Abstract
Context
Treatment with levothyroxine (LT4) that normalize serum thyrotropin (TSH) is expected to restore lipid metabolism.
Objective
To assess statin utilization in LT4-treated patients through an observational drug utilization study.
Methods
Three sites were involved: (1) 10 723 outpatients placed on LT4 during 2006-2019 identified from the Clinical Research Data Warehouse of the University of Chicago; (2) ~1.4 million LT4 prescriptions prepared by primary care physicians during January-December 2018, identified from the IQVIA™ database of medical prescriptions in Brazil; (30 ~5.4 million patient interviews during 2009-2019, including ~0.32 million patients on LT4, identified from the Fleury Group database in Brazil.
Results
On site 1, initiation of therapy with LT4 increased the frequency of statin utilization (19.1% vs 24.6%), which occurred ~1.5 years later (median 76 weeks) and, among those patients that were on statins, increased intensity of treatment by 33%, despite normalization of serum TSH levels; on site 2, after matching for sex and age, the frequency of statins prescription was higher for those patients using LT4: females, 2.1 vs 3.4% (odds ratio [OR] 1.656 [1.639-1.673]); males, 3.1 vs 4.4% (OR 1.435 [1.409-1.462]); and, on site 3, after matching for sex and age, the frequency of statin utilization was higher in those patients using LT4: females, 10 vs 18% (OR 2.02 [2.00-2.04]); males, 15 vs 25% (OR 1.92 [1.88-1.96]); all P values were <.0001.
Conclusion
Prescription and utilization of statins were higher in patients taking LT4. The reasons for this association should be addressed in future studies.
Keywords: hypothyroidism, cholesterol, statins, levothyroxine, thyroid
Hypothyroidism is the second most prevalent endocrine disease, affecting approximately 5% of the US population [1]. Treatment is straightforward, with daily tablets of levothyroxine (LT4) [2], which after absorption is activated to 3,5,3′-triiodothyronine by tissue deiodinases [3]. The dose of LT4 is adjusted at regular intervals, until serum thyrotropin (TSH) levels are within the normal reference range.
The majority of LT4-treated patients with normal TSH levels do well and remain under the care of their primary care providers [4, 5]. However, there is evidence that a fraction of LT4-treated patients remains symptomatic despite normal serum TSH levels [6-9]. Symptoms range from impaired cognition, mood, and behavior, in addition to difficulty maintaining body weight. In addition, preclinical findings in LT4-treated thyroidectomized rats indicate that serum cholesterol remains above the reference range despite normalization of serum TSH [10]. This was confirmed in patients undergoing total thyroidectomy, in whom serum cholesterol levels never returned to presurgical levels, even after 2 years on LT4 [11, 12]. A meta-analysis of more than 65 studies that included 1878 LT4-treated patients and 14 493 healthy controls revealed that LT4-treated patients have 9.6 mg/dL higher total serum cholesterol along with elevated low-density lipoprotein by ~3.3 mg/dL [13].
In 12 625 participants from the Lifelines Cohort Study, the utilization of cholesterol-lowering drugs was increased by 2-fold in 364 individuals on LT4 compared with the general population (8% vs 17%) [14]. Furthermore, the analysis of the NHANES database identified 469 LT4-treated individuals who are ~50% more likely to be on statin medications than controls matched for age, sex, ethnic background, and serum TSH levels [15].
The analysis of prescription patterns is accepted as a way to examine the rational use of medications, pharmacovigilance, evidence-based medicine, and pharmacoeconomics [16, 17]. Given the exclusive uses of LT4—for hypothyroidism—and statins—to lower serum cholesterol—here, we conducted an observational cross-sectional study of statin prescription and utilization in LT4-treated individuals in 3 different sites: a study of ~11 thousand outpatients placed on LT4, a cross-sectional analysis of ~102 million prescribed medications, and interviews with ~5.5 million patients.
Material and Methods
This is a database analysis of statin coprescription and/or co-utilization in LT4-treated patients: site 1, University of Chicago, Chicago, IL, under an institutional review board exemption IRB19-1019 to pull deidentified data; site 2, commercially available IQVIA™ (Durham NC) Brazilian database; site 3, Fleury Group, Sao Paulo, Brazil, under institutional review board approval NP-458 to pull deidentified data.
Site 1—University of Chicago
Data source
The data were from the Clinical Research Data Warehouse, containing electronic health records data of >12 million encounters for ~2.3 million patients from 2006 to present.
Study design
This was an observational cohort study of all patients with recorded prescription of LT4 within the outpatient clinic at the University of Chicago Medicine from January 1, 2006, to July 1, 2019.
Study population
Data were extracted from the Clinical Research Data Warehouse using the following inclusion criteria: >19 years old, male or female, on LT4 (Table S1 [18]), being followed in the General Internal Medicine Clinics, with at least 2 clinical encounters before LT4 therapy was initiated (window 3 years), on the day LT4 therapy was initiated (index encounter) and at least 2 times afterwards (window 3 years). Pregnant patients and patients with familial hypercholesterolemia (types I, IIa, IIb, III, IV, and V) were excluded; patients taking other thyroid-related medications such as LT3, propylthiouracil (PTU), or methimazole (MMI) were also excluded. We further considered a cohort of patients who were not prescribed levothyroxine but were prescribed statin and evaluated for at least 3 years over the study period.
Exposure, outcomes, and covariates
Exposure was prescription of LT4. The primary outcome was incidence of statin (Table S2 [18]) utilization before and after administration of LT4; as indicated, in some cases we graded intensity of statin treatment according to the American Heart Association criterion [19]: high (H), medium (M), or low (L).
Analysis
Patient characteristics (age, sex, and race) and medication history before and after initiation of LT4 prescription were analyzed. Comparing the rate of statin prescription for the same set of patients pre- and post-LT4 prescription circumvented interference of potential confounders. McNemar’s test was used to test whether the intensity of the statin regimen was affected by usage of LT4. All analyses were conducted in R Version 3.6.2, with P < .05 denoting statistical significance.
Site 2—Primary Care Physician Prescription Patterns in Brazil
Data source
The IQVIA™ Brazilian database was made available through Biolab Pharmaceutical, Sao Paulo, SP, Brazil. The deidentified data contain patient sex and prescription records from preregistered physicians with offices in 20 Brazilian states, home to approximately ~180 million people. Data were obtained from randomly selected physicians, on a weekly basis, including all types of payers, and used to estimate the totality of prescriptions in the same geographical region, with a ±11.2% CI. IQVIA databases are routinely used globally to study national prescription patterns [20].
Study design
This was an observational cross-sectional drug utilization study during January to December 2018.
Study population
Data were extracted from the database using predefined inclusion criteria: prescriptions for LT4 (Table S3 [18]), statins (Table S4 [18]), and all other medications for adult individuals >18 years old, male or female, prepared by primary care physicians; patients taking other thyroid-related medications such as LT3, PTU, or MMI were also excluded.
Exposure, outcomes, and covariates
Exposure was prescription of LT4. The primary outcome was frequency of statin coprescription. The covariates included in the models were sex and age.
Analysis
To determine the odds of statin utilization in LT4 users vs nonusers, 2 × 2 tables of LT4 and statin use were generated. The population was stratified by sex and age categories. OR point estimates and exact 95% CI were calculated using STATA (version 16.1). Statistical significance was determined via the chi-squared test.
Site 3—Fleury Group in Brazil
Data source
Data were from the Fleury Group database, containing elements of 10-minute unique patient interviews conducted by a medical assistant preceding a clinical laboratory encounter in 6 Brazilian states, home to approximately ~100 million people; the data include all payers. The database was created in October 1997 using an InterSystems Caché and Ensemble, version 1.4 (Caché, InterSystem, 2018; https://docs.intersystems.com/; November 2020), high-performance architecture that is commonly used to develop software applications for healthcare management (Cambridge, MA). The database was built using standard healthcare industry practices to ensure accuracy, completeness, and security of the data collected. Information is entered manually after an attendant has created a new entry and asked the patient about the medications in use. The information is typed in free text format and stored in a Microsoft SQL database. Within a few seconds, the data are replicated to the Cache Database—Intersytems—for permanent storage. All users have username and password, maintained by AD Windows (Active Directory). All registry changes to the database are tracked through logs and are restricted to users with high-level administrative permission. Information is kept secure through a separate network firewall, accessed only by authorized persons within the Fleury Group’s domains. Data stored in this database have been used previously in a number of clinical studies [21-23].
Study design
This was an observational cross-sectional drug utilization study during 2009-2019.
Study population
Data were extracted to identify all individuals >18 years old, male or female, referred by physicians in all specialties. Each entry corresponded to a unique patient; only the most recent dataset from each patient was retrieved. The field containing the names of medications accepts words separated by commas. Data were cleaned using a partial string-matching strategy to standardize the names and avoid loss of information. This was done using local alignment dynamic programming as implemented in the “pairwise-alignment” function from the Biostrings R package (Bioconductor project) [24] using a gap opening penalty of 2 and gap extension penalty of 0.5. The lists of medications, namely, LT4 (Table S3 [18]), statins (Table S4 [18]), and for diabetes mellitus (DM; Tables S5 [18]), were aligned against each patient’s medication string to obtain overlapping data; patients taking other thyroid-related medications such as LT3, PTU, or MMI were also excluded.
Exposure, outcomes, and covariates
Exposure was utilization of LT4 (Table S3 [18]). The primary outcome was utilization of statins (Table S4 [18]). A secondary outcome was utilization of at least 1 medication typically used to treat DM (Table S5 [18]). Covariates included in the models were sex and age.
Analysis
To determine the odds of statins and DM medication use in LT4 users vs nonusers, 2 × 2 tables for LT4 and statin use were generated. The population was stratified by sex and age category. Due to the treatment of DM as possibly confounding the association between LT4 and statins use, the population was further stratified by DM medication use. OR point estimates and exact 95% CI were calculated. Statistical significance was determined via the chi-squares test. Calculations were done using STATA (version 16.1). To determine the relationship between the use of DM medications on the odds of statin use in those on LT4, a Mantel–Haenszel (M-H) adjusted effect estimate was performed on the study population stratified by use of DM medications.
Results
In order to evaluate prescription of statins in LT4-treated patients we mined 3 different but complimentary databases and addressed the following questions: (1) Does initiation of therapy with LT4 increase the likelihood of statin coprescription? If so, how soon after LT4 is initiated are statins initiated? (2) Is the association between LT4 and statins observed outside academic medical centers?
The first question was addressed in a retrospective but longitudinal analysis of repeated encounters that took place before and after patients had been placed on LT4. The second question was addressed by looking at 2 very large databases for prescription (AQVIA) and medication utilization (Fleury), which provide complementary perspectives to prescription of medications vs patient adherence to medication. These 2 perspectives are important given that nonadherence to long-term therapies is variable and may reach 50% in some cases [25].
Site 1—LT4 and Statin Prescription Patterns in a Large Academic Medical Center
In total, 10 723 established patients that were placed on LT4 and met the inclusion and exclusion criteria were identified (Group-0). They were mostly white, non-Hispanic, or Latino women aged 59 years (Table 1) [18]. Healthcare exposure was factored in by ensuring that during the study period all patients were seen at least 2 times before and 2 times after they were placed on LT4 (Fig. 1A). In the LT4-treated patients (Group-0), statin utilization was assessed during the 3 years before the index encounter and found to be 14.3% at the index encounter; this figure increased by 3.4 percentage points after therapy with LT4 was initiated. For most patients, treatment with statins started several months after they were placed on LT4 (median, 49 weeks; 25th-75th percentile, 3-109 weeks) (Table 1).
Table 1.
Demographics, serum TSH, and statin utilization in LT4-treated patients (site 1)
| Group-0 | Group-1 | Group-2 | Group-3 | Group-4 | |
|---|---|---|---|---|---|
| — | have TSH | 0.40 < TSH < 5.0 | TSH < 0.40 | TSH > 5.0 | |
| Number of patients | 10 723 | 5112 | 3584 | 833 | 695 |
| Sex | |||||
| Female | 7562 (70%) | 3607 (71%) | 2538 (70%) | 615 (73%) | 454 (65%) |
| Race and Ethnicity | |||||
| White | 6935 (64%) | 2994 (59%) | 2100 (58%) | 544 (65%) | 350 (50%) |
| Not Hispanic or Latino | 10 192 (95%) | 4846 (95%) | 3418 (95%) | 781 (93%) | 647 (93%) |
| Age at IE | |||||
| Mean (SD) (years) | 59 (16) | 58 (16) | 59 (16) | 55 (16) | 58 (17) |
| Median (min, max) (years) | 61 (18, 92) | 60 (18, 91) | 61 (18, 91) | 56 (19, 90) | 59 (18, 91) |
| Statin utilization | |||||
| Before IE (%) | 14.3 | 18.3 | 19.1 | 15.2 | 17.7 |
| After IE (%) | 17.7 | 23.4 | 24.6 | 19.2 | 22.7 |
| Δ (percentage points) | 3.4 | 5.1 | 5.5 | 4.0 | 5.0 |
| McNemar’s chi-squared test with continuity correction | |||||
| chi-squared value | 247 | 298 | 319 | 5.6 | 42 |
| P value | <.0001 | <.0001 | <.0001 | .0183 | <.0001 |
| Serum TSH | |||||
| Median (mIU/L) (25th, 75th percentile) | N/A | 1.89 (0.71, 3.4) | 1.97 (1.1,3.0) | 0.11 (0.03, 0.25) | 8.59 (6.1, 16) |
| TSH assessment (weeks after IE) | |||||
| Median (25th, 75th percentile) | N/A | 60 (32, 90) | 61 (33, 90) | 61 (37, 90) | 56 (25, 90) |
| Started statin therapy (weeks after IE) | |||||
| Median (25th, 75th percentile) | 49 (3, 109) | 75 (18, 122) | 76 (20, 121) | 77 (3, 126) | 70 (30, 120) |
Group 0, all patients that met inclusion/exclusion criteria; Group 1, patients with serum TSH levels at or around the time therapy with statins was initiated; Group 2, patients with normal serum TSH after the IE; Group 3, patients with suppressed TSH after the IE; Group 4, patients with elevated TSH after the IE. In the control group, statin utilization increased by 1.7 percentage points on year- 2 and by 3.8 percentage points on year 3.
Abbreviations: IE, index encounter; LT4, levothyroxine; SD, standard deviation; N/A, not all values are available; TSH, thyrotropin.
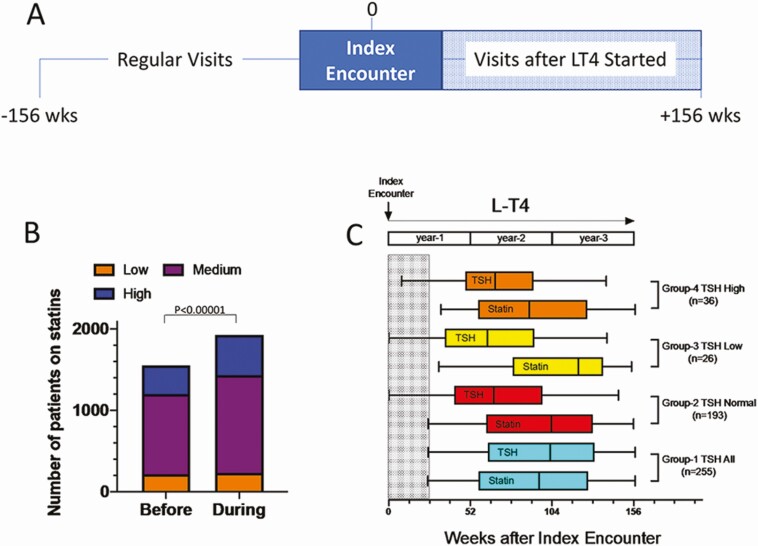
Figure 1.
(A) Timeline during which patient data were obtained at site 1; the analyses encompassed 3 years (156 weeks) prior the index encounter (start of therapy with LT4) and 3 years after LT4 was started; all patients had at least 2 clinic encounters with their providers before and 2 after therapy with LT4 was initiated. (B) Bar graph showing the total number of patients on statins before and after therapy with LT4 was initiated. Low, medium, and high indicate the intensity of treatment with statins. (C) Bar graph showing patients in each group in whom statin was initiated; the timing of stating initiation is shown along with the timing serum TSH was obtained, both measured in weeks after the index encounter; only patients in whom statins was initiated >6 months (shaded area) after index encounter were considered; 4 groups of patients are shown: Group-1, all patients that have a serum TSH on or around the time statin was initiated; Group-2, those patients with a normal serum TSH; Group-3, those patients with a suppressed serum TSH; and Group-4, those patients with a high serum TSH; the number of patients that were placed on statins after the index encounter is shown in parenthesis for each group; in all groups, statistical analysis was with McNemar’s test with a P < .01.
As expected from the age distribution of the cohort, 1536 patients in Group-0 were already on statins by the time they were placed on therapy with LT4 (Table S6 [18]). An analysis limited to these patients revealed that (1) 162 stopped taking statins after the index encounter; (2) 16 patients that were on L increased to M, 12 patients on L increased to H, whereas 132 patients remained on L; (3) 6 patients that were on M decreased to L, whereas 69 patients increased from M to H; 790 patients remained on M; (4) 5 patients that were on H decreased to L, 13 decreased to M, whereas 331 remained on H. After LT4 therapy was initiated, there was an ~33% increase in intensity of the therapy with statins (Table S6 [18] and Fig. 1B).
Because systemic thyroid status affects lipid homeostasis, we looked for serum TSH measurements that were obtained at or around the time therapy with statins was initiated, having identified 5112 individuals with available results (Group-1; Table 1). In these patients, baseline statin utilization was 18.3%, which increased by 5.1 percentage points after LT4 therapy was initiated; new statin prescriptions occurred at approximately 1.5 years after they started on LT4 (median 75 weeks) (Table 1).
Further analyses of patients in Group-1 indicated that 3584 of these patients had TSH levels within the normal range (Group-2), whereas in 833 patients serum TSH was suppressed (Group-3), and in 695 patients serum TSH remained elevated (Group-4). Nonetheless, statin utilization also increased in these groups after LT4 was initiated, by 5.5 percentage points in Group-2, by 4.0 percentage points in Group-3, and by 5.0 percentage points in Group-4. The timing of the new statin prescriptions occurred with a median of 76, 77, and 70 weeks, respectively (Table 1).
An association between therapy with LT4 and utilization of statins was assessed through McNemar’s test and found to be present in all groups (Table 1), even if we only considered patients that were placed on statins after a minimum of 6 months on LT4 (Fig. 1C). Additionally, we constructed a control population of 2062 patients who (1) were not on LT4 but (2) were on statins, were prescribed statins, or were taken off statins during the study period, (3) were evaluated for at least 3 years, and (4) were age and sex matched to a case population of patients who were both on LT4 and statin. On average, the 3-year statin utilization decreased by 3.7% in this cohort over the study period.
Site 2—Analyses of Outpatient LT4 and Statin Prescription Patterns
In total, ~102 million prescribed medications by primary care physicians for 2018 were identified; they were written for slightly more women than men, of which ~1.4 million were for LT4 (Table S7 [18]). The prescriptions for LT4 were predominantly for women (~4:1). Statins were coprescribed for 4.4% of the men and 3.4% of the women on LT4. For all prescribed medications combined, except for LT4, statins were coprescribed for 3.1% and 2.1% men and women, respectively (Table S7 [18]). The prescription of statins increased progressively with age, with women reaching a peak of ~3.3% and men of ~4.1% at ages >65 years; men exhibited a slightly faster increase than women over time (Table S7) [18]. However, in women taking LT4 the utilization of statins reached ~4.6%, and in men it reached ~5.9% (Table S7 [18]). The overall OR of statin coprescription was ~1.4 for men and ~1.7 for women (Table 2). The OR of statin prescription with LT4 was higher across all ages and both sexes. It was higher in patients aged 20-29 years, ~1.5 and ~1.9 for men and women, respectively, and down to ~1.3 and ~1.5 for patients >65 years of age (Table 2).
Table 2.
Odds ratios of statin prescription with LT4 use, stratified by age and sex (site 2)
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Age group | OR | 95% CI | P value | OR | 95% CI | P value |
| 20-29 | 1.92 | (1.80-2.04) | <.0001 | 1.54 | (1.34-1.77) | <.0001 |
| 30-39 | 1.92 | (1.85-2.00) | <.0001 | 1.42 | (1.35-1.50) | <.0001 |
| 40-54 | 1.68 | (1.66-1.71) | <.0001 | 1.42 | (1.38-1.47) | <.0001 |
| 55-64 | 1.41 | (1.39-1.44) | <.0001 | 1.46 | (1.41-1.51) | <.0001 |
| 65 years and over | 1.50 | (1.47-1.54) | <.0001 | 1.34 | (1.28-1.39) | <.0001 |
| Total | 1.66 | (1.64-1.67) | <.0001 | 1.4 | (1.41-1.46) | <.0001 |
Raw data are shown elsewhere (Table S7 [18]).
Abbreviations: LT4, levothyroxine; OR, odds ratio;
Site 3—Analyses of Interviews With Outpatients in a Large Clinical Laboratory
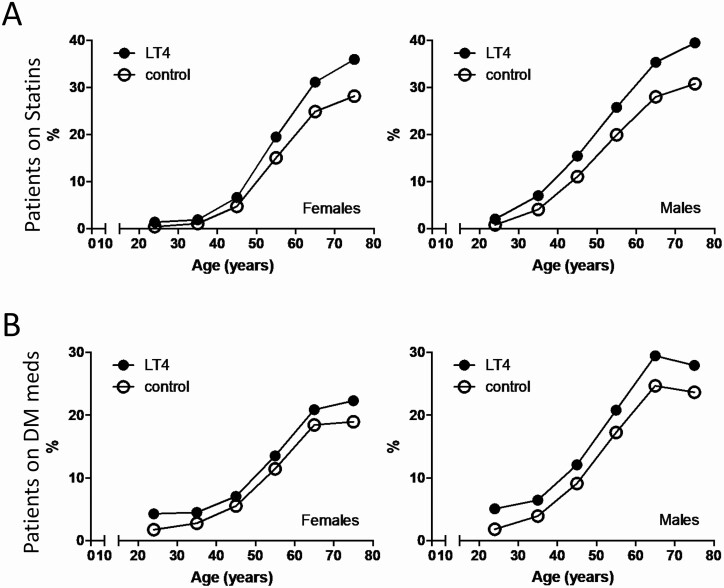
Approximately 5.4 million unique patient interviews were identified, of which ~2.5 million were on 1 or more medications (Table S8 [18]). Approximately 0.32 million patients reported being on LT4 (6.0%); ~25% of the men and ~18% of the women on LT4 were on statins (Table S8 [18]). In patients not taking LT4, utilization of statins was ~15% of the men and ~10% of the women (Table S8 [18]). The overall OR of statin utilization with LT4 was ~1.9 for men and ~2.0 for women (Table 3). The utilization of statins increased progressively with age, with women reaching a peak of ~28% and men of ~30% at ages >70 years; men exhibited a slightly faster increase than women over time (Fig. 2A). However, in women taking LT4 utilization of statin reached ~36%, and in men it reached ~40% (Fig. 2A). The OR of statin utilization with LT4 was higher in patients aged 18-30 years, ~2.6 and ~3.1 for men and women, respectively, down to ~1.5 and ~1.4 for patients >70 years of age (Table 3).
Table 3.
Odds ratios of statins use with LT4 use, stratified by age and sex (site 3)
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Age group | OR | 95% CI | P value | OR | 95% CI | P value |
| 18-30 | 3.07 | (2.69-3.50) | <.0001 | 2.61 | (2.06-3.28) | <.0001 |
| 31-40 | 1.54 | (1.59-1.85) | <.0001 | 1.78 | (1.62-1.96) | <.0001 |
| 41-50 | 1.43 | (1.37-1.49) | <.0001 | 1.46 | (1.38-1.56) | <.0001 |
| 51-60 | 1.37 | (1.33-1.40) | <.0001 | 1.39 | (1.33-1.46) | <.0001 |
| 61-70 | 1.36 | (1.33-1.39) | <.0001 | 1.40 | (1.35-1.46) | <.0001 |
| Over 70 years | 1.43 | (1.40-1.46) | <.0001 | 1.47 | (1.42-1.52) | <.0001 |
| Total | 2.02 | (2.00-2.04) | <.0001 | 1.92 | (1.88-1.96) | <.0001 |
Raw data are shown elsewhere (Table S8 [18] ).
Abbreviations: LT4, levothyroxine; OR, odds ratio.
Figure 2.
(A) Statin utilization in control and LT4-treated patients according to age group and sex in patients that are taking at least 1 DM medication; control patients are taking 1 or more medications except for LT4 (site 3). (B) Utilization of DM medications in control and LT4-treated patients according to age group and sex; control patients are taking 1 or more medications except for LT4 (site 3).
Treatment of DM includes use of statins [19]. To test whether the utilization DM medications influenced the use of statins in LT4-treated patients, we assessed their utilization of drugs typically used by patients with DM (Table S9 [18]): ~20% of the men and ~13% of the women on LT4 were on at least 1 DM medication (Table S9 [18]); in patients not taking LT4, utilization of DM medications was ~13% of the men and ~8% of the women (Fig. 2B; Table S9 [18]). The overall OR for utilization of DM medications was ~1.7 in both men and women (Table 4). The magnitude of the effect was slightly greater in younger individuals (Fig. 2B; Table S10 [18]); the OR for the utilization of DM medications with LT4 was higher in patients aged 18-30 years: ~2.9 and ~2.5 for men and women, respectively, down to ~1.3 and ~1.2 for patients >70 years (Table 4).
Table 4.
Odds ratios of at least 1 DM medication use with LT4 use, stratified by age and sex (site 3)
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Age group | OR | 95% CI | P value | OR | 95% CI | P value |
| 18-30 | 2.49 | (2.32-2.69) | <.0001 | 2.87 | (2.47-3.33) | <.0001 |
| 31-40 | 1.66 | (1.58-1.74) | <.0001 | 1.69 | (1.52-1.86) | <.0001 |
| 41-50 | 1.30 | (1.25-1.35) | <.0001 | 1.37 | (1.28-1.47) | <.0001 |
| 51-60 | 1.21 | (1.17-1.24) | <.0001 | 1.26 | (1.20-1.32) | <.0001 |
| 61-70 | 1.17 | (1.14-1.20) | <.0001 | 1.27 | (1.22-1.33) | <.0001 |
| Over 70 years | 1.22 | (1.20-1.26) | <.0001 | 1.25 | (1.20-1.30) | <.0001 |
| Total | 1.69 | (1.67-1.72) | <.0001 | 1.69 | (1.66-1.73) | <.0001 |
Raw data are shown elsewhere (Table S9 [18]).
Abbreviations; DM, diabetes mellitus; LT4, levothyroxine; OR, odds ratio.
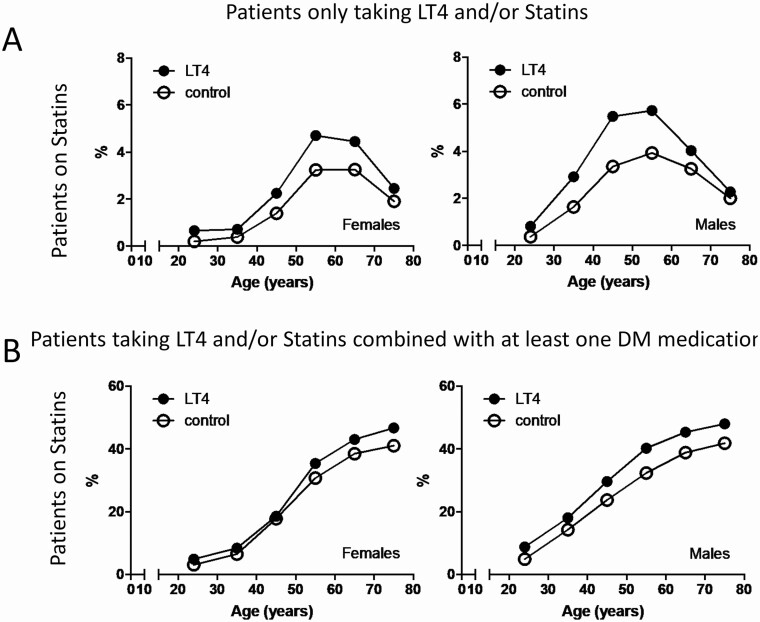
To discriminate between the effects of LT4 vs DM medication usage on statin utilization, we looked at 2 subgroups of patients: (1) those only using LT4 and/or statin and (2) all those using LT4 and/or statin combined with at least 1 DM medication.
In patients only using LT4 and/or statins (Fig. 3A), we noticed that ~3.8% of the men and ~2.8% of the women on LT4 were on statins, whereas in patients not taking LT4, utilization of statins was lower: ~2.5% of the men and ~1.5% of the women (Table S10 [18]). The overall OR of statin utilization with LT4 was ~1.9 for men and ~2.1 for women (Table S11 [18]). The effects of LT4 utilization on statin usage occurred at all ages, with a greater OR in younger patients, 2.4 in men and 3.0 in women, than in older individuals (Table S11 [18]).
Figure 3.
(A) Statin utilization in control and LT4-treated patients according to age group and sex, in patients only taking LT4 and/or statins; control patients are only taking statins (site 3). (B) Statin utilization in control and LT4-treated patients according to age group and sex, in patients that are taking at least 1 DM medication; control patients are taking at least 1 DM medication but not LT4 (site 3).
At the same time, in patients using DM medications (Fig. 3B), we noticed that ~42% of the men and ~37% of the women on LT4 were on statins, whereas in those patients not taking LT4, utilization of statins was lower: ~34% of men and ~31% of women (Table S12 [18]). The overall OR of statin utilization with LT4 was ~1.4 for men and ~1.3 for women (Tables S14 [18]). The effects of LT4 utilization on statin usage occurred at all ages, with a greater OR in younger patients, 1.9 in men and 1.6 in women, than in older individuals (Table S13 [18]).
The M-H adjusted effect estimate was performed on the study population stratified by use of DM medications. The difference between crude OR of statin use in LT4 users compared with the M-H estimate (1.79 vs 1.68) was small (<10%), suggesting the use of DM medication is not a significant confounder of the effect of LT4 use on statin use. Additionally, the M-H test of homogeneity was performed, which revealed a significant interaction between the OR of statin use and use of DM medications (P < .001). In this case, the OR of using statins in LT4 users is significantly higher in the population that does not use DM medications versus the population using DM medications. This is predictable in that patients with DM are more likely to be on a statin per clinical guidelines, thus the prevalence of statin use is greater in the population not on LT4 (control group).
Discussion
Hypothyroid patients frequently develop elevated serum cholesterol and low-density lipoprotein levels, as a result of the diminished effects of thyroid hormones in the liver. This is assumed to be fully reversed by treatment with LT4, which normalizes TSH levels and eliminates symptoms of the disease in most patients [26, 27]. Nonetheless, the utilization of cholesterol-lowering drugs has not been consistently reported in these studies. In addition, several reports with a relatively small number of participants and a metanalysis involving more than 65 studies suggest that normalization of serum cholesterol might not be achieved with LT4 alone [11-15]. Here we concluded that initiation of therapy with LT4 is strongly associated with coprescription of cholesterol-lowering therapy with statins. In addition, in those patients that were on statins, we saw that the intensity of the statin treatment increased after they were placed on LT4. The association between LT4 and statins was confirmed with the analysis of 2 large databases containing millions of prescriptions and interviews with patients.
In site 1, we analyzed data from ~10 500 patients on LT4. Our findings indicate that by and large, treatment with statins was initiated 1 to 2 years after patients had been placed on LT4. In addition, breaking down these patients according to serum TSH levels revealed that increased statin utilization was observed even in patients with a normal serum TSH, in other words the patients had reached biochemical euthyroidism. The data obtained in sites 2 and 3 support the idea that the association between LT4 and statins was not a limited finding, restricted to 1 academic medical setting. On the contrary, it was also observed in millions of individuals outside an academic medical center.
The present findings need to be considered in the light of the following important points:
Impact of healthcare exposure: in most patients, hyperlipidemia is unrelated to hypothyroidism; so that when assessment of serum lipids is triggered by identification of hypothyroidism it is conceivable that the hyperlipidemia will not be responsive to treatment with LT4, which would likely provoke statin therapy. In addition, patients on LT4 may have had more healthcare visits and/or more intensive monitoring of lipid levels. It is expected that this would naturally lead to more prescriptions for statins, and/or higher doses of statins. While these are critical points, we note that site 1 consisted of regular patients, followed at the University of Chicago for at least 3 years prior to LT4 therapy was initiated. Not only healthcare exposure was consistent before and after therapy with LT4 was initiated, but therapy with statins only started ~1.5 years later.
Control for healthcare exposure is less straightforward in sites 2 and 3. In site 2 we looked at coprescriptions by primary care physicians, so that all patients were under active ambulatory medical care as they were taking at least 1 prescription medication. At the same time, data from site 3 indicate that the effect of LT4 on statin coprescription was also present in a large subgroup of ~0.26 million patients kept on DM medications, in which LT4-treated and control patients should have had similar levels of healthcare exposure because of the higher intensity of monitoring for DM medications.
An additional important limitation of site 1 is that we could not conduct a before–after statistical analysis on a suitable control population due to lack of a proper definition of an index encounter and the presence of time-varying confounders. However, an ad hoc analysis demonstrates that on average, 3-year statin utilization decreased in a smaller set of control patients that were age and sex matched to case patients who were on both statins and LT4.
2. The absence of TSH levels on sites 2 and 3, which prevented assessment of undertreatment with LT4, that might affect 15% to 25% of all hypothyroid patients [28, 29], possibly elevating cholesterol levels and frequency of statins prescription/utilization. Nonetheless, all patients included in this study were under ongoing medical care, which makes it less likely they were undertreated. It is unlikely that LT4-treated hypothyroid patients monitored for lipids would not be monitored for TSH levels as well, which would prompt an adjustment of the LT4 dose rather than initiation of statin therapy. Indeed, it is recommended that hypothyroid patients be treated with LT4 for 3 to 4 months before starting lipid-lowering therapy, such as statins [30].
3. The small number of covariates analyzed in sites 2 and 3: Given the nature of the databases, their level of detail did not allow further refinement of the data. Thus, we could not directly consider body mass index or other confounding metabolic comorbidities, which could have affected statin utilization. Nonetheless, a large effect size such as the 1 used in the present investigation, namely 3 databases with thousands/millions of participants, can outweigh the combined effects of plausible confounders in an observational study [31]. In addition, it is accepted that even when potential confounders have not been ruled out by design, a large observed effect can also outweigh the combined effects of confounders [31]. Indeed, the OR for LT4 and statin co-utilization reached 1.9-2.0.
Alternatively, the impact of confounders in observational studies has also been addressed by assigning each participant a propensity score [31]. By analyzing the subgroup of ~0.26 million participants utilizing DM medications (site 3), we focused on patients with higher propensity to have metabolic abnormalities and found that the association of LT4 and statin coprescription persisted. At the same time, by analyzing the subgroup of ~0.05 million participants utilizing only LT4 and/or statins (site 3), the focus was on patients with lower propensity to have metabolic abnormalities. In this subgroup as well, the association of LT4 and statins coprescription still persisted.
LT4 is considered a safe and effective medication. Two large studies have found that, provided hypothyroid patients achieve normal TSH levels, mortality is similar to the background population [32, 33]. However, in these studies the use of lipid-lowering drugs was either not assessed [33] or no direct comparison in outcomes between those on and off lipid-lowering drugs was provided [32]. The present data indicate that by virtue of being more frequently coprescribed with LT4, statins might play a role in these excellent outcomes reported by these studies.
We considered the underlying factors that explain the relationship between LT4 therapy and statins. One possibility is that patients with unsuspected hypothyroidism develop hypercholesterolemia and are treated with statins, only to be diagnosed and treated for hypothyroidism at a later time. The data from site 1 are not supportive of this model, as patients were placed on statins ~1.5 years after they were started on LT4.
An alternative possibility is that treatment with LT4 normalizes TSH levels but fails to restore thyroid hormone signaling in metabolically relevant tissues, including the liver, such as seen in our preclinical model [10]. In fact, the liver and its metabolism of cholesterol is so sensitive to thyroid hormones that a liver-specific 3,5,3′-triiodothyronine analogue was successfully used to lower cholesterol levels in patients taking statins [34]. Clinical evidence that normal TSH levels do not necessarily reflect normalization of thyroid hormone signaling in all tissues was obtained in hypothyroid patients treated with replacement therapy. Whereas parameters reflecting thyroid status in the heart and skeletal muscle were promptly normalized, energy expenditure and serum cholesterol levels were not, despite near normal TSH levels [35].
An unexpected finding of the present investigation was the association between utilization of LT4 and DM medications. At face value, it suggests that the residual metabolic fingerprint of hypothyroidism could be broader than anticipated, placing LT4-treated patients at a higher risk of disruptions in energy and carbohydrate metabolism. Indeed, we have reported that, despite lower calorie intake, LT4-treated individuals exhibited ~5% higher body mass index and reported lower physical activity levels than matched controls [15]. Alternatively, we cannot exclude that medical attention, and concomitant testing, such as screening both TSH and lipid profiles at the same time, is the driver for coprescription of T4 diabetes medications. Nonetheless, patients on statins have been found to be at a higher risk of developing DM [36]. While this could be a confounding factor in the present studies, the M-H analysis indicated that the LT4 -effect on statin utilization was independent of medications for DM.
The present studies were conducted in 3 different sites, in 2 continents, and involved millions of prescribed medications and patients. The consistency of the results across the 3 sites was remarkable, supporting the idea that the present conclusions can be generalized to a larger adult population, independent of sex and age group.
Conclusion
The retrospective study of 3 independent and relatively large cohorts indicate that patients of both sexes and all adult ages on LT4 were more likely to be treated with statins. These findings call for further studies to explore whether LT4 treatment is associated with residual hypercholesterolemia to the extent intervention is required.
Acknowledgments
We are grateful to Ms. Roseli Capellari for assistance with the IQVIA™ database, and to support by the Fellowship Program, Section of Endocrinology at the University of Chicago.
Glossary
Abbreviations
- DM
diabetes mellitus
- LT4
levothyroxine
- M-H
Mantel–Haenszel
- OR
odds ratio
- TSH
thyrotropin
Additional Information
Disclosures: A.B. is a consultant for Allergan Inc and Synthonics, Inc.; the other authors have nothing to disclose.
Data Availability
All data presented in this manuscript are contained within the figures and tables presented in the manuscript.
References
- 1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jonklaas J, Bianco AC, Bauer AJ, et al. ; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bianco AC, Dumitrescu A, Gereben B, et al. . Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev. 2019;40(4):1000-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2018;391(10115):30. [DOI] [PubMed] [Google Scholar]
- 5. Jonklaas J, Bianco AC, Bauer AJ, et al. ; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf). 2002;57(5):577-585. [DOI] [PubMed] [Google Scholar]
- 7. Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf). 1988;28(3):325-333. [DOI] [PubMed] [Google Scholar]
- 8. Roberts ND. Psychological problems in thyroid disease. BTF Newsletter. 1996;18(3). [Google Scholar]
- 9. Peterson SJ, Cappola AR, Castro MR, et al. . An online survey of hypothyroid patients captured predominantly dissatisfied individuals. Thyroid. 2018;28(6):707-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Werneck de Castro JP, Fonseca TL, Ueta CB, et al. . Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Investig. 2015;125(2):769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ito M, Miyauchi A, Hisakado M, et al. . Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid. 2017;27(4):484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee YK, Lee H, Han S, et al. . Association between thyroid-stimulating hormone level after total thyroidectomy and hypercholesterolemia in female patients with differentiated thyroid cancer: a retrospective study. J Clin Med. 2019;8(8):1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAninch EA, Rajan KB, Miller CH, Bianco AC. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(12):4533-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wouters HJ, van Loon HC, van der Klauw MM, et al. . No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid. 2017;27(2):147-155. [DOI] [PubMed] [Google Scholar]
- 15. Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “Euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab. 2016;101(12):4964-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain S, Upadhyaya P, Goyal J, et al. . A systematic review of prescription pattern monitoring studies and their effectiveness in promoting rational use of medicines. Perspect Clin Res. 2015;6(2):86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Layton D, Hazell L, Shakir SA. Modified prescription-event monitoring studies: a tool for pharmacovigilance and risk management. Drug Saf. 2011;34(12):e1-e9. [DOI] [PubMed] [Google Scholar]
- 18. Idrees T, Prieto WH, Casula S, et al. . Data from: Use of statins among patients taking levothyroxine: an observational drug utilization study across sites. Dryad Digital Repository. ProMED-mail website. Deposited November 4, 2020. https://datadryad.org/stash/share/-AIZKqGacJLcLh9Q1ppHW1LaWUShuqx28V3WU_uCOBM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grundy SM, Stone NJ, Bailey AL, et al. . 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209. [DOI] [PubMed] [Google Scholar]
- 20. Furukawa NW, Zhu W, Huang YA, Shrestha RK, Hoover KW. National trends in drug payments for HIV preexposure prophylaxis in the United States, 2014 to 2018: A retrospective cohort study. Ann Int Med. 2020;173(10):799-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Candido DS, Claro IM, de Jesus JG, et al. ; Brazil-UK Centre for Arbovirus Discovery, Diagnosis, Genomics and Epidemiology (CADDE) Genomic Network . Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369(6508):1255-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baldo DC, Dellavance A, Ferraz MLG, Andrade LEC. Evolving liver inflammation in biochemically normal individuals with anti-mitochondria antibodies. Auto Immun Highlights. 2019;10(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prado MS, Dellavance A, Rodrigues SH, Marvulle V, Andrade LEC. Changes in the result of antinuclear antibody immunofluorescence assay on HEp-2 cells reflect disease activity status in systemic lupus erythematosus. Clin Chem Lab Med. 2020;58(8):1271-1281. [DOI] [PubMed] [Google Scholar]
- 24. Pagès H, Aboyoun P, Gentleman R, DebRoy S. Biostrings: Efficient manipulation of biological strings. R package version 2.58.0. 2020. https://bioconductor.org/packages/Biostrings [Google Scholar]
- 25. DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794-811. [DOI] [PubMed] [Google Scholar]
- 26. Cappola AR, Desai AS, Medici M, et al. . Thyroid and cardiovascular disease: research agenda for enhancing knowledge, prevention, and treatment. Thyroid. 2019;29(6):760-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kotwal A, Cortes T, Genere N, et al. . Treatment of thyroid dysfunction and serum lipids: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105(12):dgaa672. [DOI] [PubMed] [Google Scholar]
- 28. Vaisman F, Coeli CM, Ward LS, et al. . How good is the levothyroxine replacement in primary hypothyroidism patients in Brazil? Data of a multicentre study. J Endocrinol Invest. 2013;36(7):485-488. [DOI] [PubMed] [Google Scholar]
- 29. Okosieme OE, Belludi G, Spittle K, Kadiyala R, Richards J. Adequacy of thyroid hormone replacement in a general population. QJM. 2011;104(5):395-401. [DOI] [PubMed] [Google Scholar]
- 30. Ross DS. Lipid abnormalities in thyroid disease. In: Post TW, ed. UpToDate. UpToDate; 2020. [Google Scholar]
- 31. Nunan D, Aronson J, Bankhead C. . Catalogue of bias: attrition bias. BMJ Evid Based Med. 2018;23(1):21-22. [DOI] [PubMed] [Google Scholar]
- 32. Thayakaran R, Adderley NJ, Sainsbury C, et al. . Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ. 2019;366:l4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid. 2018;28(5):566-574. [DOI] [PubMed] [Google Scholar]
- 34. Ladenson PW, Kristensen JD, Ridgway EC, et al. . Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362(10):906-916. [DOI] [PubMed] [Google Scholar]
- 35. Ridgway EC, Cooper DS, Walker H, et al. . Therapy of primary hypothyroidism with L-triiodothyronine: discordant cardiac and pituitary responses. Clin Endocrinol (Oxf). 1980;13(5):479-488. [DOI] [PubMed] [Google Scholar]
- 36. Chogtu B, Magazine R, Bairy KL. Statin use and risk of diabetes mellitus. World J Diabetes. 2015;6(2):352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this manuscript are contained within the figures and tables presented in the manuscript.