Abstract
Background
New, sensitive diagnostic tests facilitate identification and investigation of milder forms of tuberculosis (TB) disease. We used community-based TB testing with the Xpert MTB/RIF Ultra assay (“Ultra”) to characterize individuals with previously undiagnosed TB and compare them to those from the same community who were diagnosed with TB through routine care.
Methods
We offered community-based sputum Ultra testing to adult residents of a well-defined area (population 34 000 adults) in Kampala, Uganda, via door-to-door screening and venue-based testing, then used detailed interview and laboratory testing to characterize TB-positive individuals. We compared these individuals to residents diagnosed with pulmonary TB at local health facilities and a representative sample of residents without TB (controls).
Results
Of 12 032 residents with interpretable Ultra results, 113 (940 [95% confidence interval {CI}, 780–1130] per 100 000) tested positive, including 71 (63%) positive at the lowest (trace) level. A spectrum of TB disease was observed in terms of chronic cough (93% among health facility–diagnosed cases, 77% among residents with positive community-based Ultra results at levels above trace, 33% among trace-positive community participants, and 18% among TB-negative controls), TB symptom prevalence (99%, 87%, 60%, and 38%, respectively), and C-reactive protein (75th percentile: 101 mg/L, 28 mg/L, 6 mg/L, and 4 mg/L, respectively). Community-diagnosed cases were less likely than health facility–diagnosed cases to have human immunodeficiency virus coinfection or previous TB. The specificity of Ultra was 99.4% (95% CI, 99.2%–99.5%) relative to a single spot sputum culture.
Conclusions
People with undiagnosed prevalent TB in the community have different characteristics than those diagnosed with pulmonary TB in health facilities. Newer diagnostic tests may identify a group of people with early or very mild disease.
Keywords: active case-finding, Xpert MTB/RIF Ultra, subclinical tuberculosis, prevalent tuberculosis
Community-based tuberculosis testing with Xpert MTB/RIF Ultra revealed prevalent disease that was milder and potentially more prolonged compared to routinely diagnosed tuberculosis from the same community. Trace-positive Xpert Ultra results were common, but the specificity of community-based testing exceeded 99%.
(See the Editorial Commentary by Wong on pages e1044–6.)
Tuberculosis (TB) is the leading infectious cause of mortality, killing >1.4 million people every year [1]; yet, the spectrum of TB disease remains poorly characterized. Nationally representative surveys have documented a large burden of undiagnosed prevalent TB [1], much of which is not associated with classic TB symptoms [2]. A recent trial in rural Vietnam demonstrated that universal bacteriologic testing could reduce TB burden at the community level [3], but more scalable case-finding approaches now need to be developed.
Designing targeted interventions to reduce this burden requires a better understanding of how people living with undiagnosed active TB (henceforth referred to as “prevalent TB”) differ from those who are routinely diagnosed in health facilities. New diagnostic tests for TB disease, such as Xpert MTB/RIF Ultra assay (hereafter “Ultra”), offer high diagnostic sensitivity [4, 5], and their use as research tools may facilitate a deeper understanding of the spectrum of TB disease at the community level than was previously possible.
METHODS
Study Overview
In a community-based, cross-sectional study, we performed TB testing with Ultra on sputum, through a combination of door-to-door and venue-based screening, from adult (≥15 years old) residents of a defined geographic area in Kampala, Uganda. People with a positive Ultra result formed 1 arm of a comparison between 3 populations:
Residents with a positive Ultra test in community-based testing (community-based cases).
Residents from the same community who were diagnosed with pulmonary TB through routine care at local health facilities (health-facility cases) over a 22-month period that included the 10-month community-based testing campaign.
Representative residents without TB from the same community (community controls).
The study area was a prespecified, densely populated, largely residential urban region in central Kampala, Uganda, consisting of 38 contiguous zones (the lowest administrative level in Uganda) with a combined area of 2.2 km2.
Community-Wide TB Testing
From February to November 2019, research staff moved systematically through the study area; they enumerated all households, collected household composition data from residents or neighbors, and asked adult residents to provide expectorated sputum for TB testing, regardless of symptoms or treatment history. Recruitment was attempted at all residences and at nonresidential locations such as workplaces. In parallel, 10 scheduled venue-based screening events were held in public spaces (eg, community halls or markets) selected with input from community leaders. Sputum Ultra testing was performed at Makerere University according to standard protocols [6]. The Supplementary Data describe additional details of community-based testing, population enumeration, venue-based screening events, methods to prevent duplicate enrollment, and methods to increase yield of sputum expectoration.
Community-Based Case Enrollment
Residents with a positive Ultra result in community-wide testing were referred to clinical care (with treatment decisions left to clinicians) and enrolled as community-based cases.
Community-based cases underwent human immunodeficiency virus (HIV) testing (Abbott Determine [Abbott Laboratories] with STAT Pack confirmation [ChemBio]); serum C-reactive protein (CRP) testing (i-CHROMA [Boditech, Korea] or Eurolyser [Eurolyser Diagnostica, Austria]); a standardized interview (including TB symptoms of self-reported chronic cough [≥2 weeks], fever, night sweats, or weight loss); and repeat sputum expectoration for liquid and solid culture using standard, previously described methods [7].
We performed Ultra testing or retesting of adult contacts of enrolled cases (details shown in the Supplementary Data); those with a positive result (n = 2) were also enrolled as community-based cases.
Health-Facility Case Enrollment
We also enrolled, from May 2018 through February 2020, all adult residents who were diagnosed with pulmonary TB at any of the 4 TB diagnosis and treatment centers located within the study area. An initial review of TB treatment in all nearby facilities [8] had suggested that 90% of study area residents diagnosed with TB were treated in these 4 facilities (1 public, 3 private).
Health-facility cases who agreed to participate underwent the same evaluation as community-based cases (with contact investigation performed only during the community-wide case-finding campaign). Sputum culture was performed on a new spot specimen obtained the day of enrollment.
Community Control Enrollment
For each community-based case, and for each health-facility case enrolled during the community-wide testing period, we randomly selected 1 Ultra-negative community participant (hereafter “control”) matched by zone of residence (Supplementary Data). Controls underwent the same interview, HIV testing, CRP measurement, and sputum mycobacterial culture as cases. Controls with Mycobacterium tuberculosis–positive sputum culture (n = 2) were reclassified as cases and replaced with new controls.
Statistical Methods
Binary variables were summarized as proportions with 95% binomial confidence intervals (CIs), event rates as means with 95% CIs based on Poisson-distributed counts, and other continuous variables as medians with interquartile ranges (IQRs). Comparisons used Fisher exact test for categorical variables, Wilcoxon rank-sum tests for continuous variables, and logistic regression for sex-adjusted comparisons. All analyses used R version 3.5.2 software [9].
We estimated the prevalence of Ultra-positive results (including trace-positive) among individuals who completed community-based screening with an interpretable result. We compared this result to estimates (Supplementary Table 1) of (1) age- and sex-adjusted prevalence; (2) the proportion of participants diagnosed with TB through all active case-finding activities (including contact investigation and sputum culture); (3) prevalence among all who attempted screening, including those unable to produce sputum; (4) the prevalence of Ultra-positive, culture-confirmed TB; (5) the prevalence of culture-positive TB, extrapolating the observed prevalence of positive culture among randomly selected Ultra-negative controls to the full population; and (6) the proportion of residents diagnosed with TB (including at health facilities) during the community-based case-finding period.
Finally, we estimated the specificity of Ultra relative to a single spot sputum culture, assuming that sputum culture would have been negative, if performed, for 98.6% of Ultra-negative participants who did not undergo sputum culture; the Supplementary Data describe details and sensitivity analyses.
Ethical Considerations
The Ethics Review Committee of the Makerere University School of Public Health approved the study. All participants provided informed consent/assent.
RESULTS
Results of Community-Based Ultra Testing
Of an estimated 34 135 adults who lived in the designated study area (along with 16 301 children; Supplementary Figure 1), 12 301 adults were contacted and consented to screening, of whom 12 032 (98%) successfully submitted sputum that generated a valid Ultra result (Figure 1). Of specimens with valid results, 7998 (66.5%) were collected at participants’ homes; reasons for not enrolling at home are enumerated in the Supplementary Data. Another 753 Ultra results (6.3% of all valid results) were obtained through 10 venue-based screening events, and 3281 (27.3%) were collected at other away-from-home testing locations (public or commercial spaces, homes of other residents, or venue-based screening events). Supplementary Table 2 provides age- and sex-stratified estimates of prevalence. Men were less likely than women to participate in testing overall (odds ratio [OR], 0.87 [95% CI, .84–.91]) but comprised the majority of participants at away-from-home testing locations (Supplementary Table 3).
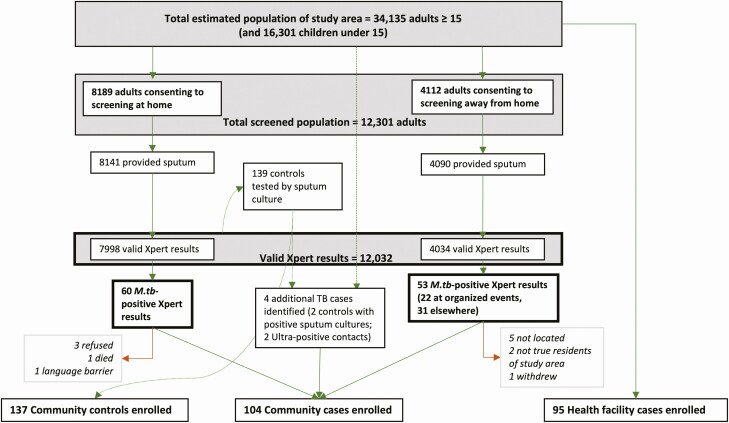
Figure 1.
Flow diagram of population tuberculosis screening and sputum Xpert MTB/RIF Ultra testing results. Community cases were drawn from those diagnosed in community-wide screening (either at home [n = 60] or in away-from-home locations [n = 51 after 2 were determined to live outside the study area]) diagnosed in the community through sputum culture (n = 1) or contact investigation (n = 3). One person died prior to enrollment (11 days after testing). Two community-based cases sought care for symptoms at local healthcare facilities while awaiting results and were also enrolled as health-facility cases (46 and 59 days after testing). Participants in boxes with bold outlines formed the basis of our primary prevalence estimate. See Supplementary Figure 1 for full flow diagram including estimation of population and all reasons for exclusion. Abbreviations: M.tb, Mycobacterium tuberculosis; TB, tuberculosis.
Of the 12 032 valid community-based Ultra results, 113 (0.94%) were positive, including 42 (37% of positives, 0.35% of all results) at levels greater than trace and 71 (63% of positives, 0.59% of all results) at the trace level. Men accounted for 58% of positive tests (OR of positive result, 1.7 [95% CI, 1.2–2.5], compared to women). A higher proportion of results were positive at venue-based screening events (22 of 753 [2.9%]) than in home-based testing (60 of 7998 [0.8%]) or other away-from-home locations (27 of 3281 [0.9%]). The prevalence among venue-based screening participants remained elevated after adjusting for age and sex (3.0% [95% CI, 1.8%–4.7%]; Supplementary Table 3). Community-based cases were found in 27 of the community’s 38 zones.
Two-thirds of submitted sputum specimens were graded as salivary, and half had volume ≤1 mL; however, these specimens had reasonable levels of positivity (0.7% for salivary vs 1.4% nonsalivary; 0.9% for ≤1 mL vs 1.1% for >1 mL; Supplementary Table 4).
Enrollment of Health Facility and Community Controls
One hundred four community-based cases were enrolled: 100 identified through community-wide testing (90% of 111 eligible Ultra-positive individuals), 2 identified through contact investigation, and 2 identified through sputum culture of Ultra-negative community controls (Figure 1). Thus, community-based testing identified 11.5 cases per month (95% CI, 9.5–13.8) during the 10 months of community-based case-finding, while local health facilities diagnosed 6.1 (95% CI 5.7–6.5) cases per month over the 22-month study period. The health facility diagnosis rate did not differ significantly for the time periods before vs during or after the case-finding campaign (Supplementary Figure 2). Of 134 eligible health-facility cases, 95 enrolled in the study (Supplementary Figure 1).
Ultra Accuracy and Community TB Prevalence
Of the community-based cases with interpretable sputum culture results (94 of 102 cases with positive Ultra results; Supplementary Table 5), 11 of 38 (29%) who were Ultra-positive at levels greater than trace had a negative culture result, compared with 48 of 56 (86%) of those with a trace-positive Ultra. Taking the single spot sputum culture as a reference, the specificity of Ultra was 99.4% (95% CI, 99.2%–99.5%) and robust to sensitivity analyses (Supplementary Table 6).
The number of Ultra-negative individuals who underwent culture was too small to estimate sensitivity with precision, but 2 (1.4% [95% CI, .07%–5.4%]) of the 139 Ultra-negative individuals enrolled as controls had a positive culture result.
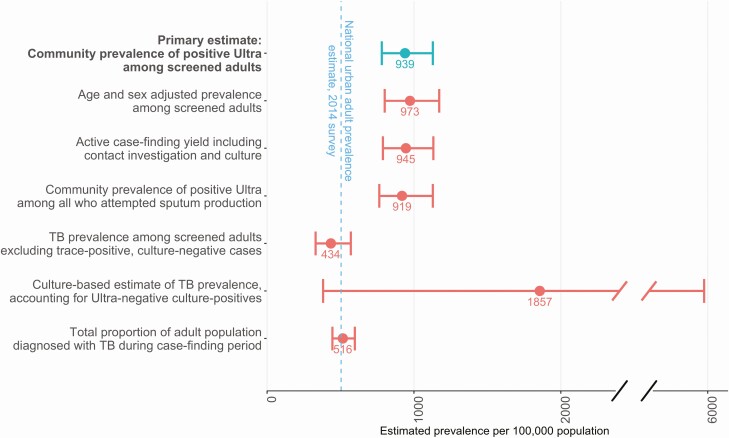
The estimated community prevalence of a positive Ultra result was 940 (95% CI, 780–1130) per 100 000 adults. If individuals with Ultra trace-positive, culture-negative sputum were excluded, the estimated prevalence of TB fell to 420 (95% CI, 320–550) per 100 000 adults. Alternative estimates of TB prevalence are shown and compared to the estimated national TB prevalence in Figure 2.
Figure 2.
Estimated prevalence of tuberculosis (TB) among adults aged ≥15 years in an urban Ugandan community. The study’s primary measure (top bar) is compared to multiple alternative definitions and to the Ugandan national prevalence survey estimate for urban areas, as detailed in Supplementary Table 1. The estimated prevalence increased slightly when adjusted for the age and sex of those screened, because younger men were both more likely to have TB and more likely to be missed by screening (Supplementary Table 3), and decreased slightly if the 70 consenting adults who were unable to produce sputum (0.6%) and the 199 with an invalid or missing Xpert MTB/RIF Ultra result (1.7%) were included. Exclusion of cases who were trace positive but culture negative shifted prevalence estimates more significantly. Conversely, including an estimate of the number who were Ultra negative but would have been culture positive increased the prevalence estimate dramatically, but our small sample size of cultures in Ultra-negative individuals is reflected in the large uncertainty around this estimate. Error bars indicate 95% confidence intervals.
Comparison of Cases by Place of Diagnosis
Relative to controls, individuals with TB were more often male (67% vs 38%, P < .0001), older (median, 31 vs 26 years, P = .001), current smokers (20% vs 7%, P = .0005), previously incarcerated (36% vs 12%, P < .0001, most for <7 nights and >3 years prior), and symptomatic (any TB symptom: 84% vs 38%, P < .0001; chronic cough: 70% vs 18%, P < .0001). These differences were present for cases identified by routine diagnosis, door-to-door screening, and away-from-home screening (Table 1), and persisted after adjusting for sex.
Table 1.
Clinical and Bacteriologic Characteristics of Community-Based Tuberculosis Cases, by Place of Diagnosis
| Place of Diagnosis of Community-Based Cases | |||
|---|---|---|---|
| Characteristic | Home (n = 59) |
Screening Event (n = 20) |
Other Location (n = 25) |
| Age, y, median (IQR) | 28 (23–34) | 31 (24–35) | 35 (25–47) |
| Sex | |||
| Male | 23 (39) | 13 (65) | 23 (92) |
| Female | 36 (61) | 7 (35) | 2 (8) |
| HIV and ART status | |||
| Negative | 50 (85) | 19 (95) | 23 (92) |
| Positive not on ART | 3 (5) | 0 (0) | 0 (0) |
| Positive on ART | 6 (10) | 1 (5) | 2 (8) |
| History of prior TB treatment | |||
| Yes | 3 (5) | 2 (10) | 2 (8) |
| No | 56 (95) | 18 (90) | 23 (92) |
| TB contact (same household or past 12 mo) | 29 (49) | 13 (65) | 10 (40) |
| Smoking status | |||
| Current smoker | 11 (19) | 4 (20) | 9 (36) |
| Former smoker | 1 (2) | 3 (15) | 3 (12) |
| Never smoker | 47 (80) | 13 (65) | 13 (52) |
| Any TB symptom | 38 (64) | 15 (75) | 20 (80) |
| Chronic cough (≥2 wk) | 23 (39) | 13 (65) | 15 (60) |
| Duration of TB symptoms, wk, median (IQR) | 2.0 (0–8.0) | 4.0 (0.75–16) | 3.0 (2.0–6.0) |
| Coughed during 5-min observation period | 8 (14) | 5 (25) | 6 (24) |
| Serum CRP, mg/L | |||
| Median (IQR) | <LLDa (2.5–9.3) | 3.6 (<LLDa–5.7) | 4.3 (<LLDa–32) |
| Missing | 3 (5.1) | 3 (15.0) | 3 (12.0) |
| Sputum smear status | |||
| Negative | 52 (88) | 16 (80) | 19 (76) |
| Positive | 7 (12) | 4 (20) | 6 (24) |
| Sputum culture result | |||
| Positive | 18 (31) | 6 (30) | 12 (48) |
| Negative | 39 (66) | 10 (50) | 11 (44) |
| Missing | 2 (3.4) | 4 (20.0) | 2 (8.0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; CRP, C-reactive protein; HIV, human immunodeficiency virus; IQR, interquartile range; LLD, lower limit of detection; TB, tuberculosis.
aBelow the lower limit of detection of ≤2.5 mg/L.
People diagnosed with TB through routine health services differed from those diagnosed through community-wide testing (as well as from controls) in terms of both HIV infection (38% prevalence among health-facility cases vs 12% among community-based cases and 10% among controls) and previous treatment for TB (24% prevalence among health-facility cases, 7% among community-based cases, and 3% among controls). Community-based cases had less contact with the healthcare system (eg, 37% visiting less than once per year) than either health-facility cases (22%) or controls (23%) (Supplementary Table 9).
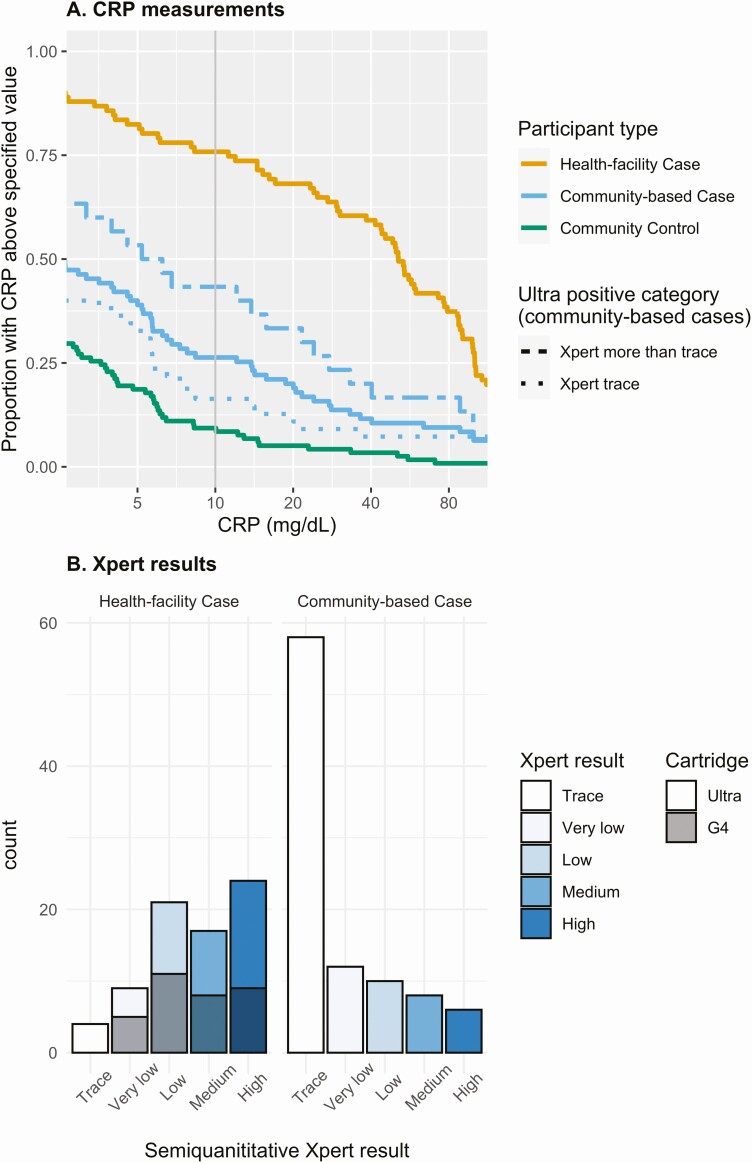
Of the community-based cases with positive Ultra results at levels greater than trace, most (87%) reported chronic cough or another characteristic TB symptom, and 95% reported a cough at the time of enrollment. These same community-based cases had similar duration of symptoms compared to health-facility cases (median, 8 [IQR, 3–16] weeks vs 8 [IQR, 4–16] weeks), similar distribution of individual symptoms (Table 2; Supplementary Table 9), and similar sputum bacillary loads (sputum smear positivity in 44% vs 55%, P = .3, with a modest tendency toward lower semi-quantitative Xpert results in community-based cases; Figure 3B, P = .06 after excluding trace-positives). However, community-based cases had much lower CRP values than health-facility cases, even when limited to community-based cases with Ultra greater than trace (median, 6 mg/L vs 51 mg/L; Figure 3A). Within each case type, symptoms and CRP values were not strongly correlated (Supplementary Table 7).
Table 2.
Clinical and Bacteriologic Characteristics of Tuberculosis Cases, by Level of Ultra Positivity
| Health-Facility Case | Community-Based Case | Community Control | |||
|---|---|---|---|---|---|
| Characteristic | (n = 95) | All Cases (n = 104)a |
Ultra More Than Trace (n = 39) | Ultra Trace (n = 63) | (n = 137) |
| Age, y, median (IQR) | 33 (27–40) | 29 (24–37) | 30 (25–36) | 28 (22–37) | 26 (22–35) |
| Sex | |||||
| Male | 65 (68) | 59 (57) | 27 (69) | 31 (49) | 45 (33) |
| Female | 30 (32) | 45 (43) | 12 (31) | 32 (51) | 92 (67) |
| HIV and ART status | |||||
| Negative | 59 (62) | 92 (88) | 34 (87) | 56 (89) | 123 (90) |
| Positive not on ART | 10 (11) | 3 (3) | 3 (8) | 0 (0) | 1 (1) |
| Positive on ART | 26 (27) | 9 (9) | 2 (5) | 7 (11) | 13 (9) |
| History of prior TB treatment | 23 (24) | 7 (7) | 2 (5) | 5 (8) | 4 (3) |
| TB contact (same household or past 12 mo) | 39 (41) | 52 (50) | 19 (49) | 32 (51) | 44 (32) |
| Smoking status | |||||
| Current smoker | 16 (17) | 24 (23) | 10 (26) | 13 (21) | 9 (7) |
| Former smoker | 21 (22) | 7 (7) | 6 (15) | 1 (2) | 4 (3) |
| Never smoker | 58 (61) | 73 (70) | 23 (59) | 49 (78) | 124 (91) |
| Any TB symptom | 94 (99) | 73 (70) | 34 (87) | 38 (60) | 52 (38) |
| Chronic cough (≥2 wk) | 88 (93) | 51 (49) | 30 (77) | 21 (33) | 24 (18) |
| Duration of TB symptoms, wk, median (IQR) | 8.0 (4.0–16) | 3.0 (0–8.8) | 8.0 (3.0–16) | 2.0 (0–4.0) | 0 (0–4.0) |
| Coughed during 5-min observation period | 45 (47) | 19 (18) | 12 (31) | 7 (11) | 9 (7) |
| Serum CRP, mg/L, median (IQR) | 51 (12–100) | 2.6 (<LLDb–13) | 6.2 (<LLDb–28) | 2.5 (<LLDb–5.7) | <LLDb (<LLDb–3.5) |
| Missing | 4 (4.2) | 9 (8.7) | 6 (15.4) | 3 (4.8) | 19 (13.9) |
| Sputum smear status | |||||
| Negative | 42 (44) | 87 (84) | 22 (56) | 63 (100) | 130 (95) |
| Positive | 50 (53) | 17 (16) | 17 (44) | 0 (0) | 1 (1) |
| Missing | 3 (3.2) | 0 (0) | 0 (0) | 0 (0) | 6 (4.4) |
| Sputum culture result | |||||
| Positive | 65 (68) | 36 (35) | 27 (69) | 8 (13) | 0 (0) |
| Negative | 23 (24) | 60 (58) | 11 (28) | 48 (76) | 121 (88) |
| Missing | 7 (7.4) | 8 (7.7) | 1 (2.6) | 7 (11.1) | 16 (11.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; CRP, C-reactive protein; HIV, human immunodeficiency virus; IQR, interquartile range; LLD, lower limit of detection; TB, tuberculosis.
aTwo community-based cases with a negative Ultra result (diagnosed based on culture) are included only in the “all cases” column.
bBelow the lower limit of detection of ≤2.5 mg/L.
Figure 3.
Distributions of serum C-reactive protein (CRP) levels and semi-quantitative Xpert MTB/RIF results, comparing people diagnosed with tuberculosis (TB) at health facilities to those diagnosed through community-based case finding. A, Inverse cumulative distribution of CRP values; the value on the y-axis indicates the proportion of participants with a CRP above a given value. For example, for a CRP cutoff of 10 mg/mL (vertical gray line), 75% of health-facility cases, 26% of community-based cases, and 9% of community controls have a CRP above the cutoff. Results for the individuals identified as community-based TB cases are shown together (solid blue lines) and stratified by level of Xpert MTB/RIF Ultra positivity (trace [dotted] or more than trace [dashed]). Health-facility cases (some of whom were testing with the older [G4] Xpert cartridge, which does not have a trace call, prior to February 2019) are plotted as a single line. B, Semi-quantitative level of all positive Xpert MTB/RIF results; for health facility cases, gray shading indicates the type of Xpert MTB/RFIF cartridge used.
Characteristics of Ultra Trace-Positive Individuals
Community-based cases with trace-positive Ultra results had similar prevalence of known TB exposure as other community-based cases, with a recent or close TB contact reported by 49% of non-trace-positive cases, 51% of trace-positive cases, and 32% of controls. For other TB risk factors, the trace-positive population was intermediate between other community-based cases and controls: for example, male sex (69% vs 49% vs 33%), current smoking (26% vs 21% vs 7%), and previous incarceration (41% vs 21% vs 12%). Trace-positive cases also had intermediate indicators of disease severity; for example, positive TB symptom screen (87% vs 60% vs 38%), chronic cough (77% vs 33% vs 18%), and CRP (75th percentile: 28 vs 5.7 vs 3.5 mg/L) (Figure 3A). Differences between trace-positive individuals and controls were still evident when analysis was limited to those with trace-positive Ultra but negative sputum culture (Supplementary Table 8).
Previous TB was also more common among trace-positive, culture-negative individuals (5/48 [10%]) than among controls (4/137 [3%]), but unlike some health facility-based populations in which half of trace-positive, culture-negative results could be attributed to previous treatment [6], previous treatment could explain only a small proportion of the trace positives in our community-based population. Trace-positive Ultra results accounted for a similar proportion of positive results in all types of community screening locations (65% at home, 64% at venue-based events, and 58% at other away-from-home locations; P = .8).
DISCUSSION
This study of >12 000 individuals tested for TB with Ultra in a community setting, in parallel with characterization of health facility–diagnosed cases and community-representative controls, provides a novel picture of the spectrum of TB disease in a high-burden community. Universal testing with a highly sensitive assay identified both highly symptomatic individuals with high bacillary burdens (37%) and people with trace-positive sputum (63%) who were largely culture negative but had characteristics that differentiated them from TB-negative controls. Compared to people diagnosed with TB in health facilities, individuals with TB in the community had lower inflammatory markers, were less likely to be HIV positive, and used healthcare resources less often. These findings illustrate, at high resolution, the spectrum of TB including subclinical and mildly symptomatic disease.
While some people with Ultra trace-positive sputum may have no TB disease (ie, represent false-positive Ultra results), trace-positive individuals were distinguished from randomly selected TB-negative controls by their TB risk profile (including male sex, smoking, and known TB contacts) and TB symptoms. Eighty percent of trace-positive individuals had a negative result on a single spot sputum culture, but culture has imperfect sensitivity as a reference standard [10], and the Ultra assay was designed to match the limit of detection of culture [11]. Thus, it is plausible that trace Ultra results identify some individuals with early, mild, or resolving TB disease whose cultures are falsely negative. Further study is needed—including radiographic characterization, additional cultures, and longitudinal follow-up—to clarify the meaning of these results.
Xpert Ultra has shown reduced specificity (96%) in clinical settings [6, 12], raising concern that Ultra might not be useful for screening general populations that have a lower pretest probability of TB. Our results provide a reassuringly higher estimate (99.4%) of the specificity of Ultra in a community-based screening context. Moreover, this estimate relative to a single culture likely underestimates the true specificity of Ultra. Although using Ultra to screen every person in a community is unlikely to be feasible at scale (without a dramatic reduction in price), our results show that Ultra can be used as a research tool to guide the development of more targeted and scalable diagnostic or preventive strategies. In addition, when paired with an appropriate triage test or targeting strategy, concerns about specificity should not preclude using Ultra in community-based active case-finding.
Our results highlight the challenges of finding TB cases in an urban setting. Due to long working hours, commutes, and migration, many residents were consistently unavailable at their homes, resulting in lower uptake of TB testing relative to what has been reported in rural Vietnam [3]. Our finding that venue-based screening events yielded >3 times as many positive results as screening the same number of people door-to-door is consistent with earlier work using a mobile testing van in Zimbabwe [13] and suggests a potential strategy for efficient active case-finding, particularly in zones with high TB notification rates [14]. In an urban setting, case-finding at community venues outside the home may identify a population that is difficult to find through door-to-door screening but epidemiologically important, both because the demographically highest-risk residents are rarely at home, and because symptomatic or high-risk participants may self-select for venue-based screening.
Our study has important limitations. First, we were unable to contact 60% of the population. This may bias our estimates of prevalence either upward (eg, because symptomatic people were more likely to participate) or downward (because low-risk individuals were at home more often). The participants nevertheless represent the population who could be contacted in a community-based active case-finding intervention. It is also encouraging that we were remarkably successful in obtaining sputum from those who participated, suggesting feasibility of sputum-based screening. Second, selected controls may be more representative of the population tested at home than of the overall community. In future studies, venue-based approaches could be used more extensively to increase population coverage and representativeness. Third, among those who submitted Ultra-positive sputum, 11 (9%) did not participate in additional data collection, and the reasons for nonenrollment are unlikely to be random (eg, more severe disease in the person who died). Finally, this study did not include longitudinal follow-up. Because clinicians chose to treat most Ultra-positive participants, it is not known what proportion would have eventually been diagnosed based on symptoms—nor what proportion would spontaneously resolve without treatment.
In summary, we used universal sputum Ultra testing in an urban community setting to characterize the spectrum of prevalent TB disease and understand how it differed from health facility–diagnosed TB in the same community. We identified individuals with prevalent TB whose high bacillary load and symptom burden were comparable to routinely notified cases, but whose potential for prolonged undiagnosed illness (due to infrequent healthcare access and lower HIV prevalence) make them an important target for active case-finding. A venue-based screening approach showed promise as a means of more efficiently identifying such individuals in the community. We also identified individuals with trace-positive Ultra results who differed from TB-negative controls in ways that might reflect early-stage TB. They would go undetected by the less sensitive screening approaches used in many previous case-finding campaigns. Together, these results show the potential of new diagnostics such as Ultra to aid in targeted case-finding among high-risk populations, and they represent an important step toward better a understanding of the epidemiological and clinical significance of the full spectrum of TB disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers R01HL138728 to D. W. D. and K08AI127908 to E. A. K.) and by the Fogarty-Fulbright Fellowship in Public Health (award number FIC D43 TW010540 to K. O. R.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Global tuberculosis report 2019. Geneva, Switzerland: WHO, 2019. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed 10 December 2019. [Google Scholar]
- 2. Frascella B, Richards AS, Sossen B, et al. . Subclinical tuberculosis disease—a review and analysis of prevalence surveys to inform definitions, burden, associations and screening methodology [manuscript published online ahead of print September 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marks GB, Nguyen NV, Nguyen PTB, et al. . Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 2019; 381:1347–57. [DOI] [PubMed] [Google Scholar]
- 4. Chakravorty S, Simmons AM, Rowneki M, et al. . The new Xpert MTB/RIF ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 2017; 8:e00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horne DJ, Kohli M, Zifodya JS, et al. . Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2019; 6:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorman SE, Schumacher SG, Alland D, et al. ; Study Team . Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018; 18:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kendall EA, Kamoga C, Kitonsa PJ, et al. . Empiric treatment of pulmonary TB in the Xpert era: correspondence of sputum culture, Xpert MTB/RIF, and clinical diagnoses. PLoS One 2019; 14:e0220251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robsky KO, Hughes S, Kityamuwesi A, et al. . Is distance associated with tuberculosis treatment outcomes? A retrospective cohort study in Kampala, Uganda. BMC Infect Dis 2020; 20:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 10. Parker RA. Implications of tuberculosis sputum culture test sensitivity on accuracy of other diagnostic modalities. Am J Respir Crit Care Med 2018; 199:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alland D, Rowneki M, Smith L, et al. . Xpert MTB/RIF Ultra: a new near-patient TB test with sensitivity equal to culture. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 23–26 February 2015. [Google Scholar]
- 12. Kendall EA, Schumacher SG, Denkinger CM, Dowdy DW. Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: a modeling study. PLoS Med 2017; 14:e1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corbett EL, Bandason T, Duong T, et al. . Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010; 376:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robsky KO, Kitonsa PJ, Mukiibi J, et al. . Spatial distribution of people diagnosed with tuberculosis through routine and active case finding: a community-based study in Kampala, Uganda. Infect Dis Poverty 2020; 9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.