Abstract
Serotonin (5-HT) appeared billions of years before 5-HT receptors and synapses. It is thus not surprising that 5-HT can control biological processes independently of its receptors. One example is serotonylation, which consists of covalent binding of 5-HT to the primary amine of glutamine. Over the past 20 years, serotonylation has been involved in the regulation of many signaling mechanisms. One of the most striking examples is the recent evidence that serotonylation of histone H3 constitutes an epigenetic mark. However, the pathophysiological role of histone H3 serotonylation remains to be discovered. All but one of the 5-HT receptors are G-protein-coupled receptors (GPCRs). The signaling pathways they control are finely tuned, and new, unexpected regulatory mechanisms are being uncovered continuously. Some 5-HT receptors (5-HT2C, 5-HT4, 5-HT6, and 5-HT7) signal through mechanisms that require neither G-proteins nor β-arrestins, the two classical and almost universal GPCR signal transducers. 5-HT6 receptors are constitutively activated via their association with intracellular GPCR-interacting proteins (GIPs), including neurofibromin 1, cyclin-dependent kinase 5 (Cdk5), and G-protein-regulated inducer of neurite outgrowth 1 (GPRIN1). Interactions of 5-HT6 receptor with Cdk5 and GPRIN1 are not concomitant but occur sequentially and play a key role in dendritic tree morphogenesis. Furthermore, 5-HT6 receptor-mediated G-protein signaling in neurons is different in the cell body and primary cilium, where it is modulated by smoothened receptor activation. Finally, 5-HT2A receptors form heteromers with mGlu2 metabotropic glutamate receptors. This heteromerization results in a specific phosphorylation of mGlu2 receptor on a serine residue (Ser843) upon agonist stimulation of 5-HT2A or mGlu2 receptor. mGlu2 receptor phosphorylation on Ser843 is an essential step in engagement of Gi/o signaling not only upon mGlu2 receptor activation but also following 5-HT2A receptor activation, and thus represents a key molecular event underlying functional crosstalk between both receptors.
Keywords: Serotonin, serotonylation, receptor, GPCR interacting protein, heteromerization
Introduction
The serotonin (5-HT) biosynthetic pathway is an ancestral biological process present in unicellular systems such as cyanobacteria, green algae, and fungi and is conserved in both invertebrates and vertebrates1. In contrast, 5-HT receptors have not been found in plants and appeared along with synapses 600 million years ago1. Therefore, it is not surprising that some 5-HT biological effects that do not require 5-HT receptors have been established during evolution. One of the most fascinating discoveries of the last 20 years is the demonstration that 5-HT can bind covalently to the primary amine of glutamine in proteins2,3. This covalent modification called serotonylation is implicated in many biological mechanisms, such as epigenetics, both at the periphery and in the brain3–7 Thus, 5-HT controls cellular signaling events by acting either extracellularly via membrane receptors or intracellularly via serotonylation, even though some serotonylation events occur extracellularly (Figure 1). This dual 5-HT control of cell signaling is shared with dopamine, histamine, and noradrenalin6,7. It illustrates the proposal from François Jacob that evolution tinkers with a limited number of disposable genes and molecules to ensure the greatest number of biological functions8,9.
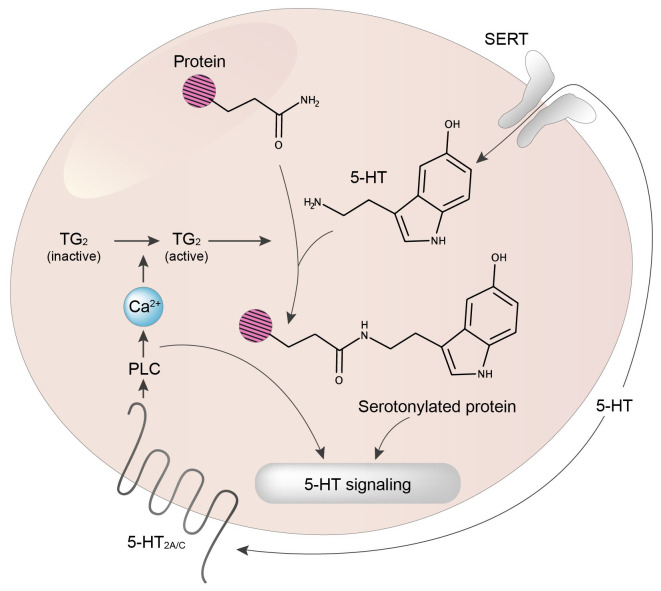
Figure 1. 5-HT signaling via G-protein-coupled receptors (GPCRs) and serotonylation.
5-HT controls cell signaling via its cognate receptors (mostly GPCRs) and intracellularly via transamidation (serotonylation) of specific proteins. Note that extracellular proteins can also be serotonylated (not illustrated). Owing to the hydrophilic nature of 5-HT, serotonylation is believed to occur only in cells expressing the serotonin transporter (SERT). In the example illustrated in the figure, 5-HT2A/C receptor stimulation by 5-HT induces activation of phospholipase C (PLC), and thereby an increase in intracellular Ca2+ concentration, a process leading to a full activation of the transglutaminase TG2.
5-HT finely controls an increasing number of functions including highly complex processes, such as anxiety, mood, learning, memory, cognition, social interactions, sleep, and appetite, but also more unexpected ones, such as shell formation in bivalves10. This large diversity of 5-HT functions has been made possible by selecting a large number of receptors that finely regulate diverse cellular signaling pathways, and 5-HT is certainly one of the neurotransmitters able to activate the largest number of receptor subtypes (17 identified in vertebrates). All are G-protein-coupled receptors (GPCRs), except for the five 5-HT3 receptors, which are cation channels11. Twenty years ago, the signaling mechanisms associated with 5-HT GPCRs were thought to be simple12. The 5-HT1 receptor family was known to be coupled to Gi proteins, thus inhibiting adenylyl cyclase (AC), the 5-HT2 family to Gq (activate phospholipase C), and 5-HT4, 5-HT6, and 5-HT7 receptors to Gs (activate AC), while the coupling mechanisms of 5-HT5 receptors remained elusive. Our current knowledge of 5-HT receptor signal transduction is now much more complex than this initial view and is in constant evolution11,13. Since 5-HT receptor-mediated signaling has been extensively reviewed elsewhere11,13–17, we will focus here on the most original and intriguing signaling mechanisms that have been recently described.
Receptor-independent 5-HT signaling: serotonylation
The covalent binding of polyamines or biogenic monoamines (serotonin, dopamine, noradrenalin) to glutamine was described a long time ago3. Enzymes responsible for this biochemical reaction, called transamidation, are transglutaminases (TGs)3. Seven TGs exhibiting mainly intracellular location have been identified, the most abundant and ubiquitous one being TG2. Blood coagulation factor XIII, once activated by thrombin during coagulation to give factor XIIIa, also displays extracellular TG activity3.
The first physiological function depending on serotonylation was described by Dale et al. in 2002–20033,5. This group of investigators showed serotonylation by factor XIIIa of several procoagulant proteins, including fibrinogen, von Willebrand factor, fibronectin, factor V, and thrombospondin on the surface of activated platelets, which leads to the accumulation of aggregated proteins in the extracellular matrix18. Fibrin is also cross-linked by factor XIII, thereby increasing clot resistance19. Following the observation that Bordetella pertussis toxin acts as a transglutaminase that covalently binds polyamines to small G-proteins20, Walther et al. discovered that small G-proteins (RhoA and Rab4) are serotonylated (likely by TG2) in platelets, a process making them constitutively active in a GTP-bound form2 (Figure 2). RhoA reorganizes the cytoskeleton, whereas Rab4 stimulates the exocytosis of α-granules, which contain proteins involved in coagulation. A rise in intracellular Ca2+ is necessary to activate TG22. This Ca2+ elevation is due, at least in part, to the activation of platelet 5-HT2A receptors21. Thus, 5-HT acts both extracellularly and intracellularly during platelet activation and the serotonin transporter (SERT) is needed for intracellular accumulation of 5-HT required for serotonylation5.
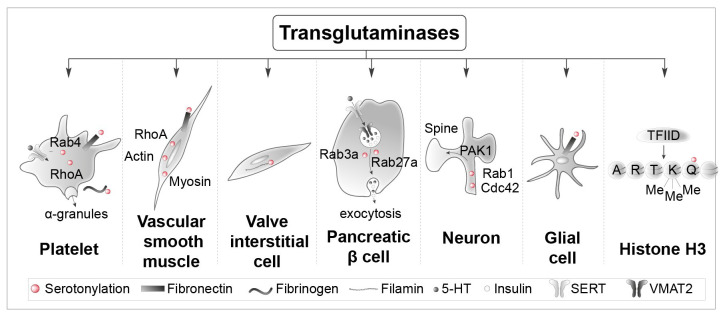
Figure 2. Specific proteins serotonylated by transglutaminases in different cell types.
Examples of intracellular and extracellular serotonylated proteins in platelets, vascular smooth muscle cells, valve interstitial cells, pancreatic β-cells, neurons, and glial cells are illustrated. The right panel shows serotonylated histone H3 at position 5 (Q5ser) by transglutaminase 2 (TG2) predominantly in combination with trimethylation of adjacent lysine (K)4, resulting in the double epigenetic mark H3K4me3Q5ser. SERT, serotonin transporter; TFIID, transcription factor II D; VMAT2, vesicular monoamine transporter 2.
Pulmonary hypertension involves proliferation and contraction of arterial smooth muscles. Proliferation of arterial smooth muscles is under the control of serotonylated RhoA22,23, whereas their contraction is modulated by serotonylated actin and myosin24 (Figure 2). Fibronectin serotonylation has also been involved in pulmonary hypertension3, a process favored by the up-regulation of TG225. The remodeling of cardiac valve interstitial cells, a heterogeneous population of cells responsible for maintaining the structural integrity and normal functioning of the valve, is key to understanding mitral and aortic valve dysfunctions in pulmonary hypertension. The role of 5-HT2B receptor activation by 5-HT in this pathology caused by fenfluramine treatment is well known, but serotonylation of filamin-A has also been implicated26 (Figure 2). Pancreatic β-cells capture extracellular 5-HT via SERT. Cytosolic 5-HT in turn accumulates with insulin in secretory vesicles through vesicular monoamine transporter 2 (VMAT2). Exocytosis of vesicles and co-release of 5-HT and insulin also require serotonylation of two small G-proteins, Rab3a and Rab27a27 (Figure 2). Stimulation of 5-HT2A/2C receptors by 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) induces Ca2+-dependent TG activation, serotonylation of Rac1 and Cdc42, and Pak1 stimulation in cortical neurons, a process leading to an increase in dendritic spine size (Figure 2)28. This identifies serotonylation as a novel signaling pathway underlying the influence of 5-HT on dendritic spine morphology and plasticity28. Fibronectin and other proteins are likewise transamidated by biogenic amines including 5-HT in glial cells (Figure 2), but few data are published on the role of this chemical modification in this cell type29.
The most exciting discovery on transamidation is its role as an epigenetic mark controlling gene expression6,7. Serotonylation of histone H3 on glutamine (Q5) has been found in several organs producing 5-HT, including the brain and the gut, and in different animal species4 (Figure 2). 5-HT and other monoamines are present in the nucleus30,31 and can thus serve as substrates for transamidation of histones. The nuclear membrane is permeable to monoamines32, which allows a rapid equilibration of extravesicular monoamines between the cytoplasm and the nucleus30. Nuclear 5-HT is mobilized and released upon stimulation of dorsal raphe-containing brain slices30.
Histone H3 serotonylation requires trimethylation of the neighboring lysine (K4) and occurs during the differentiation of human pluripotent stem cells into 5-HT-containing neurons. This histone H3 modification is enriched in gene promoters and facilitates binding of the general transcription factor TFIID and gene transcription4. Similarly, dopaminylation of Q5 of histone H3 was found during cocaine withdrawal. This promotes gene expression in ventral tegmental area (VTA) neurons, increases their excitability, and favors drug-seeking behavior in rats6,7.
G-protein-independent signaling at 5-HT G-protein-coupled receptors
Like many proteins, GPCRs can adopt different active and inactive conformations33–35. Some active conformations favor activation of one or several G-proteins, whereas others favor GPCR association with β-arrestins35. Biased ligands can stabilize either one or several G-protein-preferring conformations, or β-arrestin-preferring conformations, or conformations favoring both G-protein- and β-arrestin-dependent signaling. In addition, some GPCRs, including 5-HT receptors, trigger signaling events without any involvement of G-proteins or β-arrestins, that can thus be designated as “non-G-protein/β-arrestin” signaling. These particular signaling mechanisms can result from either receptor stabilization in a specific active conformation by binding to an agonist or, in some cases, agonist-independent (constitutive) receptor activation.
While many GPCRs are known to stimulate the MAP-kinase Erk1,2 pathway through the sequential activation of G-proteins and β-arrestins36, two 5-HT receptor subtypes have been shown to engage the Erk1,2 signaling pathway through G-protein- or β-arrestin-independent mechanisms. Stimulation of Erk1,2 by the 5-HT2C receptor does not require G-proteins and entirely depends on the concomitant recruitment of β-arrestin and calmodulin (CaM) by the receptor37. In light of the direct interaction of purified calmodulin (CaM, bound to Ca2+) with β-arrestin38 and CaM dimerization39, it has been proposed that β-arrestin might be recruited via CaM bound to the receptor, which might also stabilize the 5-HT2C receptor/β-arrestin complex (Figure 3). Consistent with this hypothesis, Erk1,2 activation by the 5-HT2C receptor is unusually long lasting (up to 3 hours) when compared to other GPCRs37. This contrasts with the activation of Erk1,2 signaling by the 5-HT4 receptor, which lasts only 20 minutes and is independent of both G-proteins and β-arrestins but requires Src activation (Figure 3)40. In the Caco-2 epithelial intestinal cell line, 5-HT4 receptor-mediated Src activation also leads to PLC/Ca2+-CaM-dependent inhibition of the Na+/H+ exchanger41.
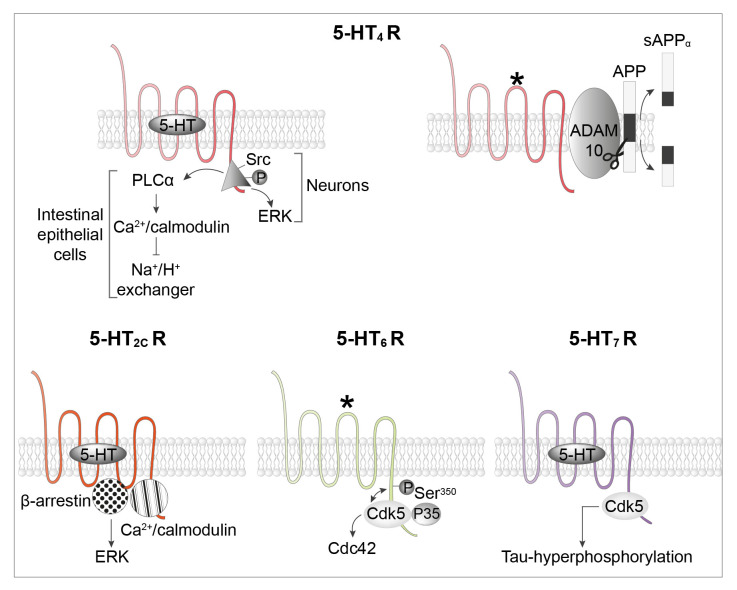
Figure 3. Non-G-protein signaling at 5-HT receptors.
Top: the 5-HT4 receptor engages Erk1,2 signaling in neurons through a Gs- and β-arrestin-independent mechanism that requires activation of the non-receptor tyrosine kinase Src. In the intestinal epithelial Caco-2 cell line, 5-HT4 receptor-mediated Src activation leads to phospholipase C (PLC)/Ca2+-calmodulin-dependent inhibition of the Na+/H+ exchanger. *Constitutively active receptors. Bottom left: the 5-HT2C receptor engages Erk1,2 signaling in neurons through a G-protein-independent, β-arrestin-dependent mechanism that requires physical association of calmodulin with the receptor’s C-terminal domain. Bottom, middle: the 5-HT6 receptor activates the cyclin-dependent kinase 5 (Cdk5)–Cdc42 signaling pathway in an agonist-independent manner through a reciprocal interplay between the receptor and associated Cdk5, by which Cdk5 bound to the receptor C-terminal domain phosphorylates the receptor on Ser350, a necessary step in Cdk5-dependent activation of Cdc42. Bottom, right: the 5-HT7 receptor binds to and activates Cdk5 signaling, a process leading to Tau hyperphosphorylation. APP, amyloid precursor protein.
The 5-HT4 receptor displays a high level of constitutive activity. Constitutively active 5-HT4 receptors directly bind to the α-secretase ADAM10 and stimulate its activity42, thus favoring non-amyloidogenic cleavage of the amyloid precursor protein (APP, Figure 3). Stimulation of ADAM10 by constitutively active 5-HT4 receptors is independent of Gs and cAMP production42. The conformation of constitutively active 5-HT4 receptors associated with ADAM10 likely differs from the conformation of constitutively active 5-HT4 receptors coupled to Gs. Indeed, whereas agonist-independent 5-HT4 receptor-operated Gs signaling is inhibited by the inverse agonists RO 116-0086 and RO 116-2617, these compounds are inactive on receptor-dependent ADAM10 activation42. Chronic administration of RS 67333, a 5-HT4 receptor agonist, or donecopride, a multi-target compound able to both inhibit acetylcholinesterase and activate 5-HT4 receptors, decreases amyloid load and Tau hyperphosphorylation as well as learning and memory deficits in mouse models of Alzheimer's disease43,44.
Native 5-HT6 receptors exhibit a high level of constitutive activity at Gs signaling. The 5-HT6 receptor also constitutively activates cyclin-dependent kinase 5 (Cdk5)/Cdc42 signaling through a mechanism involving agonist-independent association of Cdk5 and its activator p35 to the receptor C-terminus and receptor phosphorylation on a Ser residue (Ser350) by associated Cdk545 (Figure 3). This pathway is engaged by mutated 5-HT6 receptors unable to activate Gs, suggesting the presence of at least two different active receptor conformations able to activate Gs and Cdk5 signaling, respectively14,45. Agonist-independent 5-HT6 receptor-operated Cdk5 signaling finely tunes cortical neuron migration and promotes the initiation of neurite growth45–47.
Likewise, constitutively active 5-HT7 receptors directly bind to and activate Cdk5 in a G-protein-independent manner (Figure 3)48. In a mouse model of tauopathy overexpressing a human Tau mutant known to be associated with frontotemporal dementia (R406W), constitutively active 5-HT7 receptors physically associated with Cdk5 induce hyperphosphorylation of Tau and the formation of highly bundled Tau structures48, suggesting that the 5-HT7 receptor–Cdk5 signaling pathway may be a new target in tauopathies.
Constitutive activation of 5-HT6 receptor by interacting proteins
Another example of molecular tinkering of GPCRs8 is their ability to be activated by both agonists and their interaction with intracellular proteins. Twenty years ago, we described the first example of agonist-independent activation of GPCRs (the group I metabotropic glutamate receptors mGlu1 and mGlu5) by an intracellular GPCR-interacting protein (GIP), Homer1a. Homer1a is the product of an immediate early gene induced in activated neurons49. Constitutively active mGlu1/Homer1a and mGlu5/Homer1a complexes are implicated in a large series of homeostatic plasticity events50–54.
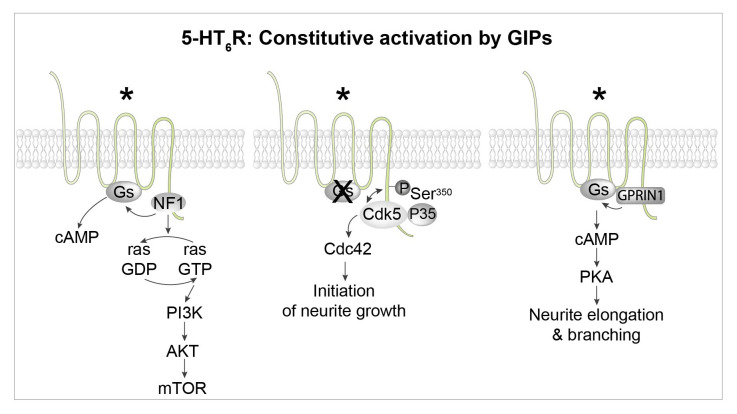
More recently, we reported the association of 5-HT6 receptor with many GIPs. These include proteins of the mechanistic target of rapamycin (mTOR) pathway (mTOR itself, Raptor, which together with mTOR is part of the mTOR complex 1, the Ras GTPase-activating protein [Ras-GAP] neurofibromin 1, and Vps34, a class III phosphatidylinositol 3‐kinase). Further studies revealed that mTOR activation by 5-HT6 receptor has a deleterious influence upon cognition in rodent models of schizophrenia and cannabis abuse during adolescence55,56. Three 5-HT6 receptor-interacting proteins were found to activate 5-HT6 receptors in an agonist-independent manner (Figure 4)13. The first one is Cdk5, as already discussed45. The second is neurofibromin 1, a protein encoded by the tumor suppressor gene NF1 that directly binds to the C-terminus of 5-HT6 receptor14,57. Mutations of the NF1 gene are responsible for neurofibromatosis type 1 (Nf1), a genetic disease characterized by skin pigmentation and benign skin tumors, low-grade tumors of the central and peripheral nervous systems, and learning and attention deficits in some patients. The binding of neurofibromin 1 to 5-HT6 receptors strongly enhances native 5-HT6 receptor constitutive activity at Gs signaling (Figure 4). Correspondingly, SB 271046, a 5-H6 receptor inverse agonist, decreases cAMP level and downstream signaling in wild-type mice but not Nf1+/− mice57. Likewise, blocking 5-HT6 receptor/neurofibromin 1 interaction by an interfering peptide strongly reduces 5-HT6 receptor constitutive activity in primary neurons. These findings demonstrate that physical interaction between neurofibromin 1 and 5-HT6 receptor enhances constitutive receptor coupling to Gs57. The third 5-HT6 receptor-interacting protein that was found to promote agonist-independent activation of Gs and cAMP production without altering the agonist-dependent response is G-protein-regulated inducer of neurite outgrowth 1 (GPRIN1)58 (Figure 4). The 5-HT6 receptor–GPRIN1 complex promotes neurite extension and branching in NG108-15 cells and mouse primary neurons through a cAMP- and PKA-dependent mechanism58.
Figure 4. Constitutive activation of 5-HT6 receptors by different GPCR-interacting proteins (GIPs).
Left: The 5-HT6 receptor binds to several proteins of the mammalian target of rapamycin complex 1 (mTORC1) pathway, including mTOR itself and the Ras GTPase-activating protein (Ras-GAP) neurofibromin 1 (NF1). Physical association of NF1 with the receptor strongly enhances constitutive activation of the Gs–adenylyl cyclase pathway by the receptor. Middle: The receptor activates the cyclin-dependent kinase 5 (Cdk5)–Cdc42 signaling pathway in an agonist-independent manner to promote the initiation of neurite growth. Dissociation of the 5-HT6 receptor–Cdk5 complex allows the recruitment of G-protein-regulated inducer of neurite outgrowth 1 (GPRIN1) by the receptor (right panel), which mediates constitutive activation of the Gs–adenylyl cyclase–protein kinase A (PKA) pathway, thereby promoting neurite elongation and branching. *Constitutively active receptor. PI3K, phosphatidylinositol 3-kinase; Ser, serine.
Agonist-independent activation of GPCRs by GIPs generally induces more prolonged receptor activation than that elicited by classical agonists. In fact, activation of a GPCR by a GIP will last as long as the protein is bound to the receptor. Accordingly, GIP-dependent GPCR activation can last hours (as shown for mGlu1/5 receptor activation by Homer1a) or even be “permanent”, such as 5-HT6 receptor constitutive activation upon association with neurofibromin 1. The reversibility of receptor activation will depend only on GIP protein turnover.
Biased agonism at 5-HT2A receptor: impact of its heteromerization with mGlu2 receptor
As already discussed, GPCRs transduce signal not only via the activation of one or several G-proteins or its binding to β-arrestins but also via non-G-protein/non-β-arrestin pathways. Different ligands of a given GPCR can preferentially stimulate either G-protein- or β-arrestin-dependent signaling, a phenomenon known as biased signaling59. The extreme situation is a ligand displaying no efficacy in promoting receptor coupling to G-proteins but serving as an agonist for β-arrestin-mediated signaling59. This phenomenon has been called “biased agonism” or “functional selectivity”60. Consequently, depending on the pattern of signaling pathways selected by a given GPCR ligand, cellular and physiological responses will differ. Functional selectivity raised great interest in the pharmaceutical industry with the perspective of developing drugs able to activate signaling pathways underlying therapeutic response but not those responsible for side effects61.
As for many GPCRs, biased ligands acting on 5-HT receptors are actively searched in order to obtain more selective drugs with fewer side effects62–65. Agonists acting at 5-HT2A receptors represent one of the most striking illustrations of functional selectivity66,67. Some 5-HT2A receptor agonists like lysergic acid diethylamide (LSD), psilocybin (“magic mushrooms” drug), or DOI (“designer drug”) trigger hallucinations, whereas its natural ligand 5-HT and other agonists like the anti-parkinsonian compound lisuride or the anti-migraine drug ergotamine do not trigger such psychoactive effects. 5-HT2A receptors are canonically coupled to both the Gαq protein family and β-arrestin and quickly desensitized upon 5-HT stimulation11. LSD is a β-arrestin-biased ligand that promotes preferential 5-HT2A receptor coupling to β-arrestin compared with 5-HT67 (Figure 5). Consistent with these findings, structural studies indicate that the conformation adopted by the structurally related 5-HT2B receptor bound to LSD slightly differs from the conformation elicited by non-hallucinogenic agonists. The most important difference is a more constrained conformation of extracellular loop 2 near the orthosteric site, which causes a more prolonged residence time of LSD, leading to a stronger and more prolonged β-arrestin recruitment68. Recently, a high-resolution structure of hallucinogen-bound 5-HT2A receptor also revealed how hallucinogens stabilize states favoring β-arrestin coupling67. Interestingly, substituting a hydrophobic residue within the intracellular loop 2, essential for coupling of various GPCRs to G-proteins (isoleucine181in 5-HT2A receptor) into glutamate, suppresses receptor coupling to Gαq while potentiating coupling to β-arrestin67 (Figure 5).
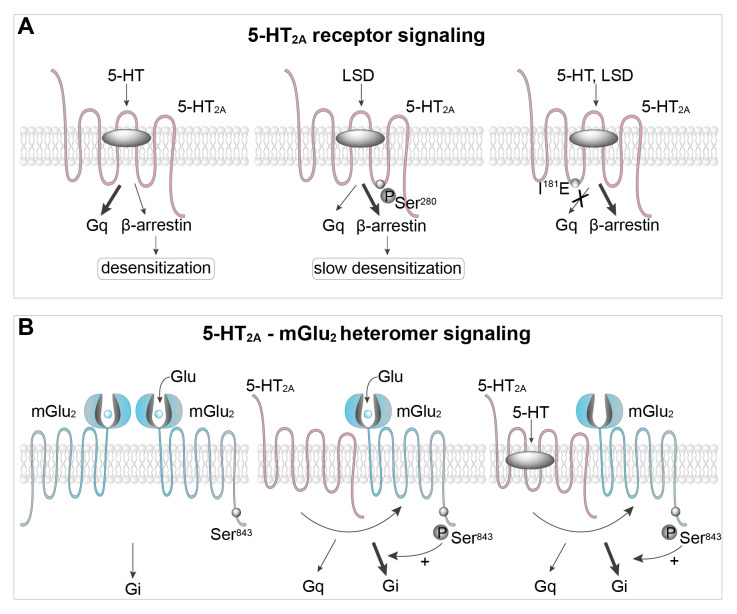
Figure 5. Biased signaling at 5-HT2A receptors and 5-HT2A/mGlu2 heteromers.
A. 5-HT2A receptor stimulation by 5-HT activates Gαq and, to a lesser extent, β-arrestin signalling and leads to 5-HT2A receptor desensitization. 5-HT2A receptor stimulation by psychedelic hallucinogens such as lysergic acid diethylamide (LSD), but not by non-hallucinogenic agonists, promotes receptor phosphorylation on serine (Ser)280, a process reducing receptor desensitization. Binding of the receptor to hallucinogenic agonists also stabilizes conformations favoring β-arrestin coupling66. Substituting isoleucine181 of the receptor to glutamate suppresses receptor coupling to Gαq while potentiating coupling to β-arrestin upon receptor activation by 5-HT or hallucinogenic agonists. B. Agonist stimulation of metabotropic glutamate receptor 2 (mGlu2) or 5-HT2A receptor within mGlu2–5-HT2A heterodimer promotes mGlu2 receptor phosphorylation on Ser843, which favors engagement of Gαi signaling.
The comparison of phosphoproteomes in 5-HT2A receptor-expressing recombinant cells challenged with either the hallucinogenic agonist DOI or the non-hallucinogenic agonist lisuride revealed that among thousands of quantified phosphorylated residues, only a few of them are specifically phosphorylated upon exposure to DOI, but not lisuride. These include a serine residue (Ser280) located in the third intracellular loop of the 5-HT2A receptor itself. The specific phosphorylation of Ser280 upon 5-HT2A receptor stimulation by hallucinogenic agonists was then established in vivo, in mouse prefrontal cortex69 (Figure 5). Further functional studies revealed that this biased phosphorylation event is responsible for a reduced desensitization of 5-HT2A receptor when stimulated by hallucinogenic vs. non-hallucinogenic agonists69. This attenuated 5-HT2A receptor desensitization following stimulation by hallucinogenic agonists results in more sustained receptor activation that might contribute, at least in part, to their psychotropic effects.
The ability of the 5-HT2A receptor to couple to Gαi and Gαs proteins in addition to Gαq is still controversial67,70. An extended analysis by Kim et al. clearly establishes that the 5-HT2A receptor is mostly coupled to the Gq protein family upon stimulation by either 5-HT or LSD67. 5-HT2A receptors can form heteromers with the mGlu2 receptor, a Gαi protein-coupled receptor71 (Figure 5). Within the heteromer, the respective coupling of each protomer to its cognate G-protein is oppositely influenced by the other protomer: while 5-HT2A receptor coupling to Gαq in response to agonist stimulation is decreased by approximately 50% within heteromers, compared with 5-HT2A receptor not associated with mGlu2 receptor, Gαi activation elicited by agonist stimulation of the mGlu2 receptor is strongly potentiated by its heteromerization with the 5-HT2A receptor72. A recent study revealed that the 5-HT2A receptor also affects mGlu2 receptor trafficking and subcellular localization through a mechanism dependent on their heterodimerization73.
We demonstrated that 5-HT2A receptor co-expression is required for the phosphorylation of the mGlu2 receptor on a serine located in its C-terminal domain (Ser843) upon mGlu2 receptor stimulation by the orthosteric agonist LY379268 in recombinant cells74. Furthermore, phosphorylation of Ser843 elicited by mGlu2 receptor stimulation is blocked by a 5-HT2A receptor antagonist (Figure 5). Corroborating these observations in cell cultures, in vivo administration of LY379268 increases mGlu2 receptor phosphorylation at Ser843 in prefrontal cortex of wild-type mice but not 5-HT2A−/− mice. Stimulation of the 5-HT2A receptor also increases phosphorylation of Ser843, an effect blocked by mGlu2 receptor antagonist, thus highlighting a sophisticated crosstalk between both receptors to promote mGlu2 Ser843 phosphorylation (Figure 5). Mutation of Ser843 into alanine strongly reduces Gαi/o signaling elicited by mGlu2 or 5-HT2A receptor stimulation in cells co-expressing both receptors74. This identifies mGlu2 Ser843 phosphorylation as a mechanism by which the 5-HT2A receptor can “hijack” Gαi signaling within 5-HT2A–mGlu2 heteromers. It has been proposed that the balance of Gαq vs. Gαi signaling at 5-HT2A–mGlu2 heteromers determines pro-psychotic vs. antipsychotic activity of ligands of each of these receptors72. Given the critical influence of Ser843 phosphorylation on Gαi/o signaling at 5-HT2A–mGlu2 heteromers, alterations of its phosphorylation level might be a key event underlying the pathogenesis of psychotic disorders such as schizophrenia as well as the behavioral effects of psychedelic drugs and antipsychotics.
5-HT receptor spatiotemporal signaling
GPCR signal transduction is not stable over time, even in the presence of constant agonist stimulation. In addition, it depends on receptor subcellular localization. Most GPCRs undergo desensitization upon agonist stimulation, leading to a decline of G-protein-dependent signaling while alternative pathways, such as the β-arrestin-dependent pathway, are enhanced9,75,76. Many GPCRs are internalized in endosomes upon prolonged agonist stimulation. Surprisingly, some GPCRs continue to transduce signal in endosomes, not only via β-arrestin but also, in some cases, via G-proteins76,77. For instance, parathormone (PTH) receptors still activate Gs and cAMP production in endosomes76.
We have recently described another example of time-dependent sequential coupling. As previously discussed, 5-HT6 receptors constitutively activate the Cdk5–Cdc42 pathway to stimulate the initiation of neurite outgrowth, and the Gs–AC pathway, via GPRIN1 physically associated with the receptor, to promote neurite extension and branching45,58. However, these two different signaling pathways are not concomitantly activated (Figure 6A). During early neuronal differentiation, Cdk5, but not GPRIN1, binds to the 5-HT6 receptor and the Cdc42 signaling pathway is switched “on”58 (Figure 6A). Subsequently, Cdk5 is released from 5-HT6 receptors, allowing recruitment of GPRIN1, activation of the Gs–AC pathway, cAMP production, and neurite extension and branching58 (Figure 6A).
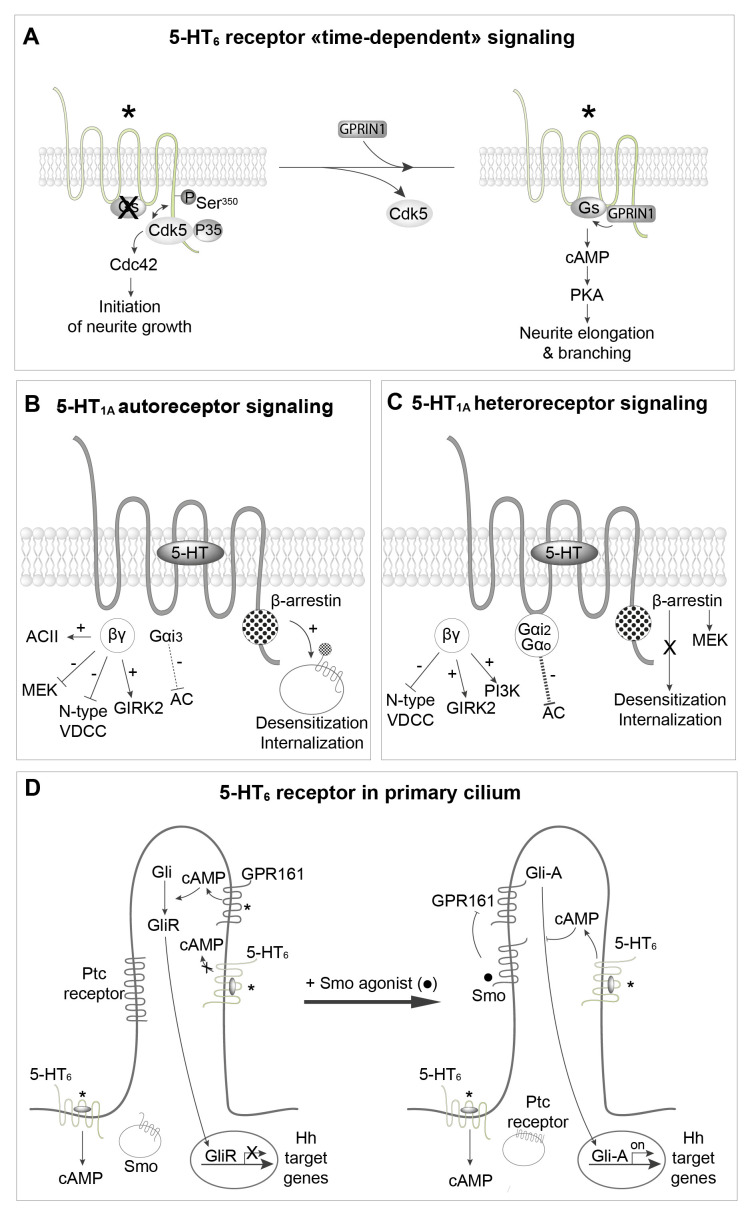
Figure 6. Spatiotemporal regulation of 5-HT receptor signaling.
A. Sequential engagement cyclin-dependent kinase 5 (Cdk5)–Cdc42 and Gs–adenylyl cyclase pathways by constitutively active 5-HT6 receptors during neuronal differentiation and dendritic tree morphogenesis. B,C. Difference in coupling properties of 5-HT1A autoreceptor and heteroreceptors and in their propensity to desensitize and internalize upon agonist stimulation. D. Spatiotemporal regulation of 5-HT6 receptor coupling to Gs in neurons. 5-HT6 receptors located in the soma, but not receptors located in the primary cilium, activate cAMP production (in absence or presence of agonist). Upon agonist stimulation, Smoothened (Smo) enters the cilium and inhibits cAMP production by constitutively active GPR161. This favors translocation of active Gli transcription factor (Gli-A) to the nucleus and the transcription of Hedgehog (Hh)-regulated genes. Concomitantly, 5-HT6 receptors located in the primary cilium become able to activate local cAMP production, which might exert a feedback inhibition of Gli-A. *Constitutively active receptors. AC, adenylyl cyclase; GIRK2, G-protein-gated inwardly rectifying potassium channel 2; GPRIN1, G-protein-regulated inducer of neurite outgrowth 1; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; Ptc, patched; Ser, serine; VDCC, voltage-dependent calcium channel.
5-HT1A receptors are particularly interesting to illustrate the impact of subcellular localization of GPCRs on their signaling specificity (Figures 6B, C). 5-HT1A autoreceptors are localized on 5-HT neurons on both cell bodies (in Raphe nucleus) and pre-synaptic terminals, whereas 5-HT1A heteroreceptors are localized post-synaptically on target neurons13. It is now well established that their signaling pathways are different13,78. 5-HT1A autoreceptors are coupled to Gαi3, whereas hippocampal heteroreceptors are coupled to Gαi2 and Gαo78. There is a consensus on the capacity of 5-HT1A receptor agonists to inhibit forskolin-stimulated AC in hippocampal membranes, whereas such an inhibition seems to depend on the agonist used in Raphe nucleus membranes78. It is likely that the presence in the Raphe nucleus of AC type II, which is known to be stimulated by βγ released from activated Gαi, masks inhibition of AC by 5-HT1A autoreceptors78.
5-HT1A autoreceptors and heteroreceptors inhibit and stimulate the Erk1,2 pathway, respectively, while in the presence of mitogen FGF2 receptors, 5-HT1A autoreceptors also stimulate the Erk1,2 pathway (Figures 6B, C). Autoreceptors and heteroreceptors also differ in their ability to desensitize. Administration of the 5-HT1A receptor agonist 8-OH DPAT as well as prolonged stimulation (10–15 days) of 5-HT1A autoreceptors elicited by fluoxetine or other specific serotonin reuptake inhibitors (SSRIs) result in desensitization and downregulation of 5-HT1A autoreceptors, whereas heteroreceptors do not desensitize78,79. The reason for this difference is unknown. 5-HT1A autoreceptors inhibit presynaptic 5-HT release. Since the kinetics of autoreceptor desensitization, observed following the administration of SSRIs is similar to that of their antidepressant effects, it has been suggested that desensitization of autoreceptors is mandatory to have sufficient 5-HT within the synapse to fully activate heteroreceptors13. Other fine differences between 5-HT1A autoreceptor and heteroreceptor signaling have been recently reviewed78.
The 5-HT6 receptor represents another intriguing example of the influence of subcellular compartmentation on 5-HT receptor signal transduction. In neurons, 5-HT6 receptors are mainly located in the primary cilium, but they are also present at the plasma membrane of the cell body58 (Figure 6D). GPRIN1, which increases 5-HT6 receptor coupling to Gs, is co-localized with the receptor in the cell body but not in the primary cilium58. Interestingly, 5-HT6 receptors stimulate cAMP production in the cell body but not in the primary cilium under basal conditions80. The more probable explanation is that coupling of 5-HT6 receptors to Gs is inhibited in the primary cilium by either a GIP or a post-translational modification, such as phosphorylation. However, no data supporting these hypotheses are so far available. In fact, a recent study suggests that 5-HT6 receptor-operated Gs signaling in the primary cilium can be finely regulated by a complex sequence of events depending on other ciliary receptors. When smoothened (Smo) receptor, a GPCR central in Hedgehog (Hh) signaling thought to decrease cAMP in the primary cilium through Gαi, is stimulated by an agonist, it enters the cilium (Figure 6D). The Hh patched (Ptc) receptor is internalized and the constitutively active Gs-coupled receptor GPR161 is inhibited. This allows activation of the Gli transcription factor (Gli-A), which translocates into the nucleus, where it induces the transcription of Hh-regulated genes81. 5-HT6 receptors concomitantly become able to activate cAMP production upon agonist receptor stimulation or as a consequence of constitutive activity, likely because they couple to Gs, even though this remains to be demonstrated. Though much work remains to be done to understand the mechanism involved, these findings indicate a fine temporal regulation of 5-HT6 receptor-operated signaling in the primary cilium. Jiang et al. also proposed that the local production of cAMP elicited by 5-HT6 receptors in the cilium exerts a local feedback inhibition of Gli-A80 (Figure 6D).
Conclusions and future directions
5-HT receptor signaling is not a closed chapter of pharmacology, and several important lines of research are still very active. One of them is the relationship between 5-HT receptor 3D structure and signaling. How many activated or inactivated conformations 5-HT receptors can adopt, what structural determinants are required for their alternative coupling to G-proteins, β-arrestins, and other signal transduction molecules, how biased agonists favor some of them, and how dimerization or heterodimerization influences signaling of 5-HT receptors, as established for the 5-HT2A–mGlu2 heterocomplex, remain important open questions that certainly warrant further exploration71,72,74. Some clues concerning the structural determinants in the 5-HT2A receptor required for hallucinogen biased actions have recently been revealed67–69. Likewise, characterizing the constitutively active conformations selected upon interactions of the 5-HT6 receptor with GIPs such as neurofibromin 1, Cdk5, and GPRIN1 might be of utmost interest given the potential of this receptor as a therapeutic target for the treatment of cognitive deficits associated with neurodevelopmental disorders and dementia45,58. Another important line of future research concerns the spatiotemporal regulation of signaling engaged by 5-HT receptors, such as the one found for the 5-HT6 receptor80.
Another avenue of research is serotonylation2–4, which has a key influence on the physiology of peripheral cells, such as platelets, pancreatic β-cells, and smooth muscle cells. Surprisingly little is known about the regulation of 5-HT neuron functional activity by this biochemical process, which has long been underestimated. It is likely that the pathophysiological influence of epigenetic mechanisms related to serotonylation will also rapidly emerge in the fields of neurology and psychiatry6,7.
The peer reviewers who approve this article are:
Mark M Rasenick, Department of Physiology and Biophysics, Department of Psychiatry, University of Illinois at Chicago, and Jesse Brown VAMC Chicago, IL, 60612, USA
Javier González-Maeso, Department of Physiology and Biophysics, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA
Amitabha Chattopadhyay, CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India
Funding Statement
The authors are supported by grants from University of Montpellier, iSITE Montpellier University of Excellence (MUSE), Centre National de la Recherche Scientifique (CNRS), Institut National pour la Santé et la Recherche Médicale (INSERM), and Agence Nationale de la Recherche (ANR, contracts n° ANR-17-CE16-0013-01, ANR-17-CE16-0010-01, and ANR-19-CE18-0018-02).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Azmitia E: Functional anatomy of the serotonergic system. In: Müller CaC, KA, editor. Handbook of Behavioral neurobiology of Serotonin. London: Academic Press, Elsevier; 2020. Reference Source [Google Scholar]
- 2. Walther DJ, Peter JU, Winter S, et al. : Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003; 115(7): 851–62. 10.1016/s0092-8674(03)01014-6 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 3. Bader M: Serotonylation: Serotonin Signaling and Epigenetics. Front Mol Neurosci. 2019; 12: 288. 10.3389/fnmol.2019.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 4. Farrelly LA, Thompson RE, Zhao S, et al. : Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019; 567(7749): 535–9. 10.1038/s41586-019-1024-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 5. Muma NA, Mi Z: Serotonylation and Transamidation of Other Monoamines. ACS Chem Neurosci. 2015; 6(7): 961–9. 10.1021/cn500329r [DOI] [PubMed] [Google Scholar]
- 6. Girault JA: Epigenetic tinkering with neurotransmitters. Science. 2020; 368(6487): 134–5. 10.1126/science.abb3533 [DOI] [PubMed] [Google Scholar]
- 7. Lepack EA, Werner CT, Stewart AF, et al. : Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science. 2020; 368(6487): 197–201. 10.1126/science.aaw8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacob F: Evolution and tinkering. Science. 1977; 196(4295): 1161–6. 10.1126/science.860134 [DOI] [PubMed] [Google Scholar]
- 9. Bockaert J, Pin JP: Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999; 18(7): 1723–9. 10.1093/emboj/18.7.1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Zhou Z, Zhang Y, et al. : Ocean acidification inhibits initial shell formation of oyster larvae by suppressing the biosynthesis of serotonin and dopamine. Sci Total Environ. 2020; 735: 139469. 10.1016/j.scitotenv.2020.139469 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 11. Marin P, Becamel C, Chaumont-Dubel S, et al. : Classification and signaling characteristics of 5-HT receptors: toward the concept of 5-HT receptosomes. In: MÜLLER CAJ, BL, editor. Handbook of Behavioral neurobiology of Serotonin. London: Academic Press; 2020; 31: 91–120. 10.1016/B978-0-444-64125-0.00005-0 [DOI] [Google Scholar]
- 12. Bockaert J, Claeysen S, Bécamel C, et al. : Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006; 326(2): 553–72. 10.1007/s00441-006-0286-1 [DOI] [PubMed] [Google Scholar]
- 13. Sharp T, Barnes NM: Central 5-HT receptors and their function; present and future. Neuropharmacology. 2020; 177: 108155. 10.1016/j.neuropharm.2020.108155 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 14. Chaumont-Dubel S, Dupuy V, Bockaert J, et al. : The 5-HT 6 receptor interactome: New insight in receptor signaling and its impact on brain physiology and pathologies. Neuropharmacology. 2020; 172: 107839. 10.1016/j.neuropharm.2019.107839 [DOI] [PubMed] [Google Scholar]
- 15. Marin P, Becamel C, Dumuis A, et al. : 5-HT receptor-associated protein networks: New targets for drug discovery in psychiatric disorders? Curr Drug Targets. 2012; 13(1): 28–52. 10.2174/138945012798868498 [DOI] [PubMed] [Google Scholar]
- 16. Wirth A, Holst K, Ponimaskin E: How serotonin receptors regulate morphogenic signalling in neurons. Prog Neurobiol. 2017; 151: 35–56. 10.1016/j.pneurobio.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 17. de Deurwaerdère P, Bharatiya R, Chagraoui A, et al. : Constitutive activity of 5-HT receptors: Factual analysis. Neuropharmacology. 2020; 168: 107967. 10.1016/j.neuropharm.2020.107967 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 18. Dale GL, Friese P, Batar P, et al. : Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002; 415(6868): 175–9. 10.1038/415175a [DOI] [PubMed] [Google Scholar]
- 19. Pisano JJ, Finlayson JS, Peyton MP, et al. : Epsilon-(gamma glutamyl) lysine in fibrin: Lack of crosslink formation in Factor 13 deficiency. Proc Natl Acad Sci U S A. 1971; 68(4): 770–2. 10.1073/pnas.68.4.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aktories K: Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Microbiol. 2011; 9(7): 487–98. 10.1038/nrmicro2592 [DOI] [PubMed] [Google Scholar]
- 21. Walther DJ, Stahlberg S, Vowinckel J: Novel roles for biogenic monoamines: From monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J. 2011; 278(24): 4740–55. 10.1111/j.1742-4658.2011.08347.x [DOI] [PubMed] [Google Scholar]
- 22. Wang HM, Wang Y, Liu M, et al. : Fluoxetine inhibits monocrotaline-induced pulmonary arterial remodeling involved in inhibition of RhoA-Rho kinase and Akt signalling pathways in rats. Can J Physiol Pharmacol. 2012; 90(11): 1506–15. 10.1139/y2012-108 [DOI] [PubMed] [Google Scholar]
- 23. Guilluy C, Eddahibi S, Agard C, et al. : RhoA and Rho kinase activation in human pulmonary hypertension: Role of 5-HT signaling. Am J Respir Crit Care Med. 2009; 179(12): 1151–8. 10.1164/rccm.200805-691OC [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 24. Watts SW, Priestley JRC, Thompson JM: Serotonylation of vascular proteins important to contraction. PLoS One. 2009; 4(5): e5682. 10.1371/journal.pone.0005682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Penumatsa KC, Fanburg BL: Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014; 306(4): L309–15. 10.1152/ajplung.00321.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayme-Dietrich E, Lawson R, Da-Silva S, et al. : Serotonin contribution to cardiac valve degeneration: New insights for novel therapies? Pharmacol Res. 2019; 140: 33–42. 10.1016/j.phrs.2018.09.009 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 27. Paulmann N, Grohmann M, Voigt JP, et al. : Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009; 7(10): e1000229. 10.1371/journal.pbio.1000229 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 28. Mi Z, Si T, Kapadia K, et al. : Receptor-stimulated transamidation induces activation of Rac1 and Cdc42 and the regulation of dendritic spines. Neuropharmacology. 2017; 117: 93–105. 10.1016/j.neuropharm.2017.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hummerich R, Thumfart JO, Findeisen P, et al. : Transglutaminase-mediated transamidation of serotonin, dopamine and noradrenaline to fibronectin: Evidence for a general mechanism of monoaminylation. FEBS Lett. 2012; 586(19): 3421–8. 10.1016/j.febslet.2012.07.062 [DOI] [PubMed] [Google Scholar]
- 30. Colgan LA, Putzier I, Levitan ES: Activity-dependent vesicular monoamine transporter-mediated depletion of the nucleus supports somatic release by serotonin neurons. J Neurosci. 2009; 29(50): 15878–87. 10.1523/JNEUROSCI.4210-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Young AB, Pert CD, Brown DG, et al. : Nuclear localization of histamine in neonatal rat brain. Science. 1971; 173(3993): 247–9. 10.1126/science.173.3993.247 [DOI] [PubMed] [Google Scholar]
- 32. Gerace L, Burke B: Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988; 4: 335–74. 10.1146/annurev.cb.04.110188.002003 [DOI] [PubMed] [Google Scholar]
- 33. Nygaard R, Zou Y, Dror RO, et al. : The dynamic process of β2-adrenergic receptor activation. Cell. 2013; 152(3): 532–42. 10.1016/j.cell.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 34. Du Y, Duc NM, Rasmussen SGF, et al. : Assembly of a GPCR-G Protein Complex. Cell. 2019; 177(5): 1232–1242.e11. 10.1016/j.cell.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 35. Suomivuori CM, Latorraca NR, Wingler LM, et al. : Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science. 2020; 367(6480): 881–7. 10.1126/science.aaz0326 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 36. DeWire SM, Ahn S, Lefkowitz RJ, et al. : Beta-arrestins and cell signaling. Annu Rev Physiol. 2007; 69: 483–510. 10.1146/annurev.physiol.69.022405.154749 [DOI] [PubMed] [Google Scholar]
- 37. Labasque M, Reiter E, Becamel C, et al. : Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell. 2008; 19(11): 4640–50. 10.1091/mbc.e08-04-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu N, Hanson SM, Francis DJ, et al. : Arrestin binding to calmodulin: A direct interaction between two ubiquitous signaling proteins. J Mol Biol. 2006; 364(5): 955–63. 10.1016/j.jmb.2006.09.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lafitte D, Heck AJ, Hill TJ, et al. : Evidence of noncovalent dimerization of calmodulin. Eur J Biochem. 1999; 261(1): 337–44. 10.1046/j.1432-1327.1999.00284.x [DOI] [PubMed] [Google Scholar]
- 40. Barthet G, Framery B, Gaven F, et al. : 5-hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol Biol Cell. 2007; 18(6): 1979–91. 10.1091/mbc.e06-12-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gill RK, Saksena S, Tyagi S, et al. : Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology. 2005; 128(4): 962–74. 10.1053/j.gastro.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 42. Cochet M, Donneger R, Cassier E, et al. : 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem Neurosci. 2013; 4(1): 130–40. 10.1021/cn300095t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baranger K, Giannoni P, Girard SD, et al. : Chronic treatments with a 5-HT 4 receptor agonist decrease amyloid pathology in the entorhinal cortex and learning and memory deficits in the 5xFAD mouse model of Alzheimer's disease. Neuropharmacology. 2017; 126: 128–41. 10.1016/j.neuropharm.2017.08.031 [DOI] [PubMed] [Google Scholar]
- 44. Rochais C, Lecoutey C, Hamidouche K, et al. : Donecopride, a Swiss army knife with potential against Alzheimer's disease. Br J Pharmacol. 2020; 177(9): 1988–2005. 10.1111/bph.14964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duhr F, Déléris P, Raynaud F, et al. : Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol. 2014; 10(7): 590–7. 10.1038/nchembio.1547 [DOI] [PubMed] [Google Scholar]
- 46. Jacobshagen M, Niquille M, Chaumont-Dubel S, et al. : The serotonin 6 receptor controls neuronal migration during corticogenesis via a ligand-independent Cdk5-dependent mechanism. Development. 2014; 141(17): 3370–7. 10.1242/dev.108043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dayer AG, Jacobshagen M, Chaumont-Dubel S, et al. : 5-HT6 Receptor: A New Player Controlling the Development of Neural Circuits. ACS Chem Neurosci. 2015; 6(7): 951–60. 10.1021/cn500326z [DOI] [PubMed] [Google Scholar]
- 48. Labus J, Röhrs KF, Ackmann J, et al. : Amelioration of Tau pathology and memory deficits by targeting 5-HT7 receptor. Prog Neurobiol. 2021; 197: 101900. 10.1016/j.pneurobio.2020.101900 [DOI] [PubMed] [Google Scholar]
- 49. Ango F, Prézeau L, Muller T, et al. : Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001; 411(6840): 962–5. 10.1038/35082096 [DOI] [PubMed] [Google Scholar]
- 50. Chokshi V, Gao M, Grier BD, et al. : Input-Specific Metaplasticity in the Visual Cortex Requires Homer1a-Mediated mGluR5 Signaling. Neuron. 2019; 104(4): 736–748.e6. 10.1016/j.neuron.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 51. Marton TM, Hussain Shuler MG, Worley PF: Homer 1a and mGluR5 phosphorylation in reward-sensitive metaplasticity: A hypothesis of neuronal selection and bidirectional synaptic plasticity. Brain Res. 2015; 1628(Pt A): 17–28. 10.1016/j.brainres.2015.06.037 [DOI] [PubMed] [Google Scholar]
- 52. Holz A, Mülsch F, Schwarz MK, et al. : Enhanced mGlu5 Signaling in Excitatory Neurons Promotes Rapid Antidepressant Effects via AMPA Receptor Activation. Neuron. 2019; 104(2): 338–352.e7. 10.1016/j.neuron.2019.07.011 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 53. Hu JH, Park JM, Park S, et al. : Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010; 68(6): 1128–42. 10.1016/j.neuron.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Diering GH, Nirujogi RS, Roth RH, et al. : Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 2017; 355(6324): 511–5. 10.1126/science.aai8355 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 55. Meffre J, Chaumont-Dubel S, Mannoury La Cour C, et al. : 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol Med. 2012; 4(10): 1043–56. 10.1002/emmm.201201410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berthoux C, Hamieh AM, Rogliardo A, et al. : Early 5-HT 6 receptor blockade prevents symptom onset in a model of adolescent cannabis abuse. EMBO Mol Med. 2020; 12(5): e10605. 10.15252/emmm.201910605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Deraredj Nadim W, Chaumont-Dubel S, Madouri F, et al. : Physical interaction between neurofibromin and serotonin 5-HT6 receptor promotes receptor constitutive activity. Proc Natl Acad Sci U S A. 2016; 113(43): 12310–5. 10.1073/pnas.1600914113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pujol CN, Dupuy V, Séveno M, et al. : Dynamic interactions of the 5-HT 6 receptor with protein partners control dendritic tree morphogenesis. Sci Signal. 2020; 13(618): eaax9520. 10.1126/scisignal.aax9520 [DOI] [PubMed] [Google Scholar]
- 59. Azzi M, Charest PG, Angers S, et al. : Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci U S A. 2003; 100(20): 11406–11. 10.1073/pnas.1936664100 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 60. Costa-Neto CM, Parreiras-E-Silva LT, Bouvier M: A Pluridimensional View of Biased Agonism. Mol Pharmacol. 2016; 90(5): 587–95. 10.1124/mol.116.105940 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 61. Smith JS, Lefkowitz RJ, Rajagopal S: Biased signalling: From simple switches to allosteric microprocessors. Nat Rev Drug Discov. 2018; 17(4): 243–60. 10.1038/nrd.2017.229 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 62. Sniecikowska J, Gluch-Lutwin M, Bucki A, et al. : Discovery of Novel pERK1/2- or β-Arrestin-Preferring 5-HT1A Receptor-Biased Agonists: Diversified Therapeutic-like versus Side Effect Profile. J Med Chem. 2020; 63(19): 10946–71. 10.1021/acs.jmedchem.0c00814 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 63. Kim Y, Kim H, Lee J, et al. : Discovery of β-Arrestin Biased Ligands of 5-HT7R. J Med Chem. 2018; 61(16): 7218–33. 10.1021/acs.jmedchem.8b00642 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 64. Gaven F, Pellissier LP, Queffeulou E, et al. : Pharmacological profile of engineered 5-HT4 receptors and identification of 5-HT4 receptor-biased ligands. Brain Res. 2013; 1511: 65–72. 10.1016/j.brainres.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 65. Vidal B, Bolbos R, Redouté J, et al. : Pharmacological MRI to investigate the functional selectivity of 5-HT1A receptor biased agonists. Neuropharmacology. 2020; 172: 107867. 10.1016/j.neuropharm.2019.107867 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 66. Poulie CBM, Jensen AA, Halberstadt AL, et al. : DARK Classics in Chemical Neuroscience: NBOMes. ACS Chem Neurosci. 2019. 10.1021/acschemneuro.9b00528 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 67. Kim K, Che T, Panova O, et al. : Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell. 2020; 182(6): 1574–1588.e19. 10.1016/j.cell.2020.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 68. Wacker D, Wang S, McCorvy JD, et al. : Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell. 2017; 168(3): 377–389.e12. 10.1016/j.cell.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 69. Karaki S, Becamel C, Murat S, et al. : Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteomics. 2014; 13(5): 1273–85. 10.1074/mcp.M113.036558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Inoue A, Raimondi F, Kadji FMN, et al. : Illuminating G-Protein-Coupling Selectivity of GPCRs. Cell. 2019; 177(7): 1933–1947.e25. 10.1016/j.cell.2019.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 71. González-Maeso J, Ang RL, Yuen T, et al. : Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008; 452(7183): 93–7. 10.1038/nature06612 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 72. Fribourg M, Moreno JL, Holloway T, et al. : Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011; 147(5): 1011–23. 10.1016/j.cell.2011.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 73. Toneatti R, Shin JM, Shah UH, et al. : Interclass GPCR heteromerization affects localization and trafficking. Sci Signal. 2020; 13(654): eaaw3122. 10.1126/scisignal.aaw3122 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 74. Murat S, Bigot M, Chapron J, et al. : 5-HT2A receptor-dependent phosphorylation of mGlu2 receptor at Serine 843 promotes mGlu2 receptor-operated Gi/o signaling. Mol Psychiatry. 2019; 24(11): 1610–26. 10.1038/s41380-018-0069-6 [DOI] [PubMed] [Google Scholar]
- 75. Ahn S, Shenoy SK, Luttrell LM, et al. : SnapShot: β-Arrestin Functions. Cell. 2020; 182(5): 1362–1362.e1. 10.1016/j.cell.2020.07.034 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 76. Sutkeviciute I, Vilardaga JP: Structural insights into emergent signaling modes of G protein-coupled receptors. J Biol Chem. 2020; 295(33): 11626–42. 10.1074/jbc.REV120.009348 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 77. Lazar AM, Irannejad R, Baldwin TA, et al. : G protein-regulated endocytic trafficking of adenylyl cyclase type 9. Elife. 2020; 9: e58039. 10.7554/eLife.58039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Albert PR, Vahid-Ansari F: The 5-HT1A receptor: Signaling to behavior. Biochimie. 2019; 161: 34–45. 10.1016/j.biochi.2018.10.015 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 79. Varrault A, Leviel V, Bockaert J: 5-HT1A-sensitive adenylyl cyclase of rodent hippocampal neurons: Effects of antidepressant treatments and chronic stimulation with agonists. J Pharmacol Exp Ther. 1991; 257(1): 433–8. [PubMed] [Google Scholar]
- 80. Jiang JY, Falcone JL, Curci S, et al. : Direct visualization of cAMP signaling in primary cilia reveals up-regulation of ciliary GPCR activity following Hedgehog activation. Proc Natl Acad Sci U S A. 2019; 116(24): 12066–71. 10.1073/pnas.1819730116 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 81. Schou KB, Pedersen LB, Christensen ST: Ins and outs of GPCR signaling in primary cilia. EMBO Rep. 2015; 16(9): 1099–113. 10.15252/embr.201540530 [DOI] [PMC free article] [PubMed] [Google Scholar]