Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) affects more than one-third of people living with human immunodeficiency virus (HIV). Nonetheless, its natural history is poorly understood, including which patients are most likely to have a progressive disease course.
Methods
We leveraged a randomized trial of the growth hormone–releasing hormone analogue tesamorelin to treat NAFLD in HIV. Sixty-one participants with HIV-associated NAFLD were randomized to tesamorelin or placebo for 12 months with serial biopsies.
Results
In all participants with baseline biopsies (n = 58), 43% had hepatic fibrosis. Individuals with fibrosis had higher NAFLD Activity Score (NAS) (mean ± standard deviation [SD], 3.6 ± 2.0 vs 2.0 ± 0.8; P < .0001) and visceral fat content (mean ± SD, 284 ± 91 cm2 vs 212 ± 95 cm2; P = .005), but no difference in hepatic fat or body mass index. Among placebo-treated participants with paired biopsies (n = 24), 38% had hepatic fibrosis progression over 12 months. For each 25 cm2 higher visceral fat at baseline, odds of fibrosis progression increased by 37% (odds ratio, 1.37 [95% confidence interval, 1.03–2.07]). There was no difference in baseline NAS between fibrosis progressors and nonprogressors, though NAS rose over time in the progressor group (mean ± SD, 1.1 ± 0.8 vs −0.5 ± 0.6; P < .0001).
Conclusions
In this longitudinal study of HIV-associated NAFLD, high rates of hepatic fibrosis and progression were observed. Visceral adiposity was identified as a novel predictor of worsening fibrosis. In contrast, baseline histologic characteristics did not relate to fibrosis progression.
Keywords: HIV, NAFLD, fibrosis, liver biopsy
In this longitudinal study of HIV-associated nonalcoholic fatty liver disease, we observed high rates of hepatic fibrosis presence and progression and identified visceral adiposity as a novel predictor of fibrosis progression.
In an era of rising rates of obesity and hepatitis C virus (HCV) cure, nonalcoholic fatty liver disease (NAFLD) has become a leading cause of liver disease among people living with human immunodeficiency virus (PLWH) [1]. More than one-third of individuals are affected with risk factors that include elevated body mass index (BMI), metabolic comorbidities, and high CD4+ T-cell count [1]. The spectrum of NAFLD is broad, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) to fibrosis. Importantly, among patients with NAFLD in the general population, the severity of fibrosis is the strongest predictor of all-cause and liver-specific mortality [2, 3]. Thus, understanding the clinical predictors of fibrosis presence and progression in human immunodeficiency virus (HIV) is imperative so that individuals with the most aggressive hepatic disease can be appropriately monitored and targeted for intervention.
Individuals with HIV/HCV coinfection have been shown to have faster progression of fibrosis as well as a higher frequency of hepatic decompensation compared to HCV-monoinfected patients [4, 5]. While these findings raise concern that the course of NAFLD in HIV may also be accelerated, its natural history among this patient population has not been previously well-defined. In this regard, studies focused on hepatic fibrosis in the setting of HIV monoinfection have most often examined a heterogeneous sample not specifically selected for NAFLD [6–9]. Furthermore, these analyses have only rarely included long-term follow-up or utilized liver biopsies that would allow for comprehensive assessment of other histologic changes [10]. While these previous studies have consistently demonstrated high rates of hepatic fibrosis in association with metabolic risk factors [6–10], the subset of patients with HIV-associated NAFLD at highest risk for disease-related morbidity remains elusive.
In the current analysis, we leveraged phenotypic data including liver biopsy samples from a recent randomized placebo-controlled trial to characterize the longitudinal course of NAFLD in HIV. This previous study demonstrated that a strategy to reduce visceral fat prevented fibrosis progression among individuals with HIV-associated NAFLD [11]. In this current analysis, we investigated for the first time the relationship of visceral fat and other clinical characteristics with liver fibrosis, particularly with respect to the natural history of fibrosis progression among placebo-treated participants undergoing serial liver biopsies. Given the high frequency of NAFLD in HIV, accurately predicting which patients will have the most severe course of disease is critically needed to optimize screening, prevention, and treatment strategies for this population.
METHODS
Study Design
We previously conducted a randomized, double-blind trial in which participants were assigned to receive the growth hormone–releasing hormone (GHRH) analogue tesamorelin 2 mg daily or identical placebo for 12 months [11]. This trial demonstrated that tesamorelin significantly reduced liver fat (primary endpoint) and prevented fibrosis progression (secondary endpoint) among PLWH [11]. In the current study, we examined the natural history of NAFLD using this study population. We assessed clinical correlates of fibrosis in the overall study sample by leveraging baseline biopsy specimens before treatment. We also identified clinical predictors of fibrosis progression by utilizing serial biopsy specimens among the subset of participants assigned to placebo. The data presented here have not been reported elsewhere.
We enrolled 61 men and women 18–70 years old who had documented HIV infection as well as hepatic steatosis defined by liver fat fraction ≥ 5% on proton magnetic resonance spectroscopy (1H-MRS). Recruitment through clinics, community health centers, and general local advertisements targeted individuals with HIV infection and known NAFLD. Enrollment criteria did not include BMI, waist circumference, or visceral adiposity cutoffs, but advertisements did seek individuals with HIV infection who had a relatively high likelihood of NAFLD, including but not limited to those with history of increased abdominal adiposity. As per the study protocol, eligibility was determined based on a well-standardized standardized definition of hepatic steatosis without regard to body composition or anthropometric measures. Participants were required to have been on stable antiretroviral therapy (ART) for ≥ 3 months with CD4+ T-cell count > 100 cells/μL and HIV RNA load < 400 copies/mL. Individuals with diabetes mellitus whose hemoglobin A1c was ≤ 7% and who did not use insulin or thiazolidinediones were eligible for inclusion. Exclusion criteria included heavy alcohol use (> 20 g daily for women or > 30 g daily for men), active hepatitis B or C as previously described [11], known cirrhosis, or stage 4 fibrosis on biopsy. Individuals with known hepatic disease besides NAFLD (eg, hemochromatosis) or exposure to certain medications associated with hepatic steatosis (eg, chronic corticosteroids) were also excluded. Participants were enrolled at the Massachusetts General Hospital (Boston, Massachusetts) and the National Institutes of Health (NIH, Bethesda, Maryland) between 20 August 2015 and 16 January 2019. The study was approved by the institutional review boards at both institutions.
Study Procedures
All study visits were conducted in a fasting state. At study screen, participants underwent detailed history, physical examination, and laboratory investigations for eligibility. Hepatic 1H-MRS was performed fasting for measurement of liver fat content with calculation of hepatic fat fraction as the area under the spectroscopic lipid peak divided by the total area of the spectroscopic lipid and water peaks combined. Abdominal magnetic resonance imaging (MRI) was performed for assessment of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) cross-sectional area at the L4 vertebral level. Cross-sectional VAT and SAT area at L4 were calculated using a semiautomated pixel thresholding to separate each compartment. Individuals who met our eligibility criteria at study screen subsequently completed a baseline assessment. Baseline evaluation included anthropometrics, liver enzymes, lipids, and inflammatory markers. Ultrasound-guided percutaneous liver biopsy was also performed on all participants, except for those with a contraindication (eg, anticoagulation). Baseline measures, including MRI/1H-MRS and liver biopsy, were repeated at 12 months following randomization. MRI/1H-MRS was also obtained at the 6-month time point. At baseline and 12 months, Fibrosis-4 Index for Liver Fibrosis (FIB-4) was calculated as a noninvasive estimate of liver fibrosis as has been previously described [12].
All liver biopsy samples were reviewed by a single expert pathologist (D. E. K., NIH) blinded to treatment. Histological scoring was performed using the Nonalcoholic Steatohepatitis Clinical Research Network scoring system [13]. The sum of grades for steatosis (grades 0–3), lobular inflammation (grades 0–3), and hepatocellular ballooning (grades 0–2) comprised the NAFLD Activity Score (NAS), and fibrosis was independently staged between 0 and 4. Fibrosis was considered to have been present if fibrosis stage was ≥ 1. Fibrosis was defined to have progressed if there was an increase in fibrosis stage between baseline and 12 months. Study procedures have been further described elsewhere [11].
Statistical Analysis
In this study, we performed 3 complementary analyses to identify clinical correlates and predictors of hepatic fibrosis presence and progression among individuals with HIV-associated NAFLD. In this regard, among all participants, baseline clinical characteristics were compared between individuals with vs without baseline hepatic fibrosis. Furthermore, in the subset of subjects randomized to placebo, we assessed for differences in baseline clinical characteristics between fibrosis progressors and nonprogressors. Last, among placebo-treated participants, we examined associations between changes in clinical characteristics and fibrosis progression over 12 months.
We compared differences between groups using a 2-tailed independent samples t test for continuous variables and χ 2 test for categorical variables. Multivariable logistic regression modeling also was performed to assess associations of key baseline variables with fibrosis progression. Odds ratios (ORs) are reported with 95% confidence intervals (CIs) and P values were determined by likelihood ratio testing. All available data were analyzed. Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were indicated as frequency (%). A critical value of P < .05 was the predefined threshold for statistical significance. Statistical analyses were performed using JMP Pro 12 (SAS Institute, Cary, North Carolina).
RESULTS
Participant Characteristics
A total of 58 participants with HIV-associated NAFLD had liver biopsy specimens available at baseline. Clinical characteristics of the overall sample are summarized in Supplementary Table 1. Study subjects (mean age, 53 ± 7 years; 81% male) had long-standing HIV infection (mean, 16 ± 9 years) that was well controlled. All participants were on stable ART with 62% receiving an integrase inhibitor–based regimen. Mean baseline liver fat content was 13.8% ± 8.6%, whereas mean NAS was 2.7 ± 1.6. A total of 59% of participants were obese. BMI was strongly correlated with SAT (r = 0.86; P < .0001) and more weakly associated with VAT (r = 0.29; P = .02) (Supplementary Figure 1).
A total of 24 participants with HIV-associated NAFLD randomized to placebo had paired liver biopsy specimens available at baseline and 12 months. Clinical characteristics in this subset were comparable to the overall study group (Supplementary Table 1).
Clinical Correlates of Baseline Hepatic Fibrosis (Overall Sample)
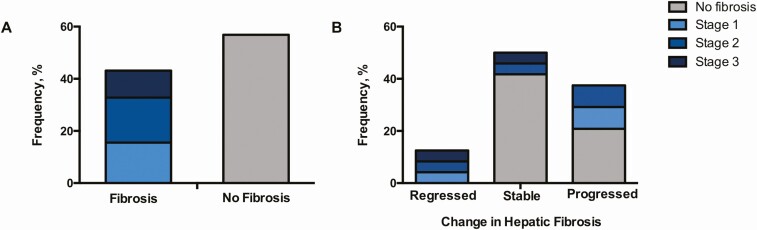
Among our overall sample with HIV-associated NAFLD, 43% had evidence of hepatic fibrosis at baseline with the following distribution by stage: stage 1, 36%; stage 2, 40%; stage 3, 24% (Figure 1A). Clinical correlates of baseline hepatic fibrosis are shown in Table 1. Individuals with and without fibrosis were of comparable age and sex. While fibrosis tended to be more common among white subjects, racial differences between groups were not statistically significant. In contrast, there was no association of fibrosis with CD4+ T-cell count, HIV RNA load, C-reactive protein (CRP), or interleukin 6.
Figure 1.
Frequency of liver fibrosis presence and progression in human immunodeficiency virus–associated nonalcoholic fatty liver disease. A, Frequency of baseline fibrosis, subdivided by stage, is shown among the overall study sample. Of note, participants with stage 4 fibrosis or clinical cirrhosis were excluded from study participation. B, Frequency of fibrosis progression and regression over 12 months is depicted in the subset of placebo-treated participants. Baseline fibrosis stage within each category is also indicated.
Table 1.
Clinical Correlates of Baseline Liver Fibrosis in Human Immunodeficiency Virus–Associated Nonalcoholic Fatty Liver Disease
| Characteristic | Fibrosis (n = 25) | No Fibrosis (n = 33) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y | 53 ± 8 | 52 ± 7 | .60 |
| Male sex, % | 88 | 76 | .24 |
| Race/ethnicity, % | .29 | ||
| Black | 20 | 36 | |
| White | 80 | 55 | |
| Hispanic | 20 | 12 | .41 |
| Smoking status, % | |||
| Current smoker | 16 | 18 | .83 |
| Ever smoker | 64 | 55 | .47 |
| HIV-related indices | |||
| Duration of HIV, y | 18 ± 9 | 15 ± 8 | .10 |
| CD4 count, cells/μL | 710 ± 285 | 802 ± 272 | .22 |
| Log HIV RNA load | 0.40 ± 0.66 | 0.39 ± 0.66 | .95 |
| NRTI use, % | 92 | 94 | .77 |
| NNRTI use, % | 56 | 27 | .03 |
| PI use, % | 20 | 27 | .52 |
| Integrase inhibitor use, % | 56 | 67 | .41 |
| Immunologic indices | |||
| CRP, mg/L | 8.0 ± 10.4 | 4.8 ± 4.7 | .11 |
| IL-6, pg/mL | 1.7 ± 1.8 | 1.4 ± 1.4 | .42 |
| Hepatic indices | |||
| Liver fat, % | 15.4 ± 10.3 | 12.6 ± 6.9 | .22 |
| NASH, % | 72 | 3 | < .0001 |
| NAS | 3.6 ± 2.0 | 2.0 ± 0.8 | < .0001 |
| Lobular inflammation | 1.5 ± 0.8 | 0.9 ± 0.2 | .0004 |
| Hepatocellular ballooning | 0.7 ± 0.8 | 0.0 ± 0.2 | < .0001 |
| Liver biopsy length, mm | 13 ± 3 | 13 ± 3 | .81 |
| ALT, U/L | 41 ± 30 | 23 ± 8 | .002 |
| AST, U/L | 44 ± 27 | 23 ± 10 | .0003 |
| FIB-4 | 1.88 ± 0.98 | 1.12 ± 0.44 | .0003 |
| Metabolic indices | |||
| BMI, kg/m2 | 31 ± 7 | 31 ± 5 | .96 |
| Waist circumference, cm | 111 ± 17 | 109 ± 10 | .48 |
| Visceral fat, cm2 | 284 ± 91 | 212 ± 95 | .005 |
| Subcutaneous fat, cm2 | 270 ± 154 | 321 ± 138 | .20 |
| Fasting glucose, mg/dL | 98 ± 22 | 94 ± 12 | .41 |
| HbA1c, % | 5.7 ± 0.6 | 5.8 ± 0.5 | .81 |
| Triglycerides, mg/dL | 151 ± 83 | 136 ± 57 | .43 |
Variables are expressed as mean ± standard deviation unless otherwise indicated. Bold text denotes statistical significance with P < .05.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; FIB-4, Fibrosis-4 Index for Liver Fibrosis; HbA1c, hemoglobin A1c; HIV, human immunodeficiency virus; IL-6, interleukin-6; NAS, Nonalcoholic Fatty Liver Disease Activity Score; NASH, nonalcoholic steatohepatitis; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
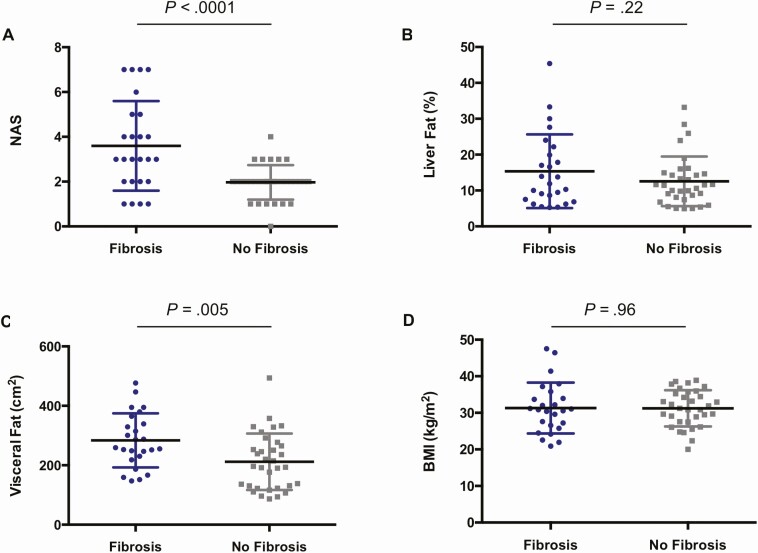
With regard to hepatic and metabolic indices, individuals with hepatic fibrosis had higher mean NAS (3.6 ± 2.0 vs 2.0 ± 0.8; P < .0001) with elevations in both lobular inflammation (1.5 ± 0.8 vs 0.9 ± 0.2; P = .0004) and hepatocellular ballooning (0.7 ± 0.8 vs 0.0 ± 0.2; P < .0001). In contrast, liver fat content was not found to differ between groups. Fibrosis also was positively associated with the noninvasive parameters alanine aminotransferase (mean, 41 ± 30 U/L vs 23 ± 8 U/L; P = .002), aspartate aminotransferase (mean, 44 ± 27 U/L vs 23 ± 10 U/L; P = .0003), and FIB-4 (mean, 1.88 ± 0.98 vs 1.12 ± 0.44; P = .0003). Notably, while VAT was higher among individuals with fibrosis (mean, 284 ± 91 cm2 vs 212 ± 95 cm2; P = .005), there were no differences between groups in BMI, waist circumference, or SAT (Figure 2).
Figure 2.
Relationship of key baseline characteristics to presence of liver fibrosis in human immunodeficiency virus–associated nonalcoholic fatty liver disease (NAFLD). A and B, In the overall sample, baseline NAFLD Activity Score (NAS) was higher in individuals with vs without baseline hepatic fibrosis (3.6 ± 2.0 vs 2.0 ± 0.8; P < .0001). Meanwhile, no difference was found between groups in baseline liver fat content. C and D, Baseline visceral fat content was higher in individuals with vs without baseline hepatic fibrosis (284 ± 91 cm2 vs 212 ± 95 cm2; P = .005), whereas there was no difference in baseline body mass index (BMI) between groups. Error bars denote mean ± standard deviation.
Clinical Predictors of Hepatic Fibrosis Progression (Placebo Group)
Over 12 months, fibrosis progressed in 38% (n = 9) of placebo-treated participants with HIV-associated NAFLD. Meanwhile, 50% (n = 12) of subjects had no change in fibrosis, whereas 13% (n = 3) experienced fibrosis regression (Figure 1B). Among all placebo-treated participants, the mean rate of fibrosis progression was 0.2 ± 0.8 stages per year. A total of 56% (n = 5) of participants with fibrosis progression had no evidence of fibrosis at baseline.
Baseline VAT was higher among those with fibrosis progression than without progression (306 ± 119 cm2 vs 212 ± 89 cm2; P = .04). An analogous relationship was also observed in a subanalysis of those without any baseline fibrosis (308 ± 120 cm2 vs 184 ± 79 cm2; P = .03). In multivariable regression modeling, each 25 cm2 higher VAT at baseline was associated with a 37% increased odds of fibrosis progression upon adjusting for baseline NAS, liver fat content, and BMI (OR, 1.37 [95% CI, 1.03–2.07]; P = .03). In contrast, baseline NAS, hepatic fat, and BMI were themselves not found to be associated with fibrosis progression in our cohort (Table 2). Likewise, baseline hepatic fibrosis, SAT, and waist circumference also did not differ in progressors vs nonprogressors. Lastly, comparisons of demographic and HIV-related characteristics were not found to be significant between groups (Supplementary Table 2).
Table 2.
Clinical Predictors of Liver Fibrosis Progression in Human Immunodeficiency Virus–Associated Nonalcoholic Fatty Liver Disease
| Baseline Parameter | Univariable Analyses (n = 24) | Multivariable Model (n = 24) | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Liver fat, % | 1.03 (.94–1.13) | .57 | 0.99 (.84–1.18) | .91 |
| NAS | 0.90 (.50–1.50) | .70 | 0.67 (.22–1.47) | .34 |
| BMI, kg/m2 | 1.03 (.89–1.21) | .70 | 1.01 (.81–1.25) | .90 |
| VAT, cm2 | 1.26 (1.02–1.69) | .03 | 1.37 (1.03–2.07) | .03 |
Odds ratios correspond to a 1-unit change in the baseline parameter, except for VAT, which is calculated based on a 25 cm2 increment. Bold text denotes statistical significance with P < .05.
Abbreviations: BMI, body mass index; CI, confidence interval; NAS, Nonalcoholic Fatty Liver Disease Activity Score; OR, odds ratio; VAT, visceral adipose tissue.
Changes in Clinical Indices With Hepatic Fibrosis Progression (Placebo Group)
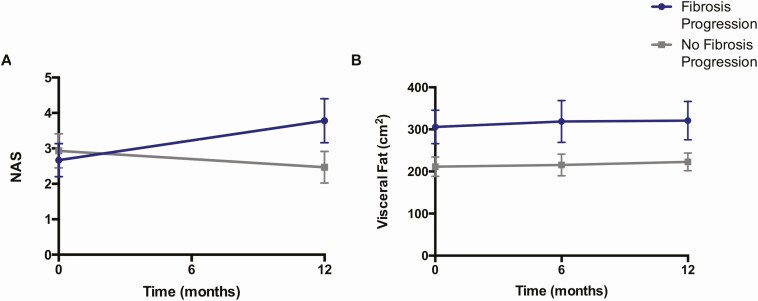
We next examined changes in clinical indices that accompanied fibrosis progression among our placebo-treated participants with HIV-associated NAFLD (Table 3). Though baseline NAS did not predict worsening of fibrosis, NAS did significantly increase among fibrosis progressors vs nonprogressors over the 12-month study period (mean, 1.1 ± 0.8 vs −0.5 ± 0.6; P < .0001) (Figure 3A). This change reflected a rise in both lobular inflammation (mean, 0.4 ± 0.5 vs −0.2 ± 0.4; P = .003) and hepatocellular ballooning (0.4 ± 0.5 vs −0.2 ± 0.6; P = .01). In contrast, the baseline difference in visceral fat between fibrosis progressors and nonprogressors remained constant over time (Figure 3B). CRP (mean, 4.0 ± 5.4 mg/L vs −0.2 ± 2.4 mg/L; P = .02) and hemoglobin A1c (mean, 0.3% ± 0.4% vs −0.1% ± 0.3%; P = .02) also were noted to increase in association with fibrosis progression. No other changes in clinical parameters were found to be significantly associated with worsening of hepatic fibrosis among our study cohort.
Table 3.
Change in Clinical Indices With Liver Fibrosis Progression in Human Immunodeficiency Virus–Associated Nonalcoholic Fatty Liver Disease
| Change in Parameter | Fibrosis Progression (n = 9) | No Fibrosis Progression (n = 15) | P Value |
|---|---|---|---|
| Change in HIV-related indices | |||
| CD4 count, cells/μL | −37 ± 124 | −37 ± 117 | > .99 |
| Log HIV RNA load | −0.04 ± 0.66 | −0.02 ± 0.87 | .95 |
| Change in immunologic indices | |||
| CRP, mg/L | 4.0 ± 5.4 | −0.2 ± 2.4 | .02 |
| IL-6, pg/mL | −0.1 ± 3.0 | −0.2 ± 1.3 | .91 |
| Change in hepatic indices | |||
| Liver fat, % | 0.8 ± 4.3 | −0.3 ± 4.3 | .54 |
| NAS | 1.1 ± 0.8 | −0.5 ± 0.6 | < .0001 |
| Lobular inflammation | 0.4 ± 0.5 | −0.2 ± 0.4 | .003 |
| Hepatocellular ballooning | 0.4 ± 0.5 | −0.2 ± 0.6 | .01 |
| ALT, U/L | 13 ± 19 | 1 ± 10 | .08 |
| AST, U/L | 6 ± 18 | −7 ± 20 | .13 |
| FIB-4 | 0.12 ± 0.40 | −0.20 ± 0.47 | .12 |
| Change in metabolic indices | |||
| BMI, kg/m2 | 0 ± 1 | 0 ± 1 | .81 |
| Waist circumference, cm | 1 ± 3 | 1 ± 3 | .83 |
| Visceral fat, cm2 | 15 ± 53 | 11 ± 33 | .83 |
| Subcutaneous fat, cm2 | 27 ± 55 | 12 ± 37 | .46 |
| Fasting glucose, mg/dL | 7 ± 13 | 4 ± 12 | .48 |
| HbA1c, % | 0.3 ± 0.4 | −0.1 ± 0.3 | .02 |
| Triglycerides, mg/dL | −11 ± 46 | −1 ± 53 | .64 |
Variables are expressed as mean ± standard deviation. Bold text denotes statistical significance with P < .05.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; FIB-4, Fibrosis-4 Index for Liver Fibrosis; HbA1c, hemoglobin A1c; HIV, human immunodeficiency virus; IL-6, interleukin 6; NAS, Nonalcoholic Fatty Liver Disease Activity Score.
Figure 3.
Visceral fat and Nonalcoholic Fatty Liver Disease (NAFLD) Activity Score (NAS) over time by fibrosis progression status in human immunodeficiency virus–associated NAFLD. A, Among placebo-treated participants, baseline NAS did not distinguish between hepatic fibrosis progressors and nonprogressors. However, over the study period, fibrosis progressors exhibited a rise in NAS, whereas nonprogressors showed a mild decline in this parameter. B, At baseline, visceral fat content differentiated hepatic fibrosis progressors vs nonprogressors. This difference in visceral fat between groups was maintained throughout the study period. Data points and error bars indicate mean ± standard error of the mean.
DISCUSSION
In this study, we demonstrated a high prevalence and progression rate of biopsy-proven liver fibrosis among a modern cohort of PLWH with well-defined NAFLD. Furthermore, we identified high visceral fat content as a novel clinical predictor of accelerated hepatic disease. Baseline histologic indices including NAS were not found to be associated with worsening fibrosis, though fibrosis progression was itself accompanied by a concurrent rise in NAS among our study sample. These findings may have important implications for the risk stratification of patients with HIV-associated NAFLD, and underscore the critical need for additional measures to prognosticate disease severity.
Among study participants with HIV-associated NAFLD, we found a striking 43% to have evidence of hepatic fibrosis on initial biopsy. In studies of NAFLD among the general population, liver fibrosis is the strongest clinical predictor of morbidity and mortality [2, 3]. Thus, a high prevalence of NAFLD [1], coupled with a high proportion of fibrosis in those with NAFLD, suggests that a large patient population of PLWH is susceptible to liver-related complications. Few studies have assessed the prevalence of hepatic fibrosis in PLWH exclusively selected for NAFLD. In 1 study of fatty liver conducted in China, the frequency of fibrosis diagnosed by transient elastography was 27% in PLWH vs 5% in matched controls [14]. The higher prevalence of fibrosis that we observed in the current analysis may relate to racial or methodologic differences between studies. An additional US-based report found the frequency of fibrosis in HIV-associated NAFLD to be 61% with a similar rate observed among the general population [15]. Notably, in this previous study, liver biopsies were obtained for clinical purposes, and thus participants may have had more advanced disease than in the current report. Our reliance on liver biopsy specimens that were obtained routinely per protocol provides a window into the natural history of HIV-associated NAFLD that has rarely been afforded by other studies.
Beyond a high prevalence of fibrosis, we also showed for the first time that individuals with HIV-associated NAFLD may experience rapid fibrosis progression. In this regard, over a 12-month period, 38% of placebo-treated participants experienced worsening of hepatic fibrosis, whereas only 13% experienced improvement. Notably, among the subset of participants with fibrosis progression, fewer than half had evidence of fibrosis at baseline; as such, absence of fibrosis at a single time point did not preclude an aggressive disease course. To our knowledge, prior studies have not evaluated changes in liver histology over time in individuals with well-defined HIV-associated NAFLD. In a systematic review of NASH in the general population, a comparable proportion of subjects were found to have progression of fibrosis on repeat biopsy, but the duration of follow-up was longer than in the current analysis [16]. When standardized by time, the mean rate of fibrosis progression in the general population with NASH was 0.03 stages/year, as compared to 0.2 stages/year in the current cohort with HIV-associated NAFLD [16].
In the current study, we demonstrated that individuals with the most profound visceral fat accumulation were at the highest risk of fibrosis presence and progression. In contrast, we did not find an association of hepatic fibrosis presence or progression with BMI, waist circumference, subcutaneous fat, or hepatic fat. Indeed, the association between baseline visceral fat and fibrosis progression persisted upon adjusting for BMI, hepatic fat, and NAS. Our findings of a relationship between visceral fat and fibrosis at baseline are consistent with cross-sectional studies in the general population [17, 18]. Meanwhile, our longitudinal analysis extends the data suggested by these previous reports, demonstrating that higher baseline visceral fat content predicts worsening of fibrosis over 1 year.
PLWH are prone to visceral adiposity due to a confluence of direct viral effects, ART toxicity, and lifestyle factors [19–21]. Importantly, pathologic gains in visceral fat have been observed even with the newest integrase inhibitor–based regimens, which were once regarded as metabolically neutral [22]. Previously, we have shown that visceral fat is higher in HIV than non-HIV among individuals with normal or overweight BMI [23]. Moreover, in the current cohort, VAT only loosely correlated with BMI, reinforcing the premise that BMI may not be an accurate gauge of visceral adiposity in this group. The dissociation of visceral fat from BMI in HIV parallels the discordant relationships of these parameters with hepatic fibrosis that we observed. Our findings suggest that detailed measures of body composition, but not BMI alone, may be useful to prognosticate disease course in patients with HIV-associated NAFLD.
In addition to its clinical utility, the emergence of visceral fat accumulation as a novel predictor of hepatic fibrosis progression may provide key insights into the pathogenesis of NAFLD in HIV. Visceral fat synthesizes and secretes cytokines that are directly delivered to the liver by the portal circulation. Accordingly, hepatic fibrosis may occur in response to this systemic proinflammatory state [17, 24]. Visceral fat may also be a key source of circulating profibrotic mediators, which in turn may directly drive end organ scarring [25]. In 2 studies of individuals without HIV infection undergoing weight loss surgery, greater macrophage infiltration in visceral but not subcutaneous fat was directly associated with a higher number of hepatic fibroinflammatory lesions and an increased stage of liver fibrosis [26, 27]. Further, in C57BL/6J mice on a high-fat diet, surgical removal of visceral epididymal white adipose tissue significantly reduced progression of steatohepatitis compared to sham surgery [28]. Taken together, this evidence suggests that therapies to reduce visceral fat may be effective to prevent progression of steatohepatitis and fibrosis in the context of HIV-associated NAFLD. Notably, in the randomized trial on which the current study is based, we found that the GHRH analogue tesamorelin, which is known to reduce visceral fat, also prevented hepatic fibrosis progression among participants with HIV-associated NAFLD [11].
Importantly, unlike visceral fat, we did not find baseline liver histology to be associated with disease course in this sample with HIV-associated NAFLD. Specifically, we showed that individuals with hepatic fibrosis at baseline had higher baseline NAS. Moreover, those with worsening of fibrosis had a greater rise in NAS over the course of the study period. However, baseline NAS was not found to distinguish hepatic fibrosis progressors from nonprogressors over the ensuing 12 months. This finding counters some reports in the general population, which have shown that the presence of NASH strongly predicts hepatic fibrosis progression [16, 29]. In addition, other indices of inflammation and markers of metabolic status including CRP and hemoglobin A1c were not useful in this study to predict fibrosis progression. Further studies are needed to assess the relative value of unique HIV parameters, such as excess visceral adiposity identified in this study, vs histologic indices to predict disease course in HIV-associated NAFLD. Our findings also highlight potential differences in NAFLD pathophysiology in PLWH vs the general population.
To our knowledge, this study comprises the first longitudinal assessment of a well-defined sample with HIV-associated NAFLD, and the first to assess serial liver biopsies to determine natural history in this group. Importantly, participants in this analysis were virologically suppressed on modern ART, and thus our findings are likely to be generalizable to contemporary PLWH. As another strength of this analysis, we evaluated hepatic fibrosis by liver biopsy rather than by noninvasive methods. In addition to serving as the gold standard for fibrosis measurement [30], the use of liver biopsy allowed us to relate other histologic indices to fibrosis presence and progression in cross-sectional and longitudinal analyses. Notably, since biopsies were obtained at a fixed interval on all study subjects per protocol, our findings were unlikely to have been influenced by ascertainment bias. As a limitation of this analysis, our sample size was relatively small. As such, we may have had limited power to detect certain differences between groups or to adjust our analyses in multivariable models; the absence of an association between a variable of interest and fibrosis presence or progression should not be interpreted as definitive evidence against such a relationship. Moreover, the overall number of women in this cohort did not allow us to investigate sex-specific differences in fibrosis progression. Biopsy length is an important factor in the diagnostic accuracy of histopathology [31]. All samples used in this study were deemed adequate for analysis by the study pathologist (D. E. K.), and sample lengths did not differ between individuals with vs without fibrosis at baseline. In addition, based on the randomized longitudinal design of this study within HIV, a non-HIV comparator group was not included, though we were able to draw inferences with respect to the general population from existing literature.
In summary, we showed high rates of hepatic fibrosis presence and progression among individuals with HIV-associated NAFLD over a 12-month period. Moreover, we identified high visceral adiposity as a novel predictor of a more aggressive course of hepatic fibrosis. Meanwhile, although worsening of fibrosis was associated with a rise in NAS, no association was observed between baseline NAS and fibrosis progression. Given the accelerated nature of NAFLD in HIV as well as the limited utility of available indices to prognosticate an individual patient’s trajectory, new biomarkers and metrics to identify those who require more intensive monitoring and therapy are critically needed. Furthermore, since assessment of visceral adiposity is not routinely available in the clinical setting, use of novel clinical methodologies to quantify visceral fat should be explored. Only by accurately risk-stratifying patients with HIV-associated NAFLD can we individualize and optimize care for this heterogeneous group of patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the participants of this study and the Nursing and Bionutrition Staff of the Massachusetts General Hospital and National Institutes of Health (NIH) Translational and Clinical Research Centers.
Financial support . This work was supported by the NIH (grant number 1KL2TR002542-01 to L. T. F.); the National Institute of Allergy and Infectious Diseases (grant number U01AI115711); the Nutrition Obesity Research Center at Harvard (grant number P30DK040561); the National Center for Advancing Translational Science (grant number 1UL1TR002541-01); and the Intramural Research Program of the NIH, National Cancer Institute. Study drug for the trial was supplied by Theratechnologies, Inc, although treatment data were not the focus of the current manuscript.
Potential conflicts of interest. L. T. F. has received consulting fees from Theratechnologies. T. L. S. has received funding from Novo Nordisk for an investigator-initiated grant unrelated to the current project. K. E. C. has received grant funding from Bristol-Myers Squibb, Novartis, and Boehringer Ingelheim as well as consulting fees from Gilead and Novo Nordisk unrelated to the current project. R. T. C. has received funding to the institution from Merck, during the conduct of the study; grants to the institution from Abbvie, Synlogic, Kaleido, Gilead, Merck, Bristol-Myers Squibb, Janssen, Boehringer, and Roche, and personal fees from Alnylam, unrelated to the current project S. K. G. has served as consultant and as a member of the Scientific Advisory Board to Theratechnologies. He also has received research support for an investigator-initiated project unrelated to the current study from Theratechnologies. He also reports grants to the institution from KOWA, Viiv, and Gilead, and personal consulting fees from Viiv, outside the submitted work. He also has received research support for an investigator-initiated project unrelated to the current study from Theratechnologies. Last, S. K. G. was the named inventor on a patent application on the effects of tesamorelin in the treatment of hepatic disease. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. [DOI] [PubMed] [Google Scholar]
- 2. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017; 65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017; 67:1265–73. [DOI] [PubMed] [Google Scholar]
- 4. Sulkowski MS, Mehta SH, Torbenson MS, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS 2007; 21:2209–16. [DOI] [PubMed] [Google Scholar]
- 5. Lo Re V 3rd, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med 2014; 160:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lemoine M, Lacombe K, Bastard JP, et al. Metabolic syndrome and obesity are the cornerstones of liver fibrosis in HIV-monoinfected patients. AIDS 2017; 31:1955–64. [DOI] [PubMed] [Google Scholar]
- 7. Lombardi R, Sambatakou H, Mariolis I, Cokkinos D, Papatheodoridis GV, Tsochatzis EA. Prevalence and predictors of liver steatosis and fibrosis in unselected patients with HIV mono-infection. Dig Liver Dis 2016; 48:1471–7. [DOI] [PubMed] [Google Scholar]
- 8. Vuille-Lessard É, Lebouché B, Lennox L, et al. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS 2016; 30:2635–43. [DOI] [PubMed] [Google Scholar]
- 9. Perazzo H, Cardoso SW, Yanavich C, et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc 2018; 21:e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivero-Juárez A, Camacho A, Merchante N, et al. Grupo para el estudio de las hepatitis víricas (HEPAVIR) de la Sociedad Andaluza de Enfermedades Infecciosas (SAEI) . Incidence of liver damage of uncertain origin in HIV patients not co-infected with HCV/HBV. PLoS One 2013; 8:e68953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stanley TL, Fourman LT, Feldpausch MN, et al. Effects of tesamorelin on non-alcoholic fatty liver disease in HIV: a randomised, double-blind, multicentre trial. Lancet HIV 2019; 6:e821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterling RK, Lissen E, Clumeck N, et al. APRICOT Clinical Investigators . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 13. Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–21. [DOI] [PubMed] [Google Scholar]
- 14. Lui G, Wong VW, Wong GL, et al. Liver fibrosis and fatty liver in Asian HIV-infected patients. Aliment Pharmacol Ther 2016; 44:411–21. [DOI] [PubMed] [Google Scholar]
- 15. Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther 2015; 41:368–78. [DOI] [PubMed] [Google Scholar]
- 16. Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009; 51:371–9. [DOI] [PubMed] [Google Scholar]
- 17. van der Poorten D, Milner KL, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008; 48:449–57. [DOI] [PubMed] [Google Scholar]
- 18. Yu SJ, Kim W, Kim D, et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine (Baltimore) 2015; 94:e2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Godfrey C, Bremer A, Alba D, et al. Obesity and fat metabolism in human immunodeficiency virus-infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis 2019; 220:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koethe JR. Adipose tissue in HIV infection. Compr Physiol 2017; 7:1339–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lake JE. The fat of the matter: obesity and visceral adiposity in treated HIV infection. Curr HIV/AIDS Rep 2017; 14:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McComsey GA, Moser C, Currier J, et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis 2016; 62:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joy T, Keogh HM, Hadigan C, et al. Relation of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr 2008; 47:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chuang JH, Wang PW, Tai MH. An adipocentric view of liver fibrosis and cirrhosis. Chang Gung Med J 2004; 27:855–68. [PubMed] [Google Scholar]
- 25. Sawaki D, Czibik G, Pini M, et al. Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation 2018; 138:809–22. [DOI] [PubMed] [Google Scholar]
- 26. Cancello R, Tordjman J, Poitou C, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006; 55:1554–61. [DOI] [PubMed] [Google Scholar]
- 27. Tordjman J, Poitou C, Hugol D, et al. Association between omental adipose tissue macrophages and liver histopathology in morbid obesity: influence of glycemic status. J Hepatol 2009; 51:354–62. [DOI] [PubMed] [Google Scholar]
- 28. Mulder P, Morrison MC, Wielinga PY, van Duyvenvoorde W, Kooistra T, Kleemann R. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int J Obes (Lond) 2016; 40:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleiner DE, Brunt EM, Wilson LA, et al. Nonalcoholic Steatohepatitis Clinical Research Network . Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2019; 2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association . American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152:1536–43. [DOI] [PubMed] [Google Scholar]
- 31. Vuppalanchi R, Unalp A, Van Natta ML, et al. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.