Abstract
Background
Proton-pump inhibitors (PPIs) have been reported to increase the risk of community-associated Clostridium difficile infection (CDI), but the association remains disputed.
Methods
A nationwide cohort study among adults in Denmark, 2010–2013, linking register data on C. difficile testing, filled prescriptions, and patient characteristics. All incident episodes of community-associated CDI (ie, positive culture, molecular assay, or toxin test in individuals without previous hospitalization in the prior 12 weeks and without a positive test for C. difficile in the prior 8 weeks) were identified in the Danish National Microbiological Database. Self-controlled case-series analyses were used to estimate incidence rate ratios (IRRs) for community-associated CDI, comparing periods with and without exposure to PPIs. By design, models took fixed confounders such as chronic disease, genetics, and socioeconomic status into account; further, time-varying confounders, including hospital stay and antibiotic and corticosteroid use were adjusted for.
Results
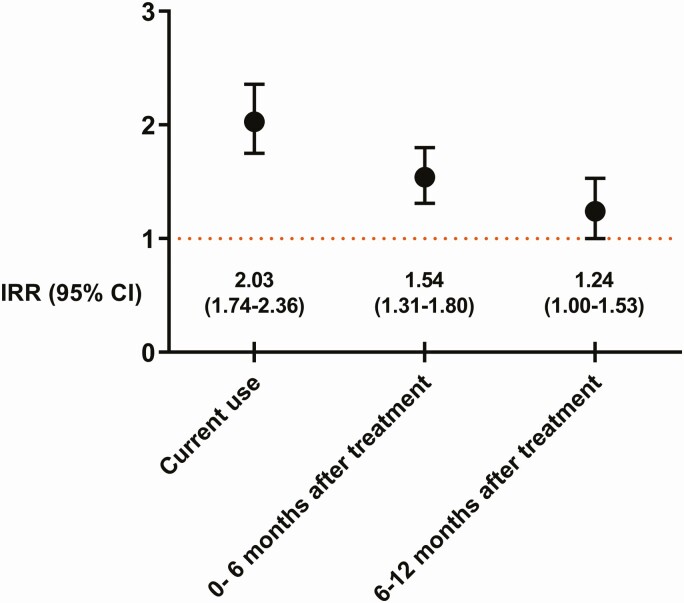
3583 episodes of community-associated CDI were identified, of which 964 occurred during current use of PPIs, 324 occurred 0–6 months after treatment cessation, 123 occurred 6–12 months after treatment cessation, and 2172 occurred during time periods without use of PPIs. The adjusted IRR was 2.03 (95% confidence interval, 1.74–2.36), comparing use of PPI with nonuse. The increased risk remained elevated in later time periods: 1.54 (1.31–1.80) for 0–6 months, 1.24 (1.00–1.53) for 6–12 months after current use.
Conclusions
Use of PPIs was associated with moderately increased risk of community-associated CDI. The risk remained elevated up to 1 year after PPI treatment had ended.
Keywords: proton-pump inhibitor, C. difficile infection, self-controlled case-series analysis, epidemiology
This study found that ongoing exposure to PPIs was associated with a doubled risk of community-acquired Clostridium difficile infection. The risk was attenuated following treatment cessation but remained significantly increased up to 1 year after PPI treatment had ended.
(See the Editorial Commentary by Villafuerte-Gálvez on pages e1090–2.)
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT?
The use of proton-pump inhibitors (PPIs) has been associated with Clostridium difficile infection (CDI) in several observational studies. The association has, however, remained controversial due to the absence of data from randomized controlled trials, considerable variability between studies, and insufficient adjustment for confounding in previous studies.
WHAT ARE THE NEW FINDINGS?
This study, on the basis of a nationwide cohort of all incident cases of community-acquired CDI, found that ongoing exposure to PPIs was associated with a doubled risk of community-acquired CDI. The risk was attenuated following treatment cessation but still remained significantly increased up to 1 year after PPI treatment had ended.
HOW MIGHT IT IMPACT ON CLINICAL PRACTICE IN THE FORESEEABLE FUTURE?
Previous observational studies have reached varying conclusions. This large study with thorough control for confounding significantly adds to the body of evidence that increased risk of CDI, even in the community setting, should be considered when prescribing PPIs, although the underlying biological mechanisms need to be explored.
Clostridium difficile, a common gram-positive, anaerobic bacterium, can cause infection (C. difficile infection [CDI]) characterized by gastrointestinal symptoms varying from life-threatening pseudo-membraneous colitis to milder diarrhea and asymptomatic carriage. Clostridium difficile infection is a major complication of antibiotic treatment, especially in the elderly and in individuals with underlying chronic diseases [1].
Clostridium difficile infection has traditionally been considered to be hospital-associated (HA-CDI; with hospital or community onset), partly due to a high density of individuals prone to CDI and the presence of spores in the hospital environment [2]. However, it is increasingly recognized that CDI can be acquired in the community setting (community-associated [CA]-CDI). It has been estimated that 20% to 30% of CDI cases in Europe and the United States may be community associated [3, 4]. In all forms of CDI, antibiotic use, advanced age, and prior hospitalization are established risk factors [2, 5]. However, reports indicate that up to one-third of CA-CDI cases had neither been hospitalized nor treated with antibiotics [3]. As additional risk factors for CA-CDI, contact with children and well as beef consumption have been suggested [6, 7].
The use of proton-pump inhibitors (PPIs) has been associated with both HA-CDI and CA-CDI in several observational studies [3, 8, 9]. The association has, however, been questioned due to the absence of data from randomized controlled trials and insufficient adjustment for confounding in previous studies [10]. Case-only designs (eg, self-controlled case-series [SCCS]), which are being increasingly used in pharmacoepidemiology, provide an alternative to more traditional designs. Self-controlled case-series compare time periods of exposure and nonexposure within individuals, thus controlling for all confounders that remain constant over the observation period, even those that may be difficult or impossible to measure (eg, general health status and frailty or genetic factors) [11, 12]. We conducted a nationwide study based on all Danish adults to evaluate the risk of CA-CDI in PPI users using an SCCS design.
METHODS
Study Population
We conducted a nationwide population-based study of all incident CA-CDI cases in Danish adults, aged 20 years and older, from 26 February 2010 to 31 December 2013, linking individual-level data from national healthcare registers.
The source population was defined using the Danish Civil Registration System, which is the main administrative population database in Denmark, comprising data on civil registry number, date of birth, residence, and vital status for the whole population. Data on filled prescriptions for PPIs (Anatomical Therapeutic Chemical [ATC] code A02BC) were obtained from the Danish National Prescription Registry, which includes information on all filled prescriptions at all pharmacies in the country [13]. Individual data on episodes of CA-CDI were obtained from the Danish Microbiology Database (MiBa). Established in 2010, MiBa is a national microbiological database comprising individual test reports from all clinical microbiological laboratories in Denmark [14]. Eligible patients were all who had an incident episode of CA-CDI during the study period.
Individual information on demographic characteristics, concomitant drug use (antibiotics and corticosteroids), healthcare use, and medical history was obtained from the Danish Civil Registration System, Danish National Prescription Registry, and the National Danish Patient Register. The information from the databases was linked using the unique civil registry number, assigned to all residents in Denmark.
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked for advice on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
The study was approved by the Danish Data Protection Agency. Ethics approval and participant informed consent are not required for register-based research in Denmark.
Community-Associated Clostridium difficile Infection
A case of CA-CDI was defined according to guidelines from the European Center for Disease Prevention and Control and the Infectious Diseases Society of America as a first positive test for C. difficile (culture, molecular assay, or toxin test), registered in MiBa 26 February 2010 to 31 December 2013, among individuals who had the sample taken in the outpatient setting or 2 or fewer days after hospital admission, who had no other positive CDI test within the previous 8 weeks, and had not had a hospital inpatient stay in the previous 12 weeks [2, 15, 16]. The MiBa was set up 1 January 2010, and we defined the study period as starting on 26 February 2010 to be able to ensure a disease-free interval of 8 weeks before the first possible date of study entry.
Proton-Pump Inhibitor Exposure
Among all cases of CA-CDI, we identified time periods of PPI exposure during the study period. New use was defined as a PPI prescription among individuals without PPI use in the prior 365 days. We defined the time period of ongoing treatment (current use) with PPIs from the first day of treatment until treatment cessation estimating treatment duration according to package size, assuming a dose regimen of 1 tablet per day. We defined 2 additional time periods after the end of estimated current use. The time period 0–6 months (0–179 days) after treatment cessation was considered indeterminate use (because of the possibility of intermittent use of prescribed medication, drug exposure might potentially continue beyond the current use period). Past use was defined as 6–12 months (180–364 days) after treatment cessation and regarded as the time period when ongoing drug exposure was unlikely. Person-time prior to an individual’s first prescription and person-time after 364 days after treatment cessation were considered unexposed time (no use). One individual patient could contribute with several episodes of exposed and unexposed time periods to the cohort.
Statistical Analyses
The SCCS method compares the number of events during exposed and unexposed person-time within the same person, in individuals with an outcome event only. Thus, confounders that remain constant over the observation period can be controlled for [11]. Models were adjusted for prespecified time-dependent potential confounders of the PPI–CA-CDI association, selected based on previous studies: hospitalization, antibiotic use, and systemic corticosteroid use [5]. We defined recent hospitalization as hospitalized time plus 90 days after discharge to account for any delayed effects of hospital admission. Recent antibiotic use was defined as prescription within the last 90 days to account for post–antibiotic treatment effects. Recent corticosteroid use was defined as prescription for oral corticosteroids within the last 90 days.
A conditional Poisson model was used to estimate the incidence rate ratio (IRR), comparing the incidence rates in each of the 3 PPI exposure time periods with the no-use time period. Patients were censored at death, emigration, or 31 December 2013, whichever came first. The IRRs for CA-CDI were also estimated in subgroups of patients, categorized according to age (<65 or ≥65 years) and sex.
In a sensitivity analysis, we both excluded all patients with any hospital admission 1 year before start of follow-up and, to minimize bias from possible informative censoring, censored patients at time of first hospital admission during follow-up. In a second sensitivity analysis, we also adjusted for antibiotic treatment for Helicobacter pylori eradication, by splitting the treatment categories into patients with PPIs alone and patients with concurrent PPI and antibiotic treatment for gastric or duodenal ulcers. For this purpose, the covariate antibiotic use was subdivided, adding a third category denoting triple therapy for H. pylori eradication (concurrent prescription for PPI + clarithromycin + amoxicillin or metronidazole).
All statistical tests were 2-sided, and 95% confidence intervals (CIs) that did not overlap by 1.0 were considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute) and Stata version 13.1 (StataCorp LP).
RESULTS
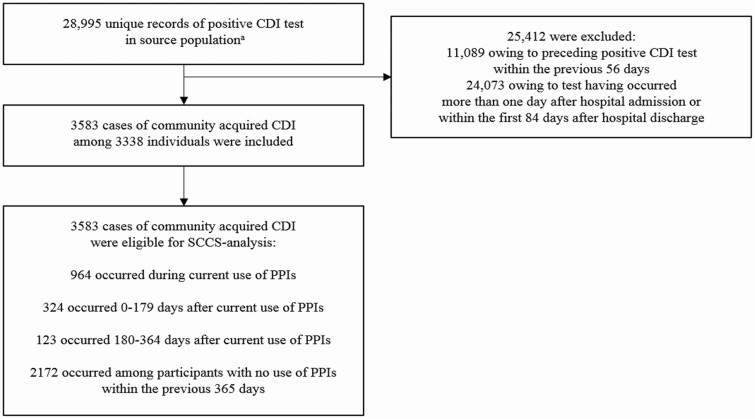
Figure 1 shows the flowchart of the study population selection. In total, we identified 28 995 unique records of a positive CDI test in the source population; 11 089 were excluded due to an additional positive CDI test within the previous 8 weeks and 24 073 were excluded due to the test having occurred 2 days or more after hospital admission or having been hospitalized within the previous 12 weeks, hence not fulfilling criteria of being community associated. The final study population consisted of 3583 cases of CA-CDI among 3338 individuals during the study period. Among included cases, the median age was 65 years (interquartile range, 44 to 80 years) and 38% were male, 54% recently filled a prescription for antibiotics, 8% for corticosteroids, and 36% were hospitalized (Table 1).
Figure 1.
Flowchart of the selection of the study population. aDanish people at least 20 years of age in 2010–2013. Abbreviations: CDI, Clostridium difficile infection; PPI, proton-pump inhibitor; SCCS, self-controlled case-series.
Table 1.
Case Characteristics of the Community-Acquired Clostridium difficile Infection Cohort
| Nonuse | Current Use of PPIs | 0–6 Months After Treatment Cessation | 6–12 Months After Treatment Cessation | |
|---|---|---|---|---|
| Number of CA-CDI events | 2172 | 964 | 324 | 123 |
| Male sex, n (%) | 845 (39) | 341 (35) | 130 (40) | 41 (33) |
| Age, mean (SD), years | 56.6 (22.0) | 71.6 (16.3) | 65.3 (19.5) | 59.1 (21.2) |
| Calendar year, n (%) | ||||
| 2010 | 386 (18) | 143 (15) | 40 (12) | 15 (12) |
| 2011 | 568 (26) | 238 (25) | 72 (22) | 34 (28) |
| 2012 | 567 (26) | 294 (30) | 100 (31) | 40 (33) |
| 2013 | 651 (30) | 289 (30) | 112 (35) | 34 (28) |
| Recent use of corticosteroids, n (%) | 126 (6) | 120 (12) | 34 (10) | 9 (7) |
| Recent use of antibiotics, n (%) | 1128 (52) | 554 (57) | 178 (55) | 66 (54) |
| Recent hospitalization, n (%) | 660 (30) | 443 (46) | 137 (42) | 45 (37) |
Abbreviations: CA-CDI, community-acquired Clostridium difficile infection; PPI, proton-pump inhibitor.
Of the 3583 episodes, during 2477 person-years of follow up 964 occurred during current use of PPIs, 324 occurred 0–6 months after treatment cessation, 123 occurred 6–12 months after treatment cessation, and 2172 occurred during time periods with no use of PPIs (Table 1). Comparing the incidence of CA-CDI during current use of PPIs with periods of nonuse, the unadjusted IRR was 2.78 (95% CI, 2.40–3.22). Adjusting for hospitalization, antibiotic use, and corticosteroid use yielded an adjusted IRR of 2.03 (95% CI 1.74–2.36). The increased risk was attenuated but remained elevated in later time periods (adjusted IRR, 1.54 [95% CI, 1.31–1.80] for 0–6 months and adjusted IRR 1.24 [1.00–1.53] for 6–12 months after current use of PPIs) (Figure 2).
Figure 2.
IRR of community-associated Clostridium difficile infection in users of proton-pump inhibitors. Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
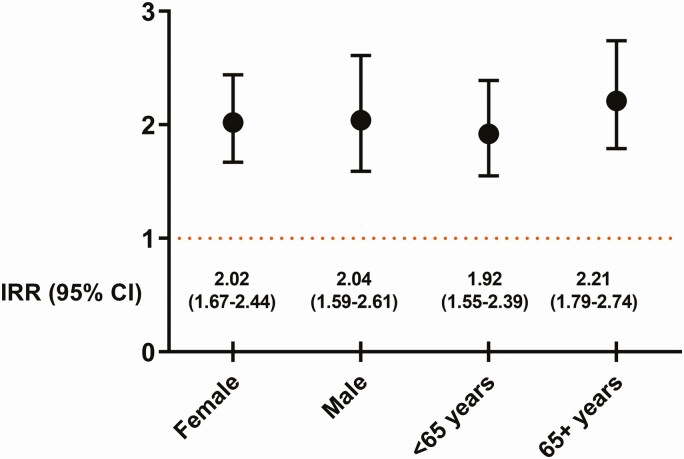
Estimates for the association between current use of PPIs and CA-CDI were similar across subgroups according to sex and age (adjusted IRR, 2.02 [95% CI, 1.67–2.44] in women and 2.04 [1.59–2.61] in men; and adjusted IRR, 1.92 [1.55–2.39] in patients aged <65 years and 2.21 [1.79–2.74] in patients aged ≥65 years) (Figure 3).
Figure 3.
IRR of community-associated Clostridium difficile infection in current users of proton-pump inhibitors according to age and gender. Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
The sensitivity analysis in which all patients with any hospital admission 1 year before the start of follow-up were excluded and in which follow-up was censored at first hospital admission during the study period yielded an adjusted IRR of 2.38 (95% CI, 1.63–3.47). In the second sensitivity analysis, in which analyses were split by triple therapy for H. pylori eradication, the adjusted IRR for PPI use alone was 2.06 (95% CI, 1.77–2.40).
DISCUSSION
In this population-based study of all incident cases of CA-CDI in Denmark during 2010–2014, we found that ongoing exposure to PPIs was associated with a doubled risk of CA-CDI. The risk was attenuated following treatment cessation but still remained significantly increased up to 1 year after PPI treatment had ended. Sixty-one percent of the CDI episodes occurred during time periods with no use of PPIs.
While a history of prior hospital admission, advanced age, and antibiotic use are well-known risk factors for CDI, the role of PPIs has remained controversial. Numerous observational studies have examined the role of PPIs in CDI. However, most studies have been conducted in the hospital setting and there has been considerable variability between studies [10]. The role of PPIs in CA-CDI has been examined in case-control studies; some report up to 3-fold increased odds ratios (ORs) [8, 17, 18], whereas other have reported neutral findings [19–22]. A recently published randomized controlled trial, mainly designed to assess cardiac safety of PPIs, reported an OR of 2.3 for CDI; however, CDI diagnosis was based on self-reporting every 6 months, and there were only 13 events of CDI during follow-up, which is why the increased risk was not statistically significant [23]. Despite this, the results support our findings.
The mechanism by which PPIs may increase the risk of CDI is not clear. Low or absent production of hydrochloric acid in gastric secretions increases the risk of enteric infections. However, disease transmission of C. difficile is mainly mediated by spores, which are not affected by gastric acidity [24, 25]. Instead, it has been proposed that PPI-induced alterations in intestinal microbiota may play an important function, by either inducing proliferation of C. difficile or by disturbing the capacity of the normal microbiome to suppress pathogen growth (colonization resistance) [10, 26, 27]; this potential explanation is, however, yet to be proved. It is also possible that the use of PPIs may increase the risk of infection with gastrointestinal pathogenic bacteria [10]. Because such infections cause diarrhea, diagnostic activity may increase and, hence, C. difficile might be demonstrated as a pure bystander rather than the actual cause of gastrointestinal illness. In this study, we did not exclude patients with polymicrobial infections, which remains a potential limitation.
In the present study, the adjusted IRR for CA-CDI was attenuated but remained elevated during both investigated time periods after treatment cessation. It is unclear whether this represents a sustained effect of PPIs after treatment discontinuation. Very few studies have explored long-term effects on the microbiome after PPI exposure. In a small study (n = 9), Seto et al [28] found decreased fecal microbial diversity during a 28-day treatment course of PPIs, which was partly reversed at follow-up after 4 weeks. In another study, based on 12 children, no relevant impact of PPIs was demonstrated on the fecal microbiome 4 weeks after discontinuation of PPIs [29].
A strength of our study is the nationwide design, including all available tests for C. difficile recorded in the microbiological laboratories in Denmark during the study period, which provides complete coverage and an unbiased selection of the cohort. The National Patient Registry covers all inpatient care in Denmark, ensuring adequate classification of HA- versus CA-CDI.
We used multiple measures to control for confounding. In the SCCS design, patients are used as their own controls, which minimizes the effect of time-fixed confounders. We also adjusted the analyses for major time-dependent confounders.
Although the SCCS design offers a powerful approach to control for confounding, we cannot rule out residual confounding. Reassuringly, the sensitivity analysis censoring on first hospitalization showed very similar results to the main analysis indicating that informative censoring was of minor importance.
An important study limitation, inherent to all database studies, is the potential misclassification of drug exposure. The Danish National Prescription Registry does not hold information on inpatient drug exposure; to take into account any delayed effects of hospital admission, we adjusted for recent hospitalization, defined as hospitalized time plus 90 days after discharge. Most PPIs are reimbursable when bought with a prescription in Denmark. A few PPIs are available over-the-counter. However, this only pertains to 2–3% of the total use of PPIs [30, 31]. We defined exposure based on filled prescriptions but did not have information on adherence to the prescribed regimen. However, previous studies have reported that filled prescriptions for PPI may be a reliable proxy for PPI use [12, 32]. Moreover, misclassification of drug exposure would, however, bias results towards the null and, if present, would not change the conclusion of the study.
The possibility that initial symptoms of CDI are misinterpreted and patients prescribed PPIs cannot be excluded. However, it is unlikely that this would lead to biased results because the symptoms of CDI (diarrhea) are distinct from the upper gastrointestinal symptoms that represent the most common indication for PPIs. Moreover, the sensitivity analyses in which the analysis was adjusted for triple therapy for H. pylori yielded very similar results to those in the primary analysis.
In conclusion, in this nationwide study in Denmark, we showed that exposure to PPIs was associated with a moderate increase in the risk of CA-CDI. The increased risk was most prominent during current PPI use but also persisted after treatment discontinuation.
Notes
Author contributions. M. I. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclaimer. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Financial support. There was no specific funding for this study. B. P. was supported by an investigator grant from the Strategic Research Area Epidemiology program at Karolinska Institutet. M. I. was supported by an investigator grant from the Swedish Government Funds for Clinical Research (ALF). A. H. reports grants from Novo Nordisk Foundation.
Potential conflicts of interest. All authors declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work. H. S. has received consulting fees from Celgene and is, outside of the submitted work, employed by IQVIA. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1. Buffie CG, Jarchum I, Equinda M, et al. . Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun 2012; 80:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freeman J, Bauer MP, Baines SD, et al. . The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev 2010; 23:529–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chitnis AS, Holzbauer SM, Belflower RM, et al. . Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 2013; 173:1359–67. [DOI] [PubMed] [Google Scholar]
- 4. Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ 2008; 179:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furuya-Kanamori L, Stone JC, Clark J, et al. . Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36:132–41. [DOI] [PubMed] [Google Scholar]
- 6. Soes LM, Holt HM, Bottiger B, et al. . Risk factors for Clostridium difficile infection in the community: a case-control study in patients in general practice, Denmark, 2009–2011. Epidemiol Infect 2014; 142:1437–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother 2008; 62:388–96. [DOI] [PubMed] [Google Scholar]
- 8. Dial S, Delaney JA, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ 2006; 175:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med 2015; 175:784–91. [DOI] [PubMed] [Google Scholar]
- 10. Villafuerte-Gálvez JA, Kelly CP. Proton pump inhibitors and risk of Clostridium difficile infection: association or causation? Curr Opin Gastroenterol 2018; 34:11–8. [DOI] [PubMed] [Google Scholar]
- 11. Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006; 25:1768–97. [DOI] [PubMed] [Google Scholar]
- 12. Othman F, Crooks CJ, Card TR. Community acquired pneumonia incidence before and after proton pump inhibitor prescription: population based study. BMJ 2016; 355:i5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011; 39:38–41. [DOI] [PubMed] [Google Scholar]
- 14. Voldstedlund M, Haarh M, Molbak K; MiBa Board of Representatives . The Danish Microbiology Database (MiBa) 2010 to 2013. Euro surveillance: bulletin Europeen sur les maladies transmissibles [European communicable disease bulletin]. 2014; 19:20667. [DOI] [PubMed] [Google Scholar]
- 15. McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK; Ad Hoc Clostridium difficile Surveillance Working Group . Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 2007; 28:140–5. [DOI] [PubMed] [Google Scholar]
- 16. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America . Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 17. Kuntz JL, Chrischilles EA, Pendergast JF, Herwaldt LA, Polgreen PM. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis 2011; 11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vesteinsdottir I, Gudlaugsdottir S, Einarsdottir R, Kalaitzakis E, Sigurdardottir O, Bjornsson ES. Risk factors for Clostridium difficile toxin-positive diarrhea: a population-based prospective case-control study. Eur J Clin Microbiol Infect Dis 2012; 31:2601–10. [DOI] [PubMed] [Google Scholar]
- 19. Kutty PK, Woods CW, Sena AC, et al. . Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis 2010; 16:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis 2006; 43:1272–6. [DOI] [PubMed] [Google Scholar]
- 21. Naggie S, Miller BA, Zuzak KB, et al. . A case-control study of community-associated Clostridium difficile infection: no role for proton pump inhibitors. Am J Med 2011; 124:276 e1–7. [DOI] [PubMed] [Google Scholar]
- 22. Guh AY, Adkins SH, Li Q, et al. . Risk factors for community-associated Clostridium difficile Infection in adults: a case-control study. Open Forum Infect Dis 2017; 4:ofx171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moayyedi P, Eikelboom JW, Bosch J, et al. . Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology 2019; 157:682–91 e2. [DOI] [PubMed] [Google Scholar]
- 24. Gurian L, Ward TT, Katon RM. Possible foodborne transmission in a case of pseudomembranous colitis due to Clostridium difficile: influence of gastrointestinal secretions on Clostridium difficile infection. Gastroenterology 1982; 83:465–9. [PubMed] [Google Scholar]
- 25. Nerandzic MM, Pultz MJ, Donskey CJ. Examination of potential mechanisms to explain the association between proton pump inhibitors and Clostridium difficile infection. Antimicrob Agents Chemother 2009; 53:4133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 2014; 146:1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blanchi J, Goret J, Mégraud F. Clostridium difficile infection: a model for disruption of the gut microbiota equilibrium. Dig Dis 2016; 34:217–20. [DOI] [PubMed] [Google Scholar]
- 28. Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome 2014; 2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castellani C, Singer G, Kashofer K, et al. . The influence of proton pump inhibitors on the fecal microbiome of infants with gastroesophageal reflux—a prospective longitudinal interventional study. Front Cell Infect Microbiol 2017; 7:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pottegård A, Broe A, Hallas J, de Muckadell OB, Lassen AT, Lødrup AB. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol 2016; 9:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haastrup P, Paulsen MS, Zwisler JE, et al. . Rapidly increasing prescribing of proton pump inhibitors in primary care despite interventions: a nationwide observational study. Eur J Gen Pract 2014; 20:290–3. [DOI] [PubMed] [Google Scholar]
- 32. Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–8. [DOI] [PubMed] [Google Scholar]