Abstract
Background & Aims
In response to tissue injury, stromal cells secrete extracellular matrix (ECM) components that remodel the tissue and lead to fibrosis. Parenchymal stellate cells are the primary contributors to fibrosis in models of hepatocellular and cholestatic injury. The liver comprises different, heterogenous compartments; stromal cells within those compartments might have unique identities and regional functions. The portal tract contains the bile duct, which is surrounded by stromal cells often called portal fibroblasts. We investigated the contributions of these cells to hepatic injury.
Methods
We performed studies with Gli1:CreERT2; Rosa26:lox-STOP-lox-tdTomato mice. Mice underwent bile duct ligation or were fed 3,5-diethoxycarbonyl-1,4-dihydrocollidine to induce cholestatic injury or were given CCL4 to induce liver fibrosis. Liver tissues were collected and analyzed by histology and immunofluorescence, and mesenchymal cells were isolated. We performed lineage tracing experiments to determine the fates of peribiliary mesenchymal cells (PMCs) that surround the bile duct after cholestatic and hepatocellular injury. We used cell sorting, combined with RNA- sequencing, to isolate stellate cells and PMCs, and identified determinants of cell identity within each population. Liver tissues were obtained from patients with primary sclerosing cholangitis, alcoholic liver disease, nonalcoholic steatohepatitis, or without disease and analyzed by quantitative reverse transcription PCR.
Results
Gli1 was a marker of mesenchymal cells that surround the biliary tree, but not epithelial cells of the canals of Hering. Lineage-traced Gli1+ PMCs proliferated and acquired a myofibroblast phenotype after cholestatic injury; Gli1+ PMCs were found only surrounding the main duct of a portal tract, but not the epithelial cells of the ductular reaction, which were instead encased by stellate cells. Compared with stellate cells, Gli1+ PMCs expressed a different subset of genes, including genes that are markers of active hedgehog signaling, Osr1 (encodes a transcription factor), and ECM-related genes. Loss of hedgehog signaling reduced expression of Osr1 and PMC-specific ECM genes. Liver tissues from patients with liver disease had increased expression of genes that define PMC identity, compared with control liver tissues.
Conclusions
In lineage-tracing studies of mice, we found that Gli1+ PMCs are a subset of stromal cells characterized by active hedgehog signaling that proliferate, acquire a myofibroblast phenotype, and surround the biliary tree in response to cholestatic injury.
Keywords: differentiation, nonparenchymal cell biology, cholangiocytes, biliary cirrhosis
Graphical Abstract

Introduction
Organ fibrosis is becoming an increasingly prevalent cause of morbidity and mortality, estimated to contribute to one-third of deaths worldwide1. The cellular sources of fibrogenic cells stem primarily from resident mesenchymal cells that transdifferentiate into myofibroblasts in response to tissue injury2. However, what heterogeneity exists in an organ’s pool of mesenchymal cells and their respective contribution to fibrosis is relatively unknown.
Liver fibrosis and its end result, cirrhosis, is the leading cause of death from liver disease. Stellate cells, which reside in the space between hepatocyte and endothelial cells, are felt to be the major fibrosing population within the liver3. Initial fate tracing studies employed a Collagen or Vimentin driven Cre, which labeled many fibrogenic cells throughout the liver 4,5 Lineage tracing of Wt1+ mesothelial cells has shown that they give rise to myofibroblasts within 150 μm of the liver capsule after injury, but do not associate with the portal tracts6. Using a constitutively-active Cre recombinase driven by lecithin-retinol acyltransferase (Lrat), parenchymal stellate cells were found to contribute to fibrosis of the liver regardless of etiology7.
Stromal cells within the portal tract have been proposed as a separate source of liver myofibroblasts; however, their fibrotic contribution and their functional relationship to stellate cells have remained a mystery due to the inability to genetically mark and lineage trace them8,9. Thy1 is expressed in mesenchymal cells around the portal tract, but also within endothelial cells and lymphocytes10. Other attempts to understand periportal mesenchymal cells rely upon indirect analysis without lineage tracing11.
Defining the role and identity of mesenchymal cells within the portal tract is a crucial step to understanding the cellular etiology underlying hepatic fibrosis.
Gli1, a transcription factor, has been shown to be expressed in stromal cells with mesenchymal stem cell-like properties (capable of trilineage differentiation) in many organs12. Hedgehog ligands activate Gli1 through Gli2 and Gli3, with Gli1 functioning to amplify existing hedgehog output and serves as a readout of active hedgehog signaling13–17. Here, we show that Gli1 is expressed within mesenchymal cells around the biliary tree without overlap with parenchymal stellate cells. We study the fates of these cells after injury, identify their functional relationship with stellate cells, determine the regulators of their cellular identity, and characterize how this cellular identity changes in human liver disease.
Methods
Mice
Gli1:LacZ, Gli3:LacZ, and Gli1:CreERT2 were generously donated by Alexandra Joyner (Memorial Sloan Kettering Cancer Center) and described previously18–20. SHH.LacZ was generously donated by Chitra Dahia (Hospital for Special Surgery) and described previously21. Rosa26:lox-STOP-lox-tdTomato22 was generously obtained from Bisen Ding (Mt Sinai). Rosa26:lox-STOP-lox-SmoM2 and Smof/f were obtained from Jackson Labs (Stock Nos 005130 and 004526, respectively) and were described previously23,24. All experiments were performed using male and female mice at 8–10 weeks of age. For lineage tracing experiments, mice received four daily intraperitoneal injections of tamoxifen (Sigma) at a dose of 100 mg/kg. For recombination in Rosa26:lox-STOP-lox-SmoM2 and Smof/f lines, mice received three daily intraperitoneal injections of tamoxifen at a dose of 100 mg/kg. Bile duct ligations were performed as previously described25. For CCL4 experiments, mice received biweekly injections of 25% CCL4 (Sigma), diluted in corn oil at a dose of 2 pl/g, for a total of 4 weeks. 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC; Sigma-Aldrich) was mixed with 5053, Purina Picolab Rodent Diet 20 (Envigo) and given for 21 days. Animals were randomly assigned to groups. Blinding could not be performed given the nature of the experiments. All animal experiments were performed on at least two separate occasions. All animal experiments procedures, breeding, and ethical use were performed in accordance with the guidelines set by the Institutional Animal Care and Use Committee at Weill Cornell Medical College.
Histology and Immunofluorescence
Freshly isolated tissue was fixed in 4% paraformaldehyde (PFA), cryoprotected in 30% sucrose, embedded in OCT (Tissue-Tek; Sakura Finetek), and sectioned at 8 pm. Sections were incubated with primary antibodies (Supplementary Table 1) using standard protocols; secondary Alexa Fluor antibodies (Thermo) were used for detection. For LacZ staining, PFA-fixed cryosections were incubated in 5-bromo-4-chloro-3-indolyl-p-D-galactopyranoside (X-gal) overnight and then counterstained with nuclear fast red. Fluorescent images were taken with a Leica SP5 confocal microscope to determine colocalization and analyzed using ImageJ software (NIH). Brightfield images were taken with a Zeiss AxioPlan. For cell counting experiments, images were taken from at least five separate portal tracts for each animal, and counting was performed manually using ImageJ.
RNA isolation and qPCR
Human liver tissue samples were acquired from the Liver Tissue Cell Distribution System Minneapolis, Minnesota, which was funded by NIH Contract #HHSN276201200017C. Human and murine samples were first homogenized in TRIzol (Ambion), mixed with chloroform at a 1:5 ratio, and centrifuged at 4 °C, 1,200 g, for 15 minutes. The aqueous supernatant was collected, mixed 1:1 with 100% ethanol, and extracted using Zymo direct-zol RNA miniprep kit according to the manufacturer’s instructions (Zymo Research). Reverse transcription was performed with iScript cDNA synthesis Kit (Bio-Rad). qPCR was performed upon a Bio-Rad CFX384 RealTime System using Luna qPCR Master Mix (NEB) with primers in Supplementary Table 2. Data was normalized to GAPDH and expressed relative to the sample with the lowest expression. All qPCR experiments were performed in technical triplicates with multiple biological replicates.
RNAscope in situ hybridization
7 μm fixed cryosections were prepared according to manufacturer’s instructions (Advanced Cell Diagnostics). The 2.5 HD Reagent Red Kit was used for detection with probes directed against IHH (NM_010544.2, region 990–2336), SHH (NM_009170.3, region 307–1197), and dapB (negative control).
Proliferation Assays
To measure cell proliferation, EdU (Lumiprobe, 10540) was dissolved in PBS at a concentration of 4 mg/ml and 1 mg was injected into each mouse daily for four days. Detection was performed with “click” chemistry using a Sulfo-Cyanine5 azide (Lumiprobe, A3330) as previously described26. Briefly, sections were incubated for 15 minutes in a solution containing 100 mM Tris buffer, 100 mM ascorbic acid stock, 2 mM CuSO4 stock, 10 μl of azide dye to detect EdU. Images were taken from at least five separate portal tracts and quantified using ImageJ.
Isolation of Hepatobiliary Mesenchymal Cells
Cell isolation was performed as described with the following modifications27. The liver was retrograde perfused at a rate of 3.5 ml/minute with liver perfusion media (Gibco, 17701038) for 3 minutes, 0.5 mg/ml of Pronase (Sigma) in DMEM for 4 mins and 0.5mg/ml Collagenase IV (Gibco) in DMEM for 4 minutes. Liver was minced and further digested in 0.6 mg/ml of Pronase, 0.4 mg/ml Collagenase IV, 1% DNAase (Roche) in DMEM for 20 mins in shaker set at 38 °C and 60 RPM. After straining in a 70 micron filter, cells were spun down at 55 g for 5 minutes to pellet hepatocytes. Supernatant was spun at 580 g for 5 minutes and resuspended in MACs buffer and subjected to a 12% OptiPrep (Sigma) gradient spun at 1450 g for 18 minutes. Cells at the interface were taken and washed in MACs buffer and strained again prior to flow sorting on a BD FACS Aria II.
Analysis of RNA-Seq Data
The Smart-seq2 protocol28 was used to prepare full length cDNA libraries from sorted Gli1+ PMCs and stellate cells before and after bile-duct ligation with an average input of ~1,000 cells per sample. Libraries for Illumina sequencing were generated using NEBNext® Ultra™ II FS DNA. Libraries were pooled together and sequenced at an average depth of 30 million reads per sample on an Illumina HiSeq 4000. The QC was performed for each read file using Fastqc. Gencode mouse genome version mm10 was used for alignment. STAR aligner version 2.5.2b was used to align and remove duplicates, and the read counts per gene for each dataset were calculated using FeatureCounts tool. DEseq2 was used to perform differential gene expression analysis. We used a 1-fold change (log2), FDR of 0.05, and a Benjamini-Hochberg adjusted p-value cutoff of < 0.01 to determine significant differentially expressed genes reported here.
Statistical analysis
Animal studies were used throughout the majority of this work. For animal studies involving cell counting, at least 3 or more separate animals were used and measurements were made on at least 5 different portal tracts with 10 measurements made from a given animal. For human qPCR data, we used all samples available to us. For parametric data involving cell counting experiments and qPCR, significance was analyzed using a one-tailed unpaired Student’s t-test performed in Microsoft Excel.
Data availability
Sequencing data generated can be downloaded from the Gene Expression Omnibus under accession number GSE135993. All other data are available from the corresponding authors upon reasonable request.
Results
Gli1 is Expressed in Peribiliary Mesenchymal Cells that do not Extend into the Canals of Hering
Using a Gli1:LacZ reporter, we found that Gli1 marked mesenchymal cells concentrically wrapped around large and small bile ducts of the biliary tree (Figure 1A–C). To lineage trace these cells, adult Gli1:CreERT2; Rosa26:lox-STOP-lox-tdTomato mice were pulsed with tamoxifen, and labeled cells were analyzed ten days after the last dose. In the absence of tamoxifen, only rare (~0.1%) recombination could be seen (Supplementary Figure 1). Using the biliary markers EPCAM and KRT19, Gli1+ cells were tightly wrapping around cholangiocytes of peripheral small ducts and large hilar ducts (Figure 1D and Supplementary Figure 2A–C). In the extrahepatic biliary tree, numerous Gli1+ cells were embedded within the mesenchyme containing the ductal lumen and peribiliary glands (Figure 1E). CD31+ endothelial cells could be seen intermingling with Gli1+ cells without detectable overlap in large and peripheral ducts (Figure 1F–I). Gli1+ PMCs were also negative for the macrophage marker F4/80, confirming that Gli1 marks mesenchymal cells around the bile duct (Supplementary Figure 2D–I). The portal tracts of the intrahepatic biliary tree typically contain a main bile duct that terminates into the canals of Hering, which connects the biliary tree to the hepatic parenchyma. The epithelia of the canals still retain EPCAM positivity and are thought to be a source of progenitor cells29–32. Gli1 labeling of cells did not extend into the EPCAM+ epithelia of the canals of Hering, and Gli1+ cells decreased along the ductular tract leading to the canals (Figure 1J and Supplementary Figure 2J). Stellate cells express desmin within the hepatic parenchyma, where no recombined cells could be seen, confirming Gli1 specificity to non-stellate mesenchymal cells (Supplementary Figure 3A–C). Gli1+ PMCs could be found to colocalize with desmin at ~8.8% and with alpha smooth muscle actin (α-SMA), a myofibroblast marker, at ~1.0% (Figure 3H, I and Supplementary Figure 3D–O). Six months after labeling, these cells remained within the portal mesenchyme and were not seen to differentiate into cholangiocytes (Supplementary Figure 4).
Figure 1. Gli1 marks mesenchymal cells surrounding main duct cholangiocytes.
(A-C) LacZ expression is detected within mesenchymal cells surrounding small and large bile ducts within Gli1:LacZ reporter mice (n = 3). (D) After tamoxifen induced recombination in Gli1:CreERT2; Rosa26:lox-STOP-lox-tdTomato mice, tdTomato+ cells can be seen wrapping around EPCAM+ cholangiocytes of hilar ducts. (E) Gli1+ mesenchymal cells are seen within the cystic duct of the extrahepatic biliary tree intermingled with CD31+ endothelial cells (F, G). Zoom of (E), where CD31+ endothelial cells (arrowheads) are separate from Gli1+ mesenchymal cells. (H) A few labeled mesenchymal cells can be seen around a peripheral bile duct. (I) Zoom of peripheral bile duct from (H). (J) Gli1+ mesenchymal cells are seen extending to the smaller branches of the biliary tree (arrowhead), but are not observed adjacent to EPCAM+ cells of the canals of Hering (arrow). (n = 5 for D-J). Scale bars, 100 μm.
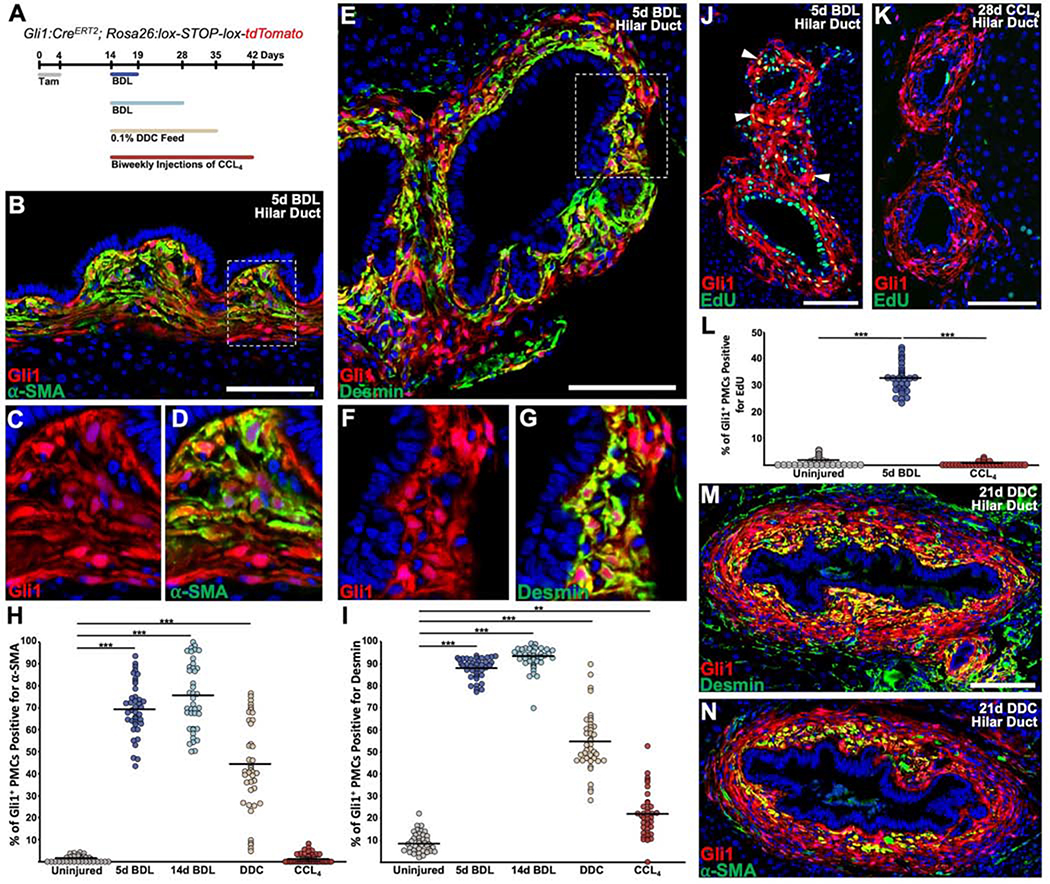
Figure 3. Cholestatic injury causes activation and proliferation of Gli1+ PMCs around hilar ducts.
(A) Experimental scheme. Tamoxifen was administered daily for 4 doses, followed by a 10 day washout. Subsequently, animals underwent the described injuries and were analyzed at the indicated timepoint. (B) α-SMA was strongly induced within Gli1+ PMCs 5 days post BDL, particularly within mesenchymal cells adjacent to cholangiocytes. (C, D) Zoom of dash box in (B), where Gli1+ PMCs are seen to colocalize with α-SMA. (E) Desmin was induced within Gli1+ PMCs 5 days post BDL in a similar pattern to α-SMA. (F, G) Zoom of dash box in (B), where Gli1+ PMCs are seen to colocalize with desmin. (H, I) Quantification of the percentages of Gli1+ PMCs that are positive for α-SMA (H) or desmin (I). (J) Gli1+ PMCs incorporate EdU 5 days post BDL (arrowhead). (K) Gli1+ PMCs do not show EdU incorporation after CCL4. (L) Percentage of Gli1+ EdU+ PMCs 5 days post BDL and 28 days post CCL4. (M, N) After 21 days of 0.1% DDC diet, large ducts have numerous layers of Gli1+ PMCs, with desmin and α-SMA restricted primarily to the innermost layer of mesenchymal cells (n = 4 for all experiments). ***P<0.001; **P<0.05. Scale bars, 100 μm.
Cholangiocyte Expression of Indian Hedgehog Decreases from Large to Small Ducts
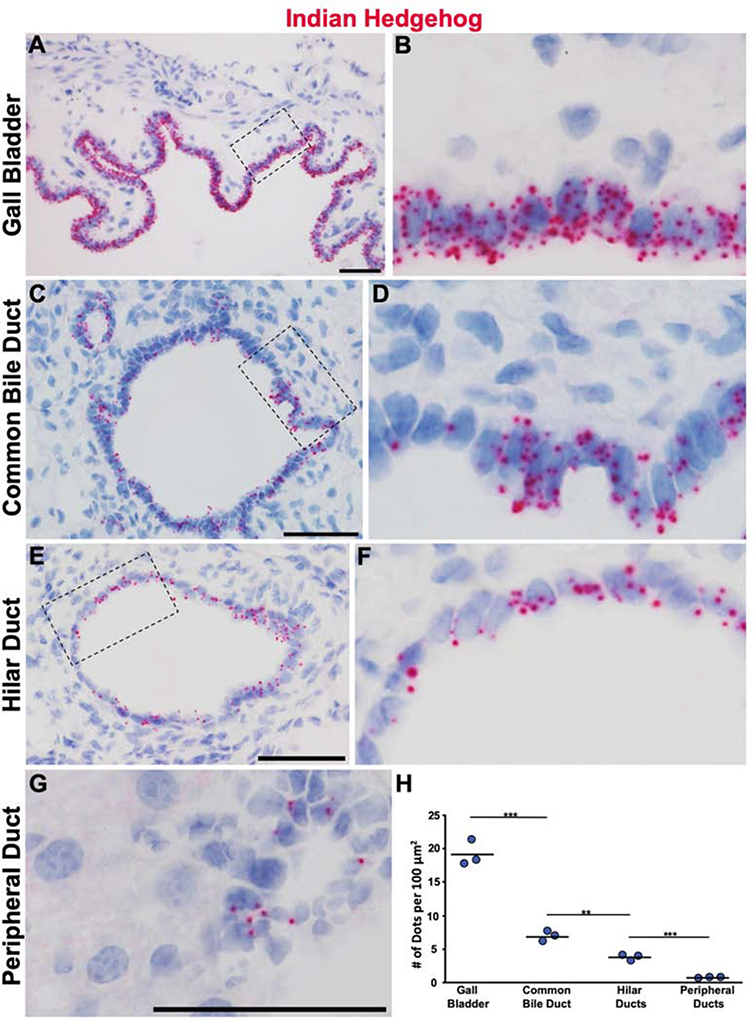
The presence of Gli1 suggests active hedgehog signaling, and its expression is used as a method to determine where active hedgehog signaling is taking place as it functions to amplify existing hedgehog signaling13–17. Of the three hedgehog ligands, Sonic hedgehog (Shh) is the most widely expressed and studied13,17. However, it has been reported that hedgehog signaling is relatively inactive within the uninjured adult liver with little to no ligand expression33–35. To determine the source of hedgehog ligand, we used a LacZ reporter driven by Shh and found no expression around the portal tract or parenchyma, which we confirmed with in situ hybridization (Supplementary Figure 5). However, cholangiocytes did show strong expression of Indian hedgehog (Ihh) visualized by in situ hybridization. This expression was the highest within the gallbladder, decreasing from the common bile duct (CBD) to the hilar ducts and was lowest in peripheral ducts (Figure 2). These data illustrate that IHH is the primary hedgehog ligand secreted from cholangiocytes to the surrounding mesenchyme.
Figure 2. IHH secretion from cholangiocytes decreases along the biliary tree.
(A) The expression of Ihh is highest within the epithelium of the gallbladder detected using in situ hybridization. (B) Zoom of dashed box in (A). (C) Compared to the gallbladder, the murine CBD has less in situ signal. (D) Zoom of dashed box in (C). (E) Hilar ducts appear to have slightly less Ihh compared to the CBD. (F) Zoom of dash box in (E). (G) A peripheral duct with very little, but detectable Ihh in situ signal. (H) In situ dots were counted and expressed as a function of cholangiocyte area (n = 3 for all). ***P<0.001; **P<0.05. Scale bars, 50 μm.
Genetically Tagged Gli1+ PMCs Proliferate and Acquire a Myofibroblast Phenotype after Cholestatic Injury
To determine the fate of labeled Gli1+ PMCs after cholestatic injury, Gli1:CreERT2; Rosa26:lox-STOP-lox-tdTomato mice were pulsed with tamoxifen, recovered for 10 days to allow tamoxifen washout, and then underwent bile duct ligation (BDL) (Figure 3A). Five days after injury, Gli1+ PMCs showed evidence of myofibroblast transdifferentiation with increased expression and colocalization with α-SMA, whose expression was greatest in cells closest to the biliary epithelium in hilar ducts (Figure 3B–D). In a similar manner, there was increased expression and colocalization of the stellate cell marker desmin in Gli1+ PMCs, also primarily within the inner most layer of cells (Figure 3E–G). Five days after BDL, ~70% and ~88% of these cells colocalized with α-SMA and desmin, respectively (Figure 3H, I). The expression pattern remained largely the same 14 days after BDL (Supplementary Figure 6). Unlike the large hilar ducts of the liver, the smaller peripheral bile ducts did not display this level of desmin and α-SMA staining (Supplementary Figure 7A–F). As Gli1+ PMCs appeared to expand around the injured bile ducts, we assayed for the rate of proliferation of Gli1+ PMCs by giving 4 days of daily 5-ethynyl-2-deoxyuridine (EdU) after BDL and analyzed animals 1 day after the last dose. An average of 32% of Gli1+ PMCs were visualized with EdU incorporation within the hilar bile ducts (Figure 3J, L). While the PMCs of peripheral smaller ducts did not show significant staining with desmin and α-SMA, they still proliferated at an average of 19% (Supplementary Figure 7G–I).
To test the activation of Gli1+ PMCs in another cholestatic liver injury model, tamoxifen labeled Gli1:CreERT2; Rosa26:lox-STOP-lox-tdTomato mice were given a three week diet of 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC). Similar to BDL, large bile ducts from DDC-fed animals displayed expansion of Gli1+ PMCs in an onion-skin pattern, which expressed desmin and α-SMA in the inner most layer (Figure 3H, I, M, N). Unlike BDL and DDC, which are portal based injuries, chronic injury to hepatocytes surrounding the central vein via carbon tetrachloride (CCL4) injections did not result in the same levels of activation or proliferation of Gli1+ PMCs as cholestatic injury (Figure 3H, I, K, L, and Supplementary Figure 8).
Lineage Traced Gli1+ PMCs Encase Main Bile Ducts, but not Epithelia of the Ductular Reaction
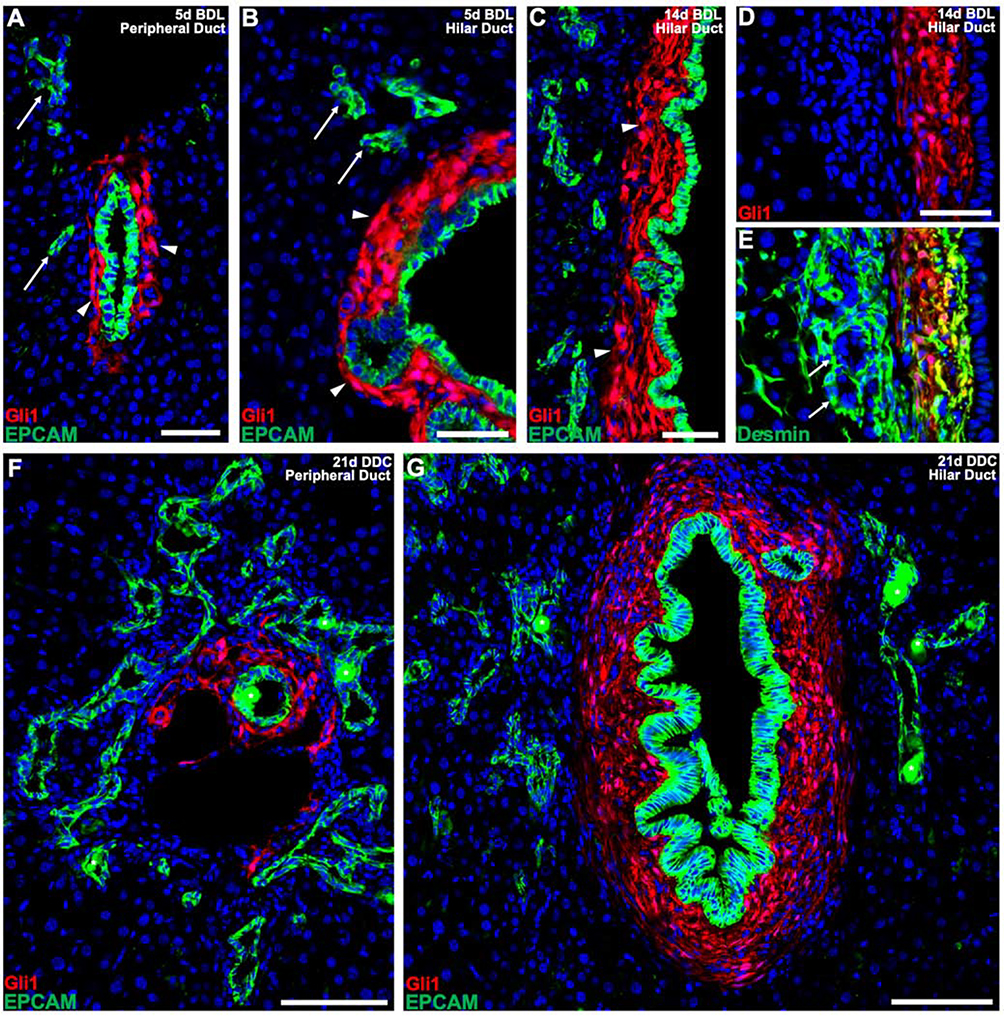
During cholestatic injury, a phenomenon known as the ductular reaction occurs, where reactive bile ductules are seen adjacent to the portal tract. It is debated whether these reactive ductules stem from cholangiocytes themselves or whether they derive from hepatocytes and/or hepatic progenitors that transdifferentiate to acquire biliary-like features29,32,36–42. We sought to determine how Gli1+ PMCs respond to the ductular reaction by assessing their lineage fates in relationship to reactive ductules. 5 days after BDL, EPCAM+ epithelia could be seen adjacent to Gli1+ PMC encased ducts, forming ductular structures near peripheral and hilar ducts (Figure 4A, B). By 14 days post BDL, we found that the main bile ducts within hilar and peripheral portal tracts were surrounded by Gli1+ PMCs; however, the reactive ductules were surrounded by desmin+ stellate cells that were negative for Gli1 (Figure 4C–E and Supplementary Figure 9A–C). Similarly, after 21 days of DDC, Gli1+ PMCs within peripheral and hilar ducts retained the same lineage pattern, with reactive ductules containing no adjacent Gli1+ PMCs (Figure 4F, G and Supplementary Figure 9D–F).
Figure 4. Gli1+ PMCs do not wrap around epithelial cells of the ductular reaction.
(A, B) 5 days after BDL, Gli1+ PMCs continue to expand around cholangiocytes of main peripheral (A) and hilar (B) ducts (arrowheads) with EPCAM+ cells of the ductular reaction forming without any communicating Gli1+ PMCs (arrows). (C) 14 days after BDL, a clear distinction between Gli1+ PMCs of the bile duct (arrowheads) and EPCAM+ cells of the ductular reaction is still detected. (D, E) Cells of the ductular reaction are surrounded by desmin+ stellate cells. (F, G) After 21 days of DDC, Gli1+ PMCs consistently surround the main peripheral (F) and hilar (G) ducts, without surrounding E PCAM+ cells of the ductular reaction. (n = 4 for all experiments). * denotes autofluorescent porphyrin pigment plugs. Scale bars, 50 μm (A-E); 100 μm (F, G).
The Cellular Identity of Gli1+ PMCs is Distinct from Stellate Cells
These results show that Gli1+ PMCs proliferate and acquire a myofibroblast phenotype in response to cholestatic injury, demonstrating a compartmentalized response to injury and distinguishing them from parenchymal stellate cells. To understand global similarities and differences between Gli1+ PMCs and stellate cells, we performed RNA-sequencing on these populations from uninjured animals and animals five days after BDL with injury utilized to identify genes from Gli1+ PMCs that could contextually change. Stellate cells could be isolated via their retinoid autofluorescence27 and flow cytometry analysis showed that stellate cells and tdTomato expressing Gli1+ PMCs were distinct from one another (Figure 5A). Via principle component analysis, injury altered global gene expression, but Gli1+ PMCs and stellate cells continue to remain distinct from one another accounting for 81% of the variance (Figure 5B and Supplementary Figure 10A). To identify which factors could be driving their individual cellular identities, we analyzed differential gene expression in stellate cells compared to Gli1+ PMCs in BDL injured and uninjured livers. Using a False Discovery Rate (FDR) of 0.05, and a Benjamini-Hochberg adjusted p-value of < 0.01, we found 2523 genes that had at least a 1-fold change in expression between stellate cells and Gli1+ PMCs (Figure 5C). We also examined which genes within a given cell population maintained their differential expression after injury, revealing 592 genes in Gli1+ PMCs and 336 genes within stellate cells that had at least a 1-fold change in expression and a Benjamini-Hochberg adjusted p-value < 0.01 (Figure 5D). Mining these two types of analyses, we found genes involved in retinoid metabolism such as β-carotene 15,15’-oxygenase 1 (Bco1) and Lrat as well as neuronal genes were highly enriched in stellate cells, confirming the specificity of the analysis (Figure 5C, E)43. Of these genes that maintained their differential expression after injury, 93 of them related to the gene ontology term ECM, depicting a number of differentially expressed ECM genes that defined the parenchyma and portal tract compartments (Supplementary Figure 10B). In addition, genes involved in the process of fibrogenesis, such as α-SMA, Col1a1, Col1a2, Timp1, and Lox were significantly upregulated in Gli1+ PMCs compared to stellate cells 5 days post BDL, showing that they are a much more potent fibrogenic population in the liver than previously believed (Figure 5G).
Figure 5. Gli1+ PMCs have a unique transcriptomic signature driven by hedgehog signaling.
(A) After 4 daily doses of tamoxifen and an additional 10 day washout, isolated cells underwent flow sorting or BDL for 5 days and then flow sorting. Of the live nonparenchymal population, stellate cells made up 6.8% of cells, whereas Gli1:tdTomato+ cells made up a smaller fraction at 0.5%. (B) Principle component analysis. (C) Volcano plot, showing differential gene expression between flow sorted stellate cells and Gli1+ PMCs. 2523 genes were differentially expressed using an FDR of 0.05, a Benjamini-Hochberg adjusted p-value < 0.01, with a 1-fold log2 change. (D) 336 genes in stellate cells and 592 genes in Gli1+ continued to maintain high differential expression after injury. qPCR analysis of fibrogenic genes shows that Gli1+PMCs markedly induce fibrogenic genes after BDL compared to stellate cells (p < 0.01 for uninjured Gli1 compared to uninjured stellate cells and injured Gli1 compared to injured stellate cells for all 5 genes). (E, F) Heatmaps of identified genes that maintain differential expression in stellate cells (E) and Gli1+ PMCs (F) from uninjured (U1–2) and injured (B1–2) samples. (G) qPCR analysis of fibrogenic genes shows that Gli1+ PMCs markedly induce fibrogenic genes after BDL compared to stellate cells (p < 0.01 for uninjured Gli1 compared to uninjured stellate cells and injured Gli1 compared to injured stellate cells for all 5 genes). (H) Boxplots of Log2 fold change of gene expression values between Gli1+ PMCs compared to stellate cells shows that genes in the Hedgehog pathway (BSID 137950) are significantly upregulated (bars represent 1.5 times the width of a quartile in either direction. Two-sided unpaired Wilcoxon ranked sum, p=0.0384). (I) The expression differential for known hedgehog target genes within the hedgehog signaling gene set between Gli1+ PMCs compared to stellate cells. **P<0.05. Scale bars, 50 pm.
Besides hedgehog signaling, Gli1+ PMCs maintained expression of the transcription factor Odd skipped-related 1 (Osr1), which had virtually no detection within stellate cells (Figure 5F). Osr1 was shown in mouse embryonic limb muscle connective tissue cells to control the expression of a number of ECM related genes, such as Mfap5 and Col6a5, which were also genes that were expressed more specifically in Gli1+ PMCs compared to stellate cells (Figure 5C, F and Supplementary Figure 9B)44.
Active Hedgehog Signaling Defines PMCs during Homeostasis and Injury
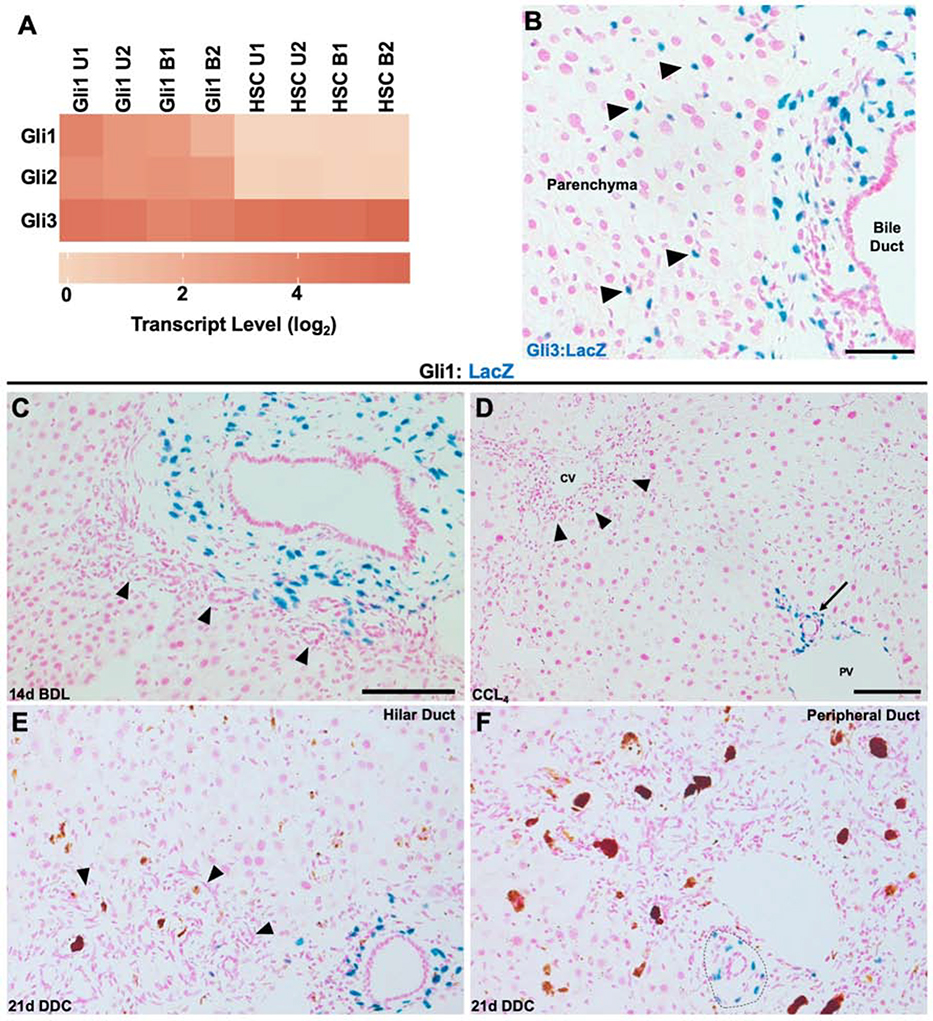
Using Gene Set Enrichment Analysis (GSEA), we found that PMCs were enriched for the hedgehog signaling pathway compared to stellate cells (Figure 5H, I). The transcriptional effectors for hedgehog signaling are the Gli transcription factors. Interestingly, we found that Gli1+ PMCs contained transcripts for Gli1, Gli2, and Gli3, whereas stellate cells expressed only Gli3 (Figure 6A). Gli1 is thought to primarily function as an amplifier of existing hedgehog signaling, whereas Gli2 functions largely as a transcriptional activator and Gli3 largely as a transcriptional repressor13,17. To confirm the transcriptomic analysis, we utilized a Gli3:LacZ animal and found positive mesenchymal cells around the portal tract in addition to scattered stellate cells located within the parenchyma (Figure 6B). Furthermore, our RNA-seq detected Hedgehog interacting protein (Hhip) as maintaining differential expression between stellate cells and Gli1+ PMCs, having much higher expression within stellate cells (Figure 5C, E). Hhip functions as an inhibitor of hedgehog signaling by binding hedgehog ligands45.
Figure 6. Cholestatic and hepatocellular injury does not significantly change the location of Gli1 expression.
(A) Heatmap of Gli transcription factors, showing specificity of Gli1 and Gli2 to PMCs and Gli3 to both cell types. (B) Gli3:LacZ reporter showing positive cells around the bile duct, but also stellate cells within the hepatic parenchyma (arrowheads) (n = 3). (B) 14 days after BDL, Gli1 expression is localized around the main duct and its small branches, but does not extend to the cells of the ductular reaction (arrowhead). (C) After 28 days of biweekly CCL4 injections, Gli1 expression remains around the bile duct (arrow) near the portal vein (PV). (D) Gli1 expression remains around a hilar bile duct and is not significantly induced around cells of the ductular reaction (arrowhead) after 21 days of DDC. (E) Similarly, DDC does not induce Gli1 expression within cells of the ductular reaction in peripheral ducts, but remains around the main duct (dashed outline). Scale bars, 100 μm (E and F scaled at same ratio as C).
To see whether hedgehog signaling continued to identify mesenchymal cells around the bile duct after injury, Gli1:LacZ animals underwent BDL, DDC, and CCL4 treatments and were assessed for regional changes in Gli1 expression. Gli1 expression remained around the main duct in a portal tract in all injury models (Figure 6C–F). After BDL, there was no Gli1 expression around reactive ductules (Figure 6C). After 21 days of DDC, there were rare Gli1 expressing cells around reactive ductules (Figure 6E, F).
Hedgehog Signaling Regulates Expression of Osr1 and Portal ECM genes, but is not Required for Injury Activation of PMCs
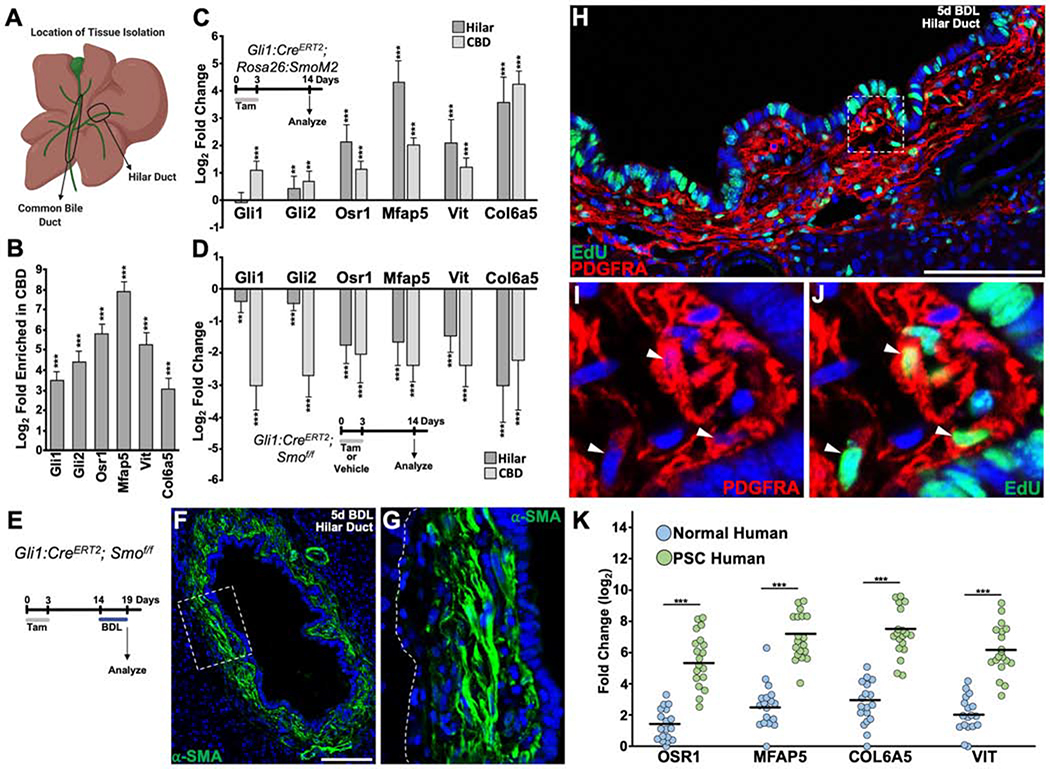
As the presence of active hedgehog signaling appeared to be a major difference between PMCs and stellate cells, we asked whether hedgehog signaling controlled the expression of Osr1 and the PMC-specific ECM genes Mfap5, Col6a5, and Vit. To determine the cell-autonomous role of hedgehog signaling in peribiliary mesenchyme, we targeted Smoothened (Smo), which functions to transduce hedgehog signaling, activating Gli transcription factors13. The CBD has a much higher density of hedgehog signaling compared to the hilar area, and both were used to study low and high signaling areas (Figure 7A, B). We used Gli1:CreERT2; Rosa26:lox-STOP-lox-SmoM2 animals to overexpress a constitutively active form of Smo in Gli1+ PMCs and found increases in the expression of Gli2, Osr1, Mfap5, Col6a5, and Vit in both the CBD and hilar region of the liver compared to Gli1:CreERT2 control animals that were given tamoxifen (Figure 7C). Loss of Smo function through the use of Gli1:CreERT2; Smof/f animals caused a decrease in Gli1, Gli2, Osr1, Mfap5, Col6a5, and Vit in both the CBD and hilar region of the liver compared to Gli1:CreERT2; Smof/f control animals that were given vehicle (Figure 7D). To determine if hedgehog signaling was required for injury induced activation and proliferation of PMCs, Gli1:CreERT2; Smof/f animals underwent BDL (Figure 7E). 5 days after BDL, α-SMA staining was still seen in peribiliary mesenchyme (Figure 7F, G). Despite loss of Smo, PMCs still incorporated EdU at an average of ~30%, when PDGFRA (Platelet-derived growth factor receptor a) was used to highlight them (Figure 7H–J).
Figure 7. Hedgehog Signaling Controls Expression of Osr1 and Portal ECM genes, but is not Required for Proliferation and Activation of PMCs.
(A) Cartoon depicting location of tissue harvest for qPCR analysis. (B) Compared to the hilar region of the liver, the CBD contains a higher density of Gli1, Gli2, and genes associated with PMC identity (n = 4). (C) Relative gene expression from hilar and CBD regions of the liver in which PMCs overexpress SmoM2, compared to Gli1:CreERT2 control animals (n = 4 for each group). (D) Relative gene expression from hilar and CBD regions of the liver in which PMCs lack Smo compared to vehicle control animals (n = 4 for each group). (E) Experimental timeline. (F) α-SMA expression 5 days after BDL where Gli1+ PMCs have loss of Smo. (G) Zoom of dashed box in (F) showing that mesenchyme closest to cholangiocytes have strongest expression of α-SMA. Dotted line indicates border between biliary mesenchyme and hepatic parenchyma. (H) EdU incorporation 5 days after BDL after conditional loss of Smo. (I, J) Zoom of dashed box in (H) showing PDGFRA+ PMCs positive for EdU incorporation (arrowheads) (n = 3). (K) Relative gene expression of human OSR1, MFAP5, COL6A5, and VIT in liver samples from 18 normal patients and 19 patients with PSC. Black bars indicate mean. **P<0.05; ***P<0.001. Scale bars, 100 μm.
To determine whether the cellular identity of murine PMCs are important in human liver disease, we analyzed human liver samples with Primary Sclerosing Cholangitis (PSC), Alcoholic Liver Disease (ALD), and Nonalcoholic Steatohepatitis (NASH). Osr1 and the corresponding ECM genes were all significantly upregulated in diseased liver samples compared to normal human liver (Figure 7K and Supplementary Figure 11).
Discussion
A major focus of antifibrotic therapy in chronic liver disease has revolved around stellate cells, despite the fact that multiple mesenchymal cell types exist within the liver. Little progress has been made to understand what makes each mesenchymal population distinct and how these populations behave after injury. Temporally controlled fate-tracing approaches allow high resolution discernment of cell behavior over time, which has not been precisely determined for portal mesenchymal cells, contributing to controversy surrounding their role8,9. In this work, we describe a number of significant findings that deepen our understanding of the heterogeneity of liver mesenchymal cells, their contributions to fibrosis, and their cellular identities. We identify mesenchymal cells surrounding the extrahepatic and intrahepatic biliary tree by virtue of active hedgehog signaling. Using genetic lineage tracing, we find that these cells adopt a myofibroblast phenotype in response to cholestatic injury and proliferate around the biliary tree. The idea that these peribiliary mesenchymal cells are potent fibrogenic cells is exemplified by their greater expression of fibrogenic genes at baseline and after cholestatic injury in comparison to stellate cells.
Fibrosis during cholestasis starts around the biliary tree and then spreads out into the area of the ductular reaction. The relative contributions of portal mesenchymal cells and stellate cells to fibrosis as cholestatic injury becomes chronic have been debated. Our lineage tracing demonstrates that PMCs proliferate around the main bile ducts within a portal tract, with stellate cells encasing epithelia of the ductular reaction, forming another layer around the portal tract. These results depict that PMCs and stellate cells have regionalized roles during cholestatic liver disease, where contribution of PMCs is greatest during early cholestasis, but further fibrotic contributions come from stellate cells in murine models.
Besides fibrosis, we were able to understand a wealth of biology about mesenchymal cells in the liver. Bile flows from the hepatocyte canaliculi through the canals of Herring and into the biliary tree. Where stellate cells end and Gli1+ PMCs begin appears to depend upon their relative location within the liver. As cholangiocyte derived hedgehog is needed for a component of peribiliary mesenchymal cellular identity, location adjacent to cholangiocytes is key. This is true in other endodermally derived organs such as the intestine, respiratory tract, bladder, and prostate, where epithelial derived hedgehog ligands cause expression of Gli1 in adjacent stromal cells46. Interestingly, Gli1+ PMCs decrease along the small ductules and are not found around epithelia of the canals of Hering. As the biliary branches approach the canals, there is less cholangiocyte IHH and increased exposure to Hhip from stellate cells, creating a fine-tuned regulation of hedgehog signaling at this interface. These data suggest that the presence of active hedgehog signaling within an adjoining mesenchymal cell is a means to differentiate a main bile duct from the canals of Hering.
Using RNA-seq to compare the transcriptomes of PMCs to stellate cells, we demonstrate how distinct these two mesenchymal populations are from each other. Of the genes that were distinct, we found the transcription factor Osr1 and the associated ECM genes Mfap5, Col6a5, and Vit to be very specific for Gli1+ PMCs, with virtually no expression in stellate cells. These genes depend on hedgehog signaling, as blocking hedgehog signaling caused their reduction and upregulating hedgehog signaling increased their expression. Notably, Gli2−/−, Gli3+/+ double mutants lose Osr1 expression in splanchnic mesoderm, which is induced by foregut epithelial SHH47 Our results also indicate that Osr1 is downstream of hedgehog signaling in the mature biliary tree. Isolation of interstitial muscle connective tissue cells from Osr1−/− embryonic mutants exhibited a reduction in multiple ECM genes, including Mfap5 and Col6a544. Assuming that embryonic gene regulation continues to hold true in adults, it would suggest that biliary epithelial hedgehog ligands cause activation of Gli transcription factors in PMCs, leading to transcription of Osr1, which regulates production of ECM genes. Further in vivo experimentation will be needed to determine if such is the case in the mature biliary tree.
While hedgehog signaling, and its downstream targets, appears to distinguish PMCs from stellate cells, hedgehog signaling is dispensable for injury induced proliferation and activation of PMCs. Our results suggest that hedgehog signaling is controlling the expression of a number of ECM genes. From these findings, we infer that PMCs need hedgehog signaling for portal matrix homeostasis; however, the genetic programs that are activated after cholestatic injury are not dependent upon hedgehog. It will be important to understand how PMCs incorporate signals from hedgehog and injury to alter the composition of the ECM and determine why such a dichotomy would exist, if at all.
Importantly, we show that the transcripts that define murine PMCs are also upregulated in PSC, NASH, and ALD. Murine models of liver injury differ from humans, particularly when it comes to duration and pathophysiology. Ductular injury with a ductular reaction is seen in cholestatic models in mice; however, it is seen in almost all types of human chronic liver disease including ALD and NASH32. We speculate that portal and biliary injury in ALD and NASH could cause an increased expression of these transcripts that we report here. The precise contributions of mesenchymal populations to human fibrosing liver disease are difficult to define. The architecture of the human liver differs subtly from the murine liver in that the vessels of the portal tract are embedded in a thicker matrix and fibrous septa surround the hepatic lobules. These architectural differences might influence the types of mesenchymal cells and their properties. Indeed, a recent single cell sequencing of normal and cirrhotic human liver uncovered 4 types of mesenchymal cells48. In scar-associated mesenchymal cells, a subfraction was identified by the expression of OSR1. If and how hedgehog signaling controls expression of OSR1 in human livers will need to be determined to understand how human liver fibrosis is generated. Doing so can give better insights into the cellular origins of human hepatic fibrosis. Our findings provide an important step toward understanding the relationship between different mesenchymal cell populations within the liver. They also provide an important foundation for appreciating compartmentalized cellular activity in response to cholestatic injury, which will be important for the development of antifibrotic and regenerative therapies.
Supplementary Material
What you need to know.
Background and Context
Development of liver fibrosis involves activation and proliferation of parenchymal stellate cells, but it is not clear what other mesenchymal cells types contribute to the fibrotic process.
New Findings
The authors identified and characterized a subset of mesenchymal cells around the biliary tree. Lineage tracing depicted their expansion around the original biliary tree after cholestatic injury, with stellate cells enveloping nearby reactive ductules.
Limitations
Studies were performed in mice and human tissue samples. Studies are needed to determine how different mesenchymal cells contribute to fibrosis in human liver.
Impact
Peribiliary mesenchymal cells and stellate cells perform regional functions during development of fibrosis. These cell types might serve as targets for development of anti-fibrotic agents.
Lay Summary
The authors identified a population of liver cells that proliferate and promote development of fibrosis in mice with liver injuries.
Acknowledgements
We would like to thank the staff of the Weill Cornell Genomics, Flow Cytometry Core, and Imaging Core facilities. We would also like to thank Drs. Praveen Raju and Baolin Wang for helpful discussions and comments. Lastly, we would like to thank Dr. Alexandra Joyner for graciously sharing reagents with us. Cartoons and graphical abstract created with BioRender.
Grant Support: V.G. is a Weill Cornell Department of Medicine Fund for the Future awardee, supported by the Kellen Foundation. R.E.S is supported by 1K08DK101754, DOD grant W81XWH1810237, and R01CA234614 and is an Irma Hirschl Trust Research Award Scholar.
Abbreviations
- ECM
extracellular matrix
- PMCs
peribiliary mesenchymal cells
- LRAT
lecithin-retinol acyltransferase
- GLI
glioma-associated oncogene
- OSR1
odd-skipped related transcription factor 1
- PSC
primary sclerosing cholangitis
- NASH
nonalcoholic steatohepatitis
- ALD
alcoholic liver disease
- EPCAM
epithelial cell adhesion molecule
- KRT19
keratin 19
- Smo
smoothened
- α-SMA
alpha smooth muscle actin
- IHH
indian hedgehog
- SHH
sonic hedgehog
- BDL
bile duct ligation
- EdU
9, 5-ethynyl-2- deoxyuridine
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- FDR
false discovery rate
- Bco1
β-carotene 15,15’-oxygenase 1
- CCL4
carbon tetrachloride
- Col
collagen
- TIMP
tissue inhibitor of metalloproteinases
- LOX
lysyl oxidase
- WT1
Wilms tumor 1
- MFAP
microfibril associated protein
- PDGFRA
platelet-derived growth factor receptor A
- CBD
common bile duct
- VIT
Vitrin
- qPCR
quantitative polymerase chain reaction
- Hhip
hedgehog interacting protein
Footnotes
Disclosures: The authors declare no competing interests.
Author names in bold designate shared co-first authorship
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rockey DC, Bell PD, Hill JA. Fibrosis — A Common Pathway to Organ Injury and Failure. N Engl J Med 2015;372:1138–1149. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL, Sheppard D, Duffield JS, et al. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci Transl Med 2013;5:1–17. [DOI] [PubMed] [Google Scholar]
- 3.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14:397–411. [DOI] [PubMed] [Google Scholar]
- 4.Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of Hepatic Stellate Cells During Liver Fibrosis Resolution in Mice. Gastroenterology 2012;143:1073–1083.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci U S A 2013;110:2324–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 2013;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology 2009;51:1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells RG. The Portal Fibroblast: Not Just a Poor Man’s Stellate Cell. Gastroenterology 2014;147:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsumata LW, Miyajima A, Itoh T. Portal fibroblasts marked by the surface antigen Thy1 contribute to fibrosis in mouse models of cholestatic liver injury. Hepatol Commun 2017;1:198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwaisako K, Jiang C, Zhang M, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A 2014;111:E3297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramann R, Schneider RK, DiRocco DP, et al. Perivascular gli1(+) progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015;16:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong JH, Siebold C, Rohatgi R. Biochemical mechanisms of vertebrate hedgehog signaling. Development 2019;146:166892–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Platt KA, Censullo P, et al. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 1997:2537–2552. [DOI] [PubMed] [Google Scholar]
- 15.Hynes M, Stone DM, Dowd M, et al. Control of Cell Pattern in the Neural Tube by the Zinc Finger Transcription Factor and Oncogene Gli-1. Neuron 1997;19:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Grindley JC, Bellusci S, Perkins D, et al. Evidence for the Involvement of the Gli Gene Family in Embryonic Mouse Lung Development. Dev Biol 1997;188:337–348. [DOI] [PubMed] [Google Scholar]
- 17.Hui C-C, Angers S. Gli Proteins in Development and Disease. Annu Rev Cell Dev Biol 2011;27:513–537. [DOI] [PubMed] [Google Scholar]
- 18.Bai CB, Auerbach W, Lee JS, et al. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 2002;129:4753–4761. [DOI] [PubMed] [Google Scholar]
- 19.Garcia ADR, Petrova R, Eng L, et al. Sonic Hedgehog Regulates Discrete Populations of Astrocytes in the Adult Mouse Forebrain. J Neurosci 2010;30:13597–13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn S, Joyner AL. Dynamic Changes in the Response of Cells to Positive Hedgehog Signaling during Mouse Limb Patterning. Cell 2004;118:505–516. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Reyes LE, Verbitsky M, Blesa J, et al. Sonic Hedgehog Maintains Cellular and Neurochemical Homeostasis in the Adult Nigrostriatal Circuit. Neuron 2012;75:306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2009;13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong J, Mao J, Tenzen T, et al. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 2004;18:937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long F, Zhang XM, Karp S, et al. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 2001;128:5099–5108. [DOI] [PubMed] [Google Scholar]
- 25.Tag CG, Sauer-Lehnen S, Weiskirchen S, et al. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp 2015:e52438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 2008;105:2415–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mederacke I, Dapito DH, Affo S, et al. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc 2015;10:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picelli S, Faridani OR, Bjorklund AK, et al. Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc 2014;9:171–181. [DOI] [PubMed] [Google Scholar]
- 29.Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739–1745. [DOI] [PubMed] [Google Scholar]
- 30.Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec 2008;291:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemaigre FP. Development of the biliary tract. Mech Dev 2003:81–87. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Marzioni M, Meng F, et al. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019;69:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado MV, Diehl AM. Hedgehog signalling in liver pathophysiology. J Hepatol 2018;68:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omenetti A, Yang L, Li Y-X, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest 2007;87:499–514. [DOI] [PubMed] [Google Scholar]
- 35.Omenetti A, Popov Y, Jung Y, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut 2008;57:1275–1282. [DOI] [PubMed] [Google Scholar]
- 36.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 2005;41:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Font-Burgada J, Shalapour S, Ramaswamy S, et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell 2015;162:766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarlow BD, Pelz C, Naugler WE, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 2014;15:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanger K, Zong Y, Maggs LR, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev 2013;27:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jörs S, Jeliazkova P, Ringelhan M, et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J Clin Invest 2015;125:2445–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banales JM, Huebert RC, Karlsen T, et al. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol 2019;16:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaub JR, Huppert KA, Kurial SNT, et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 2018;557:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallecillo-Garcia P, Orgeur M, Hofe-Schneider vom S, et al. Odd skipped-related 1 identifies a population of embryonic fibro-adipogenic progenitors regulating myogenesis during limb development. Nat Commun 2017:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature 1999;397:617–621. [DOI] [PubMed] [Google Scholar]
- 46.Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 2014;141:3445–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han L, Xu J, Grigg E, et al. Osr1 functions downstream of Hedgehog pathway to regulate foregut development. Dev Biol 2017;427:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran P, Dobie R, Wilson-Kanamori JR, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data generated can be downloaded from the Gene Expression Omnibus under accession number GSE135993. All other data are available from the corresponding authors upon reasonable request.