SUMMARY
The domestication syndrome refers to a set of traits that are the by-products of artificial selection for increased tolerance toward humans [1–3]. One hypothesis is that some species, like humans and bonobos, “self-domesticated” and have been under selection for that same suite of domesticated phenotypes [4–8]. However, the evidence for this has been largely circumstantial. Here, we provide evidence that, in marmoset monkeys, the size of a domestication phenotype—a white facial fur patch—is linked to their degree of affiliative vocal responding. During development, the amount of parental vocal feedback experienced influences the rate of growth of this facial white patch, and this suggests a mechanistic link between the two phenotypes, possibly via neural crest cells. Our study provides evidence for links between vocal behavior and the development of morphological phenotypes associated with domestication in a nonhuman primate.
In Brief
Like humans and bonobos, marmoset monkeys share a suite of phenotypes associated with the domestication syndrome. Ghazanfar et al. show that the size of their white facial fur patch—a common domestication phenotype—is correlated with vocal behavior. They then reveal that the two traits are causally linked during development.
RESULTS
Domesticated mammals have a set of characteristics in common that are rarely observed together in their wild counterparts [1–3, 9]. These traits include depigmentation, more frequent and non-seasonal estrous cycles, reduced sexual dimorphism, and expanded time windows of behavioral development; collectively, they are referred to as the “domestication syndrome” [10, 11]. The traits comprising the domestication syndrome were not each selected for but were the by-products of selection for increased tolerance toward humans [1, 2].
Some hypothesize that species can “self-domesticate”: changes in niches and social organization (among other things) can result in species being more tolerant of conspecifics or another species with whom they share a habitat [4]. Humans [5, 6] and bonobos [7, 8] are thought to have undergone selection favoring increased in-group tolerance (see [12] for a review). Relative to chimpanzees, bonobos exhibit less inter- and intragroup aggression [13] and increased social tolerance [14]. They also exhibit expanded developmental windows and juvenilized patterns of cognition [15], a juvenilized craniofacial morphology [16], and depigmented lips.
The evidence that some wild species have been under selection for domesticated phenotypes is preliminary. There are no data linking the degree of affiliative or tolerant behavior an individual exhibits with the presence or magnitude of other domestication traits [10, 11]. Moreover, there is a lack of evidence connecting behavioral phenotypes with morphological ones during development, even though certain types of developmental mechanisms are central to the hypothesis [10]. Here, we introduce marmoset monkeys (Callithrix jacchus) as a candidate domestication-syndrome species and demonstrate (1) a relationship between the magnitude of an affiliative behavior and a morphological phenotype and (2) a causal connection between them during development.
Marmoset monkeys exhibit a high degree of social tolerance and prosociality [17]. Like humans, they exhibit allomaternal care of infants [18], food sharing with unrelated group members [19], and affiliative vocal exchanges [20, 21] (for reviews, see [22, 23]). Marmosets take turns vocalizing, exhibiting contingent and repeated exchanges of vocalizations between two individuals for an extended time period [21]. Marmosets also exhibit a number of domestication syndrome traits: apparent depigmentation (a prominent white patch of fur on their foreheads; Figure 1A); non-seasonal breeding [24]; a lack of sexual dimorphism [25]; and an expanded developmental time window (relative to other nonhuman primates) during which they exhibit vocal production learning as infants [26, 27]. In human prelinguistic vocal learning, turn-taking behavior serves as a learning mechanism: parents provide contingent responses to their offspring to spur the development of an infant’s vocalizations [28]. Marmoset monkeys also show this social reinforcement strategy during vocal development [26, 27].
Figure 1. The Probability of Vocal Responses by Marmoset Monkeys Is Related to the Size of Their White Facial Patch.
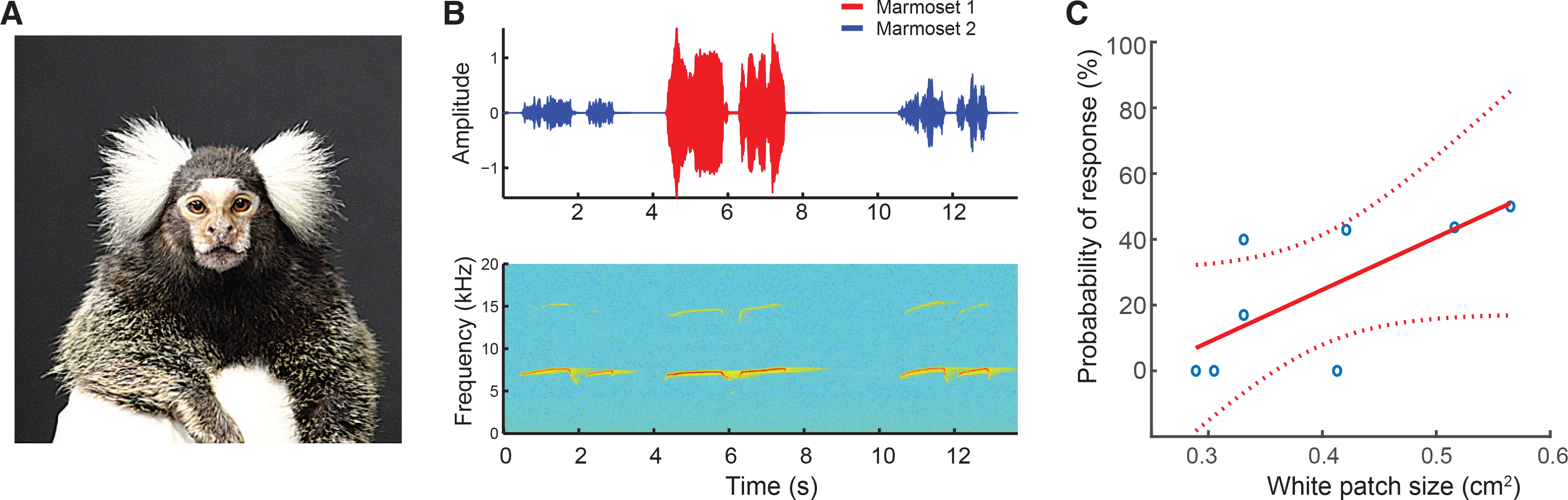
(A) Adult common marmosets all exhibit a prominent white facial patch on their foreheads. We measured their area using image analysis software.
(B) Example of a vocal exchange between two adult marmosets. Top panel shows the time-amplitude waveforms, and the bottom panel shows the spectrogram.
(C) Correlation between white patch size and probability to respond to a conspecific call. Red solid line is the regression line, the blue circles are the data points, and the dotted red lines show the 95% confidence interval.
See Figure S1.
We investigated whether there was a relationship between the vocal exchanges and a morphological feature considered one of the most specific markers of domestication in many species [2]: the white patch on the forehead. Hypothesizing that vocal cooperation and depigmentation are linked domestication phenotypes, we predicted that the size of the white patch should be positively correlated with the probability of responding to conspecific contact calls (Figure 1B). We found a significant positive relationship between white patch size and percent of contact calls to which the subject responded (n = 8 subjects; Spearman rho = 0.847; p = 0.013; Figure 1C). Body weight was not correlated to either white patch size (Spearman rho = −0.587; p = 0.134) or vocal responses (Spearman rho = −0.293; p = 0.483; Figure S1). Moreover, the correlation between white patch size and rate of vocal exchange remained significant and positive after controlling for the body weight (partial Spearman rho = 0.872; p = 0.011). We also found that there was no correlation between vocal output per se—i.e., spontaneous contact calling with no other conspecific within ear shot and the size of the white patch (Spearman rho = 0.563; p = 0.154).
The correlation between the size of the white patch and the degree of cooperative vocal behavior suggests a pleiotropic mechanism. One hypothesis posits that the domestication syndrome reflects partial loss-of-function mutations related to neural crest cell development [3]; the neural crest is a population of pluripotent cells that migrate to various parts of the body during development and whose derivatives include melanocytes, secretory cells (such as those of the adrenal glands), the cells that make up the bones, cartilage, and connective tissues of the head (including the larynx), and neurons of the autonomic nervous system [29]. Wilkins et al. [3] suggest that selection pressures on tameness acted via a reduction in the size of the adrenal gland (which plays a role in stress responses) and that a smaller neural crest cell population would lead to a smaller adrenal gland while simultaneously reducing the population size of other neural-crest-derived cells (e.g., less pigmentation through a reduction in melanocyte numbers). Applied to marmosets, a prediction of the hypothesis is that the white facial patch is white due to a reduction in melanocytes [30, 31]. We tested whether melanocytes were reduced or absent, consistent with the neural crest cell hypothesis. The white patches, along with some of the surrounding blackish fur, were dissected from two adult marmosets. These tissues were stained with a melanocyte marker (Trp1; green) and a nuclear marker (DAPI; blue). Figure 2 shows that, in both animals, hair follicles in the white patches contain far fewer melanocytes than those follicles in the darker fur.
Figure 2. The White Facial Patch Contains a Paucity of Melanocytes Relative to the Adjacent Dark Fur.
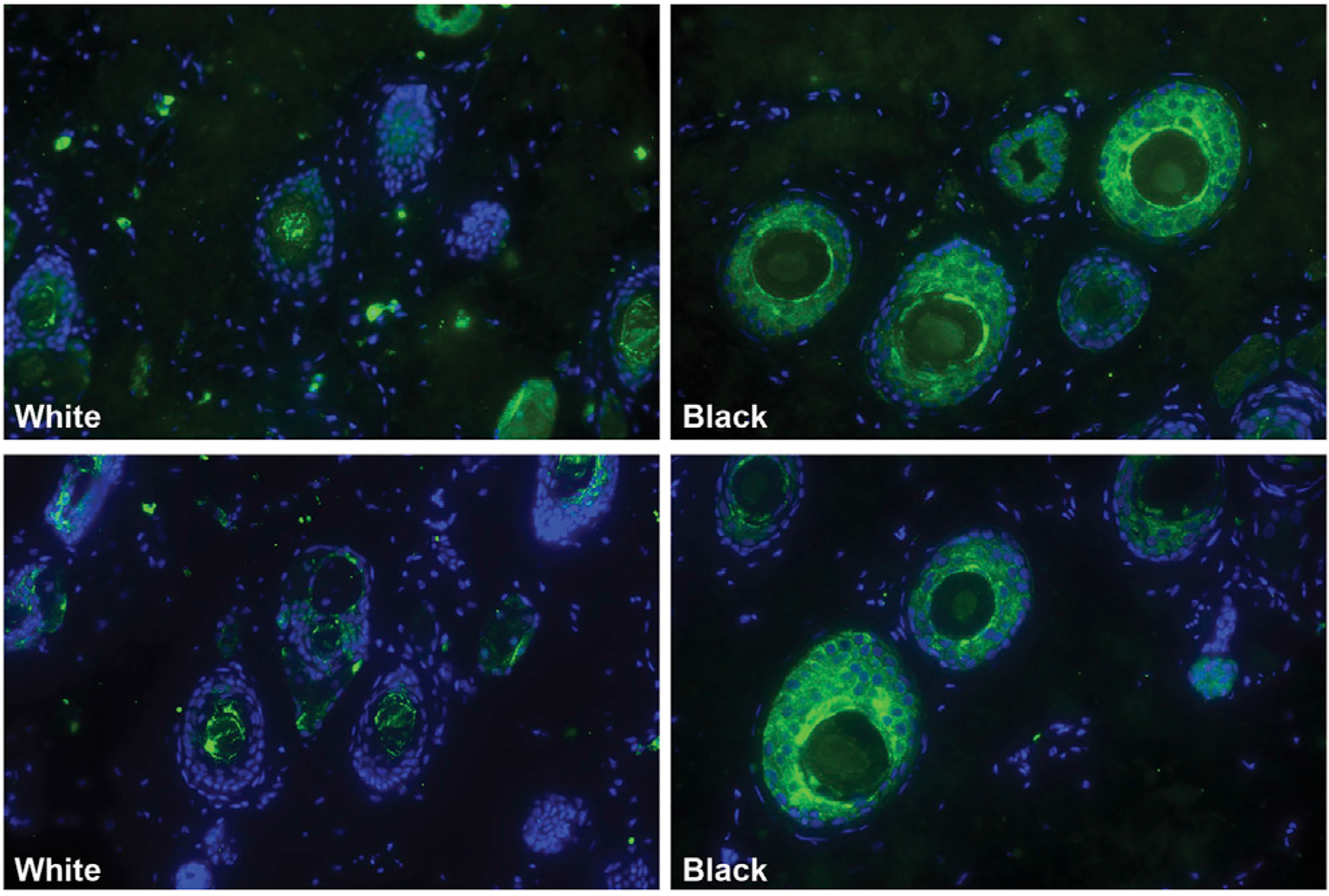
This supports the neural crest cell hypothesis for domestication phenotypes. Green indicates the presence of melanocytes (Trp1 antibody); the blue is a nuclear stain. Samples from two different marmosets are depicted, one per row.
The neural crest cell hypothesis also predicts that the white patch on the head and vocal behavior are developmentally linked. If true, then changing the trajectory of vocal development should also change face patch development. Infant marmoset monkeys produce immature-sounding contact calls that, over the course of 2 months, transform into mature-sounding versions. In a published study of three pairs of dizygotic twins (6 infants from 3 different sets of parents, with each twin pair consisting of a male and a female) and starting at postnatal day 1 (P1), we provided one randomly selected twin the best possible simulated “parent,” who gave 100% vocal feedback via a computer-controlled closed-loop playback system when the infant produced an immature contact call. The other twin received vocal feedback to only 10% of the contact calls it produced [27] (Figure S2). Each experimental session lasted 40 min; the infants were otherwise with their families for the remaining ~23 h each day. These conditioning sessions occurred almost every day until P60 and revealed that infants who received greater contingent feedback from parents developed their mature contact calls faster [27].
In the same set of infants, we tracked the development of the white face patch from P1 to P157, sampling through color-calibrated digital photos about twice a week. We assessed whether the development of the white facial patch—like vocal production—was also influenced by contingent parental feedback. We systematically measured the size of white patch using a computer imaging technique based on a pulse-coupled neural network (PCNN), which segments images into clusters of similar pixels (Figure 3A). The PCNN-segmented images were standardized for size and orientation, and the maximum length (horizontal), height (vertical), and area of the patch were calculated in pixels. These measurements were used to generate growth curves estimating patch size on each postpartum day for each infant. Figures 3B and 3C show the relationship between the white patch size and age and body size (weight) for the three pairs of twins. We fitted a multiple linear regression model to the data with white patch size as the dependent variable and the postnatal day, body weight, sex, feedback condition (high versus low rate of contingent parental vocal responses), and family as independent variables (Table 1). We also included in the regression the interaction between postnatal day with sex, condition, and family. The fitted model had an adjusted R2 of 0.988. The rate of white patch development was significantly faster for the marmosets that received vocal feedback 100% versus 10% of the time (n = 315 days, 6 subjects; p < 0.001; difference between conditions is ~0.1 mm2/30 days), supporting the hypothesis that the amount of parental vocal feedback can indeed change the developmental trajectory of the white facial patch (Figure 3D). Family, sex, and body weight also influenced the rate of white patch development (n = 315 days, 6 subjects; p < 0.001 for all factors). Because body weight and postnatal day are highly correlated, we also fitted the linear regression model without body weight as a factor (Table S1). The results are consistent overall: vocal feedback condition significantly affects the rate of the white patch development (n = 315 days, 6 subjects; p = 0.006). To further support our claims, we also fitted a linear mixed-effect model using family as grouping variable (Table S2). We again find that rate of white patch growth is significantly affected by vocal feedback condition (n = 315; p = 0.005).
Figure 3. Contingent Vocal Feedback from Parents Influences the Rate of White Patch Development in Infants.
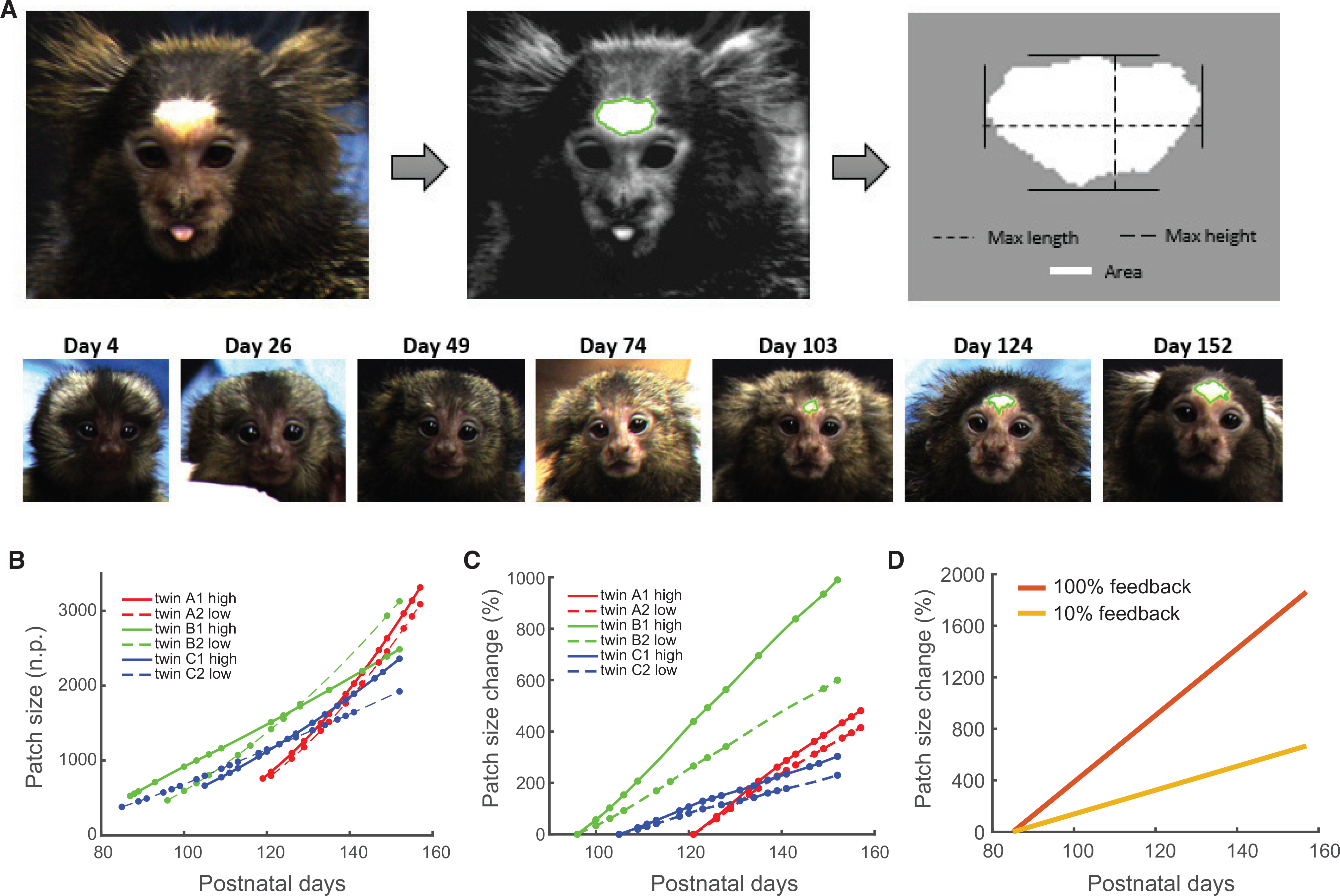
(A) White patch measurements were made semi-automatically using pulse-coupled neural networks. These captured the initial appearance and growth of the patch in infant marmosets.
(B) Graph representing the change in white patch size during development. Data are plotted from the first day of detection. White patch size is calculated in normalized pixel units (n.p.); circles show the dates when data were collected.
(C) Percentage change in white patch size as a function of contingency and after correcting for sex and body weight. The percentage is calculated comparing the sex and body-weight-correct white patch sizes with the size at days 121 (twin A), 96 (twin B), and 105 (twin C), respectively. Circles show the dates where data were collected.
(D) Predicted patch size change for each condition (100% and 10% feedback). The percentage change is estimated based on the white patch sizes estimated by the regression at postnatal day 85 for each group.
See Figure S2.
Table 1.
Parental Vocal Feedback Influences Size of White Facial Patch
| SumSq | DF | F | p Value | |
|---|---|---|---|---|
| Post-natal day (PND) | 3.5812e+06 | 1 | 636.61 | <0.001 |
| Body size | 8.6966e+05 | 1 | 154.6 | <0.001 |
| Sex | 25,522 | 1 | 4.5369 | 0.033 |
| Condition | 18,168 | 1 | 3.2297 | 0.073 |
| Family | 1.6221e+07 | 2 | 1,441.6 | <0.001 |
| PND:sex | 3.057e5+06 | 1 | 543.51 | <0.001 |
| PND:condition | 2.1365e+0.5 | 1 | 37.98 | <0.001 |
| PND:family | 2.799e+06 | 2 | 247.01 | <0.001 |
| Error | 1.7101e+06 | 304 | ||
DISCUSSION
Critics of the domestication syndrome have pointed out that there is little evidence for trait associations at the individual level [10, 11]. Our results show that the degree of affiliative vocal behavior in marmoset monkeys is linked to the size of the white patch of fur on their head, that the fur is white because of a paucity of melanocytes, and that experimentally influencing vocal development modulates the rate of white patch development. The latter suggests that rearing experience can influence the adult endpoints observed in Figure 1. These and other phenotypes exhibited by marmoset monkeys suggest that they may be the result of selection pressures that lead to the domestication phenotype.
Domestication in other species is linked both empirically and theoretically to changes in vocal behavior and vocal learning. Foxes selected for tameness have altered vocalizations in response to the presence of humans [32]. The Bengalese finch (Lonchura striata var. domestica), a domesticated lineage derived from the white-rumped munia (Lonchura striata), learns and produces a more-complex song, is less constrained in what it learns, and retains greater song plasticity in adulthood compared to its wild counterpart [33]. In the human lineage, it has been proposed that selection for the domestication syndrome also facilitated communicative learning and hence language evolution [34, 35].
One hypothesis suggests that selection by humans for increased tameness in animals acted via a reduction in the size of the adrenal gland, with a smaller neural crest cell population as one mechanism [3]. Because the neural crest contributes to many phenotypes, any selective pressure on that cell population will inadvertently affect other phenotypes derived from it. A number of genetic changes could potentially lead to this hypofunction of the neural crest cell population [3], and some genes are linked to both domestication and changes in the neural crest [36–39]. What evidence connects the neural crest to vocal behavior? The larynx is an anatomical structure derived from neural crest cells [40], and there is evidence for at least one link between larynx size and the domestication syndrome: bonobos show a reduction in the size of their larynx when compared to the larynx of the closely related chimpanzee [41]. Hypofunction of the neural crest cell population during embryonic development potentially explains this reduced larynx size.
Our findings in marmoset monkeys are consistent with selection for the domestication syndrome via the neural crest cell population, because the size of the white facial patch was positively correlated with affiliative vocal behavior and exhibited a paucity of neural-crest-cell-derived melanocytes. We also showed that the growth of the white patch accelerates with greater vocal feedback; this feedback also accelerates vocal production learning [27]. In addition, we know that the marmoset larynx, which is also neural crest derived [40], is still developing during this time [42]. How can we relate these postnatal developmental processes to neural crest cell hypofunction when neural crest migration is largely an embryonic phenomenon? One odd feature of embryonic development in marmosets is that the twin embryos stop growing for a time interval such that they lag behind the development of other nonhuman primates by about 3 weeks [43, 44]. The result is altricial offspring relative to other nonhuman primates. At birth, this is reflected in poor locomotor skills [45–47]. It may be that neural-crest-cell-related developmental events that are typically embryonic in other primates are occurring in early postnatal life in marmoset monkeys. Another possibility is that, although the initial size and migration of the neural crest cell may occur embryonically, their differentiation and survival are modulated postnatally. A reduction in glucocorticoid concentrations adversely affects the survival of neural-crest-derived cells [48]; glucocorticoid concentrations are reduced during vocal interactions [24]. Thus, vocal interactions during development may be an experiential means for reducing the number of neural-crest-derived cells.
We speculate that the putative selection pressure leading to the domestication phenotype in marmoset monkeys and humans is cooperative breeding. Humans and most species in the callitrichine subfamily of monkeys (to which marmosets belong) are the only primates to exhibit cooperative breeding in which both parents, older siblings, and unrelated conspecifics help care for infants [49]. For marmoset monkeys, the strategy evolved alongside obligate production of dizygotic twins. For humans, it may be necessary because we produce such extremely altricial offspring. In both cases, the energetic costs of caring for one or more infants exceed the capacity of a single parent [50, 51]. Conversely, Wrangham posits that linguistic capacity (and the attendant ability for a group to conspire against an overly aggressive individual) rather than cooperative breeding is the likely pressure for domestication [52]. We counter that the large modern human brain likely resulted in increased pressure for more intensive cooperative breeding, perhaps requiring more individuals to help care for altricial infants. The larger brains of H. sapien infants increase both their energetic requirements and the likelihood of an earlier birth in a more altricial state [53], and the proposed evolutionary timing of birthing difficulties (<500,000 years ago) [54] coincides with the timing of the appearance of domestication phenotypes in humans [52].
A similar pressure may have been applied to marmosets when they started twinning: two growing fetal brains are more energetically costly than a single brain [55], and marmosets exhibit birthing difficulties in captivity [56]. All marmoset species produce twins and presumably adopt a cooperative breeding strategy, and most, but not all, exhibit a white forehead patch. The Maués marmoset (Mico mauesi), for instance, lacks a white patch. Another callitrichine, Callimico (Goeldi’s monkey), is a monophyletic genus most closely related to marmosets that does not produce twins; their production of singleton infants is a derived phenotype [57]. Yet this species still exhibits cooperative care of infants [58] (albeit perhaps not at the same level as in other callitrichines) [59] and lacks a white facial patch. One approach for further testing of our ideas would be to compare the development of vocal behavior in species lacking the white face patch and/or twinning with those of the common marmoset. Another approach might be to make comparisons with species outside the subfamily. Squirrel monkeys (Saimiri spp.), for example, are also small in size and produce singleton offspring, which are large relative to other species and result in birthing difficulties [55, 60]. Moreover, squirrel monkeys exhibit partial cooperative care: related and unrelated adult females care for each other’s infants [61].
There are multiple caveats to our study which we hope will encourage replication. We use only a small number of animals. Our sample size in the adult study (n = 8; Figure 1) precluded us from a more-refined analysis of vocal exchanges; we could not ascertain whether marmosets show a bias toward responding to some individuals versus others. We know from our previous, slightly larger study of vocal interactions that marmosets will exchange vocalizations with any other marmoset [21]. However, other studies suggest that marmosets, based on their contact call acoustics, have the potential to discriminate between individuals (and may bias their vocal responses accordingly) [62–64]. Another caveat is that we used animals born and raised in captivity [65]. Our monkeys are descended from a population subset that was “trappable” in the wild; individuals with particular traits are more likely to be caught. Captivity also affects genetic background and social experience [65]. Nonetheless, the behaviors (vocal exchanges) and the white facial patch on which we focus are both present in wild marmosets.
In summary, our study provides experimental evidence that affiliative behavior can be directly linked to the emergence of phenotypes associated with domestication in a cooperatively breeding nonhuman primate. The potential involvement of neural crest cells provides a mechanism by which behavioral experience can be linked to the emergence of morphological phenotypes associated with domestication. This in turn provides new insights into how selection on correlated phenotypes may have acted during human evolution, as hominins became increasingly reliant on cooperative networks for survival and reproduction.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources should be directed to the lead contact, Asif Ghazanfar (asifg@princeton.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data and the code to analyze them are available in DRYAD (https://doi.org/10.5061/dryad.51c59zw65)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All experiments were approved by, and performed in compliance with, Princeton University Institutional Animal Care and Use Committee and its guidelines (protocol # 1908–18). The subjects were captive common marmosets (Callithrix jacchus) housed at Princeton University. The colony room is maintained at a temperature of approximately 27°C and 50%–60% relative humidity, with 12L:12D light cycle. The marmosets live in family groups; all were born in captivity. They had ad libitum access to water and were fed daily with standard commercial chow supplemented with fruits and vegetables.
METHOD DETAILS
Facial image collection
For adult marmoset monkeys (n = 8), head-on photographs were used to assess white patch size. Photographs were taken while the animals were anesthetized during a routine physical exam performed by university veterinary staff. A Canon T2i camera with a standard Canon EF-S lens with focal length of 18–55mm captured the images. Lighting and image resolution were controlled: One experimenter would hold each marmoset, while another would hold up a ColorChecker Passport (color standard) and a ruler in the same frame and level to the marmoset’s forehead. All photos were shot and stored in the raw image format. Because adult patches are large, easy to measure, and do not change over time, we took a simple approach to their measurement. The white patch of fur on each head was outlined digitally in each photograph by hand using the ImageJ program [66]. ImageJ calculated the area and Feret’s diameter (the maximum diameter) of the patch.
For infant marmoset monkeys (n = 6), starting on postnatal day 1, they were removed from their homecage and photographed in a separate room using a Canon EOS Rebel T2i DSLR with a Canon EF-S 18–55mm IS lens, with images collected in RAW format. An X-Rite ColorChecker Passport Photo color standard was placed next to the infant in each image and was later used to calibrate the images. Pictures were taken at least twice a week during their first 5 months of postnatal life. One experimenter manually held the infant and ColorChecker standard facing the camera while the second experimenter took the picture. In parallel, these same infants participated in a vocal learning experiment approximately every day for their first 2 months of postnatal life [27].
Because there were many more infant images than the eight adult images, and because the white patch needed to be tracked over time to measure growth, image processing and analysis for infant photos were implemented in MATLAB (version R2017a) using previously reported [67, 68] and custom written code. RAW images were converted to linear TIFFs using dcraw (Coffin 2012). Color constancy was implemented by re-scaling image color channels based on reflectance measured from the white patch of the ColorChecker standard in each image (the adjacent method [69, 70]). The images were cropped to include only the subject’s head and converted to greyscale (Figure 3A).
For analysis, we used a pulse-coupled neural network (PCNN) to extract primate face patches [67]. The algorithm for generating segmented images using PCNNs requires a “link” parameter that specifies the local connectivity of each pixel. This controls how large the extracted regions are. In our images, variability in lighting was such that no single link value worked for every photo without the PCNN incorrectly estimating the size of the white face patch. Therefore, all images were run through the PCNN using three different link parameters (link = 6, 7, 8). By default, the middle value (link = 7) was used, but where one of the two other values performed significantly better, that value was used instead. All PCNNs were run for ten iterations to generate images with the forehead patch segmented from the surrounding regions of the face.
The PCNN segmented images were rotated/rescaled so that the outer corners of eyes were horizontal and 200 pixels apart (standardized for size/orientation). In this way, we accounted for changes in white patch size that were due to differences in head size. The PCNN image region corresponding to the white patch was selected when present (when absent, measurements = 0). The maximum length (horizontal), height (vertical) and area of the patch were calculated in pixel units (Figure 3A). Local regression (loess) smoothing was used to generate a general patch growth curve for each metric for each animal. Loess smoothing was implemented using the loess function in R (Version 3.3.3), with span = 0.8 and served to predict/interpolate values for all days between the first day that the white patch was detected until ~150 postnatal days.
The collection of facial images for adults versus infants was separated by almost 3 years; they were taken under different photographic conditions. We did not anticipate the connection between the studies at the time. Unfortunately, this precluded us from using the PCNN on adult facial images as we did for the infants.
Vocal data collection
Adult vocal behavioral data were collected from the same 8 animals used for facial photos. Two types of behaviors were recorded under controlled conditions: spontaneous vocalizations produced when the animal was alone, and vocal exchanges produced when two animals were in auditory but not visual contact. We acquired vocal exchanges produced by marmosets paired in various combinations (e.g., cagemate pairs and non-cagemate pairs with none related to each other). Although marmosets have various distinct vocalizations produced in a number of different contexts [71], 99.9% of calls recorded under these conditions were contact “phee” calls [21].
Measuring vocal exchanges
We ran each adult marmoset in two experimental conditions: alone and paired. In the alone condition, each marmoset was placed alone in the testing room and the vocalizations were recorded. In the paired condition, two animals were placed in the same room and the vocalizations were recorded. All sessions lasted either 15 or 30 minutes. Each animal was tested only once a day and subjects were run on the two conditions in randomized order. The experimental room measured 2.5 m × 2.5 m with walls covered in sound attenuating foam. Two tables (.66 m in height) were positioned at diagonally opposite corners of the room. The animals were placed–one on each table in the paired condition–in prism-shaped testing boxes made of plexiglas and wire (.30 m × 0.30 m × 0.35 m). The testing corner was counterbalanced across each monkey and sessions. A speaker was positioned at a third corner equidistant from both testing corners and pink noise was broadcast at ~45 dB in order to mask occasional noises produced external to the testing room. Digital recorders (ZOOM H4n Handy Recorder) were placed directly in front of each testing box at a distance of 0.76 m. Audio signals were acquired at a sampling frequency of 96 kHz. An opaque cloth occluder divided the room in two and prevented the subjects from getting visual cues from each other during the course of the experiment. Each testing box was thoroughly wiped down between each test session to eliminate odors left by previous subjects. For the paired condition, the experimenter ensured that each of the paired marmosets had no visual contact with each other, from the time of removal from the home environment until the end of experiment. Once the subjects were in place, the experimenter turned on both recorders and left the room.
Each adult marmoset paired with one of our 8 subjects was selected randomly from our colony. We calculated vocal exchange from 49 sessions in paired conditions. Each session was analyzed to report the response probability (measure of cooperation) for each marmoset. Time intervals between calls were calculated as the difference between the beginning of the subsequent call and the end of the previous call. Calls from different individuals less than 12 s apart were considered response calls. Intervals between calls from different marmosets that were 0 s or less (negative intervals) indicated an overlapping call. As vocal exchanges require cooperation and consist of minimal overlaps [21], overlapping calls were not considered response calls. The probability of response for each subject was calculated as the average of response probabilities of all sessions from the same subject.
Phee call detection
A custom made MATLAB routine automatically detected the onset and offset of any acoustic signal that differed from the background noise at specific frequency range. To detect the differences, we band-passed the entire recording signal between 5 and 8kHz. This corresponds to the fundamental frequency of marmoset phee calls. We then compared the amplitude of the signal at this frequency band for the periods without call and during a call. A simple threshold was enough to distinguish both periods. Onset-offset gaps longer than one second indicated separate calls, whereas gaps shorter or equal to one second indicated syllables from the same call. After this procedure, we manually verified for each call whether the automatic routine correctly identified single phee calls or combined multiple calls, using the one-second separation criteria. For the paired dataset, we had to compare the amplitude of the band-passed signal recorded from the two microphones in the room to determine which of the marmoset was producing a call. When the same call recorded from opposing corners of the room was compared, the amplitude was larger for the microphone at the same corner of the caller.
Immunohistochemistry
Skin tissues from black and white patches were dissected post-mortem from animals that were euthanized for health-related reasons; all euthanasia decisions are made by the university veterinary staff. These tissues were fixed overnight with 4% Paraformaldehyde, and embedded in paraffin. Samples were sectioned and deparaffinized by incubation in sequential series of Xylene, Ethanol (100%, 95%, 70%, 50%) and ddH2O. Antigen unmasking was carried by boiling slides at 100°C in Citrate buffer for 20 min. Tissues were blocked with 3% BSA dissolved in 1xPBT (1xPBS + 0.05% Tween) and incubated with anti-Tyrp1 antibody (1:200; kind gift of Dr. Vince Hearing) overnight at 4°C. The following day, tissues were washed with 1xPBT and incubated with Alexa 488 goat anti rabbit antibody (Molecular Probes) for 1hr. Following a series of washes with 1xPBT, nuclei were stained with DAPI and slides were cover-slipped and imaged with a Nikon NiE Upright microscope. For negative controls, the same procedure was followed but no primary antibody was used.
QUANTIFICATION AND STATISTICAL ANALYSIS
We used MATLAB to calculate the statistics. We used the functions corr and partialcorr to calculate the correlations and partial correlation, respectively. We used the function fitlm and fitlme to calculate the multiple linear regression and linear mixed effect model. Categorial variables (Sex = {male/female}, Condition = {high/low contingency rate}, Family = {family 1/2/3}) were converted to dummy variables. Family and Sex uniquely specifies the identity of the subject. We considered three different models of the data. Model 1: Using the Wilkinson notation, we have
WhitePatchSize ~(1+PND)*(Condition + Sex + Family) + BodyWeight.
Here, we are using the fixed effect model following the recommendation to avoid specifying random effects when the number of levels are less than five. Model 2: WhitePatchSize ~(1+PND)*(Condition + Sex + Family). Using this model, we verified if the collinearity between BodyWeight and PND could influence the result. Model 3: WhitePatchSize ~(1+PND)*(Condition + Sex) + (PND|Family). Despite the small number of levels (three), we nevertheless tested if considering Family as a random effect would significantly affect the result; it did not (Table S2). Details regarding the number of subjects, means, confidence intervals, p values, etc. can be found in the Results text and in Tables 1, S1, and S2.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| melanocyte marker (Tyrp1) | Vince Hearing Lab, NIH | N/A |
| nuclear marker (DAPI) | Southern Biotech | N/A |
| Alexa 488 goat anti rabbit antibody | Molecular Probes | ab150077 |
| Deposited Data | ||
| Raw and analyzed | This paper | https://doi.org/10.5061/dryad.51c59zw65 |
| Experimental Models: Organisms/Strains | ||
| Common marmoset monkey (Callithrix jacchus) | Texas Biomedical Research Institute | http://txbiomed.org/SNPRC/resources.aspx |
| Common marmoset monkey (Callithrix jacchus) | Princeton Neuroscience Institute | N/A |
| Software and Algorithms | ||
| Analysis code | This paper | https://doi.org/10.5061/dryad.51c59zw65 |
| ImageJ | [66] | https://imagej.nih.gov/ij/ |
| Pulse-coupled neural networks | [67] | https://royalsocietypublishing.org/doi/suppl/10.1098/rspb.2014.2284 |
Highlights.
Marmoset monkeys exhibit a number of domestication phenotypes
White facial patch size correlates with vocal responses
The development of patch is influenced by parental vocal feedback
Paucity of melanocytes in patch supports neural crest hypothesis
ACKNOWLEDGMENTS
For insightful discussions, we thank Laurie Santos, Richard Schneider, Cassie Stoddard, Bridgett vonHoldt, and the Evolution Group at Princeton. We thank Hopi Hoekstra for the suggestion of the melanocyte staining and are grateful to our colleagues Ricardo Mallarino and Yisi Zhang for helping us in that regard. This work was supported by an NIH-NINDS grant to A.A.G. (R01NS054898).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.cub.2020.09.049.
REFERENCES
- 1.Belyaev D, and Trut L (1989). The convergent nature of incipient forms and the concept of destabilizing selection. In Vavilov’s heritage in modern biology, Ovchinnikov YA, and Rapoport IA, eds. (Moscow: Nauka; ), pp. 155–169. [Google Scholar]
- 2.Trut L, Oskina I, and Kharlamova A (2009). Animal evolution during domestication: the domesticated fox as a model. BioEssays 31, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins AS, Wrangham RW, and Fitch WT (2014). The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeder MA (2006). Central questions in the domestication of plants and animals. Evol. Anthropol. 15, 105–117. [Google Scholar]
- 5.Darwin C (1871). The Descent of Man, and Selection in Relation to Sex (Murray; ). [Google Scholar]
- 6.Boas F (1938). The Mind of Primitive Man, Revised Edition (MacMillan; ). [Google Scholar]
- 7.Wrangham R, and Pilbeam D (2002). African apes as time machines. In All Apes Great and Small, Galdikas BMF, Briggs NE, Sheeran LK, Shapiro GL, and Goodall J, eds. (Springer; ), pp. 5–17. [Google Scholar]
- 8.Hare B, Wobber V, and Wrangham R (2012). The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Anim. Behav. 83, 573–585. [Google Scholar]
- 9.Darwin CR (1868). Variation of Plants and Animals under Domestication (John Murray; ). [Google Scholar]
- 10.Sánchez-Villagra MR, Geiger M, and Schneider RA (2016). The taming of the neural crest: a developmental perspective on the origins of morphological covariation in domesticated mammals. R. Soc. Open Sci. 3, 160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lord KA, Larson G, Coppinger RP, and Karlsson EK (2020). The history of farm foxes undermines the animal domestication syndrome. Trends Ecol. Evol. 35, 125–136. [DOI] [PubMed] [Google Scholar]
- 12.Hare B (2017). Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annu. Rev. Psychol. 68, 155–186. [DOI] [PubMed] [Google Scholar]
- 13.Gruber T, and Clay Z (2016). A comparison between bonobos and chimpanzees: A review and update. Evol. Anthropol. 25, 239–252. [DOI] [PubMed] [Google Scholar]
- 14.Hare B, Melis AP, Woods V, Hastings S, and Wrangham R (2007). Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623. [DOI] [PubMed] [Google Scholar]
- 15.Wobber V, Wrangham R, and Hare B (2010). Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Curr. Biol. 20, 226–230. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman DE, Carlo J, Ponce de León M, and Zollikofer CP (2007). A geometric morphometric analysis of heterochrony in the cranium of chimpanzees and bonobos. J. Hum. Evol. 52, 647–662. [DOI] [PubMed] [Google Scholar]
- 17.Burkart JM, Allon O, Amici F, Fichtel C, Finkenwirth C, Heschl A, Huber J, Isler K, Kosonen ZK, Martins E, et al. (2014). The evolutionary origin of human hyper-cooperation. Nat. Commun. 5, 4747. [DOI] [PubMed] [Google Scholar]
- 18.Erb WM, and Porter LM (2017). Mother’s little helpers: what we know (and don’t know) about cooperative infant care in callitrichines. Evol. Anthropol. 26, 25–37. [DOI] [PubMed] [Google Scholar]
- 19.Burkart JM, Fehr E, Efferson C, and van Schaik CP (2007). Other-regarding preferences in a non-human primate: common marmosets provision food altruistically. Proc. Natl. Acad. Sci. USA 104, 19762–19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JY, Takahashi DY, and Ghazanfar AA (2015). Cooperative vocal control in marmoset monkeys via vocal feedback. J. Neurophysiol. 114, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi DY, Narayanan DZ, and Ghazanfar AA (2013). Coupled oscillator dynamics of vocal turn-taking in monkeys. Curr. Biol. 23, 2162–2168. [DOI] [PubMed] [Google Scholar]
- 22.Borjon JI, and Ghazanfar AA (2014). Convergent evolution of vocal cooperation without convergent evolution of brain size. Brain Behav. Evol. 84, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkart J, Guerreiro Martins E, Miss F, and Zürcher Y (2018). From sharing food to sharing information: cooperative breeding and language evolution. Interact. Stud. 19, 136–150. [Google Scholar]
- 24.Sussman RW (2000). Primate Ecology and Social Structure: New World Monkeys (Pearson Custom; ). [Google Scholar]
- 25.Araújo A, Arruda MF, Alencar AI, Albuquerque F, Nascimento MC, and Yamamoto ME (2000). Body weight of wild and captive common marmosets (Callithrix jacchus). Int. J. Primatol. 21, 317–324. [Google Scholar]
- 26.Takahashi DY, Fenley AR, Teramoto Y, Narayanan DZ, Borjon JI, Holmes P, and Ghazanfar AA (2015). Language development. The developmental dynamics of marmoset monkey vocal production. Science 349, 734–738. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi DY, Liao DA, and Ghazanfar AA (2017). Vocal learning via social reinforcement by infant marmoset monkeys. Curr. Biol. 27, 1844–1852.e6. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein MH, and Schwade JA (2008). Social feedback to infants’ babbling facilitates rapid phonological learning. Psychol. Sci. 19, 515–523. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert SF (2006). Developmental Biology, Eighth Edition (Sinauer; ). [Google Scholar]
- 30.Ducrest A-L, Keller L, and Roulin A (2008). Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol. Evol. 23, 502–510. [DOI] [PubMed] [Google Scholar]
- 31.Caro T, and Mallarino R (2020). Coloration in mammals. Trends Ecol. Evol. 35, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gogoleva SS, Volodin IA, Volodina EV, Kharlamova AV, and Trut LN (2011). Explosive vocal activity for attracting human attention is related to domestication in silver fox. Behav. Processes 86, 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki K, Ikebuchi M, Bischof H-J, and Okanoya K (2014). Behavioral and neural trade-offs between song complexity and stress reaction in a wild and a domesticated finch strain. Neurosci. Biobehav. Rev. 46, 547–556. [DOI] [PubMed] [Google Scholar]
- 34.Thomas J, and Kirby S (2018). Self domestication and the evolution of language. Biol. Philos. 33, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benítez-Burraco A, and Kempe V (2018). The emergence of modern languages: has human self-domestication optimized language transmission? Front. Psychol. 9, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benítez-Burraco A, Theofanopoulou C, and Boeckx C (2018). Globularization and domestication. Topoi 37, 265–278. [Google Scholar]
- 37.Pendleton AL, Shen F, Taravella AM, Emery S, Veeramah KR, Boyko AR, and Kidd JM (2018). Comparison of village dog and wolf genomes highlights the role of the neural crest in dog domestication. BMC Biol. 16, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanella M, Vitriolo A, Andirko A, Martins PT, Sturm S, O’Rourke T, Laugsch M, Malerba N, Skaros A, Trattaro S, et al. (2019). Dosage analysis of the 7q11.23 Williams region identifies BAZ1B as a major human gene patterning the modern human face and underlying self-domestication. Sci. Adv. 5, eaaw7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theofanopoulou C, Gastaldon S, O’Rourke T, Samuels BD, Martins PT, Delogu F, Alamri S, and Boeckx C (2017). Self-domestication in Homo sapiens: insights from comparative genomics. PLoS ONE 12, e0185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabler JM, Rigney MM, Berman GJ, Gopalakrishnan S, Heude E, Al-Lami HA, Yannakoudakis BZ, Fitch RD, Carter C, Vokes S, et al. (2017). Cilia-mediated Hedgehog signaling controls form and function in the mammalian larynx. eLife 6, e19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grawunder S, Crockford C, Clay Z, Kalan AK, Stevens JMG, Stoessel A, and Hohmann G (2018). Higher fundamental frequency in bonobos is explained by larynx morphology. Curr. Biol. 28, R1188–R1189. [DOI] [PubMed] [Google Scholar]
- 42.Zhang YS, Takahashi DY, Liao DA, Ghazanfar AA, and Elemans CPH (2019). Vocal state change through laryngeal development. Nat. Commun. 10, 4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marroig G, and Cheverud JM (2001). A comparison of phenotypic variation and covariation patterns and the role of phylogeny, ecology, and ontogeny during cranial evolution of new world monkeys. Evolution 55, 2576–2600. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery SH, and Mundy NI (2013). Parallel episodes of phyletic dwarfism in callitrichid and cheirogaleid primates. J. Evol. Biol. 26, 810–819. [DOI] [PubMed] [Google Scholar]
- 45.Gustison ML, Borjon JI, Takahashi DY, and Ghazanfar AA (2019). Vocal and locomotor coordination develops in association with the autonomic nervous system. eLife 8, e41853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Fang Q, and Gong N (2014). Motor assessment of developing common marmosets. Neurosci. Bull. 30, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz-Darken N, Braun KM, and Emborg ME (2016). Neurobehavioral development of common marmoset monkeys. Dev. Psychobiol. 58, 141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doupe AJ, Landis SC, and Patterson PH (1985). Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J. Neurosci. 5, 2119–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burkart JM, Hrdy SB, and Van Schaik CP (2009). Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186. [Google Scholar]
- 50.Leutenegger W (1980). Monogamy in callitrichids: a consequence of phyletic dwarfism? Int. J. Primatol. 1, 95–98. [Google Scholar]
- 51.Dunsworth HM, Warrener AG, Deacon T, Ellison PT, and Pontzer H (2012). Metabolic hypothesis for human altriciality. Proc. Natl. Acad. Sci. USA 109, 15212–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wrangham RW (2019). Hypotheses for the evolution of reduced reactive aggression in the context of human self-domestication. Front. Psychol. 10, 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin RD (1996). Scaling of the mammalian brain: the maternal energy hypothesis. Physiology 11, 149–156. [Google Scholar]
- 54.Dunsworth H, and Eccleston L (2015). The evolution of difficult childbirth and helpless hominin infants. Annu. Rev. Anthropol. 44, 55–69. [Google Scholar]
- 55.Leutenegger W (1976). Allometry of neonatal size in eutherian mammals. Nature 263, 229–230. [DOI] [PubMed] [Google Scholar]
- 56.Phillips IR (1976). The reproductive potential of the common cotton-eared marmoset (Callithrix jacchus) in captivity. J. Med. Primatol. 5, 49–55. [DOI] [PubMed] [Google Scholar]
- 57.Harris RA, Tardif SD, Vinar T, Wildman DE, Rutherford JN, Rogers J, Worley KC, and Aagaard KM (2014). Evolutionary genetics and implications of small size and twinning in callitrichine primates. Proc. Natl. Acad. Sci. USA 111, 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porter LM, and Garber PA (2009). Social behavior of callimicos: mating strategies and infant care. In The Smallest Anthropoids, Ford SM, Porter LM, and Davis LC, eds. (Springer; ), pp. 87–101. [Google Scholar]
- 59.Masataka N (1981). A field study of the social behavior of Goeldi’s monkeys (Callimico goeldii) in North Bolivia. I. Group composition, breeding cycle, and infant development. Kyoto U. Overs. Res. Rep. New World Monk. 2, 23–32. [Google Scholar]
- 60.Goss CM, Popejoy L, Fusiler JL, and Smith TM (1968). Observations on the relationship between embryological development, time of conception, and gestation. In The Squirrel Monkey, Rosenblum LA, and Cooper RW, eds. (Academic; ), pp. 171–191. [Google Scholar]
- 61.Williams L, Gibson S, McDaniel M, Bazzel J, Barnes S, and Abee C (1994). Allomaternal interactions in the Bolivian squirrel monkey (Saimiri boliviensis boliviensis). Am. J. Primatol. 34, 145–156. [DOI] [PubMed] [Google Scholar]
- 62.Jorgensen DD, and French JA (1998). Individuality but not stability in marmoset long calls. Ethology 104, 729–742. [Google Scholar]
- 63.Norcross JL, and Newman JD (1993). Context and gender-specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. Am. J. Primatol. 30, 37–54. [DOI] [PubMed] [Google Scholar]
- 64.Zürcher Y, Willems EP, and Burkart JM (2019). Are dialects socially learned in marmoset monkeys? Evidence from translocation experiments. PLoS ONE 14, e0222486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webster MM, and Rutz C (2020). How STRANGE Are Your Study Animals? (Nature Publishing Group; ). [DOI] [PubMed] [Google Scholar]
- 66.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen WL, and Higham JP (2015). Assessing the potential information content of multicomponent visual signals: a machine learning approach. Proc. Biol. Sci. 282, 20142284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dubuc C, Winters S, Allen WL, Brent LJ, Cascio J, Maestripieri D, Ruiz-Lambides AV, Widdig A, and Higham JP (2014). Sexually selected skin colour is heritable and related to fecundity in a non-human primate. Proc. Biol. Sci. 281, 20141602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bergman TJ, and Beehner JC (2008). A simple method for measuring colour in wild animals: validation and use on chest patch colour in geladas (Theropithecus gelada). Biol. J. Linn. Soc. 94, 231–240. [Google Scholar]
- 70.Stevens M, Stoddard MC, and Higham JP (2009). Studying primate color: towards visual system-dependent methods. Int. J. Primatol. 30, 893–917. [Google Scholar]
- 71.Bezerra BM, and Souto A (2008). Structure and usage of the vocal repertoire of Callithrix jacchus. Int. J. Primatol. 29, 671. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and the code to analyze them are available in DRYAD (https://doi.org/10.5061/dryad.51c59zw65)


