Abstract
Background
We report results of years 2 and 3 of consecutive cluster-randomized controlled trials of trivalent inactivated influenza vaccine (IIV3) in Senegal.
Methods
We cluster-randomized (1:1) 20 villages to annual vaccination with IIV3 or inactivated poliovirus vaccine (IPV) of age-eligible residents (6 months–10 years). The primary outcome was total vaccine effectiveness against laboratory-confirmed influenza illness (LCI) among age-eligible children (modified intention-to-treat population [mITT]). Secondary outcomes were indirect (herd protection) and population (overall community) vaccine effectiveness.
Results
We vaccinated 74% of 12 408 age-eligible children in year 2 (June 2010–April 11) and 74% of 11 988 age-eligible children in year 3 (April 2011–December 2011) with study vaccines. Annual cumulative incidence of LCI was 4.7 (year 2) and 4.2 (year 3) per 100 mITT child vaccinees of IPV villages. In year 2, IIV3 matched circulating influenza strains. The total effectiveness was 52.8% (95% confidence interval [CI], 32.3–67.0), and the population effectiveness was 36.0% (95% CI, 10.2–54.4) against LCI caused by any influenza strain. The indirect effectiveness against LCI by A/H3N2 was 56.4% (95% CI, 39.0–68.9). In year 3, 74% of influenza detections were vaccine-mismatched to circulating B/Yamagata and 24% were vaccine-matched to circulating A/H3N2. The year 3 total effectiveness against LCI was −14.5% (95% CI, −81.2–27.6). Vaccine effectiveness varied by type/subtype of influenza in both years.
Conclusions
IIV3 was variably effective against influenza illness in Senegalese children, with total and indirect vaccine effectiveness present during the year when all circulating strains matched the IIV3 formulation.
Clinical Trials Registration
Keywords: influenza vaccine, Africa, children, vaccine effectiveness, cluster-randomized trial
Inactivated influenza vaccine given to children in Senegal as part of a multiyear village-randomized trial provided moderate total and indirect protection against laboratory-confirmed influenza illness in year 2 but was not protective during year 3.
In 2009, we initiated a project to define the potential for pediatric influenza vaccination to reduce disease in low-resource African populations. We conducted annual cluster-randomized controlled trials (CRCTs), administrating seasonal, trivalent inactivated influenza vaccine (IIV3) or inactivated poliovirus vaccine (IPV) to children aged 6 months through 10 years with the aim of estimating the total, indirect, and population effectiveness of IIV3. Total effectiveness in immunized individuals measures both direct protection of immunization and indirect protection, or herd immunity, conferred by reduced exposure to persons with infections. Population (or overall) effectiveness is the measure of the effectiveness of immunization as experienced by unvaccinated and vaccinated persons.
During study year 1 (2009–2010), IIV3 total effectiveness, indirect effectiveness, and population effectiveness against influenza A/H3N2, the predominant circulating strain, were 43.6% (95% confidence interval [CI], 18.6–60.9), 15.4% (95% CI, −22.0–41.3), and 31.7% (95% CI, 6.0–50.3), respectively [1]. In January 2010, the 2009 H1N1 influenza pandemic virus (A/H1N1pdm) arrived in Senegal. There was no significant effectiveness of the seasonal IIV3 against A/H1N1pdm illness. Here, we report CRTC results of years 2 and 3 after A/H1N1pdm had been incorporated into IIV3 formulations.
METHODS
Study Design
We conducted 2 double-blind, IPV-controlled, parallel CRCTs in 2010–2011 and 2011 in the area of the Niakhar Demographic Surveillance System (DSS). Neither IIV3 nor IPV was available in this community prior to the start of our project, although trivalent oral poliovirus vaccine was part of the routine childhood immunization schedule. Unless otherwise specified, all study standards, procedures (including informed consent), definitions, and sample size calculations previously described for year 1 also applied to years 2 and 3 [1]. Prior to year 1, we randomly allocated each of 20 geographically contiguous villages of the Niakhar DSS [2] to have children targeted for study vaccination allocated at a 1:1 ratio of IIV3 or IPV. Villages were not re-randomized thereafter. Upon completion of each annual vaccination campaign, we monitored all residents in the study area, whether vaccinated or not, for influenza illness by active surveillance (weekly visits to each residential compound) and enhanced passive surveillance (health post–based assessments). From 3 January 2011 to 18 February 2011 we did not conduct active surveillance in the community due to a health worker job action, but health post–based surveillance continued. Study surveillance used standardized case definitions and methods [1]. The Senegal National Ethics Committee for Health Research and the Western Institutional Review Board (United States) approved this study. The trial is registered at ClinicalTrials.gov (NCT00893906).
Participants
The Niakhar DSS regularly updates a census of all residents of the 20 villages included in this CRCT [2]. At the start of each annual vaccination campaign, a healthy child currently 6 months through 10 years of age was eligible to receive study vaccine if the child’s family planned to stay in the study area during the next 12 months and a parent/guardian was willing to provide written informed consent. Children were ineligible if they had a history of hypersensitivity to any study vaccine or vaccine components. A current febrile illness (>37.5°C axillary) was a temporary exclusion criterion. All residents in the study area were eligible to participate in influenza surveillance if they provided written informed consent at the time of illness identification.
Interventions
The IIV3 and IPV product information is presented in Table 1. Study vaccine–naive children aged 6 months through 8 years were offered 2 age-appropriate doses of the same vaccine 1 month apart. All children aged 9 through 10 years and all children younger than 11 years who had previously received study vaccine were offered 1 dose. Vaccine recipients were monitored for 1 month after vaccination for serious adverse events (SAEs).
Table 1.
Annual Influenza Vaccine Formulations and Match to Circulating Influenza Viruses, by Study Year
| Study Year 1 (2009) [1] | Study Year 2 (2010) | Study Year 3 (2011) | |
|---|---|---|---|
| IIV3 formulation | 2008–2009 Northern Hemispherea | 2010 Southern Hemisphere | 2011 Southern Hemisphere |
| A/H1N1 | A/Brisbane/59/2007 (H1N1)-like | A/California/7/2009 (H1N1)-like | A/California/7/2009 (H1N1)-like |
| A/H3N2 | A/Brisbane/10/2007 (H3N2)-like | A/Perth/16/2009 (H3N2)-like | A/Perth/16/2009 (H3N2)-like |
| B | B/Florida/4/2006-like (B/Yamagata lineage) |
B/Brisbane/60/2008-like (B/Victoria lineage) |
B/Brisbane/60/2008-like (B/Victoria lineage) |
| IIV3 product (lot) | Vaxigrip, Sanofi Pasteur (D5813 and D9672) |
Vaxigrip, Sanofi Pasteur (G7051-1 and G5171-1) |
Vaxigrip, Sanofi Pasteur (G7111-3 and G0382-2) |
| IPV product (lot) | IMOVAX Polio, Sanofi Pasteur (B0283) |
IMOVAX Polio, Sanofi Pasteur (D0238-1) |
IMOVAX Polio, Sanofi Pasteur (D6082-2) |
| Influenza detections among age-eligible residents of IPV villages, n | 585 | 217 | 192 |
| A/H1N1pdm, n (%) | 115 (19.7)b | 32 (14.7) | 2 (1.0) |
| A/H3N2, n (%) | 481 (82.2) | 55 (25.3) | 50 (26.0) |
| B, n (%) | 3 (0.5) | 134 (61.8) | 142 (74.0)b |
| Antigenic characterization,c total N | 30 | 33 | 38 |
| A/H1N1, n | 0 | 11 A/California/7/2009 (H1N1)-like | 6 A/California/7/2009 (H1N1)-like |
| A/H3N2, n | 30 A/Perth/16/2009 (H3N2)-likeb | 8 A/Perth/16/2009 (H3N2)-like | 14 A/Perth/16/2009 (H3N2)-like |
| B, n | 0 | 14 B/Brisbane/60/2008-like (B/Victoria lineage) | 18 B/Wisconsin/01/2010-like (B/Yamagata lineage)b |
| Comment | Only H3N2 isolates were characterized, and all were found to be A/Perth/16/2009-like, mismatched from study vaccine strains. H1N1pdm detections by RT-PCR used primers specific to A/California/7/2009 (H1N1)-like viruses, indicating mismatch from IIV3 strains. | All characterized influenza virus isolates were antigenically similar to IIV3 strains. | All characterized influenza A virus isolates were antigenically similar to IIV3 strains. All characterized influenza B virus isolates were antigenically similar to B/Wisconsin/01/2010-like virus from the B/Yamagata lineage, indicating mismatch from IIV3 influenza B strain. |
Abbreviations: IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; RT-PCR, reverse transcription–polymerase chain reaction.
aIdentical to the 2009 Southern Hemisphere formulation.
bVaccine mismatch.
cSubset of all influenza detections in the study.
Outcomes
The individual-level primary outcome was laboratory-confirmed influenza illness (LCI) caused by any type/subtype. Secondary outcomes were vaccine effectiveness by influenza type/subtype. Specimens were collected from persons meeting these criteria—among children younger than 2 years: sudden onset of fever (>37.5°C axillary) or subjective (parent-reported) feverishness, plus at least 1 of cough, sore throat, nasal congestion, rhinorrhea, or difficulty breathing; and among children 2 years and older: sudden onset of fever (>37.5°C axillary) or subjective (parent- or participant-reported) feverishness, plus at least 1 of cough or sore throat [1]. Laboratory confirmation of influenza illness was done by reverse transcription–polymerase chain reaction (RT-PCR) in nasal and oropharyngeal swab specimens combined into a single vial of transport media after collection. A subset of clinical specimens was antigenically characterized at the US Centers for Disease Control and Prevention (CDC).
Allocation and Blinding
Before study year 1, we performed restricted, stratified randomization at the village level before seeking individual-level informed consent and delivery of study vaccine, as previously described [1]. All subjects and study staff involved in the evaluation of clinical and safety outcomes were blinded to the village-level allocation.
Statistical Methods
We calculated the total effectiveness of IIV3 in reducing rates of LCI among vaccinated children from IIV3 villages compared with among vaccinated children from IPV villages. As we did for the year 1 analysis [1], we developed a logistic regression model fit to the individual-level data via generalized estimating equations to account for within-village correlation (clustering) and assuming exchangeable correlation matrices [3]. Participants could not contribute more than 1 outcome per year to the analyses. We used Stata, version 11 (StataCorp LP, College Station, TX), and R version 3.1.1 for analyses [4]. As secondary objectives, we estimated indirect and population effectiveness using a similar analytic approach. We calculated indirect effectiveness of IIV3 in reducing rates of LCI among persons who were not vaccinated (children <6 months of age and children and adults >10 years of age) from IIV3-allocated villages compared with among persons who were not vaccinated from IPV-allocated villages. We calculated population effectiveness of IIV3 in reducing rates of LCI among all persons (of any age) from IIV3-allocated villages compared with among all persons from IPV-allocated villages. The prespecified analyses were estimates of these 3 effects against the primary endpoint by study year.
We used a modified intention-to-treat (mITT) approach for primary total effectiveness analyses, in which an age-eligible child was included if a parent/guardian provided informed consent and the child was enrolled for vaccination. We also used a per-protocol approach for total effectiveness analyses in which enrolled, age-eligible children were included if they received the protocol-specified course of study vaccine and contributed at least 1 day of study follow-up time. We estimated indirect effectiveness among all residents who were not age-eligible to receive vaccine at the start of each annual vaccination campaign. We estimated the population effectiveness among all residents of the study villages. We assessed each effectiveness parameter for primary and secondary endpoints. Exploratory analyses estimated the per-protocol total and indirect effectiveness by prespecified age groups.
RESULTS
The 20 villages randomized at the beginning of our project participated in study years 2 and 3 with the same vaccine allocation (Figure 1) [1]. At the start of annual vaccination, 12 408 children were age-eligible for year 2 and 11 988 were age-eligible for year 3 (Figure 2). Vaccination campaigns occurred between 3 June and 9 July 2010 and between 26 April and 30 May 2011. In both years, we vaccinated 74% of eligible children (Figure 2). The study arms had similar distributions of baseline characteristics (Table 2).
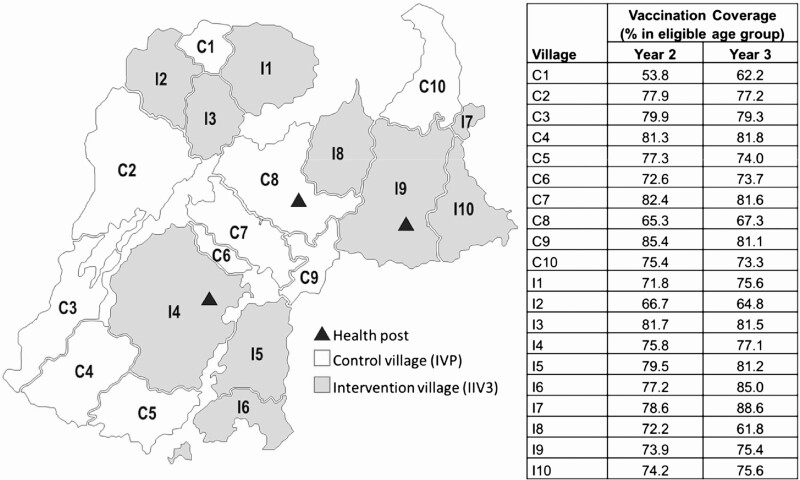
Figure 1.
Geographic distribution of 20 villages randomized to IIV3 and IPV campaigns (map) and the achieved village-level vaccination coverage (%) of the protocol-specified regimen among age-eligible children 6 months through 10 years of age during the year 2 and 3 vaccination campaigns (table), Niakhar Demographic Surveillance System [2]. Note: Villages assigned to IIV3 are shaded gray and labeled with the prefix “I” for intervention, and villages assigned to IPV were shaded white and labeled with the prefix “C” for control. Enhanced, passive surveillance was conducted in the 3 health posts marked on the map with solid triangles. Abbreviations: IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine.
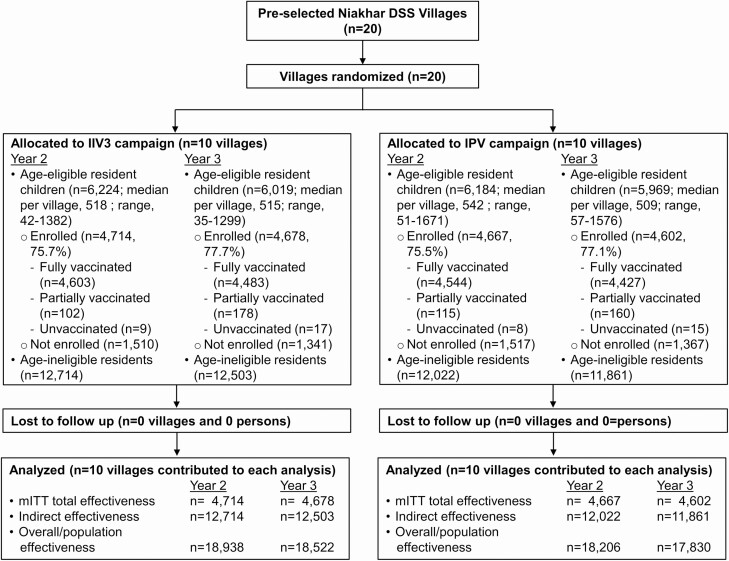
Figure 2.
Study profile for years 2 and 3. Note: The profiles are designed for the primary objective of total effectiveness and the secondary objectives of indirect and population effectiveness. The mITT population consisted of children 6 months through 10 years of age who were enrolled in the vaccine component of the study. Abbreviations: DSS, Demographic Surveillance System; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; mITT, modified intention-to-treat.
Table 2.
Baseline Demographic Characteristics of Each Study Group, by Study Year
| Year 2 | Year 3 | |||
|---|---|---|---|---|
| IIV3 arm (n = 10 villages) | IPV arm (n = 10 villages) | IIV3 arm (n = 10 villages) | IPV arm (n = 10 villages) | |
| Cluster-level characteristics | ||||
| Mean village population size (SD) | 1894 (1230) | 1821 (1381) | 1852 (1195) | 1783 (1334) |
| Residents per compound (SD) | 12.8 (15.4) | 13.1 (16.8) | 19.7 (17.4) | 20.7 (19.0) |
| Individual-level characteristics | ||||
| Total population (all ages) | 18 938 | 18 206 | 18 522 | 17 830 |
| Sex, n (%) | ||||
| Male | 9269 (48.9) | 8930 (49.0) | 9172 (49.5) | 8879 (49.8) |
| Female | 9623 (50.8) | 9221 (50.6) | 9348 (50.5) | 8942 (50.2) |
| Unknown | 46 (0.2) | 55 (0.3) | 2 (0.0) | 5 (0.0) |
| Mean age of population (SD), years | 22.7 (19.5) | 22.4 (19.4) | 23.7 (19.5) | 22.4 (19.4) |
| Age-eligible children, n (%) | 6224 | 6184 | 6019 | 5969 |
| 6–35 months | 1818 (29.2) | 1835 (29.7) | 1702 (28.3) | 1625 (27.2) |
| 3–5 years | 1866 (30.0) | 1801 (29.1) | 1809 (30.1) | 1804 (30.2) |
| 6–8 years | 1553 (25.0) | 1599 (25.9) | 1539 (25.6) | 1572 (26.3) |
| 9–10 years | 987 (15.9) | 949 (15.3) | 969 (16.1) | 968 (16.2) |
| Information on enrollment and vaccination | ||||
| Number of age-eligible children, n (%)enrolled (percent of all age-eligible children) | 4714 (75.7) | 4667 (75.5) | 4678 (77.7) | 4602 (77.1) |
| 6–35 months | 1163 (64.0) | 1179 (64.3) | 1059 (62.2) | 978 (60.2) |
| 3–5 years | 1498 (80.3) | 1436 (79.7) | 1519 (84.0) | 1533 (85.0) |
| 6–8 years | 1280 (82.4) | 1318 (82.4) | 1329 (86.4) | 1349 (85.8) |
| 9–10 years | 773 (78.3) | 734 (77.3) | 771 (79.6) | 742 (76.7) |
| Number receiving dose 1 (% of those enrolled) | 4705 (99.8) | 4659 (99.8) | 4661 (99.6) | 4587 (99.7) |
| 6–35 months | 1163 (100.0) | 1179 (100.0) | 1055 (99.6) | 974 (99.6) |
| 3–5 years | 1495 (99.8) | 1431 (99.7) | 1516 (99.8) | 1531 (99.9) |
| 6–8 years | 1276 (99.7) | 1318 (100.0) | 1324 (99.6) | 1344 (99.6) |
| 9–10 years | 771 (99.7) | 731 (99.6) | 766 (99.4) | 738 (99.5) |
| Number fully dosed per protocol (% of those enrolled) | 3832 (97.4) | 3813 (97.1) | 3717 (79.5) | 3689 (80.2) |
| 6–35 months | 1090 (93.7) | 1098 (93.1) | 942 (89.0) | 885 (90.5) |
| 3–5 years | 1478 (98.7) | 1413 (98.4) | 1472 (96.9) | 1482 (96.7) |
| 6–8 years | 1264 (98.8) | 1302 (98.8) | 1303 (98.0) | 1322 (98.0) |
| 9–10 years | 771 (99.7) | 731 (99.6) | 766 (99.4) | 738 (99.5) |
Abbreviations: IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine.
Surveillance
We conducted year 2 surveillance from 19 July 2010 to 22 April 2011 (Figure 3). At the time we began year 2 surveillance, influenza B was already circulating in the study area. We conducted year 3 influenza surveillance from 6 June 2011 to 16 December 2011. Likewise, influenza A/H3N2 was already circulating when year 3 surveillance began. Influenza A/H1N1pdm, A/H3N2, and B were each detected in both years 2 and 3. For both years, influenza transmission started before calendar week 28, extended past week 48, and peaked between weeks 40 and 44. Of the 5582 clinical specimens collected during year 2 community surveillance, influenza was detected in 584 (10.5%): 98 for A/H1N1pdm, 134 for A/H3N2, 348 for B, and 4 for both B and A/H3N2. Of the 3642 clinical specimens collected from the residents of study villages during the year 3 community surveillance, influenza was detected in 746 (20.5%): 25 for A/H1N1pdm alone; 230 for A/H3N2; 482 for B; 2 for both A/H3N2 and A/H1N1pdm; 6 for both B and A/H3N2; and 1 for A/H1N1pdm, A/H3N2, and B. Antigenic characterization of a subset of clinical specimens annually indicated that, in year 2, all isolates assessed were antigenically similar to IIV3 strains and, in year 3, influenza B viruses (Yamagata lineage) were antigenically different from IIV strains (Victoria lineage) and A/H1N1pdm and A/H3N2 were antigenically similar to IIV3 strains (Table 1).
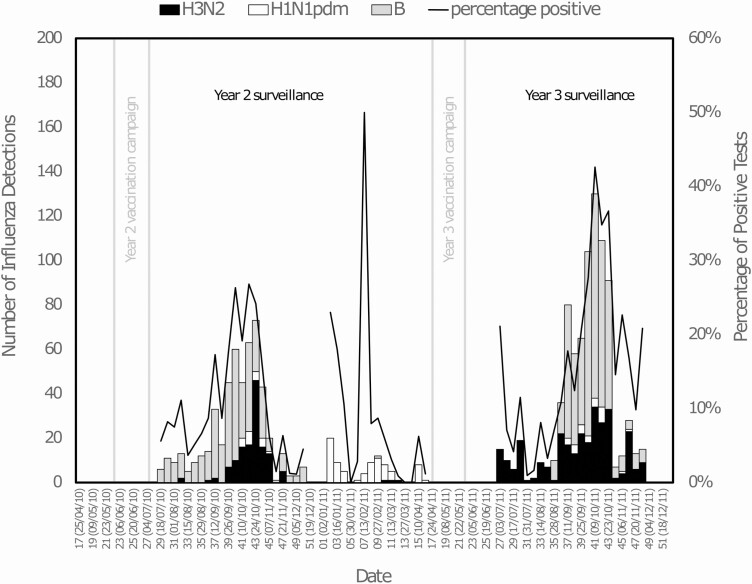
Figure 3.
Number of specimens positive for influenza by type and subtype, from week 29 of 2010 to week 48 of 2011. Note: The stacked vertical bars (left y axis) plot the weekly number of study specimens positive for influenza, by type and subtype as determined by RT-PCR of clinical specimens collected during illness. The black line in the plot traces the percentage of specimens that are positive for any influenza by RT-PCR (right y axis). Vertical lines delineate the vaccination campaign (light gray) for each study season. Cases consistent with laboratory-confirmed influenza illness are associated with calendar week (Sunday to Saturday) within which the first symptoms occurred. Abbreviation: RT-PCR, reverse transcription–polymerase chain reaction.
Total Vaccine Effectiveness
For year 2 in the mITT population, 105 cases of LCI occurred in children in the IIV3 villages (cumulative incidence of 2.23 per 100) and 217 occurred in children in the IPV villages (cumulative incidence of 4.65 per 100) (Table 3). For year 3 in the mITT population, 206 cases of LCI occurred in children in the IIV3 villages (cumulative incidence of 4.40 per 100) and 192 occurred in children in the IPV villages (cumulative incidence of 4.17 per 100) (Table 4). Estimated mITT total effectiveness against all strains was 52.8% (95% CI, 32.3–67.0) for year 2 and −14.5% (95% CI, −81.2–27.6) for year 3.
Table 3.
Year 2 Total Effectiveness of IIV3 in Preventing Laboratory-Confirmed Influenza Illness, by Type/Subtype and Age Group
| IIV3 Villages | IPV Villages | ||||||
|---|---|---|---|---|---|---|---|
| Cases,a n | nb | Cumulative Incidencec | Cases,a n | nb | Cumulative Incidencec | Adjusted VET,d % (95% CI) | |
| VET (mITT) | |||||||
| Any influenza | 105 | 4714 | 2.23 | 217 | 4667 | 4.65 | 52.8 (32.3–67.0) |
| A/H3N2 | 25 | 4714 | 0.53 | 55 | 4667 | 1.18 | 57.7 (20.6–77.5) |
| A/H1N1 | 13 | 4714 | 0.28 | 32 | 4667 | 0.69 | 61.4 (21.0–81.1) |
| B | 67 | 4714 | 1.42 | 134 | 4667 | 2.87 | 49.7 (23.2–67.0) |
| VET (PP) | |||||||
| A/H3N2 | 24 | 4603 | 0.52 | 52 | 4544 | 1.14 | 57.6 (16.4–78.5) |
| 6–35 months | 9 | 1090 | 0.83 | 26 | 1098 | 2.37 | 65.8 (19.2–85.5) |
| 3–5 years | 13 | 1478 | 0.88 | 17 | 1413 | 1.20 | 29.1 (−60.5–68.7) |
| 6–8 years | 2 | 1264 | 0.16 | 5 | 1302 | 0.38 | 64.1 (−79.1–92.8) |
| 9–10 years | 0 | 771 | 0.00 | 4 | 731 | 0.55 | … e |
| A/H1N1 | 12 | 4603 | 0.26 | 31 | 4544 | 0.68 | 63.2 (21.1–82.8) |
| 6–35 months | 6 | 1090 | 0.55 | 9 | 1098 | 0.82 | 36.1 (−76.2–76.8) |
| 3–5 years | 5 | 1478 | 0.34 | 9 | 1413 | 0.64 | 48.6 (−86.5–85.8) |
| 6–8 years | 1 | 1264 | 0.08 | 7 | 1302 | 0.54 | 88.6 (−4.1–98.7) |
| 9–10 years | 0 | 771 | 0.00 | 6 | 731 | 0.82 | … e |
| B | 66 | 4603 | 1.43 | 129 | 4544 | 1.43 | 47.6 (18.0–66.5) |
| 6–35 months | 27 | 1090 | 2.48 | 52 | 1098 | 4.74 | 48.7 (12.8–69.8) |
| 3–5 years | 20 | 1478 | 1.35 | 53 | 1413 | 3.75 | 68.5 (34.1–84.9) |
| 6–8 years | 13 | 1264 | 1.03 | 13 | 1302 | 1.00 | −4.8 (−151.2–56.3) |
| 9–10 years | 6 | 771 | 0.78 | 11 | 731 | 1.50 | 68.3 (−133.1–95.7) |
Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; mITT, modified intention-to-treat; PP, per protocol; VET, total vaccine effectiveness.
aOnly the first episode is counted where more than 1 instance of an endpoint is recorded during the same study year.
bNumber of children followed.
cPer 100 persons through the entire surveillance period: 2010–2011, 19 July 2010 through 22 April 2011; 2011–2012, 6 June 2011 through 16 December 2011.
dEstimated using a logistic regression model fit using generalized estimating equations, assuming an exchangeable correlation matrix to account for within-village correlation of participant observations.
eNot calculable.
Table 4.
Year 3 Total Effectiveness of IIV3 in Preventing Laboratory-Confirmed Influenza Illness, by Type/Subtype and Age Group
| IIV3 Villages | IPV Villages | ||||||
|---|---|---|---|---|---|---|---|
| Cases,a n | nb | Cumulative Incidencec | Cases,a n | nb | Cumulative Incidencec | Adjusted VET,d % (95% CI) | |
| VET (mITT) | |||||||
| Any influenza | 206 | 4678 | 4.40 | 192 | 4602 | 4.17 | −14.5 (−81.2–27.6) |
| A/H3N2 | 84 | 4678 | 1.80 | 50 | 4602 | 1.09 | -82.9 (−288.6–13.9) |
| A/H1N1 | 5 | 4678 | 0.11 | 2 | 4602 | 0.04 | −253.6 (−4982.3–75.4) |
| B | 122 | 4678 | 2.61 | 142 | 4602 | 3.09 | 11.6 (−48.7–47.4) |
| VET (PP) | |||||||
| A/H3N2 | 80 | 4483 | 1.78 | 50 | 4427 | 1.13 | −73.1 (−262.8–17.4) |
| 6–35 months | 33 | 942 | 3.50 | 22 | 885 | 2.49 | −45.2 (−248.5–39.5) |
| 3–5 years | 32 | 1472 | 2.17 | 16 | 1482 | 1.08 | −95.2 (−395.7–23.1) |
| 6–8 years | 8 | 1303 | 0.61 | 8 | 1322 | 0.61 | 5.7 (−1077.1–92.4) |
| 9–10 years | 7 | 766 | 0.91 | 4 | 738 | 0.54 | −141.8 (−695.5–26.5) |
| A/H1N1 | 4 | 4483 | 0.09 | 2 | 4427 | 0.05 | −170.2 (−5804.4–87.6) |
| 6–35 months | 1 | 942 | 0.11 | 0 | 885 | 0.00 | … e |
| 3–5 years | 2 | 1472 | 0.14 | 1 | 1482 | 0.07 | −243.5 (−23 494.2–95.0) |
| 6–8 years | 1 | 1303 | 0.08 | 0 | 1322 | 0.00 | … e |
| 9–10 years | 0 | 766 | 0.00 | 1 | 738 | 0.14 | … e |
| B | 117 | 4483 | 2.61 | 137 | 4427 | 3.09 | 11.8 (−52.4–48.9) |
| 6–35 months | 46 | 942 | 4.88 | 32 | 885 | 3.62 | −32.9 (−127.7–22.4) |
| 3–5 years | 43 | 1472 | 2.92 | 55 | 1482 | 3.71 | 21.9 (−24.2–50.9) |
| 6–8 years | 20 | 1303 | 1.53 | 37 | 1322 | 2.80 | 44.3 (−39.2–77.7) |
| 9–10 years | 8 | 766 | 1.04 | 13 | 738 | 1.76 | 38.0 (−424.2–92.7) |
Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; mITT, modified intention-to-treat; PP, per protocol; VET, total vaccine effectiveness.
aOnly the first episode is counted where more than 1 instance of an endpoint is recorded during the same study year.
bNumber of children followed.
cPer 100 persons through the entire surveillance period: 2010–2011, 19 July 2010 through 22 April 2011; 2011–2012, 6 June 2011 through 16 December 2011.
dEstimated using a logistic regression model fit using generalized estimating equations, assuming an exchangeable correlation matrix to account for within-village correlation of participant observations.
eNot calculable.
Type/Subtype Total Vaccine Effectiveness
For year 2, 25 cases of A/H3N2 LCI occurred in the mITT population of IIV3 villages (cumulative incidence of 0.5 cases per 100) and 55 such outcomes occurred in IPV villages (cumulative incidence of 1.18 cases per 100) for an A/H3N2-specific mITT total effectiveness of 57.7% (95% CI, 20.6–77.5) (Table 3). Thirteen cases of A/H1N1pdm LCI occurred in IIV3 villages (cumulative incidence of 0.28 per 100) and 32 such outcomes occurred in IPV villages (cumulative incidence of 0.69 per 100) during year 2 for an A/H1N1pdm-specific mITT total effectiveness of 61.4% (95% CI, 21.0–81.1). Total effectiveness analysis against influenza B LCI involved 67 outcomes in IIV3 villages (cumulative incidence of 1.42 per 100) and 134 outcomes in IPV villages (cumulative incidence of 2.87 per 100) for an effect of 49.7% (95% CI, 23.2–67.0).
For year 3, the mITT total effectiveness estimates against A/H3N2 and against A/H1N1pdm were negative and not statistically significant (Table 4). For year 3, 84 A/H3N2 LCIs occurred in the mITT population of IIV3 villages (cumulative incidence of 1.80 cases per 100) and 50 such outcomes occurred in IPV villages (cumulative incidence of 1.09 cases per 100) for an A/H3N2-specific mITT total effectiveness of −82.9% (95% CI, −288.6–13.9) (Table 4). Five cases of A/H1N1pdm LCI occurred in IIV3 villages (cumulative incidence of 0.11 per 100) and 2 such outcomes occurred in IPV villages (cumulative incidence of 0.04 per 100) for an A/H1N1pdm-specific mITT total effectiveness of −253.6% (95% CI, −4982.3–75.4). The mITT total effectiveness analysis against influenza B LCI comprised 122 outcomes in IIV3 villages (cumulative incidence of 2.61 per 100 children) and 142 outcomes in IPV villages (cumulative incidence of 3.09 per 100 children) for an estimated effect of 11.6% (95% CI, −48.7–47.4). For any influenza and specific measures for A/H3N2, A/H1N1pdm, and B, all mITT total effectiveness estimates were comparable in magnitude to their corresponding per-protocol estimates.
Indirect Vaccine Effectiveness
For year 2, 12 cases of A/H3N2 LCI occurred among residents of IIV3 villages not age-eligible for study vaccine (cumulative incidence of 0.09 per 100) and 28 such outcomes occurred in IPV villages (cumulative incidence of 0.23 per 100) for an indirect effectiveness of 56.4% (95% CI, 39.0–68.9) (Table 5). The strongest level of indirect protection during year 2 was observed among adults aged 18–49 years old. For year 2, no statistically significant indirect protection was observed for A/H1N1 or B, and for year 3 no statistically significant indirect protection was observed for any of the vaccine strains (Table 6).
Table 5.
Year 2 Indirect Effectiveness of IIV3 in Preventing Laboratory-Confirmed Influenza Illness Among Unvaccinated Residents, by Type/Subtype and Age Group
| IIV3 Villages | IPV Villages | ||||||
|---|---|---|---|---|---|---|---|
| Cases,a n | nb | Cumulative Incidencec | Cases,a n |
nb | Cumulative Incidencec | Adjusted VEI,d % (95% CI) | |
| Any influenza | 82 | 12 714e | 0.64 | 96 | 12 022e | 0.80 | 15.4 (−44.3–50.4) |
| <6 months | 20 | 928 | 2.16 | 29 | 842 | 3.44 | 36.0 (−17.7–65.2) |
| 11–17 years | 23 | 2784 | 0.83 | 21 | 2723 | 0.77 | −5.5 (−177.7–59.9) |
| 18–49 years | 32 | 6847 | 0.47 | 36 | 6471 | 0.56 | 13.2 (−94.7–61.2) |
| 50–64 years | 5 | 1333 | 0.38 | 6 | 1213 | 0.49 | 11.2 (−263.3–78.3) |
| > 64 years | 2 | 812 | 0.25 | 4 | 765 | 0.52 | … |
| A/H3N2 | 12 | 12 714e | 0.09 | 28 | 12 022e | 0.23 | 56.4 (39.0–68.9) |
| <6 months | 4 | 928 | 0.43 | 7 | 842 | 0.83 | 47.7 (−1786.7–98.6) |
| 11–17 years | 2 | 2784 | 0.07 | 4 | 2723 | 0.15 | 48.2 (−208.8–91.3) |
| 18–49 years | 5 | 6847 | 0.07 | 12 | 6471 | 0.19 | 58.4 (14.5–79.8) |
| 50–64 years | 0 | 1333 | 0.00 | 3 | 1213 | 0.25 | … |
| > 64 years | 1 | 812 | 0.12 | 2 | 765 | 0.26 | … |
| A/H1N1 | 18 | 12 714e | 0.14 | 18 | 12 022e | 0.15 | 0.0 (−149.3–59.9) |
| <6 months | 3 | 928 | 0.32 | 5 | 842 | 0.59 | 28.7 (−63.1–68.8) |
| 11–17 years | 4 | 2784 | 0.14 | 4 | 2723 | 0.15 | 6.4 (−13 537.1–99.4) |
| 18–49 years | 7 | 6847 | 0.10 | 8 | 6471 | 0.12 | 18.6 (−169.0–75.4) |
| 50–64 years | 4 | 1333 | 0.30 | 1 | 1213 | 0.08 | −447.7 (−15 033.4–80.2) |
| > 64 years | 0 | 812 | 0.00 | 0 | 765 | 0.00 | … |
| B | 52 | 12 714e | 0.41 | 52 | 12 022e | 0.43 | −0.2 (−93.6–48.1) |
| <6 months | 13 | 928 | 1.40 | 17 | 842 | 2.02 | 22.4 (−35.7–55.6) |
| 11–17 years | 17 | 2784 | 0.61 | 14 | 2723 | 0.51 | −14.2 (−158.3–49.5) |
| 18–49 years | 20 | 6847 | 0.29 | 17 | 6471 | 0.26 | −11.7 (−210.1–59.7) |
| 50–64 years | 1 | 1333 | 0.08 | 2 | 1213 | 0.16 | 64.0 (−283.8–96.6) |
| > 64 years | 1 | 812 | 0.12 | 2 | 765 | 0.26 | … |
Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; VEI, indirect vaccine effectiveness among age-ineligible.
aOnly the first episode is counted where more than 1 instance of an endpoint is recorded during the same study year.
bNumber of children followed.
cPer 100 persons through the entire surveillance period: 2010–2011, 19 July 2010 through 22 April 2011; 2011–2012, 6 June 2011 through 16 December 2011.
dEstimated using a logistic regression model fit using generalized estimating equations, assuming an exchangeable correlation matrix to account for within-village correlation of participant observations.
eIncludes individuals whose age was not reported: year 2 (IIV3, 10: IPV, 8) and year 3 (IIV3, 2: IPV, 1). No laboratory-confirmed influenza illness was detected among these individuals during year 2 or year 3.
Table 6.
Year 3 Indirect Effectiveness of IIV3 in Preventing Laboratory-Confirmed Influenza Illness Among Unvaccinated Residents, by Type/Subtype and Age Group
| IIV3 Villages | IPV Villages | ||||||
|---|---|---|---|---|---|---|---|
| Cases,a n | nb | Cumulative Incidencec | Cases,a n |
nb | Cumulative Incidencec | Adjusted VEI,d % (95% CI) | |
| Any influenza | 110 | 12 503e | 0.88 | 125 | 11 861e | 1.05 | 20.1 (−23.9–48.5) |
| <6 months | 20 | 854 | 2.34 | 29 | 859 | 3.38 | 24.6 (−26.0–54.9) |
| 11–17 years | 23 | 2788 | 0.82 | 21 | 2639 | 0.80 | −0.1 (−113.7–53.1) |
| 18–49 years | 50 | 6761 | 0.74 | 53 | 6394 | 0.83 | 11.8 (−26.0–38.2) |
| 50–64 years | 15 | 1318 | 1.14 | 18 | 1221 | 1.47 | 30.0 (−150.3–80.4) |
| > 64 years | 2 | 780 | 0.26 | 4 | 747 | 0.54 | … |
| A/H3N2 | 53 | 12 503e | 0.42 | 63 | 11 861e | 0.53 | 20.7 (−64.2–61.8) |
| <6 months | 14 | 854 | 1.64 | 24 | 859 | 2.79 | 40.9 (−38.0–74.7) |
| 11–17 years | 11 | 2788 | 0.39 | 10 | 2639 | 0.38 | 4.9 (−213.1–71.1) |
| 18–49 years | 20 | 6761 | 0.30 | 18 | 6394 | 0.28 | −4.9 (−89.4–41.9) |
| 50–64 years | 6 | 1318 | 0.46 | 4 | 1221 | 0.33 | −18.7 (−587.7–79.5) |
| > 64 years | 2 | 780 | 0.26 | 7 | 747 | 0.94 | … |
| A/H1N1 | 4 | 12 503e | 0.03 | 5 | 11 861e | 0.04 | 46.1 (−2338.0–98.8) |
| <6 months | 0 | 854 | 0.00 | 1 | 859 | 0.12 | … |
| 11–17 years | 2 | 2788 | 0.07 | 1 | 2639 | 0.04 | … |
| 18–49 years | 2 | 6761 | 0.03 | 3 | 6394 | 0.05 | 36.8 (−3984.8–99.0) |
| 50–64 years | 0 | 1318 | 0.00 | 0 | 1221 | 0.00 | … |
| > 64 years | 0 | 780 | 0.00 | 0 | 747 | 0.00 | … |
| B | 74 | 12 503e | 0.59 | 84 | 11 861e | 0.71 | 19.1 (−25.8–48.0) |
| <6 months | 17 | 854 | 1.99 | 18 | 859 | 2.10 | 13.9 (−111.5–65.0) |
| 11–17 years | 15 | 2788 | 0.54 | 13 | 2639 | 0.49 | −6.8 (−109.8–45.6) |
| 18–49 years | 29 | 6761 | 0.43 | 33 | 6394 | 0.52 | 20.1 (−27.5–50.0) |
| 50–64 years | 9 | 1318 | 0.68 | 14 | 1221 | 1.15 | 45.6 (−36.8–78.4) |
| > 64 years | 4 | 780 | 0.51 | 6 | 747 | 0.80 | … |
Abbreviations: CI, confidence interval; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; VEI, indirect vaccine effectiveness among age-ineligible.
aOnly the first episode is counted where more than 1 instance of an endpoint is recorded during the same study year.
bNumber of children followed.
cPer 100 persons through the entire surveillance period: 2010–2011, 19 July 2010 through 22 April 2011; 2011–2012, 6 June 2011 through 16 December 2011.
dEstimated using a logistic regression model fit using generalized estimating equations, assuming an exchangeable correlation matrix to account for within-village correlation of participant observations.
eIncludes individuals whose age was not reported: year 2 (IIV3, 10: IPV, 8) and year 3 (IIV3, 2: IPV, 1). No laboratory-confirmed influenza illness was detected among these individuals during year 2 or year 3.
Population Vaccine Effectiveness
The year 2 population effectiveness against LCI was 36.0% (95% CI, 10.2–54.4) for any influenza (IIV3, 226 cases; IPV, 344 cases), 54.7% (95% CI, 22.0–73.6) for A/H3N2 (IIV3, 46 cases; IPV, 91 cases), 32.3% (95% CI, −22.3–62.6) for A/H1N1pdm (IIV3, 40 cases; IPV, 57 cases), and 29.3% (95% CI, −9.2–54.3) for B (IIV3, 140 cases; IPV, 205 cases). The year 3 population effectiveness against LCI was −2.5% (95% CI, −53.9–31.7) for any influenza (IIV3, 388 cases; IPV, 393 cases), −25.4% (95% CI, −117.0–27.6) for A/H3N2 (IIV3, 156 cases; IPV, 127 cases), −19.5% (95% CI, −691.3–82.0) for A/H1N1pdm (13 cases in each arm), and 9.4% (95% CI, −47.5–44.3) for B (IIV3, 227 cases; IPV, 258 cases).
Safety
For years 2 and 3 combined, 3 SAEs were identified among vaccinees within 1 month of the final dose. One occurred among IIV3 recipients and 2 among IPV recipients. All SAEs were deemed unrelated to the administered vaccine by the investigator.
DISCUSSION
Here, we report results of CRCTs of IIV3 versus IPV from years 2 and 3 of a multiyear project designed to evaluate the impact of a pediatric influenza vaccination program on population-level disease incidence in rural Africa. In our Senegalese population, A/H1N1pdm, A/H3N2, and B influenza strains circulated from 2010 through 2011 [5–7]. Among children aged 6 months to 10 years in the IPV control villages, LCI was common, with a cumulative incidence of first infection of 4.65% and 4.17% during study years 2 and 3, respectively.
In year 2, we estimated 52.8% total effectiveness of IIV3 against LCI among age-eligible vaccinated children. The CDC antigenic characterization of a subset of influenza specimens identified that all isolates tested were antigenically similar to IIV3 strains. Total effectiveness estimates were similar for each of the IIV3 components: 61.4% for A/H1N1pdm, 57.5% for A/H3N2, and 49.7% for B. Our estimates of total effectiveness in Senegal are similar to direct effectiveness reported by other studies conducted in the 2010–2011 Northern Hemisphere season [8–13]. In the United States, for example, IIV3 direct effectiveness in children aged 6 months through 8 years was estimated to be 60% for A/H1N1, 66% for A/H3N2, and 62% for B [14].
In year 3, we found no significant total effectiveness of IIV3 against LCI. That year, 74.0% of all antigenically characterized specimens from IPV villages were from the B/Yamagata lineage, which was antigenically distinct from the B/Victoria vaccine strain. The 2011 Southern Hemisphere and the 2011–2012 Northern Hemisphere IIV3 formulations were the same, and reports for the 2011–2012 season from elsewhere reported poor effectiveness against B/Yamagata strains [8, 11, 15–25]. In Senegal, the predominance of circulating B/Yamagata viruses could partially explain the overall poor effectiveness [21]. However, CDC antigenic characterization of a subset of A/H3N2 isolates in Senegal found them all to be similar to the study vaccine strain, A/Perth/16/2009 (H3N2)-like. Thus, strain mismatch does not appear to be the cause for the lack of effectiveness for A/H3N2 in year 3 of this study. In the United States during 2011–2012, effectiveness against this strain was 39% despite an antigenic match between vaccine and circulating strains, although some genetic variation among circulating A/H3N2 isolates was observed [26–28]. Possible reasons for recent poor effectiveness against A/H3N2 strains seen here and in other trials include antigenic drift, deleterious effects due to prior vaccination, egg adaptation, and/or decreased immunogenicity [26, 29–31]. While immune responses to influenza A strains were robust in the Senegalese children during the first year of our study [32], we did not determine vaccine immunogenicity in years 2 and 3.
In year 2, when IIV3 was well matched with circulating strains, it provided 56% indirect protection against A/H3N2 LCI among persons who were not age-eligible to receive study vaccine. Evidence for indirect effectiveness in our study was strongest among adults aged 18–49 years, suggesting that this is a group highly exposed to vaccinated children. The lack of indirect effects for A/H1N1 or B in year 2 or for any vaccine strain in year 3 suggests that vaccination of larger proportions of the population and/or a more effective vaccine may be needed to reduce spread of infection. In rural Senegal, large, extended families live in close quarters in densely grouped compounds and likely experience high contact rates [33].While indirect effects must be interpreted in the context of the living conditions and social patterns that affect transmission, we do not believe that these patterns changed appreciably between study years. Our estimate of total effectiveness is consistent with reports from trials in non-African settings [34–38]. An IIV3 CRCT conducted in India during the same time period reported a statistically significant household-level indirect effectiveness of 38% against LCI in 2011–2012 [37].
Several study limitations should be noted. The small proportion of RT-PCR–positive specimens that were antigenically characterized limited our ability to make definitive conclusions about antigenic match between vaccine strains and circulating viruses. We had a lower cumulative incidence of LCI in years 2 and 3 compared with that observed in year 1 (15% among age-eligible children in the control group). During the periods of active surveillance, the intensive nature of the CRCT procedures suggests that the cumulative incidence seen in both years reflected the true nature of local influenza transmission and not bias due to decreased case capture.
Annual pediatric immunization campaigns with IIV3 reduced the risk of LCI among vaccinated children in 2 of the 3 study years. As in high-resource settings, influenza vaccine effectiveness was highly variable from year to year. Further, the study was the first to demonstrate the indirect effects of an influenza vaccination program in an African setting. Such an approach leverages existing childhood vaccination infrastructures to protect at-risk adult groups without immunization platforms [39]. Our year 3 experience with an influenza B lineage mismatch between vaccine and circulating viruses supports the transition from trivalent to quadrivalent influenza vaccines containing both B lineages [40]. Influenza immunization in developing countries would be more feasible with improved influenza vaccines that provide broader and more durable protection.
Notes
Author contributions. J. C. V. and K. M. N. conceived the study. A. D., J. C. V., K. M. N., J. R. O., J. D. S., M. E. H., K. E. L., and M.-A. W. designed the trial. A. D., O. M. D., M. N. N., J. C. V., J. R. O., J. D. S., K. E. L., and M.-A. W. developed study methods and data collection instruments. J. D. S. and M. E. H. designed the randomization, and J. D. S. and J. C. V. performed the randomization. A. D., and B. D. collected the data and biological specimens. M. N. N. performed RT-PCR assays. D. J. R. O. and K. M. N. served as medical monitors for PATH. K. D. C. L. designed and coordinated data management. J. D. S., M. E. H., and J. C. V. designed the statistical analyses. J. D. S. performed the statistical analyses, and M. E. H. and J. C. V. verified their accuracy. A. D. served as the study principal investigator in Senegal and led the team at Institut de Recherche Pour le Développement, which administers the Niakhar DSS. O. M. D. led the team at Institut Pasteur de Dakar, which houses Senegal’s National Influenza Center. J. C. V. served as the primary investigator for the Cooperative Agreement between PATH and the US Centers for Disease Control and Prevention, and M. A. W. served as its program officer. J. C. V., K. M. N., J. R. O., and J. D. S. drafted the manuscript. A. D., O. M. D., M. N. N., J. D. S., J. R. O., K. D. C. L., K. E. L., M. E. H., M. A. W., K. M. N., and J. C. V. critically revised the manuscript. All authors had full access to study data, opportunity to review drafts, and approved the final version submitted for publication.
Acknowledgments. This study was a collaboration of PATH (Seattle, WA, USA, and Dakar, Senegal), Institut de Recherche Pour le Développement (IRD; Dakar, Senegal), the Institut Pasteur de Dakar (IPD; Dakar, Senegal), and the US Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA). Supporting PTH in fulfilling its sponsor obligations, the Agence Africaine De Recherche en Sante Humaine conducted site monitoring and the Fred Hutchinson Cancer Research Center conducted statistical analyses for vaccine effectiveness. The authors are sincerely thankful to all the families who participated in this trial and to the full research teams at IRD and IPD in Senegal. They are most grateful to Sanofi Pasteur for donating the study vaccines. The authors thank Dr Kathryn Edwards for serving as the study Independent Safety Monitor and Dr Xiyan Xu of the CDC for antigenic characterization of submitted influenza-positive specimens.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of any collaborating institution or the study funders.
Financial support. This work was funded through US Centers for Disease Control and Prevention Cooperative Agreement U01IP000174 with PATH. Sanofi Pasteur donated study vaccines but had no other role in the study. Co-authors at the Fred Hutchinson Cancer Research Center were partially funded by National Institutes of Health grant R37 AI032042.
Potential conflicts of interest. K. M. N. receives salary support as the principal investigator on a National Institutes of Health (NIH)–funded contract (Collaborative Influenza Vaccine Innovation Center) and as a co-principal investigator on the NIH-funded Vaccine Treatment and Evaluation Unit. J. R. O. reports travel fees and honoraria from Pfizer and Seqirus and travel fees from Sanofi, outside the submitted work. M. E. H. and J. D. S. report grants from the National Institute of Allergy and Infectious Diseases during the conduct of the study. J. D. S. is currently employed by the US Department of Veterans Affairs. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Diallo A, Diop OM, Diop D, et al. Effectiveness of seasonal influenza vaccination in children in Senegal during a year of vaccine mismatch: a cluster-randomized trial. Clin Infect Dis 2019; 69:1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delaunay V, Douillot L, Diallo A, et al. Profile: the Niakhar Health and Demographic Surveillance System. Int J Epidemiol 2013; 42:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCullagh P. Generalized linear models. New York: Routledge, 2018. [Google Scholar]
- 4. R Core Team. R: a language and environment for statistical computing. Available at: https://www.R-project.org/. Accessed 28 March 2018.
- 5. Gasparini R, Bonanni P, Amicizia D, et al. Influenza epidemiology in Italy two years after the 2009–2010 pandemic: need to improve vaccination coverage. Hum Vaccines Immunother 2013; 9:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emukule GO, Khagayi S, McMorrow ML, et al. The burden of influenza and RSV among inpatients and outpatients in rural Western Kenya, 2009–2012. PLoS One 2014; 9:e105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiménez-Jorge S, Savulescu C, Pozo F, et al. cycEVA Study Team; Spanish Influenza Sentinel Surveillance System . Effectiveness of the 2010-11 seasonal trivalent influenza vaccine in Spain: cycEVA study. Vaccine 2012; 30:3595–602. [DOI] [PubMed] [Google Scholar]
- 9. Martinez-Baz I, Martinez-Artola V, Reina G, et al. Effectiveness of the trivalent influenza vaccine in Navarre, Spain, 2010–2011: a population-based test-negative case-control study. BMC Public Health 2013; 13:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pebody RG, Andrews N, Fleming DM, et al. Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom. Epidemiol Infect 2013; 141:620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramsay LC, Buchan SA, Stirling RG, et al. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med 2019; 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skowronski DM, Chambers C, De Serres G, et al. Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J Infect Dis 2017; 215:1059–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Syrjanen RK, Jokinen J, Ziegler T, et al. Effectiveness of pandemic and seasonal influenza vaccines in preventing laboratory-confirmed influenza in adults: a clinical cohort study during epidemic seasons 2009–2010 and 2010–2011 in Finland. PLoS One 2014; 9:e108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Treanor JJ, Talbot HK, Ohmit SE, et al. US Flu-VE Network . Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi WS, Noh JY, Baek JH, et al. Suboptimal effectiveness of the 2011–2012 seasonal influenza vaccine in adult Korean populations. PLoS One 2015; 10:e0098716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu C, He Q, Li Z, et al. Seasonal influenza vaccine effectiveness among children, 2010–2012. Influenza Other Respir Viruses 2013; 7:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson ML, Jackson LA, Kieke B, et al. Incidence of medically attended influenza infection and cases averted by vaccination, 2011/2012 and 2012/2013 influenza seasons. Vaccine 2015; 33:5181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacIntosh VH, Tastad KJ, Eick-Cost AA. Mid-season influenza vaccine effectiveness 2011–2012: a Department of Defense Global, Laboratory-based, Influenza Surveillance System case-control study estimate. Vaccine 2013; 31:1651–5. [DOI] [PubMed] [Google Scholar]
- 19. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puig-Barbera J, Garcia-de-Lomas J, Diez-Domingo J, et al. Influenza vaccine effectiveness in preventing influenza A(H3N2)-related hospitalizations in adults targeted for vaccination by type of vaccine: a hospital-based test-negative study, 2011–2012 A(H3N2) predominant influenza season, Valencia, Spain. PLoS One 2014; 9:e112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 22. Suzuki M, Minh LN, Yoshimine H, et al. Vaccine effectiveness against medically attended laboratory-confirmed influenza in Japan, 2011–2012 season. PLoS One 2014; 9:e88813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson MG, Clippard J, Petrie JG, et al. Influenza vaccine effectiveness for fully and partially vaccinated children 6 months to 8 years old during 2011–2012 and 2012–2013: the importance of two priming doses. Pediatr Infect Dis 2016; 35:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torner N, Martínez A, Basile L, et al. Influenza vaccine effectiveness assessment through sentinel virological data in three post-pandemic seasons. Hum Vaccin Immunother 2015; 11:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2013; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010–2011 season. Clin Infect Dis 2012; 55:332–42. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2011–2012 Northern Hemisphere influenza season. Releve Epidemiologique Hebdomadaire 2011; 86:86–90. [PubMed] [Google Scholar]
- 29. Skowronski DM, De Serres G. Role of egg-adaptation mutations in low influenza A(H3N2) vaccine effectiveness during the 2012–2013 season. Clin Infect Dis 2018; 67:1474–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cobey S, Gouma S, Parkhouse K, et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012–2013. Clin Infect Dis 2018; 67:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker L, Wharton SA, Martin SR, et al. Effects of egg-adaptation on receptor-binding and antigenic properties of recent influenza A (H3N2) vaccine viruses. J Gen Virol 2016; 97:1333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niang M, Deming ME, Goudiaby D, et al. Immunogenicity of seasonal inactivated influenza and inactivated polio vaccines among children in Senegal: results from a cluster-randomized trial. Vaccine 2020; 38:7526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Potter GE, Wong J, Sugimoto J, et al. Networks of face-to-face social contacts in Niakhar, Senegal. PLoS One 2019; 14:e0220443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16:1–14. [DOI] [PubMed] [Google Scholar]
- 35. Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010; 303:943–50. [DOI] [PubMed] [Google Scholar]
- 36. Mertz D, Fadel SA, Lam PP, et al. Herd effect from influenza vaccination in non-healthcare settings: a systematic review of randomised controlled trials and observational studies. Euro Surveill 2016; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sullender WM, Fowler KB, Gupta V, et al. Efficacy of inactivated trivalent influenza vaccine in rural India: a 3-year cluster-randomised controlled trial. Lancet Glob Health 2019; 7:e940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin JK, Heywood AE, Georgousakis M, et al. Systematic review and meta-analysis of indirect protection afforded by vaccinating children against seasonal influenza: implications for policy. Clin Infect Dis 2017; 65:719–28. [DOI] [PubMed] [Google Scholar]
- 39. Williams SR, LeBuhn HM, Driscol AJ, Neuzil KM, Chen WH, Ortiz JR. Which countries have adult vaccine programs? A global review of national adult influenza and pneumococcal vaccine policies. In: Annual Meeting of the American Thoracic Society. Philadelphia, PA: American Thoracic Society, 2020. [Google Scholar]
- 40. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]