Abstract
Background
Metabolic acidosis is a common problem in haemodialysis patients, but acidosis overcorrection has been associated with higher mortality. There is no clear definition of the optimal serum bicarbonate target or dialysate bicarbonate. This study analysed the impact of reducing dialysate bicarbonate from 35 to 32 mEq/L on plasma bicarbonate levels in a cohort of patients treated with online haemodiafiltration (OL-HDF).
Methods
We performed a prospective cohort study with patients in a stable chronic OL-HDF programme for at least 12 months in the Hospital Clinic of Barcelona. We analysed pre- and post-dialysis total carbon dioxide(TCO2) before and after dialysate bicarbonate reduction from 35 to 32 mEq/L, as well as the number of patients with a pre- and post-dialysis TCO2 within 19–25 and ≤29 mEq/L, respectively, after the bicarbonate modification. Changes in serum sodium, potassium, calcium, phosphorous and parathyroid hormone (PTH) were also assessed.
Results
We included 84 patients with a 6-month follow-up. At 6 months, pre- and post-dialysis TCO2 significantly decreased (26.78 ± 1.26 at baseline to 23.69 ± 1.92 mEq/L and 31.91 ± 0.91 to 27.58 ± 1.36 mEq/L, respectively). The number of patients with a pre-dialysis TCO2 >25 mEq/L was significantly reduced from 80 (90.5%) to 17 (20.2%) and for post-dialysis TCO2 >29 mEq/L this number was reduced from 83 (98.8%) to 9 (10.7%). PTH significantly decreased from 226.09 (range 172–296) to 182.50 (125–239) pg/mL at 6 months (P < 0.05) and post-dialysis potassium decreased from 3.16 ± 0.30 to 2.95 ± 0.48 mEq/L at 6 months (P < 0.05). Sodium, pre-dialysis potassium, calcium and phosphorous did not change significantly.
Conclusions
Reducing dialysate bicarbonate concentration by 3 mEq/L significantly and safely decreased pre- and post-dialysis TCO2, avoiding acidosis overcorrection and improving secondary hyperparathyroidism control. An individualized bicarbonate prescription (a key factor in the adequate control of acidosis) according to pre-dialysis TCO2 is suggested based on these results.
Keywords: bicarbonate, dialysate, hyperparathyroidism, online haemodiafiltration, total carbon dioxide
INTRODUCTION
The aim of dialysate fluid is to normalize pre-dialysis plasma electrolytes and provide toxin and phosphate removal [1]. Among the various components of dialysis fluid, bicarbonate is the fundamental compound for the correction of metabolic acidosis, an intrinsic condition in end-stage kidney disease (ESKD).
Current evidence shows that uncorrected metabolic acidosis is an independent risk factor for mortality in patients on haemodialysis [1, 2]. However, no adequately powered clinical trials have examined the associations among different dialysate bicarbonate concentrations and outcomes, resulting in reliance on observational data [3]. Therefore, the optimal bicarbonate value to be achieved with dialysis remains unclear [3], as does the impact of metabolic alkalosis due to intradialysis overcorrection [4–6].
One of the most important factors determining pre- and post-dialysis bicarbonate concentration is dialysate bicarbonate concentration [7]. The 2000 National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines recommend a high dialysate bicarbonate concentration (≥38 mEq/L) to achieve a pre-dialysis serum bicarbonate level (≥22 mEq/L) [8]. This recommendation has resulted in a gradual increase in dialysate bicarbonate concentration over time [3, 9–11]. Although higher bicarbonate concentrations have some potential benefits on bone metabolism and hyperparathyroidism control [12, 13], some studies have questioned the safety of the KDOQI recommendations, reporting an association between high dialysate bicarbonate and an increased risk of all-cause mortality [9, 14]. This higher mortality is due to infections and cardiovascular hospitalization [14], as well as to other adverse effects such as greater haemodynamic instability [15], cardiac arrhythmias [16, 17] and calcium phosphate precipitation in soft tissues [18]. Therefore it seems reasonable not to exceed a post-dialysis bicarbonate concentration of 28 mEq/L [1, 19], a threshold that is usually reached or exceeded with a standard dialysate bicarbonate level of 35 mEq/L [1].
Online haemodiafiltration (OL-HDF) has shown multiple benefits due to its ability to reach high convective and diffusive doses and improve dialysis adequacy [20–22]. These enhancements have also increased delivery of bicarbonate from the dialysate to the patient, achieving higher plasma bicarbonate levels and thus obtaining better acidosis control [2]. However, this increased bicarbonate release can lead to overcorrection of acidosis and significant adverse effects [14, 17, 23].
At present, the dialysate bicarbonate concentration can be chosen according to the desired plasma bicarbonate target [1]. In our centre, we have used a dialysate bicarbonate of 35 mEq/L for >10 years. However, because of growing evidence of potential risks associated with overcorrection of acidosis, we hypothesized that these risks could be reduced by lowering dialysate bicarbonate to reduce plasma bicarbonate. Therefore the aim of the present study was to evaluate the impact of dialysate bicarbonate optimization from 35 to 32 mEq/L on plasma bicarbonate levels in a cohort of patients treated with OL-HDF. We also evaluated the impact of this optimization on potassium control and phosphorus and calcium metabolism.
MATERIALS AND METHODS
Study design and population
This prospective, single-centre, observational study was conducted in a population of patients in a stable OL-HDF programme with a regular prescription of dialysate bicarbonate concentration of 35 mEq/L who were switched to a lower bicarbonate prescription of 32 mEq/L. We included patients ≥18 years of age in a stable chronic OL-HDF programme for at least 12 months at the time of study initiation. We excluded patients <18 years old, those with a percutaneous catheter (not tunnelled) as vascular access, those included in a living donor kidney transplantation programme, those with immunosuppressive treatment and those with significative residual kidney function (defined as residual diuresis >100 mL/24 h and urea clearance >2.5 mL/min/1.72 m2 [24]).
The primary outcome of the study was to evaluate changes in plasma bicarbonate levels (pre- and post-dialysis) after the dialysate bicarbonate prescription was reduced from 35 to 32 mEq/L and to analyse the effectiveness of this change in achieving pre- and post-dialysis bicarbonate values within the range suggested as desirable by previous studies [i.e. between 19–25 and ≤29 mEq/L for pre- and post-dialysis total carbon dioxide(TCO2), respectively] [1, 9, 19]. Thus, after the change in prescription, the patients were followed up for 6 months, with monthly measurement of TCO2 levels and comparison of the results with the baseline TCO2 (calculated as the mean of the monthly TCO2 values during the 6 months before study initiation). Serum bicarbonate was measured indirectly through TCO2, which is usually measured in epidemiological studies on the acid–base balance [2]. TCO2 has shown a good correlation with serum bicarbonate and, in most conditions, is approximately 1–1.2 mEq/L higher than the serum bicarbonate measured in the same sample [9, 19]. The use of TCO2 allows serum bicarbonate to be indirectly measured by obtaining venous blood samples without the need to perform a blood gas analysis but preserves accuracy in estimating plasma bicarbonate [9].
As a secondary outcome, we analysed the impact of reducing the dialysate bicarbonate concentration on control of potassium and phosphorus and calcium metabolism. To do this, monthly measurements were made of serum potassium (pre- and post-dialysis), calcium (pre- and post-dialysis) and intact parathyroid hormone (iPTH, pre-dialysis) during the 6-month follow-up after the dialysate change. These values were compared with the mean of the monthly measurements made during the 6 months prior to dialysate optimization. Calcium was expressed as corrected calcium by albumin according to the formula: total calcium + (4 − albumin) × 0.8, expressing calcium in mg/dL and albumin in g/dL. Throughout the follow-up, the patients received standard treatment with calcimimetics, phosphate binders (calcium and non-calcium) and alfacalcidiol, with dose titration according to the analytical parameters and the criteria of the treating physician. All these treatments and their changes were recorded at baseline and during the follow-up.
Dialysis parameters
In all patients, post-dilutional OL-HDF with the CorDiax 5008 monitor (Fresenius Medical Care, Bad Homburg, Germany) was used throughout the study. Baseline OL-HDF parameters consisted of bicarbonate buffer at a concentration of 35 mEq/L, 1.4–1.8 m2 high-flux synthetic dialysers, blood flow (Qb) 416 ± 33 mL/min (range 350–500), dialysate flow (Qd) 378 ± 41 mL/min and infusion flow (Qi) according to the automated infusion system. The average time per session was 332 ± 80 min (range 240–480): 66 patients (78.6%) were dialysed during 240–300 min sessions three times a week (standard dialysis group) and 18 patients (21.4%) during 480-min every-other-day nocturnal sessions (nocturnal dialysis group).
Regarding dialysate composition, the baseline dialysate bicarbonate concentration was 35 mEq/L (‘standard bicarbonate’ dialysate) and at the beginning of the study was switched to 32 mEq/L. However, due to the proportioning of the concentrate in the dialysis machine, the lower bicarbonate concentration setting led to an increase in all acid concentrate components as a result of the decreased base concentrate proportion to maintain the preset sodium concentration and conductivity, which in our case was 140 mEq/L and 14.0 mS/cm, respectively. Nevertheless, when the dialysate bicarbonate concentration was reduced to 32 mEq/L, conductivity significantly increased to 14.1 mS/cm (‘reduced bicarbonate–standard sodium’ dialysate). Therefore, to maintain conductivity at 14.0 mS/cm, we also reduced dialysate sodium to 139 mEq/L (‘reduced bicarbonate and sodium’ dialysate). Because of this change, we monitored serum sodium monthly and compared any changes with the baseline concentration (as a mean of the monthly values for the 6 months before the beginning of the study; Table 1). After optimization of the dialysate bicarbonate concentration, none of these dialysate parameters were modified during the study (Table 1).
Table 1.
Dialysate composition according to dialysis bicarbonate concentration
| Dialysate parameter | Standard bicarbonatea | Reduced bicarbonate–standard sodiumb | Reduced bicarbonate and sodiumc |
|---|---|---|---|
| Bicarbonate (mEq/L) | 35 | 32 | 32 |
| Sodium (mEq/L) | 140 | 140 | 139 |
| Potassium (mEq/L) | 1.50 | 1.54 | 1.52 |
| Calcium (mmol/L) | 1.5 | 1.54 | 1.53 |
| Magnesium (mmol/L) | 0.50 | 0.51 | 0.50 |
| Chlorine (mEq/L) | 106.50 | 109.54 | 108.52 |
| Acetate (mmol/L) | 4.00 | 4.11 | 4.07 |
| Glucose (g/L) | 1.00 | 1.02 | 1.01 |
| Conductivityd (mS/cm) | 13.84 (14.0) | 13.94 (14.1) | 13.84 (14.0) |
Standard bicarbonate: dialysate with a standard bicarbonate concentration of 35 mEq/L.
Reduced bicarbonante–standard sodium: dialysate with a reduced bicarbonate concentration of 32 mEq/L but a standard sodium concentration of 140 mEq/L.
Reduced bicarbonate and sodium: dialysate with reduced bicarbonate and sodium concentrations of 32 and 139 mEq/L, respectively. This was the dialysate composition used for the present study.
Conductivity values correspond to the measured or real ones while values in parentheses correspond to the set values.
The local ethics committee approved the study and it was conducted according to the Declaration of Helsinki. All the patients gave their written informed consent to participate in the study.
Statistical analysis
The results are expressed as the arithmetic mean ± standard deviation (SD), number(%) or median [interquartile range (IQR)]. Each patient served as his/her own control. The Student t-test and analysis of variance test (repeated measures) were used in the analysis of quantitative variables. The results were corroborated with a linear mixed model with Bonferroni adjustment for multiple comparisons. For non-parametric variables, the Friedman’s test followed by post hoc Wilcoxon’s test was used. The McNemar test was used in the analysis of qualitative variables. P-values <0.05 were considered statistically significant. All statistical analyses were performed using the SPSS Statistical Package version 23 (IBM, Armonk, NY, USA). Graphics were made using GraphPad Prism software (version 6; GraphPad Software, San Diego, CA, USA).
RESULTS
Baseline characteristics
Of the 95 eligible patients at the beginning of the study, 11 were excluded because they did not complete the follow-up after the change in dialysate bicarbonate. The reasons for exclusion were kidney transplantation (eight patients) and death during follow-up (three patients). The causes of death were an intestinal obstruction due to lymphoma (already diagnosed before the beginning of the study), acute cholecystitis treated conservatively and a sudden death unrelated to the haemodialysis sessions. There were no statistically significant differences between excluded patients and those included in the study.
Of the 84 patients analysed, 63 (75%) were male, with a mean age of 66.7 ± 14.8 years and in a stable chronic haemodialysis programme for an average of 60.6 ± 56.7 months. Of these, 3 (4%), 14 (17%) and 49 (58%) patients underwent sessions of 4, 4.5 and 5-h thrice-weekly sessions, respectively (standard dialysis group, 66 patients). The remaining 18 (21%) were on 8-h nocturnal every-other-day sessions (nocturnal dialysis group). The most frequent cause of ESKD was vascular (26.2%) followed by undiagnosed ESKD (17.9%; Table 2). No patient was under treatment with oral bicarbonate supplements or other extradialysis bicarbonate supplementation during baseline and follow-up.
Table 2.
Baseline characteristics of the included patients
| Characteristics | Patients (n = 84) |
|---|---|
| Male | 63 (75) |
| Age (years) | 66.7 ± 14.8 |
| Haemodialysis vintage (months), mean ± SD | 60.6 ± 56.7 |
| Vascular access | |
| Native AVF | 68 (81) |
| Prosthetic AVF | 8 (9.5) |
| Catheter | 8 (9.5) |
| Haemodialysis parameters, mean ± SD | |
| Blood flow (mL/min) | 416 ± 33 |
| Dialysate flow (mL/min) | 378 ± 41 |
| Time per session (min), mean ± SD | 332 ± 80 |
| 240 | 3 (4) |
| 270 | 14 (17) |
| 300 | 49 (58) |
| 480 | 18 (21) |
| ESKD aetiology | |
| Chronic glomerulonephritis | 13 (15.5) |
| Tubulointerstitial nephritis | 4 (4.8) |
| Vascular | 22 (26.2) |
| Polycystic kidney disease | 9 (10.7) |
| Diabetic nephropathy | 11 (13.1) |
| Systemic | 5 (6) |
| Urological | 1 (1.2) |
| Kidney tumour | 4 (4.8) |
| Undiagnosed | 15 (17.9) |
| Comorbidities | |
| Smoking habit | 14 (17) |
| Diabetes mellitus | 37 (44) |
| Dyslipidaemia | 49 (58) |
| Hypertension | 46 (54.8) |
| Ischaemic heart disease | 28 (33) |
| Cerebrovascular accident | 8 (9.5) |
| Peripheral artery disease | 21 (25) |
| COPD | 14 (17) |
| Chronic HBV infectiona | 6 (5) |
| Chronic HCV infectionb | 5 (4) |
| Chronic HIV infectiona | 7 (6) |
| Chronic treatment | |
| Hypoglycaemic agents | 32 (38) |
| Oral antidiabetic agents | 10 (12) |
| Insulin | 22 (26) |
| Hypolipidaemic agents | 49 (58) |
| Antiplatelet agents | 43 (51) |
| Antihypertensive drugs | 46 (54.8) |
| Phosphorus–calcium complementary treatment | |
| Alfacalcidiol | 37 (44) |
| Etelcalcetide | 26 (31) |
| Phosphate binders | |
| With calcium | 36 (42.9) |
| Without calcium | 15 (17.9) |
Values are presented as n (%) unless stated otherwise.
All patients were under treatment with a controlled viral load.
All patients were treated and cured with direct-acting antiviral agents before the study.
AVF, arteriovenous fistula; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Change of TCO2 after dialysate bicarbonate optimization
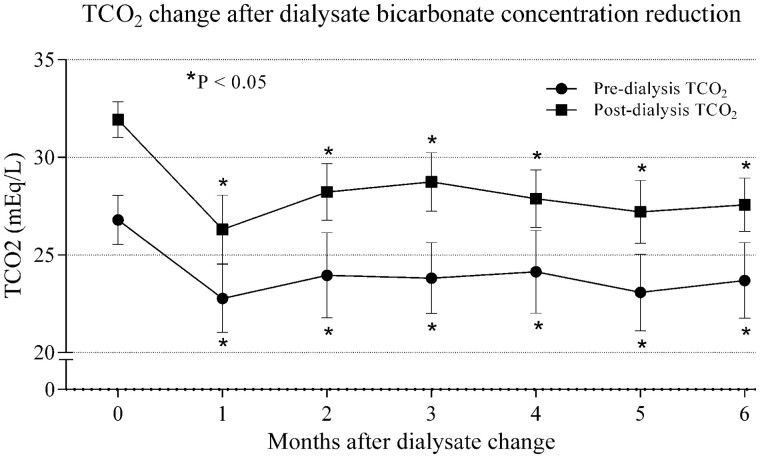
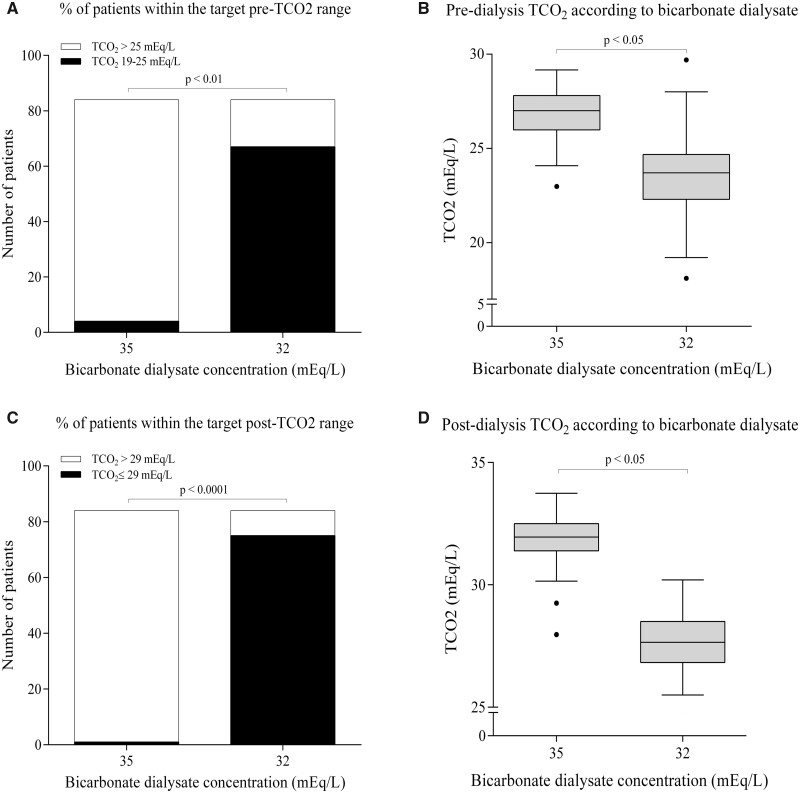
After the reduction of bicarbonate in the dialysate from 35 to 32 mEq/L, pre-dialysis TCO2 significantly decreased from 26.79 ± 1.26 at baseline to 23.81 ± 1.81 and 23.69 ± 1.92 mEq/L at 3 and 6 months, respectively (P < 0.001; Figure1). There was a tendency towards higher TCO2 values in the nocturnal dialysis group at baseline (26.69 ± 1.24 versus 27.17 ± 1.29 mEq/L) and at 3 months (23.64 ± 1.79 versus 24.44 ± 1.79 mEq/L), but at 6 months (23.69 ± 1.97 versus 23.68 ± 1.80 mEq/L) there was little difference between the standard and nocturnal dialysis groups (P > 0.05). The mean reduction in pre-dialysis TCO2 at 6 months was 3.10 ± 1.84 mEq/L. Among the 84 patients included, pre-dialysis TCO2 was >25 mEq/L in 80 patients (90.5%) at baseline but only 17 patients (20.2%) at 6 months after bicarbonate reduction, while levels ranged from 19 to 25 mEq/L in the remaining 67 patients (79.77%; P < 0.001; Figure 2A and B).
FIGURE 1.
TCO2 change after dialysate bicarbonate concentration reduction. *P < 0.05 with respect to baseline.
FIGURE 2.
Pre- and post-dialysis TCO2 according to dialysate bicarbonate. (A) Number of patients with a pre-dialysis TCO2 of 19–25 mEq/L and >25 mEq/L before and after dialysate bicarbonate concentration optimization. (B) Pre-dialysis TCO2 at baseline and at 6 months after dialysate bicarbonate optimization. (C) Number of patients with a post-dialysis TCO2 ≤29 mEq/L and >29 mEq/L before and after dialysate bicarbonate concentration optimization. (D) Post-dialysis TCO2 at baseline and at 6 months after dialysate bicarbonate optimization.
Post-dialysis TCO2 significantly decreased from 31.92 ± 0.91 to 28.73 ± 1.51 and 27.57 ± 1.36 mEq/L at 3 and 6 months, respectively (P < 0.001; Figure 1). Baseline values were significantly higher in the nocturnal dialysis group (31.74 ± 0.87 versus 32.56 ± 0.73 mEq/L, P < 0.05), without differences at 3 months (28.49 ± 1.46 versus 28.63 ± 1.71 mEq/L) and at 6 months (27.57 ± 1.39 versus 27.65 ± 1.28 mEq/L) between the standard and nocturnal dialysis groups, respectively (P > 0.05). The mean reduction in post-dialysis TCO2 at 6 months after bicarbonate adjustment was 4.33 ± 1.44 mEq/L. Post-dialysis TCO2 was >29 mEq/L in 83 patients (98.8%) at baseline but only 9 patients (10.7%) at 6 months after bicarbonate optimization. Post-dialysis TCO2 was ≤29 mEq/L in the remaining 75 patients (89.3%; P < 0.001; Figure 2B and C).
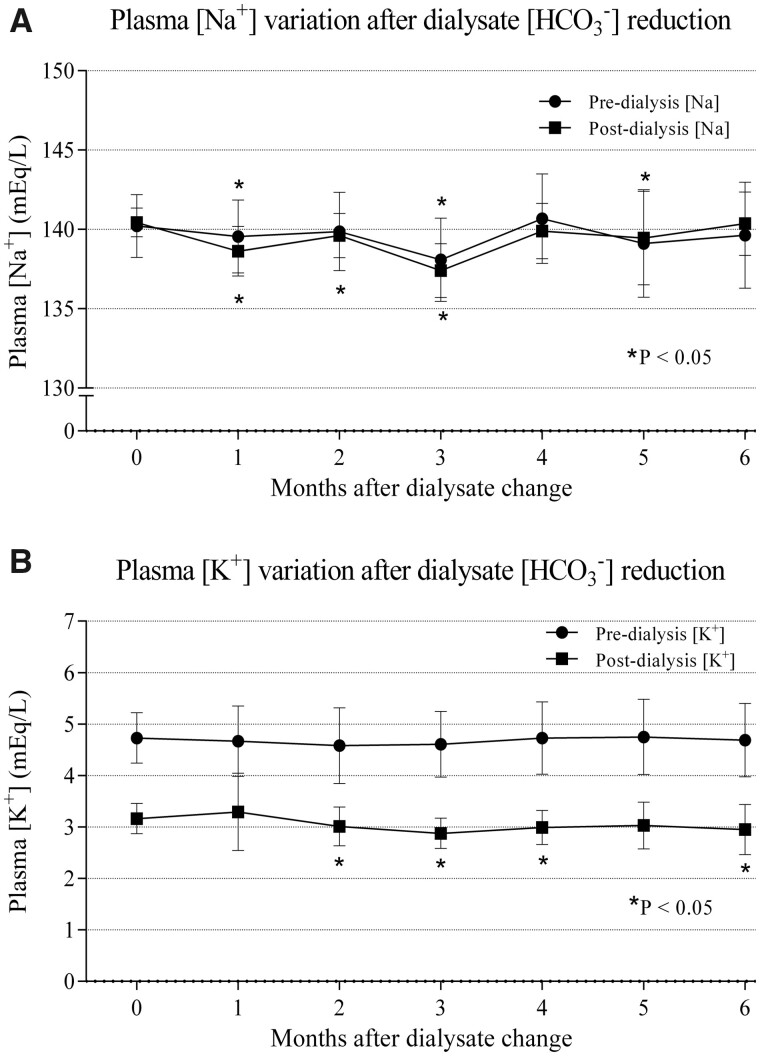
Sodium and potassium changes
After the reduction indialysate bicarbonate and sodium concentrations, pre-dialysis plasma sodium significantly decreased from 140.22 ± 1.98 at baseline to 138.08 ± 2.62 mEq/L at 3 months (P < 0.001), although this decrease was not significant at 6 months (139.63 ± 3.34 mEq/L, P = 0.18; Figure 3A). Post-dialysis sodiumsignificantly decreased from 140.44 ± 0.90 at baseline to 137.40 ± 1.71 mEq/L at 3 months (P < 0.001). Nevertheless, this decrease was not significant at 6 months (140.36 ± 1.99 mEq/L, P > 0.05; Figure 3A). There were not significant differences in pre- and post-dialysis sodiumbetween standard and nocturnal dialysis groups in any of the analysed periods (data not shown).
FIGURE 3.
Plasma sodium and potassium concentration variations after dialysate bicarbonate change. (A) Plasma sodium concentration variation after dialysate bicarbonate change. (B) Plasma potassium concentration variation after dialysate bicarbonate change. *P < 0.05 with respect to baseline.
Pre-dialysis plasma potassiumshowed no statistically significant differences compared with baseline values at any of the time points analysed after the dialysate bicarbonate change (baseline 4.73 ± 0.50 versus 4.61 ± 0.63 and 4.69 ± 0.72 mEq/L at 3 and 6 months, respectively, P > 0.05; Figure 3B). However, post-dialysis plasma potassiumsignificantly decreased, which was evident from the second month after the dialysate bicarbonate change (3.16 ± 0.30 at baseline versus 2.88 ± 0.30 and 2.95 ± 0.48 mEq/L at 3 and 6 months, respectively, P < 0.05; Figure 3B). There were not significant differences in pre- and post-dialysis potassiumbetween standard and nocturnal dialysis groups in any of the analysed periods (data not shown).
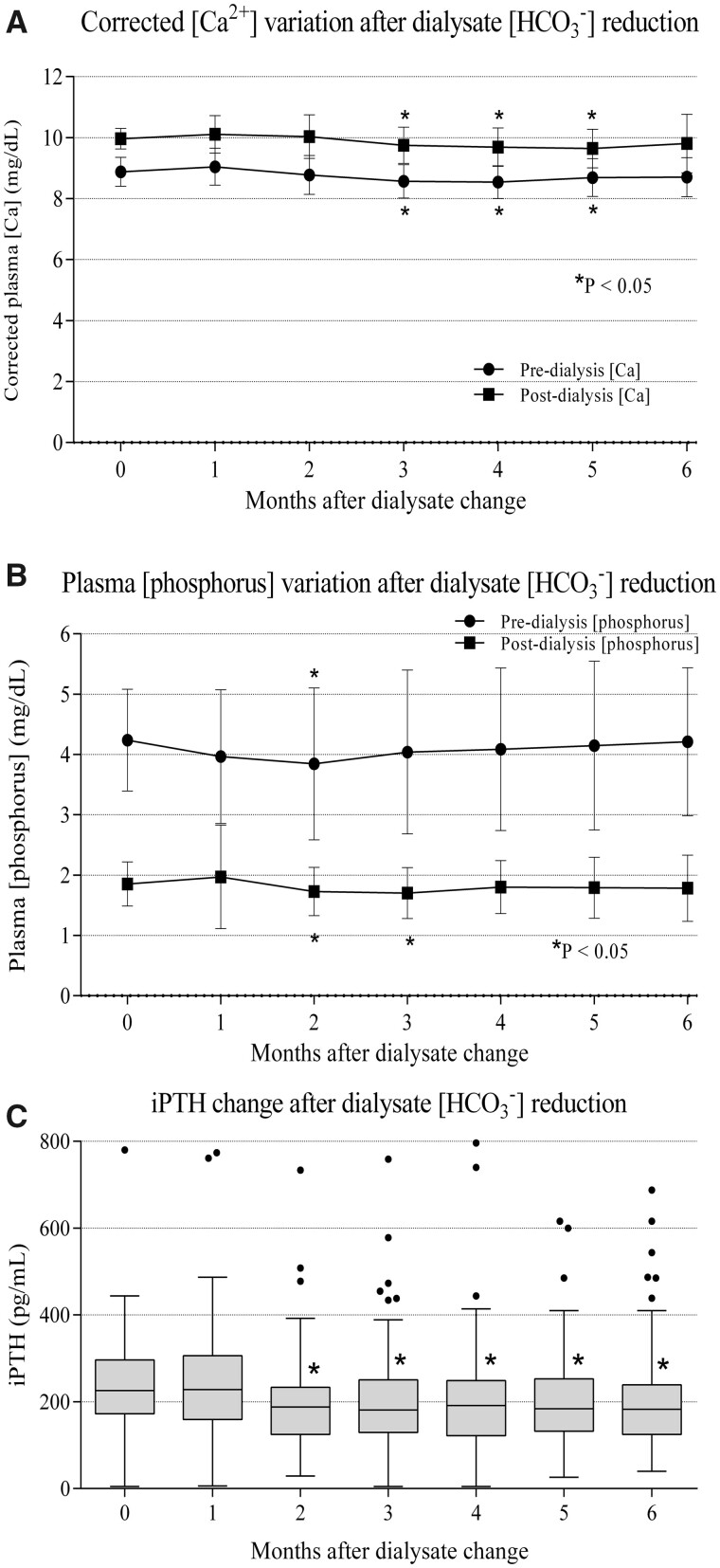
Calcium, phosphate and PTH changes
After the change of bicarbonate in the dialysate, pre-dialysis plasma calcium (corrected for albumin) tended to increase in the first month (8.87 ± 0.47 at baseline versus 9.05 ± 0.60 mg/dL at the first month, P = 0.05). However, plasma calcium levels decreased after the second month, and this decrease became significant in the third month (8.87 ± 0.47 at baseline versus 8.57 ± 0.54 at 3 months, P < 0.05), although afterwards calcium levels progressively stabilized close to baseline values (8.71 ± 0.64 mg/dL at 6 months, P > 0.05; Figure 4A). Nevertheless, in post-dialysis plasma calcium, a significant reduction was only evident from the third to fifth months (9.97 ± 0.33 at baseline versus 9.75 ± 0.59 at 3 months, P < 0.05). At 6 months, post-dialysis plasma calcium reached values close to baseline (9.81 ± 0.96 mg/dL, P > 0.05; Figure 4A). There were no significant differences in pre- and post-dialysis calcium between the standard and nocturnal dialysis groups in any of the analysed periods (data not shown).
FIGURE 4.
Phosphorus–calcium metabolism changes after dialysate bicarbonate reduction. (A) Calcium variation after dialysate bicarbonate change. Calcium is corrected by plasma albumin. (B) Plasma phosphorus variation after dialysate bicarbonate change. (C) iPTH variation after dialysate bicarbonate change. *P < 0.05 with respect to baseline.
Pre-dialysis phosphorus concentration did not significantly change in the periods analysed, except in the second month (4.24 ± 0.85 at baseline versus 3.96 ± 1.11 mg/dL at 2 months, P < 0.05; Figure 4B). Pre-dialysis phosphorous concentrations were significantly lower in the nocturnal dialysis group in the analysed periods (3.76 ± 1.08 versus 4.37 ± 0.72 mg/dL at baseline, 3.38 ± 1.50 versus 4.22 ± 1.27 mg/dL at Month 3 and 3.53 ± 1.28 versus 4.39 ± 1.15 mg/dL at Month 6 for the nocturnal and standard dialysis groups, respectively, P < 0.05). Post-dialysis phosphorus significantly increased in the first month (1.85 ± 0.37 at baseline versus 1.96 ± 0.86 mg/dL, P < 0.05), followed by a significant decrease at 2 and 3 months (1.85 ± 0.37 at baseline versus 1.72 ± 0.40 and 1.70 ± 0.42 mg/dL at 2 and 3 months, respectively, P < 0.05). After the fourth month, post-dialysis phosphorus again increased up to values that were not significantly different from those at baseline (1.85 ± 0.37 at baseline versus 1.80 ± 0.44 and 1.78 ± 0.54 mg/dL at 4 and 6 months, respectively, P > 0.05; Figure 4B). There were no significant differences in post-dialysis phosphorous between the standard and nocturnal dialysis groups for any of the analysed periods.
After the change in dialysate bicarbonate, iPTH significantly decreased compared with baseline values [226.09 (IQR 172–296) pg/mL at baseline versus 181.00 (IQR 129–250) and 182.5 (IQR 125–239) pg/mL at 3 and 6 months, respectively, P < 0.05; Figure 4C]. There were no significant differences in iPTH between standard and nocturnal dialysis groups for any of the analysed periods (data not shown). Doses of etelcalcetide, alfacalcidiol and phosphate binders, as well as convective volume, did not change significantly during the follow-up, although there was a tendency to decrease etelcalcetide and non-calcium phosphate binder doses (Table 3). Convective volume was significantly higher in the nocturnal dialysis group compared with the standard dialysis group for all the moments analysed: 36.06 ± 4.64 versus 52.32 ± 8.56 L (baseline), 36.41 ± 4.81 versus 51.34 ± 8.84 L (Month 1), 36.37 ± 5.74 versus 50.87 ± 3.45 L (Month 2), 36.86 ± 4.33 versus 51.76 ± 7.68 L (Month 3), 36.83 ± 3.96 versus 54.23 ± 6.35 L (Month 4), 36.65 ± 4.50 versus 47.84 ± 8.91 L (Month 5) and 36.25 ± 4.41 versus 51.71 ± 7.71 L (Month 6) for the standard and nocturnal dialysis groups, respectively.
Table 3.
Phosphorus–calcium metabolism treatment and albumin changes during follow-up
| Baseline | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | |
|---|---|---|---|---|---|---|---|
| Convective volume (L) | 40.94 ± 9.91 | 40.88 ± 9.21 | 40.72 ± 8.58 | 41.33 ± 8.79 | 41.98 ± 9.34 | 40.01 ± 8.31 | 40.89 ± 9.05 |
| Albumin (g/L) | 38.89 ± 3.22 | 38.98 ± 3.07 | 39.58 ± 3.34 | 37.85 ± 3.12* | 38.79 ± 3.50 | 38.02 ± 3.49 | 38.37 ± 3.69 |
| Etelcalcetide (mg/day), median (IQR) | 1.7 (0.7–2.8) | 1.5 (0.9–2.8) | 1.7 (1.1–3.2) | 1.3 (0.7–3.2) | 1.1 (0.7–3.0) | 1.1 (0.7–2.9) | 1.2 (0.7–3.5) |
| Alfacalcidiol (µg/week) | 2.91 ± 1.48 | 2.68 ± 1.28 | 2.72 ± 1.31 | 2.56 ± 1.39 | 2.80 ± 1.55 | 2.92 ± 1.86 | 2.56 ± 1.57 |
| Phosphate binders | |||||||
| With calcium (g/day), median (IQR) | 1.3 (1.1–2.1) | 1.4 (0.9–2.1) | 1.3 (0.9–2.0) | 1.5 (0.8–2.1) | 1.4 (0.8–2.1) | 1.5 (0.8–2.1) | 1.3 (0.8–2.1) |
| Without calcium (g/day), median (IQR) | 4.1 (1.6–5.5) | 3.3 (0.8–6.4) | 2.4 (0.8–6.4) | 2.4 (0.8–6.4) | 2.0 (0.8–6.4) | 2.0 (0.8–6.4) | 2.4 (0.9–6.4) |
Values are presented as mean ± SDunless stated otherwise.
P < 0.05 with respect to baseline values.
DISCUSSION
In this prospective, observational study, we evaluated the impact of reducing dialysate bicarbonate concentration from 35 to 32 mEq/L on pre- and post-dialysis TCO2 (as an indirect measure of plasma bicarbonate concentration) and found a significant decrease versus baseline values after this dialysate adjustment. This change had little effect on sodium, potassium and phosphorus–calcium control. However, it slightly reduced post-dialysis potassium plasma concentration (without affecting pre-dialysis values) and significantly decreased iPTH without sustained changes in calcium and phosphorus levels.
In patients with ESKD, it is essential that renal replacement therapy correct metabolic acidosis to avoid the adverse effects of this condition [1, 2]. However, the optimal pre- and post-dialysis bicarbonate target is not clearly defined, and a wide range of dialysate bicarbonate concentrations are used around the world, usually chosen empirically [25]. Moreover, some studies have reported an increase in mortality associated with excessive bicarbonate correction: the Dialysis Outcomes and Practice Patterns Study (DOPPS) showed a U-shaped curve, with an increase in the relative risk of mortality for pre-dialysis bicarbonate values <17 mEq/L and >27 mEq/L, with the lowest mortality risk in patients with a pre-dialysis bicarbonate of 18–23 mEq/L (equivalent to TCO2 between 19 and 24 mEq/L) [9]. Therefore, some studies recommend not exceeding pre- and post-dialysis bicarbonate levels of 24 and 28 mEq/L (equivalent to a TCO2 of 25 and 29 mEq/L, respectively), a threshold that is usually reached or exceeded with a standard dialysate bicarbonate level of 35 mEq/L. This is especially true with OL-HDF due to the higher bicarbonate delivery achieved by this type of dialysis modality [1, 2, 8, 10]. Other studies suggest a desirable pre-dialysis bicarbonate concentration between 18 and 26 mEq/L [19].
In the present cohort of patients treated with OL-HDF, we decided to choose a narrower and more accurate target as a reference, based on available evidence (19–25 and ≤29 mEq/L of TCO2 for pre- and post-dialysis values, respectively) [1, 19]. After this adjustment, the reduction in dialysate bicarbonate concentration from 35 to 32 mEq/L significantly decreased pre- and post-dialysis TCO2 to a mean value of 23.69 and 27.57 mEq/L, respectively, at 6 months. This value was within the range proposed as a target in the present study (i.e. between 19–25 and ≤29 mEq/L for pre- and post-dialysis TCO2, respectively) [1]. More importantly, pre-dialysis TCO2 was >25 mEq/L in 90.5% of the patients at baseline but only 20.2% of the patients after we reduced the dialysate bicarbonate concentration by 3 mEq/L. Post-dialysis TCO2 was >29 mEq/L in 98.8% of the patients at baseline but only 10.7% after dialysate bicarbonate optimization.
When we compared pre- and post-TCO2 values between the standard and nocturnal dialysis group, there were no differences at the end of the follow-up despite a significantly higher convective volume per session in the nocturnal dialysis group, which is probably justified by the higher baseline TCO2 in this group. However, it should be noted that despite starting from higher TCO2 values, the higher convective volume allowed the nocturnal dialysis group to reach pre- and post-TCO2 values similar to the standard dialysis group at the end of the follow-up.
Importantly, we did not individualize the dialysate bicarbonate concentration based on pre-dialysis TCO2 in each patient, which may explain the persistence of a pre- and post-dialysis TCO2 above the target range (i.e. 20.2 and 10.7%, respectively) in a significant percentage of patients. In fact, baseline pre-dialysis TCO2 values were significantly higher in patients not achieving the target range of TCO2 after dialysate optimization than in those who reached the target. These patients would probably benefit from an even greater reduction in dialysate bicarbonate, thus highlighting the importance of dialysate individualization. Therefore, taking into account that the mean reduction in pre-dialysis TCO2 after the reduction of bicarbonate concentration in the dialysate from 35 to 32 mEq/L was 3.10 mEq/L, we can estimate that the expected reduction in pre-dialysis TCO2 is approximately 1.033 mEq/L per each 1 mEq/L decrease of dialysate bicarbonate.
Based on this approach, we suggest individualized adjustment of bicarbonate concentration in the dialysate according to the pre-dialysis value of TCO2 with a pre-dialysis target between 19 and 25 mEq/L (Table 4). With this individualization, we speculate that a higher percentage of patients in our cohort would probably have reached the pre-dialysis range of TCO2 marked as posing a lower mortality risk (i.e. 19–25 mEq/L of TCO2) [9, 19].
Table 4.
Suggested dialysate bicarbonate concentration according to pre-dialysis TCO2
| Pre-dialysis TCO2 (mEq/L)a | Suggested dialysate bicarbonate concentration (mEq/L)b | Expected pre-dialysis TCO2 after the adjustment (mEq/L)a |
|---|---|---|
| 20 | 39 | 24.12 |
| 21 | 38 | 24.09 |
| 22 | 37 | 24.06 |
| 23 | 36 | 24.03 |
| 24 | 35 | 24.00 |
| 25 | 34 | 23.96 |
| 26 | 33 | 23.93 |
| 27c | 32c | 23.90c |
| 28 | 31 | 23.86 |
| 29 | 30 | 23.83 |
| 30 | 29 | 23.80 |
Bicarbonate (mEq/L) = TCO2 (mEq/L) – 1.
Suggested bicarbonate concentration reduction is made based on a desirable pre-dialysis TCO2 between 19 and 25 mEq/L (arbitrary target ∽24 mEq/L) and supposing that pre-dialysis TCO2 (before the dialysate optimization) has been obtained with a dialysate bicarbonate concentration of 35 mEq/L.
Values obtained from the present study. The other measures are extrapolated from the equation TCO2ad = 1.03 · (dyHCO3 – 35) + TCO2ba, where TCO2ad is TCO2 after adjustment, dyHCO3 is target dialysate bicarbonate and TCO2ba is TCO2 before adjustment.
An important issue to consider is that the reduction in dialysate bicarbonate concentration increased conductivity from 14 to 14.1 mS/cm (while dialysate sodium remained unchanged at 140 mEq/L) due to the concentrate proportioning in the dialysis machine. As previously reported, high conductivities based on high dialysate sodium prescriptions increase blood pressure [26], water intake [27] and interdialysis weight gain [28]. Therefore, as presented in Table 1, to avoid increasing conductivity when we reduced dialysate bicarbonate, we decreased the sodium concentration in the dialysate from 140 to 139 mEq/L, achieving again a conductivity of 14.0 mS/cm. This change in the dialysate sodium concentration was associated with a significant decrease in the pre- and post-dialysis plasma sodium concentration during the first 3 months, although plasma values subsequently stabilized close to baseline, with no statistically significant differences at 6 months of follow-up. This new increase in plasma sodium values is probably secondary to the passage of free water from the intravascular to the interstitial space and an inverse sodium diffusive passage from the interstitial to the intravascular space in favour of a concentration gradient [29]. Maintaining these slightly lower plasma sodium values (avoiding extremely low sodium values) reached at 6 months can be potentially beneficial, since it could improve interdialysis weight gain and blood pressure control and avoid the adverse effects of excessively low sodium concentrations (as intradialysis hypotension) [30]. However, solid studies that specifically analyse the benefits of slightly decreasing sodium concentration due to dialysate bicarbonate adjustment on weight and blood pressure control are needed.
In addition to the impact of reducing dialysate bicarbonate on TCO2, we secondarily analysed the consequences of this change on potassium and phosphorus–calcium metabolism control. In terms of potassium clearance, we found that reducing dialysate bicarbonate from 35 to 32 mEq/L significantly decreased post-dialysis potassium without affecting pre-dialysis values. However, in a randomized controlled trial, Heguilén et al. [31] demonstrated an association between a higher dialysate bicarbonate concentration and a faster decrease in intradialytic plasma potassium concentration. This discrepancy has two possible explanations: first, in one of the intervention arms in the study by Heguilén et al. [31], the difference between the two dialysate bicarbonate concentrations was 8 mEq/L (27 versus 35 mEq/L), whereas in the present study the difference was only 3 mEq/L (35 versus 32 mEq/L). Second, certain studies have suggested that alkalosis induced by higher bicarbonate levels in the dialysate may compromise the net depuration of potassium during haemodialysis, given that the intracellular shift in potassium forms a ‘potassium pool’ that cannot be cleared through the dialysate [18, 32]. In fact, Heguilén et al. [31] reported that potassium removal was greater in those patients with a lower dialysate bicarbonate concentration. In addition, the potassium concentration 1 h after the haemodialysis session was lower for lower dialysate bicarbonate concentrations (especially 27 mEq/L), although these differences were not statistically significant, probably due to the small number of patients studied [31]. In the present study, the use of OL-HDF with higher blood flow, more time per haemodialysis session and consequently higher dialysis dose, together with the greater amount of potassium available for its elimination (due to lower intracellular uptake) explains the increase in potassium removal and therefore the decrease in post-dialysis plasma potassium concentration associated with the reduction of dialysate bicarbonate concentration. An important issue to consider due to this reduction in post-dialysis potassium is the potential increased risk of ventricular arrhythmias and sudden death. In our cohort, no cardiorespiratory arrest potentially associated with arrhythmic events due to hypokalaemia was observed: although there was a significant decrease in post-dialysis values of potassium, this decrease was relatively small (about 0.20 mEq/L in 6 months) and always remained >2.5 mEq/L, which is the threshold below which hypokalaemia-associated arrhythmias occur [33]. In addition, this decrease was temporary since pre-dialysis values have always remained within the normal range. Therefore the authors think that the associated risk with the post-dialysis potassium value decrease is unlikely to cause arrhythmias or other potassium-related complications. Nevertheless, it should be noted that the follow-up of the study was only 6 months, so studies with longer follow-up that provide stronger data on the safety of bicarbonate change in dialysate and levels of post-dialysis potassium are needed.
In terms of phosphorus–calcium metabolism, Havlin et al. [13] recently published a study comparing the impact of two dialysate bicarbonate concentrations (26 versus 32 mEq/L) on iPTH and plasma calcium control. In that study, the decrease in the dialysate bicarbonate concentration to 26 mEq/L significantly reduced post-dialysis iPTH compared with the concentration of 32 mEq/L, given that the greater intradialysis alkalinization entails a decrease in ionic calcium and, as a consequence, an increase in iPTH. According to these results, in the present study we also observed a significant decrease in iPTH levels after reducing the concentration of dialysate bicarbonate. However, Havlin et al. [13] evaluated iPTH variation in only two haemodialysis sessions in 10 patients but did not examine the impact of dialysate bicarbonate variation on iPTH in the long-term. In our study, our follow-up lasted up to 6 months after the dialysate adjustment and our sample of patients was larger than that in the study by Havlin et al. [13]. Moreover, we observed a tendency to decrease etelcalcetide doses, which probably is secondary to the iPTH decrease after the dialysate bicarbonate concentration change.
In our study, both pre- and post-dialysis calcium concentrations slightly decreased, although these changes were not statistically significant at the end of the follow-up. This maintenance of calcium concentrations can be explained by the increment in dialysate calcium, which is invariably associated with the decrease in dialysate bicarbonate due to the need to maintain the acid/base proportionality during dialysis (Table 1) and also due to the decrease we found in iPTH levels. In the case of phosphorus, the change in dialysate bicarbonate caused no significant variations that persisted throughout the follow-up, despite a tendency to decrease in the dose of non-calcium phosphorus binders.
Finally, when we analysed differences in sodium, potassium, calcium, phosphorus and iPTH between the standard and nocturnal dialysis groups, we found no statistically significant differences except for the pre-dialysis phosphorus, which was significantly lower in the nocturnal dialysis group both at baseline and during the study. This fact is expected due to the higher dialysis dose that these patients receive and that is already reflected in the baseline phosphorus values.
In conclusion, optimization of the dialysate bicarbonate concentration from 35 to 32 mEq/L significantly and safely reduced pre- and post-dialysis TCO2 in patients treated with OL-HDF, improving control of the acid–base balance and secondary hyperparathyroidism, but avoiding overcorrection of acidosis and without negatively affecting control of potassium and phosphorus–calcium. More importantly, individualization of bicarbonate prescription is a key factor in the adequate control of acidosis in patients with ESKD on dialysis. Therefore, as an illustrative guideline, we recommend an individualized dialysate bicarbonate concentration based on pre-dialysis TCO2 in patients treated with OL-HDF. Nevertheless, more studies are needed to confirm the validity of these recommendations and evaluate the long-term impact of dialysate bicarbonate concentration adjustment.
ACKNOWLEDGEMENTS
We would like to express our gratitude to all participating patients as well as to all the staff of the Dialysis Section of Hospital Clínic of Barcelona for their collaboration in this study and enthusiasm.
AUTHORS’ CONTRIBUTIONS
E.M.-M. collected, analysed and interpreted the data and prepared the manuscript. D.R.-E. collected the data. J.B., L.R., E.H.-L., M.X., M.A.-G., N.F., and M.V. analysed and interpreted the data. J.L.B. proportioned and collected the data. N.R. proportioned, collected and analysed the data. F.M. collected, analysed and interpreted the data and prepared the manuscript. Statistical analysis was performed by E.M.-M. and F.M. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
The authors declare no financial support for the project. F.M. has received consultancy fees and lecture fees from Amgen, Baxter, BBraun, Fresenius Medical Care, Medtronic and Nipro. The other authors declare no conflicts of interest.
REFERENCES
- 1. Locatelli F, La Milia V, Violo L et al. Optimizing haemodialysate composition. Clin Kidney J 2015; 8: 580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abramowitz MK. Bicarbonate balance and prescription in ESRD. J Am Soc Nephrol 2017; 28: 726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGill RL, Weiner DE. Dialysate composition for hemodialysis: changes and changing risk. Semin Dial 2017; 30: 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gennari FJ. Very low and high predialysis serum bicarbonate levels are risk factors for mortality: what are the appropriate interventions? Semin Dial 2010; 23: 253–257 [DOI] [PubMed] [Google Scholar]
- 5. Mcgill RL, Weiner DE. Dialysate composition for hemodialysis: changes and changing risk. Semin Dial 2017; 30: 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basile C, Rossi L, Lomonte C. Dialysate bicarbonate concentration: too much of a good thing? Semin Dial 2018; 31: 576–582 [DOI] [PubMed] [Google Scholar]
- 7. Lin S-H, Lin Y-F, Chin H-M et al. Must metabolic acidosis be associated with malnutrition in haemodialysed patients? Nephrol Dial Transplant 2002; 17: 2006–2010 [DOI] [PubMed] [Google Scholar]
- 8. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 Suppl 2): S1–S140 [DOI] [PubMed] [Google Scholar]
- 9. Bommer J, Locatelli F, Satayathum S et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44: 661–671 [PubMed] [Google Scholar]
- 10. Bozikas A, Kiriakoutzik I, Petrou I et al. Aiming for the optimal bicarbonate prescription for maintenance hemodialysis therapy in end-stage renal disease. Hemodial Int 2019; 23: 173–180 [DOI] [PubMed] [Google Scholar]
- 11. Wu DY, Shinaberger CS, Regidor DL et al. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol 2006; 1: 70–78 [DOI] [PubMed] [Google Scholar]
- 12. Graham KA, Hoenich NA, Tarbit M et al. Correction of acidosis in hemodialysis patients increases the sensitivity of the parathyroid glands to calcium. J Am Soc Nephrol 1997; 8: 627–631 [DOI] [PubMed] [Google Scholar]
- 13. Havlin J, Vankova S. Intradialytic alkalinization is a neglected factor affecting calcium mass balance and parathyroid hormone level during haemodiafiltration. Clin Kidney J 2019; 12: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tentori F, Karaboyas A, Robinson BM et al. Association of dialysate bicarbonate concentration with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2013; 62: 738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gabutti L, Ferrari N, Giudici G et al. Unexpected haemodynamic instability associated with standard bicarbonate haemodialysis. Nephrol Dial Transplant 2003; 18: 2369–2376 [DOI] [PubMed] [Google Scholar]
- 16. Rhee CM, Chou JA, Kalantar-Zadeh K. Dialysis prescription and sudden death. Semin Nephrol 2018; 38: 570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Iorio B, Torraca S, Piscopo C et al. Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis: pilot study of single dialysis effects. J Nephrol 2012; 25: 653–660 [DOI] [PubMed] [Google Scholar]
- 18. Basile C, Rossi L, Lomonte C. The choice of dialysate bicarbonate: do different concentrations make a difference? Kidney Int 2016; 89: 1008–1015 [DOI] [PubMed] [Google Scholar]
- 19. Fishbane S, Mathew AT, Gennari FJ et al. Acid-base assessment of patients receiving hemodialysis. What are our management goals? Semin Dial 2018; 31: 382–387 [DOI] [PubMed] [Google Scholar]
- 20. Locatelli F, Marcelli D, Conte F et al. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. Kidney Int 1999; 55: 286–293 [DOI] [PubMed] [Google Scholar]
- 21. Maduell F, del Pozo C, Garcia H et al. Change from conventional haemodiafiltration to on-line haemodiafiltration. Nephrol Dial Transplant 1999; 14: 1202–1207 [DOI] [PubMed] [Google Scholar]
- 22. Carracedo J, Merino A, Nogueras S et al. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: a prospective, crossover study. J Am Soc Nephrol 2006; 17: 2315–2321 [DOI] [PubMed] [Google Scholar]
- 23. Gabutti L, Ross V, Duchini F et al. Does bicarbonate transfer have relevant hemodynamic consequences in standard hemodialysis? Blood Purif 2005; 23: 365–372 [DOI] [PubMed] [Google Scholar]
- 24. Fernández-Lucas M, Teruel-Briones JL, Gomis-Couto A et al. Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen. Nefrologia 2012; 32: 767–776 [DOI] [PubMed] [Google Scholar]
- 25. Sargent JA, Marano M, Marano S, et al. Changing dialysate composition to optimize acid-base therapy. Semin Dial 2019; 32: 248–254 [DOI] [PubMed] [Google Scholar]
- 26. Chen Z, Sun F, Shen Y et al. Impact of dialysate sodium concentration lowering on home blood pressure variability in hemodialysis patients. Ther Apher Dial 2019; 23: 153–159 [DOI] [PubMed] [Google Scholar]
- 27. Santos SFF, Peixoto AJ. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munoz Mendoza J, Bayes LY, Sun S et al. Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice-weekly in-center nocturnal hemodialysis: a quality improvement study. Am J Kidney Dis 2011; 58: 956–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterns RH. General principles of disorders of water balance (hyponatremia and hypernatremia) and sodium balance (hypovolemia and edema). UpToDate 2019. https://www.uptodate.com/contents/general-principles-of-disorders-of-water-balance-hyponatremia-and-hypernatremia-and-sodium-balance-hypovolemia-and-edema#!
- 30. Dunlop JL, Vandal AC, Marshall MR. Low dialysate sodium levels for chronic haemodialysis. Cochrane Database Syst Rev 2019; 2019; CD011204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heguilen RM, Sciurano C, Bellusci AD et al. The faster potassium-lowering effect of high dialysate bicarbonate concentrations in chronic haemodialysis patients. Nephrol Dial Transplant 2005; 20: 591–597 [DOI] [PubMed] [Google Scholar]
- 32. Fissell R, Hakim RM. Improving outcomes by changing hemodialysis practice patterns. Curr Opin Nephrol Hypertens 2013; 22: 675–680 [DOI] [PubMed] [Google Scholar]
- 33. Kardalas E, Paschou SA, Anagnostis P et al. Hypokalemia: a clinical update. Endocr Connect 2018; 7: R135–R146 [DOI] [PMC free article] [PubMed] [Google Scholar]