Abstract
Background
The impact of low levels of human immunodeficiency virus (HIV) RNA (low-level viremia [LLV]) during combination antiretroviral therapy (cART) on clinical outcomes is unclear. We explored the associations between LLV and all-cause mortality, AIDS, and serious non-AIDS events (SNAEs).
Methods
We grouped individuals starting cART 1996–2017 (identified from the Swedish InfCare HIV register) as virologic suppression (VS; <50 copies/mL), LLV (repeated viral load, 50–999 copies/mL), and nonsuppressed viremia (NSV; ≥1000 copies/mL). Separately, LLV was subdivided into 50–199 and 200–999 copies/mL (reflecting different definitions of virologic failure). Proportional-hazard models (including sex, age, pre-ART CD4 count and viral load, country of birth, injection drug use, treatment experience and interruptions, and an interaction term between viremia and time) were fitted for the study outcomes.
Results
A total of 6956 participants were followed for a median of 5.7 years. At the end of follow-up, 60% were categorized as VS, 9% as LLV, and 31% as NSV. Compared with VS, LLV was associated with increased mortality (adjusted hazard ratio [aHR], 2.2; 95% confidence interval [CI], 1.3–3.6). This association was also observed for LLV 50–199 copies/mL (aHR, 2.2; 95% CI, 1.3–3.8), but was not statistically significant for LLV 200–999 copies/mL (aHR, 2.1; 95% CI, .96–4.7). LLV 50–999 copies/mL was not linked to increased risk of AIDS or SNAEs, but in subanalysis, LLV 200–999 copies/mL was associated with SNAEs (aHR, 2.0; 95% CI, 1.2–3.6).
Conclusions
In this population-based cohort, LLV during cART was associated with adverse clinical outcomes.
Keywords: HIV, low-level viremia, mortality, serious non-AIDS events, antiretroviral therapy
In retrospective analysis of the Swedish HIV cohort, low-level viremia 50–999 copies/mL was independently associated with increased mortality. Participants with HIV RNA of 200–999 copies/mL had increased risk of serious non-AIDS events compared with those with virologic suppression.
Although survival continues to improve for people living with human immunodeficiency virus (HIV) who receive combination antiretroviral therapy (cART), their overall mortality remains elevated compared with the general population [1]. With reduced incidence of AIDS, noncommunicable diseases represent an increasing proportion of deaths among people living with HIV (PLHIV), in whom several noncommunicable diseases are more prevalent than in the general population [2]. The reasons for this increased risk are complex and not fully characterized. Three separate mechanisms can be considered: higher rates of conventional risk factors (such as smoking) [3], antiretroviral drug toxicity [4], and HIV-related chronic immune activation and inflammation [5].
Persistent suppression of viral replication to undetectable levels is achieved in most persons receiving cART. However, in 3–10% of cART recipients, low levels of HIV RNA remain detectable, a phenomenon commonly referred to as low-level viremia (LLV) [6]. Whereas high levels of HIV RNA are strongly associated with death, AIDS, and serious non-AIDS events (SNAEs) [7], the impact of LLV on clinical outcomes is debated [8–13]. Most studies demonstrate an increased risk of subsequent virologic failure associated with a viral load (VL) of 200 copies/mL or higher [8, 9]. This is currently the definition of virologic failure in high-income countries [14], whereas repeated VL measurements of 1000 copies/mL or more define failure in World Health Organization (WHO) guidelines [15]. For LLV of 50–199 copies/mL the risk of virologic failure is less apparent [8, 16] and increased risk of adverse clinical events has previously not been reported.
We aimed to determine the associations between LLV (defined as 50–999 copies/mL and subdivided into 50–199 and 200–999 copies/mL) and all-cause mortality, AIDS-defining conditions, and SNAEs among adults receiving cART.
METHODS
Study Population
Participants were identified from the InfCare HIV register, a nationwide observational cohort including more than 99.9% of PLHIV in Sweden [17]. For this study, individuals who started cART (complete list of regimens in the Supplementary Material) in January 1996 or later were included if they were 15 years or older when starting cART, had a personal identity number, and had 2 or more VL results available 6 or more months after cART initiation. The data from InfCare HIV were last updated in June 2017. All data were pseudo-anonymized prior to analysis.
Outcome Definitions
Data on overall mortality and causes of death were based on the Swedish Cause of Death Register, which includes date and cause of death for all deceased persons in Sweden, coded according to the International Classification of Diseases (ICD).
The following conditions were considered as SNAEs: cardiovascular diseases, venous thromboembolic disease, pulmonary arterial hypertension, chronic kidney disease, decompensated liver disease, and non–AIDS-defining malignancies. Codes for AIDS-defining conditions and SNAEs were searched in the Patient Register, which covers all in-patient care in Sweden. In addition, the Cause of Death Register was used to identify SNAEs and AIDS that may have caused death without prior hospitalization. A complete list of ICD codes used for classification is provided as Supplementary Material.
Study Design
Participants were followed from the start of cART until reaching the respective clinical outcomes with administrative censoring 14 June 2017. Individuals without VL data for more than 12 months were considered lost to follow-up. All participants were categorized by their longitudinal viremia profiles 6 months or more after starting cART using the following definitions: (1) virologic suppression (VS), defined as VL less than 50 copies/mL (including cases with 1 or several episodes of a single VL of 50–999 copies/mL if preceded and followed by a VL <50 copies/mL); (2) LLV of 50–999 copies/mL, defined as 2 or more consecutive VLs of 50–999 copies/mL, at least 1 month apart; and (3) nonsuppressed viremia (NSV), defined as 1 or more VLs of 1000 copies/mL or more. In a separate analysis, LLV was subdivided into LLV of 50–199 copies/mL (≥2 consecutive VLs of 50–199 copies/mL at least 1 month apart) and LLV of 200–999 copies/mL (≥2 consecutive VLs of 50–999 copies/mL, with ≥1 VL 200–999 copies/mL). The cutoff of 50 copies/mL was chosen to account for VL assays used during the first part of the study period. Results obtained with VL assays reported as less than 500 copies/mL were considered as VS.
Statistical Analysis
The study size was determined by the number of cases in Sweden during the study period. Median and interquartile range (IQR) were used to summarize numerical values, and frequencies and percentage to summarize categorical variables. Patient characteristics were compared using Pearson’s χ 2 and Kruskal-Wallis tests for categorical and continuous variables, respectively.
We fitted 3 separate Cox proportional-hazard models to determine the associations between viremia category and all-cause mortality, first AIDS-defining condition, and first-ever SNAE. A time-updated measure of viremia was used, so that participants were reclassified if they developed viremia during follow-up. Reclassification was only done to higher viremia strata. The proportional hazard assumption was checked graphically and by Schoenfeld residuals. Since the proportional hazard assumption was violated for NSV in the model for all-cause mortality (but not in the model for SNAEs), we included an interaction term between viremia category and time. In multivariable analysis, the following variables were included to adjust for potential confounders: sex, age (defined as age at outcome event), CD4 count and VL before initiation of any ART, birth in Sweden, injection drug use, exposure to antiretroviral drugs prior to cART, and documented treatment interruptions during follow-up. Since treatment recommendations have changed during the study period and more potent antiretroviral agents have become available, a sensitivity analysis was performed restricted to participants starting cART after January 2005. To test the robustness of the model, we also modeled age using restricted cubic splines. To rule out that a possible effect of viremia on the risk of an adverse outcome was dependent on type of cART regimen, we conducted a subanalysis restricted to participants not changing treatment during follow-up, including type of regimen (protease inhibitors [PIs], yes/no; non-nucleoside reverse transcriptase inhibitors [NNRTI], yes/no; integrase strand transfer inhibitors [INSTIs], yes/no; and abacavir, yes/no) as covariates. In order to assess the importance of viremia persistence among participants with LLV of 50–199 copies/mL, we performed a subanalysis separating persons with less than 25% versus those with more than 25% VL measurements of more than 50 copies/mL. Cumulative viral burden was calculated as viremia copy-years using the trapezoidal rule [18].
Missing data were handled using a “complete case” approach and the number of missing values is reported for each step of the analysis. Statistical analyses were performed using Stata SE 15 (StataCorp). The study was approved by the Lund Regional Ethics Committee, Sweden (2017/1023). Data linkage was performed by Statistics Sweden and the National Board of Health and Welfare.
RESULTS
Patient Characteristics
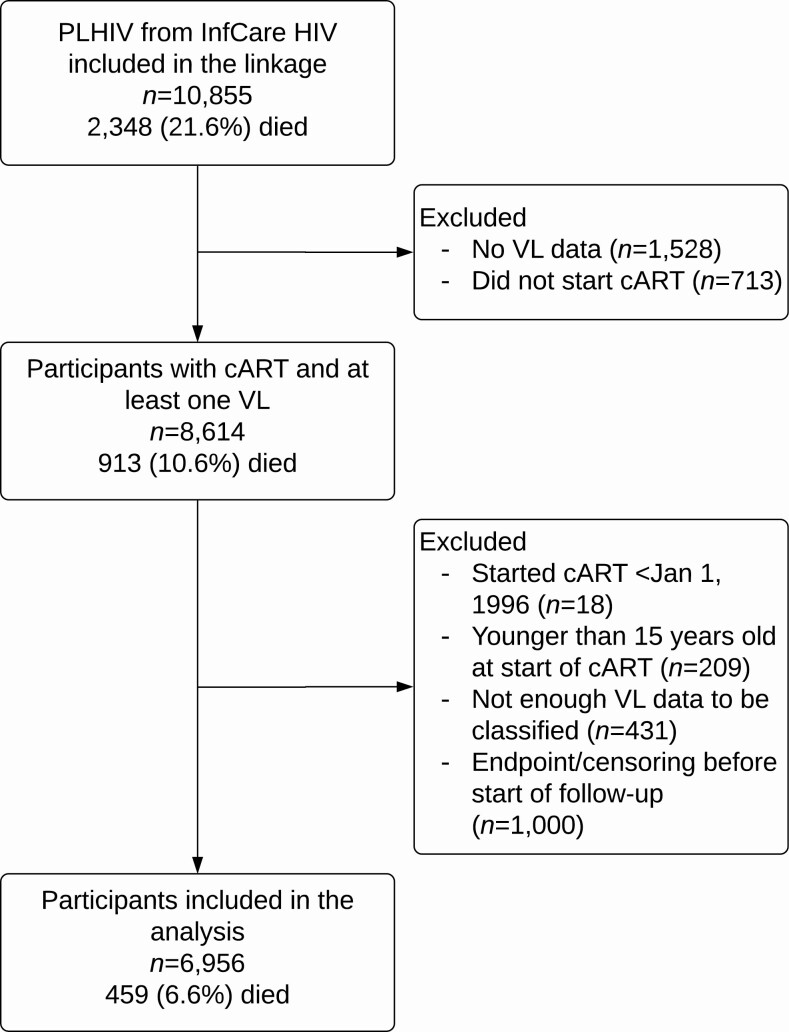
Of 10 855 PLHIV in the InfCare HIV cohort, 3899 (36%) were excluded (Figure 1). The 6956 included participants had lower overall mortality compared with the entire cohort (7% vs 22%, respectively), and most excluded individuals were diagnosed earlier than included participants (median year of diagnosis, 1994 vs 2005).
Figure 1.
Exclusion flow diagram. The number of deaths in the group included in the final analysis only counts those who died during follow-up (no loss to follow-up). Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; PLHIV, people living with human immunodeficiency virus; VL, viral load.
Demographic and clinical data for participants stratified by their final viremia category are presented in Table 1. The majority were male (63%) with a median age at cART initiation of 37 years. Individuals with LLV during cART had a higher median VL before receiving any ART than persons with VS (P < .001). Compared with other viremia profiles during cART, participants with VS were more likely to be included at a later time point (P < .001) and were less likely to initiate cART with PI-based regimens (P < .001). Furthermore, exposure to antiretroviral drugs prior to cART was lower among individuals with VS compared with those with LLV (P < .001).
Table 1.
Characteristics of Study Participants Identified From the Swedish InfCare HIV Cohort
| LLV | ||||||
|---|---|---|---|---|---|---|
| Overall (N = 6956) | Virologic Suppression (n = 4177; 60%) | 50–199 Copies/mL (n = 339; 5%) | 200–999 Copies/mL (n = 258; 4%) | Nonsuppressed Viremia (n = 2182; 31%) | P a | |
| Males, n (%) | 4396 (63) | 2649 (63) | 244 (72) | 179 (69) | 1324 (61) | <.001 |
| Age at start of cART, years | 37 (31–45) | 38 (31–46) | 39 (33–47) | 39 (33–49) | 36 (31–44) | <.001 |
| Median year of HIV diagnosis | 2005 | 2008 | 2006 | 2003 | 1996 | <.001 |
| Median year of start of cART | 2007 | 2010 | 2007 | 2005 | 1999 | <.001 |
| Country of birth, n (%) | <.001 | |||||
| Sweden | 2643 (38) | 1482 (35) | 151 (45) | 106 (41) | 904 (41) | |
| Outside Sweden | 4152 (60) | 2582 (62) | 182 (54) | 149 (58) | 1239 (57) | |
| Unknown | 5 (0) | 1 (0) | 0 | 0 | 4 (0) | |
| Country of birth missing | 156 (2) | 112 (3) | 6 (2) | 3 (1) | 35 (2) | |
| Transmission group, n (%) | <.001 | |||||
| Heterosexual | 3566 (51) | 2189 (52) | 153 (45) | 133 (52) | 1091 (50) | |
| Homosexual/bisexual | 2332 (34) | 1417 (34) | 129 (38) | 83 (32) | 703 (32) | |
| Injection drug use | 396 (6) | 156 (4) | 20 (6) | 15 (6) | 205 (9) | |
| Otherb | 531 (8) | 310 (7) | 33 (10) | 26 (10) | 162 (7) | |
| Transmission group missing | 131 (2) | 105 (3) | 4 (1) | 1 (0) | 21 (1) | |
| VL before ART initiation,c copies/mL | 73 000 (18 050–242 000) [28% missing] | 58 400 (15 150–196 000) [21% missing] | 230 000 (66 000–750 000) [21% missing] | 194 500 (55 600–541 000) [31% missing] | 80 000 (20 900–246 000) [43% missing] | <.001 |
| CD4 cell count before ART initiation,c cells/mm3 | 240 (140–360) [18% missing] | 252 (146–370) [17% missing] | 189 (90–290) [13% missing] | 200 (90–280) [17% missing] | 240 (150–350) [19% missing] | <.001 |
| Ever HCV positive, n (%) | 651 (9) | 308 (7) | 38 (11) | 28 (11) | 277 (13) | <.001 |
| HCV status missing | 975 (14) | 589 (14) | 42 (12) | 35 (14) | 309 (14) | |
| Ever HBsAg positive, n (%) | 243 (3) | 137 (3) | 11 (3) | 10 (4) | 85 (4) | .78 |
| HBV status missing | 3695 (53) | 2220 (53) | 174 (51) | 144 (56) | 1157 (53) | |
| Type of first cART regimen, n (%) | ||||||
| Including PIs | 4040 (58) | 1874 (45) | 220 (65) | 169 (66) | 1777 (81) | <.001 |
| Including NNRTI | 2475 (36) | 1874 (45) | 102 (30) | 84 (33) | 415 (19) | <.001 |
| Including INSTIs | 588 (8) | 523 (13) | 26 (8) | 12 (5) | 27 (1) | <.001 |
| Including abacavir | 1414 (20) | 1097 (26) | 58 (17) | 44 (17) | 215 (10) | <.001 |
| Switched treatment during follow-up, n (%) | 2368 (34) | 600 (14) | 87 (26) | 75 (29) | 1606 (74) | <.001 |
| Treatment experienced at start of cART, n (%) | 1331 (19) | 337 (8) | 63 (19) | 63 (24) | 868 (40) | <.001 |
| Total viremia copy-years from start to end of follow-up, log10 copy × year/mL | 2.4 (1.6–4.0) | 1.6 (1.1–2.1) | 2.2 (1.9–2.5) | 2.5 (2.2–2.8) | 4.4 (3.6–5.1) | <.001 |
Values are n (%) or median (interquartile range) unless otherwise indicated. Participants are grouped by the last viremia category they belonged to during follow-up.
Abbreviations: ART, antiretroviral therapy; cART, combination antiretroviral therapy; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; LLV, low-level viremia; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load.
a P values are the results of Kruskal-Wallis tests for continuous variables and Pearson χ 2 tests for categorical variables.
bIncluding blood products, mother-to-child, and unknown.
cRefers to any antiretroviral treatment before start of cART.
Viremia Profiles During Combination Antiretroviral Therapy
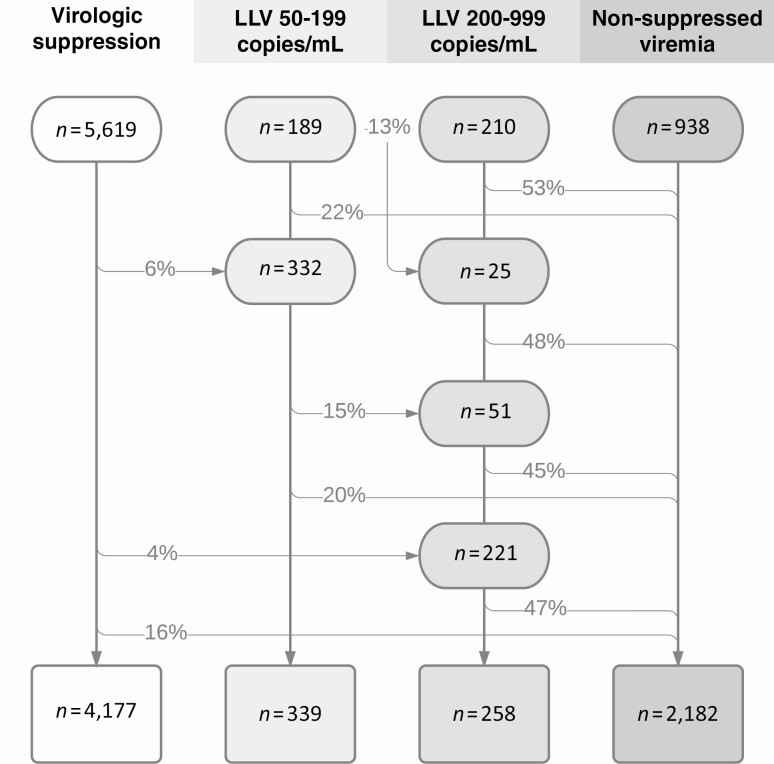
During follow-up, 953 participants (14%) met the criteria for LLV of 50–999 copies/mL; 521 belonged to the LLV 50–199 copies/mL category at some time point and 508 to LLV of 200–999 copies/mL. The following distribution of viremia categories was found at the end of follow-up: 60% VS, 9% LLV of 50–999 copies/mL (5% LLV of 50–199 and 4% LLV of 200–999 copies/mL), and 31% NSV (Figure 2). Of 5169 individuals with VS, 1808 (35%) had 1 or more isolated VL of 50–999 copies/mL. The median interval between VL results was 120 days (IQR, 78–175 days), and participants had a median of 15 (IQR, 6–29) VL measurements during follow-up. Of 143 347 VLs included in the final analysis, 1094 measurements of undetectable VL (0.8%) in 668 individuals were performed with assays having a lower detection limit of 500 copies/mL.
Figure 2.
Flow of patients through viremia categories during follow-up. The percentages represent the proportion of participants who were reclassified to the respective category. Reclassification was possible only to higher viremia strata. Abbreviation: LLV, low-level viremia.
Counted from 6 months after starting cART, participants with LLV had a median cumulative exposure to viremia, measured as viremia copy-years, of 2.2 (IQR, 1.9–2.5) log10 copy × year/mL for LLV of 50–199 copies/mL and 2.5 (IQR, 2.2–2.8) log10 copy × year/mL for LLV of 200–999 copies/mL (Table 1).
All-Cause Mortality and Viremia Profiles During Combination Antiretroviral Therapy
During 49 986 person-years of follow-up (median, 5.7; maximum, 20.7 years), 459 deaths were registered. The most frequently stated cause of death (31%) was from HIV/AIDS, followed by cardiovascular disease and non-AIDS malignancy (Table 2).
Table 2.
Cause of Death by Viremia Category at Time of Death
| LLV | |||||
|---|---|---|---|---|---|
| Overall (N = 459) | Virologic Suppression (n = 142; 31%) | 50–199 Copies/mL (n = 31; 7%) | 200–999 Copies/mL (n = 16; 3%) | Nonsuppressed Viremia (n = 270; 59%) | |
| Underlying cause of death | |||||
| HIV/AIDS | 144 (31) | 39 (27) | 2 (6) | 5 (31) | 98 (36) |
| Cardiovascular death | 84 (18) | 32 (23) | 8 (26) | 3 (19) | 41 (15) |
| Non-AIDS malignancy | 84 (18) | 25 (18) | 7 (23) | 1 (6) | 51 (19) |
| Violent/accidental death | 63 (14) | 19 (13) | 7 (23) | 3 (19) | 34 (13) |
| Other conditionsa | 59 (13) | 15 (11) | 4 (13) | 3 (19) | 37 (14) |
| Unknown | 15 (3) | 7 (5) | 3 (10) | 0 | 5 (2) |
| Cause of death missing | 10 (2) | 5 (4) | 0 | 1 (6) | 4 (1) |
Data are presented as n (%).
Abbreviations: HIV, human immunodeficiency virus; LLV, low-level viremia.
aLiver diseases (n = 15), pulmonary diseases (n = 12), non-AIDS infections (n = 12), gastrointestinal diseases (n = 6), neurological conditions (n = 5), diabetes mellitus (n = 4), rheumatological conditions (n = 2), urological conditions (n = 1), hyperlipidemia (n = 1), and bipolar disorder (n = 1).
In unadjusted analysis including an interaction term between viremia category and time, participants with LLV had significantly higher mortality compared with those with VS (crude hazard ratio [HR] for LLV of 50–999 copies/mL, 2.6; 95% CI, 1.8–3.7). Adjustment for potential confounders resulted in slightly decreased HR for LLV, although with retained statistical significance (Table 3). When analyzing the LLV groups separately, LLV of 50–199 copies/mL had an adjusted HR (aHR) of 2.2 (95% CI, 1.3–3.8) and LLV of 200–999 copies/mL had an aHR of 2.1 (95% CI, .96–4.7) (Table 4). The interaction term between viremia category and time had an aHR of .86 (95% CI, .76–.98), suggesting a decreasing influence of viremia category with increasing follow-up time (Supplementary Table 1). Low-level viremia was also associated with increased all-cause mortality in participants starting cART after January 2005 (n = 3186) (aHR, 3.2; 95% CI, 1.5–6.7) for LLV of 50–999 copies/mL compared with VS (Supplementary Table 2). Adjusting for type of cART regimen did not change the impact of LLV on all-cause mortality in a subanalysis of participants not switching treatment (n = 3207); likewise, neither PIs, NNRTI, INSTIs, or abacavir were associated with increased all-cause mortality (Supplementary Table 3). In a subanalysis of participants in the LLV 50–199 category with regard to proportions of VL measurements of 50 copies/mL or higher (<25% vs ≥25%), only those with 25% or more detectable VL had significantly increased mortality (aHR, 3.3; 95% CI, 1.8–6.4). Besides viremia profiles, the following were significantly associated with all-cause mortality: older age, male sex, higher pre-ART CD4 cell counts, injection drug use, and treatment interruptions (Supplementary Table 1).
Table 3.
Cox Regression Models for All-Cause Mortality, AIDS, and Serious Non-AIDS Events by Viremia Category
| Unadjusted Model | Unadjusted Model With Time Interaction | Fully Adjusted Modela | |
|---|---|---|---|
| All-cause mortality, n | 6956 | 6956 | 4541 |
| Virologic suppression | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| LLV of 50–999 copies/mL | 1.7 (1.2–2.4) | 2.6 (1.8–3.7) | 2.2 (1.3–3.6) |
| Nonsuppressed viremia | 2.5 (2.0–3.1) | 6.6 (4.2–10.6) | 7.7 (3.8–15.6) |
| AIDS, n | 6823 | 6823 | 4440 |
| Virologic suppression | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| LLV of 50–999 copies/mL | .45 (.11–1.9) | .84 (.19–3.7) | No event |
| Nonsuppressed viremia | 4.6 (2.9–7.3) | 20.1 (8.3–48.6) | 23.9 (6.3–90.1) |
| Serious non-AIDS events, n | 6884 | 6884 | 4486 |
| Virologic suppression | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| LLV of 50–999 copies/mL | 1.2 (.92–1.6) | 1.6 (1.2–2.2) | 1.2 (.78–1.8) |
| Nonsuppressed viremia | 1.5 (1.3–1.8) | 2.9 (1.9–4.3) | 2.8 (1.6–5.2) |
Values are hazard ratios with 95% confidence intervals.
Abbreviations: ART, antiretroviral therapy; LLV, low-level viremia; Ref, reference; VL, viral load.
aAdjusted for age, sex, CD4 count and VL before start of ART, injection drug use, born in Sweden, treatment experience, and treatment interruptions. Including an interaction term between viremia category and time.
Table 4.
Cox Regression Models for All-cause Mortality and Serious Non-AIDS Events by Viremia Category, LLV Subdivided Into 50–199 And 200–999 copies/mL
| Unadjusted Model | Unadjusted Model With Time Interaction | Fully Adjusted Modela | |
|---|---|---|---|
| All-cause mortality, n | 6956 | 6956 | 4541 |
| Virologic suppression | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| LLV of 50–199 copies/mL | 2.3 (1.5–3.3) | 2.9 (1.9–4.3) | 2.2 (1.3–3.8) |
| LLV of 200–999 copies/mL | 1.2 (.68–2.0) | 2.0 (1.1–3.7) | 2.1 (.96–4.7) |
| Nonsuppressed viremia | 2.5 (2.0–3.1) | 6.3 (3.9–10.1) | 7.7 (3.7–15.8) |
| Serious non-AIDS events, n | 6884 | 6884 | 4486 |
| Virologic suppression | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| LLV of 50–199 copies/mL | 1.1 (.76–1.6) | 1.3 (.91–2.0) | .86 (.50–1.5) |
| LLV of 200–999 copies/mL | 1.3 (.92–1.9) | 2.0 (1.3–3.1) | 2.0 (1.2–3.6) |
| Nonsuppressed viremia | 1.5 (1.3–1.8) | 3.1 (2.1–4.7) | 3.3 (1.8–6.0) |
Values are hazard ratios with 95% confidence intervals.
Abbreviations: ART, antiretroviral therapy; LLV, low-level viremia; Ref, reference; VL, viral load.
aAdjusted for age, sex, CD4 count and VL before start of ART, injection drug use, born in Sweden, treatment experience, and treatment interruptions. Including, including an interaction term between viremia category and time.
Incident AIDS and Viremia Profiles During Combination Antiretroviral Therapy
Ninety-four participants developed at least 1 AIDS-defining condition during follow-up. Compared with individuals with VS, persons with NSV had an increased risk of AIDS (aHR, 23.9; 95% CI, 6.3–90.1), whereas this risk was not elevated for participants with LLV (Table 3).
Incident Serious Non-AIDS Events and Viremia Profiles During Combination Antiretroviral Therapy
In total, 684 participants experienced at least 1 SNAE during 47 247 person-years of follow-up (median, 5.5; maximum, 20.7 years). Nonsuppressed viremia, but not LLV of 50–999 copies/mL, was associated with a higher risk of SNAEs (aHR, 2.8; 95% CI, 1.6–5.2) (Table 3). Separate analysis of the 2 LLV categories showed that only LLV of 200–999 copies/mL was associated with SNAEs (aHR, 2.0; 95% CI, 1.2–3.6) (Table 4). This relationship was similar in a sensitivity analysis restricted to participants starting cART after January 2005 (n = 3145) (Supplementary Table 2). Type of cART was not associated with SNAEs, and in a subanalysis of individuals without cART regimen switch during follow-up (n = 3168) the relationship between LLV of 200–999 copies/mL and SNAEs was independent of regimen type (Supplementary Table 3). The most common type of SNAE was cardiovascular disease (n = 357), followed by non-AIDS malignancy (n = 197) (Supplementary Table 4).
DISCUSSION
In this nationwide Swedish cohort, LLV of 50–999 copies/mL during cART was independently associated with increased all-cause mortality. Furthermore, persons with LLV in the higher stratum (200–999 copies/mL) had a higher risk of SNAEs, whereas LLV was not linked to AIDS-defining conditions.
Several factors could explain the discordant results previously reported on associations between LLV and mortality [8–13, 19, 20], such as study design, population characteristics, and duration of follow-up. Furthermore, varying criteria for virologic treatment failure have resulted in different definitions of LLV. Since our study is based on a cohort with follow-up since 1996, we chose to define LLV using an upper limit of 1000 copies/mL (which is also in agreement with WHO guidelines [15]). To account for the lower threshold of virologic failure currently used in high-income countries, we performed subanalyses for LLV of 50–199 and 200–999 copies/mL. Interestingly, the reported association with mortality was also statistically significant for LLV of less than 200 copies/mL. In a separate subanalysis, the association with mortality was restricted to participants with 25% or more of VL of 50 copies/mL or greater, supporting the existence of a relationship between persistent LLV and mortality.
Our finding of increased mortality for persons with LLV is in agreement with a Spanish study that observed a higher risk for the composite endpoint all-cause mortality/AIDS for persons with LLV of 200–499 copies/mL [10]. In contrast, the Antiretroviral Therapy Cohort Collaboration Study did not observe higher risk of death for either LLV of 50–199 or 200–499 copies/mL [8], similar to 3 smaller studies [12, 13, 20]. Compared with all prior studies, our cohort had longer follow-up, increasing the chance of detecting a difference in mortality. The proportion of participants meeting criteria for LLV was comparatively high (7.5% vs 3.5% [8] and 4.0% [10] for LLV 50–199 copies/mL). Since our participants were identified from a nationwide cohort, selection bias is likely to be low.
The impact of viremia could also be estimated by measuring cumulative exposure defined as viremia copy-years [18]. This variable has been associated with both elevated mortality [21] and SNAEs [22]. Different methods have been used to calculate cumulative viremia. While most studies have used the linear scale, Sempa et al [23] suggested that the logarithmic scale is more predictive. To our knowledge, only 1 study has focused specifically on LLV and mortality using viremia copy-years, with no association observed [20]. An important caveat of the copy-year variable is the high dependence on timing and frequency of sampling; consequently, levels of viremia copy-years can usually not be compared between studies [24]. For these reasons, and since our aim was to explore the effects of LLV specifically, we chose a time-updated classification based on different cutoffs used for definition of virologic failure (1000 and 200 copies/mL) instead of copy-years to assess viremia exposure.
Previous studies have not found associations between LLV and SNAEs [10, 11]. Compared with these, we used a wider definition of SNAEs, including venous thromboembolic disease and pulmonary arterial hypertension, conditions for which HIV is a known risk factor [25, 26]. Like the analysis of mortality, the longer follow-up period in our study could increase the chance of revealing an association between LLV and SNAEs.
Low-level viremia during ART can be due to separate mechanisms: release of viral particles from latently infected cells and active replication [27]. Interestingly, different profiles of inflammatory cytokines have been associated with 2 distinct HIV env sequence patterns, monotypic and diverse LLV [28]. Biological pathways might therefore explain an association between LLV and adverse clinical outcomes. For example, the role of chronic inflammation in the pathogenesis of atherosclerosis may be of particular importance in PLHIV [5]. Based on trials of structured treatment interruptions, HIV replication has been associated with inflammation as well as increased risk of SNAEs and death [7, 29]. Moreover, several studies have found higher levels of blood biomarkers reflecting cellular immune activation [30], inflammation, innate immunity, coagulation, and cardiovascular risk [31–33] in ART recipients with LLV compared with those with undetectable viremia.
The potential relation between non-AIDS morbidity and antiretroviral drugs has been extensively studied. Specifically, an increased risk of cardiovascular events has been reported for regimens including PIs and for recent exposure to abacavir [4]. Due to the long follow-up in our cohort, many participants changed regimens, and the specific impact of certain drugs on long-term outcomes is therefore difficult to elucidate. Nonetheless, in a subanalysis of participants not switching treatment, the effects of LLV on the risk of mortality and SNAEs were independent of cART regimen.
If individuals with LLV have increased long-term risk of adverse clinical outcomes, could ART modification abrogate this? Several studies have indicated that residual viremia measured with ultrasensitive assays is not affected by treatment intensification [34, 35]. In persons with LLV, however, resistance-guided treatment modification has led to better virologic control [36, 37], but whether this also results in improved clinical outcomes is unknown. In our cohort, relatively low proportions of participants with detectable viremia switched regimens, both for LLV and NSV. This was due to either subsequent loss to follow-up or resuppression without regimen modification (data not shown).
Our study has certain limitations. First, the classification of viremia is based on VL measurements from clinical care, with a median interval between samples of 120 days; thus, shorter episodes of LLV might have been missed. A substantial proportion (35%) of participants categorized as VS had isolated elevated VL results. Whereas these probably represent blips, some of these could be transient episodes of LLV. Second, residual confounding could occur if unmeasured factors are associated with both LLV and the study outcomes. We have controlled for potential confounders but lack information on some factors associated with death and morbidity in PLHIV, such as smoking and socioeconomic background [3, 38]. Still, to our knowledge, these conditions have not been linked to LLV. Third, data originating from the initial years of our study may have limited relevance in relation to currently recommended regimens. Nevertheless, our findings are robust in sensitivity analysis restricted to participants starting cART 2005 and later. Last, ICD classification has been reported to overestimate HIV as an underlying cause of death among PLHIV [39], which explains why the number of HIV-related deaths exceeds the number of AIDS diagnoses.
In conclusion, we observed increased mortality for participants with LLV of 50–999 copies/mL during cART, which was also found in the subset of persons with LLV of 50–199 copies/mL. In addition, individuals with LLV of 200–999 copies/mL had an elevated risk of SNAEs compared with those with virologic suppression. These findings add to mounting evidence that LLV is associated with worse clinical outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: This data was presented in part at the Conference on Retroviruses and Opportunistic Infections 2020, Boston MA, 8–11 March 2020, abstract 152.
Acknowledgments. This study benefited from data provided by the InfCare human immunodeficiency virus quality assurance register.
Disclaimer. The funders had no impact on the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Physicians Against AIDS Research Foundation (grant number FOa2019-0006; to O. E.); the Gilead Nordic Fellowship (grant number 2018004132; to P. B.); the Swedish State under the agreement between the Swedish government and the county councils, the ALF-agreement (grant numbers ALFSUS- 40103 [to P. B.] and ALFGBG-717531 [to M. G.]); Region Skåne (grant number REGSKANE821541; to P. B.); and the Swedish Research Council (grant number 2016-01675; to A. S.).
Potential conflicts of interest. M. G. reports grants from Gilead, personal fees from Gilead, personal fees from Janssen, personal fees from GlaxoSmithKline/ViiV, and personal fees from Merck Sharp & Dohme Corp. outside the submitted work. P. B. reports grants from Gilead Nordic Fellowship during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Croxford S, Kitching A, Desai S, et al. . Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2017; 2:e35–46. [DOI] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R, et al. . Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 3. Helleberg M, Afzal S, Kronborg G, et al. . Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis 2013; 56:727–34. [DOI] [PubMed] [Google Scholar]
- 4. Bavinger C, Bendavid E, Niehaus K, et al. . Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One 2013; 8:e59551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection: a systematic review. PLoS One 2016; 11:e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother 2014; 58:3585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strategies for Management of Antiretroviral Therapy Study G, El-Sadr WM, Lundgren J, et al. . CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 8. Vandenhende MA, Ingle S, May M, et al. ; Antiretroviral Therapy Cohort Collaboration (ART-CC) . Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 2015; 29:373–83. [DOI] [PubMed] [Google Scholar]
- 9. Elvstam O, Medstrand P, Yilmaz A, Isberg PE, Gisslén M, Björkman P. Virological failure and all-cause mortality in HIV-positive adults with low-level viremia during antiretroviral treatment. PLoS One 2017; 12:e0180761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernal E, Gómez JM, Jarrín I, et al. ; CoRIS Study Group . Low-level viremia is associated with clinical progression in HIV-infected patients receiving antiretroviral treatment. J Acquir Immune Defic Syndr 2018; 78:329–37. [DOI] [PubMed] [Google Scholar]
- 11. Zhang S, van Sighem A, Kesselring A, et al. . Episodes of HIV viremia and the risk of non-AIDS diseases in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr 2012; 60:265–72. [DOI] [PubMed] [Google Scholar]
- 12. Zhang S, van Sighem A, Gras L, et al. . Clinical significance of transient HIV type-1 viraemia and treatment interruptions during suppressive antiretroviral treatment. Antivir Ther 2010; 15:555–62. [DOI] [PubMed] [Google Scholar]
- 13. Eastburn A, Scherzer R, Zolopa AR, et al. . Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One 2011; 6:e26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 28 March2020.
- 15. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 28 March 2020.
- 16. Joya C, Won SH, Schofield C, et al. . Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis 2019; 69:2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Häggblom A. Antiretroviral drug resistance and treatment outcomes of human immunodeficiency virus type 1, implications for low and middle income countries. Stockholm: Karolinska Institute, 2016. [Google Scholar]
- 18. Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol 2010; 171:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JS, Cole SR, Richardson DB, et al. ; Center for AIDS Research Network of Integrated Clinical Systems . Incomplete viral suppression and mortality in HIV patients after antiretroviral therapy initiation. AIDS 2017; 31:1989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quiros-Roldan E, Raffetti E, Castelli F, et al. . Low-level viraemia, measured as viraemia copy-years, as a prognostic factor for medium-long-term all-cause mortality: a MASTER cohort study. J Antimicrob Chemother 2016; 71:3519–27. [DOI] [PubMed] [Google Scholar]
- 21. Mugavero MJ, Napravnik S, Cole SR, et al. ; Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort Study . Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis 2011; 53:927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kowalkowski MA, Day RS, Du XL, Chan W, Chiao EY. Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. J Acquir Immune Defic Syndr 2014; 67:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sempa JB, Dushoff J, Daniels MJ, et al. . Reevaluating cumulative HIV-1 viral load as a prognostic predictor: predicting opportunistic infection incidence and mortality in a Ugandan cohort. Am J Epidemiol 2016; 184:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lesosky M, Glass T, Rambau B, Hsiao NY, Abrams EJ, Myer L. Bias in the estimation of cumulative viremia in cohort studies of HIV-infected individuals. Ann Epidemiol 2019; 38:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fultz SL, McGinnis KA, Skanderson M, Ragni MV, Justice AC. Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans. Am J Med 2004; 116:420–3. [DOI] [PubMed] [Google Scholar]
- 26. Quezada M, Martin-Carbonero L, Soriano V, et al. . Prevalence and risk factors associated with pulmonary hypertension in HIV-infected patients on regular follow-up. AIDS 2012; 26:1387–92. [DOI] [PubMed] [Google Scholar]
- 27. Tobin NH, Learn GH, Holte SE, et al. . Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 2005; 79:9625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bull ME, Mitchell C, Soria J, et al. . Monotypic low-level HIV viremias during antiretroviral therapy are associated with disproportionate production of X4 virions and systemic immune activation. AIDS 2018; 32:1389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calmy A, Gayet-Ageron A, Montecucco F, et al. ; STACCATO Study Group . HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS 2009; 23:929–39. [DOI] [PubMed] [Google Scholar]
- 30. Karlsson AC, Younger SR, Martin JN, et al. . Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS 2004; 18:981–9. [DOI] [PubMed] [Google Scholar]
- 31. Falasca F, Di Carlo D, De Vito C, et al. . Evaluation of HIV-DNA and inflammatory markers in HIV-infected individuals with different viral load patterns. BMC Infect Dis 2017; 17:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elvstam O, Medstrand P, Jansson M, Isberg PE, Gisslén M, Björkman P. Is low-level HIV-1 viraemia associated with elevated levels of markers of immune activation, coagulation and cardiovascular disease? HIV Med 2019; 20:571–80. [DOI] [PubMed] [Google Scholar]
- 33. Bastard JP, Soulié C, Fellahi S, et al. . Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther 2012; 17:915–9. [DOI] [PubMed] [Google Scholar]
- 34. Rasmussen TA, McMahon JH, Chang JJ, et al. . The effect of antiretroviral intensification with dolutegravir on residual virus replication in HIV-infected individuals: a randomised, placebo-controlled, double-blind trial. Lancet HIV 2018; 5:e221–30. [DOI] [PubMed] [Google Scholar]
- 35. McMahon D, Jones J, Wiegand A, et al. . Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis 2010; 50:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swenson LC, Cobb B, Geretti AM, et al. ; International Viral Load Assay Collaboration . Comparative performances of HIV-1 RNA load assays at low viral load levels: results of an international collaboration. J Clin Microbiol 2014; 52:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McConnell MJ, Mier-Mota J, Flor-Parra F, et al. . Improved viral suppression after treatment optimization in HIV-infected patients with persistent low-level viremia. J Acquir Immune Defic Syndr 2011; 58:446–9. [DOI] [PubMed] [Google Scholar]
- 38. Sobrino-Vegas P, Rodríguez-Urrego J, Berenguer J, et al. ; CoRIS Study Group . Educational gradient in HIV diagnosis delay, mortality, antiretroviral treatment initiation and response in a country with universal health care. Antivir Ther 2012; 17:1–8. [DOI] [PubMed] [Google Scholar]
- 39. Kowalska JD, Friis-Møller N, Kirk O, et al. ; CoDe Working Group; D:A:D Study Group . The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology 2011; 22:516–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.