of favipiravir-RTP, Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Peng Q, Peng R, Yuan B, Wang M, Zhao J, Fu L, Qi J, and Shi Y (2021) Structural basis of SARS-CoV-2 polymerase inhibition by favipiravir, Innovation (N Y) 2, 100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen J, Malone B, Llewellyn E, Grasso M, Shelton PMM, Olinares PDB, Maruthi K, Eng ET, Vatandaslar H, Chait BT, Kapoor TM, Darst SA, and Campbell EA (2020) Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex, Cell 182, 1560–1573 e1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xia S, and Konigsberg WH (2014) RB69 DNA polymerase structure, kinetics, and fidelity, Biochemistry 53, 2752–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lo MK, Albarino CG, Perry JK, Chang S, Tchesnokov EP, Guerrero L, Chakrabarti A, Shrivastava-Ranjan P, Chatterjee P, McMullan LK, Martin R, Jordan R, Gotte M, Montgomery JM, Nichol ST, Flint M, Porter D, and Spiropoulou CF (2020) Remdesivir targets a structurally analogous region of the Ebola virus and SARS-CoV-2 polymerases, Proc Natl Acad Sci U S A 117, 26946–26954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Watson ZL, Ward FR, Meheust R, Ad O, Schepartz A, Banfield JF, and Cate JH (2020) Structure of the bacterial ribosome at 2 A resolution, Elife 9, E60482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Breyne S, Vindry C, Guillin O, Conde L, Mure F, Gruffat H, Chavatte L, and Ohlmann T (2020) Translational control of coronaviruses, Nucleic Acids Res 48, 12502–12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Banerjee AK, Blanco MR, Bruce EA, Honson DD, Chen LM, Chow A, Bhat P, Ollikainen N, Quinodoz SA, Loney C, Thai J, Miller ZD, Lin AE, Schmidt MM, Stewart DG, Goldfarb D, De Lorenzo G, Rihn SJ, Voorhees RM, Botten JW, Majumdar D, and Guttman M (2020) SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses, Cell 183, 1325–1339 e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang Y, Hussain S, Wang H, Ke M, and Guo D (2009) Translational control of the subgenomic RNAs of severe acute respiratory syndrome coronavirus, Virus Genes 39, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


