Abstract
Animal disease surveillance encompasses systematic collection of long-term data on disease events, risk factors and other relevant parameters followed by analyzing the same with reference to temporal and spatial characteristics to arrive at a conclusion so that necessary preventive measures can be taken. In India, the animal disease surveillance is done through National Animal Disease Reporting System, which is a web-based information technology system for disease reporting from States and Union Territories with the aim to record, monitor livestock disease situation and to initiate the preventive and curative action in a swift manner during disease emergencies. National Animal Disease Referral Expert System is a dynamic geographic information system and remote sensing-enabled expert system that captures an incidence of 13 economically important livestock diseases from all over the country and also provides livestock disease forecasting. The laboratories under State and Central governments, several research institutes under the Indian Council of Agricultural Research and veterinary colleges are involved in livestock disease diagnosis including zoonotic diseases. An integrated surveillance system is necessary for early detection of emerging/zoonotic diseases in humans. This review provides information on disease reporting and surveillance systems in animal health sector and the need for One Health approach to improve and strengthen the zoonotic disease surveillance system in India.
Keywords: Animal disease surveillance, epidemiology, forewarning, India, informatics, National Animal Disease Referral Expert System (NADRES), surveillance, zoonoses
India is home to 536.76 million livestock and 851.81 million poultry birds1. Livestock sector contributes 4.9 per cent of the GDP (gross domestic product) and nearly 28.4 per cent to agricultural GDP of the nation2. Globally, India is the largest producer of milk (187.75 million MT) and produced 103 billion eggs and 8.1 million tons of meat during the year 2018-20192. Even though good growth has been achieved in the livestock sector, the animal diseases are a stumbling block for the efficient growth of livestock sector. Further, the zoonotic diseases also contribute significantly for reduced growth of this sector. By virtue of close contact with animals due to traditional husbandry practices with limited biosecurity and biosafety measures, the risk of zoonotic disease transmission to humans could be high.
Zoonotic diseases are the global health threats arising from complex interaction of human, animal and environment. There are about 1415 species of infectious agents that are pathogenic to humans, of which nearly 60 per cent are zoonotic. It is also alarming that 75 per cent of the emerging pathogens are zoonotic in nature3,4. In addition, food-borne diseases and antimicrobial resistance (AMR) have also added the burden on existing health system and the economy of the nation. Considering the re-emergence of old diseases and emergence of new diseases, a One Health approach is the need of the hour5. Since several emerging diseases have animals as reservoirs, the disease surveillance in animals has the added advantage of protecting humankind by early detection, prevention and control of zoonotic diseases. Hence, the current review discusses about the animal disease surveillance infrastructure, functional mechanism and gaps in animal disease surveillance programmes in India. It also highlights the scope to integrate keeping the human disease surveillance system with the focus on zoonotic diseases.
Animal disease surveillance system in India
The livestock disease surveillance is planned with the objectives of early warning of disease events, to assess the effectiveness of intervention measures and to determine the disease-free areas or freedom from infection. The data obtained through such surveillance programme will provide sufficient evidence in evaluating the national disease control and eradication programmes6.
The Department of Animal Husbandry and Dairying (DAHD) under the Ministry of Fisheries, Animal Husbandry and Dairying, Government of India, supports the States in the matters related to livestock health and diseases through various animal disease control programmes and animal disease reporting system. Among the various animal disease control programmes, the Foot and mouth disease (FMD) and Brucellosis control programme are implemented nationally while other diseases such as Classical swine fever and Peste des petits ruminants are implemented regionally2.
The country is equipped with robust veterinary infrastructure consisting of 12,076 veterinary hospitals/polyclinics, 25,571 veterinary dispensaries, 28,168 veterinary aid centres (Stockmen centres/Mobile dispensaries), to ensure the health of livestock and poultry by providing timely veterinary services thereby complementing the accelerated growth of livestock sector7. Additionally, the veterinary teaching hospitals also provide diagnostic and therapeutic services.
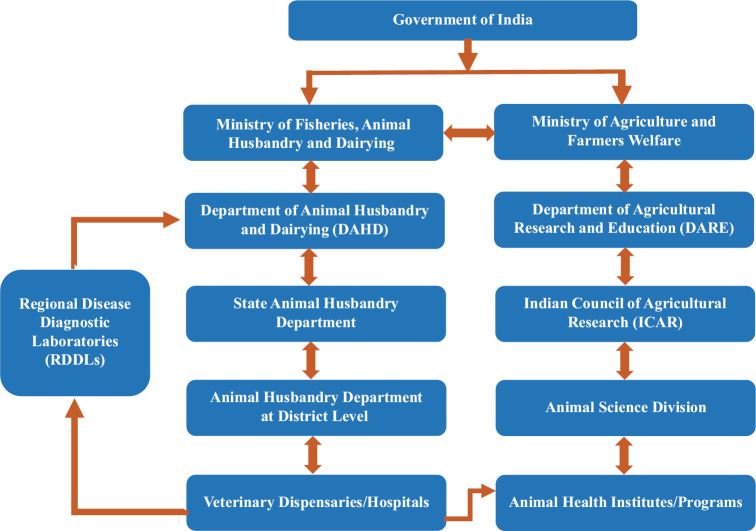
Disease diagnostic laboratories at State and district levels that are under the purview of respective State Animal Husbandry and Veterinary Services Departments also play a pivotal role in the country's animal disease diagnosis and control activities. In addition to the district and State-level livestock diseases diagnostic laboratories, there is one Central and five Regional Disease Diagnostic Laboratories (RDDLs) for referral services2 (Fig. 1). Largely, the livestock health sector depends on government infrastructure and services; however, the poultry sector has many private companies which are into contract farming that includes health services. Apart from diagnostic laboratories under the State and Central government, private animal disease diagnostic laboratories also exist in the country.
Fig. 1.
Organizational structure of the Animal Health Research Institutes under Indian Council of Agricultural Research and Department of Animal Husbandry and Dairying for animal disease surveillance. Source: Refs8,9.
National Animal Disease Reporting System (NADRS)
The reporting of animal diseases in the country is governed by the DAHD, Ministry of Fisheries, Animal Husbandry and Dairying, Government of India. National Animal Disease Reporting System (NADRS) is a web-based information technology system for reporting the diseases from the field level from States and Union Territories (UTs). The primary objective of the NADRS is to record and monitor livestock disease situation in the country with the aim of initiating the preventive and curative action in a swift manner during disease emergencies2. The NADRS considers village as an epidemiological unit for the purpose of reporting of outbreaks. This platform uses computerized system of animal disease reporting, linking each block, district- and State-level headquarters to the Central Disease Reporting and Monitoring at New Delhi. In addition, this network also has been linked to the animal disease diagnostic laboratories from the district level onwards. Further, to analyze the information captured at block level and through various reports, the Department has further reoriented the application on the technical and operational fronts and launched modified version NADRS 2.0 application2.
National Animal Disease Referral Expert System (NADRES)
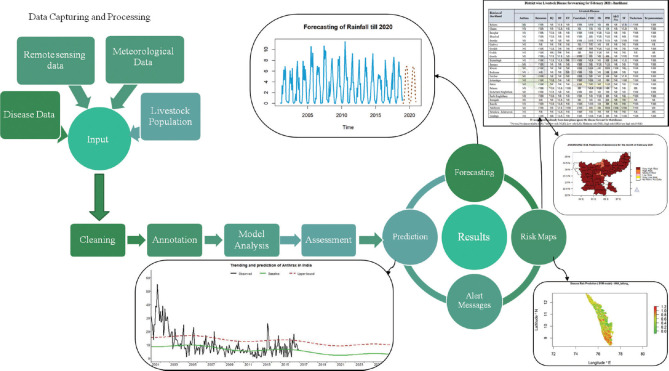
National Animal Disease Referral Expert System (NADRES) is a dynamic geographic information system and remote sensing-enabled expert system which is based on animal disease information collected and collated along with risk factor data of 652 (out of 735) districts of the country over a long period of time10 (Fig. 2). Indian Council of Agricultural Research (ICAR)-National Institute of Veterinary Epidemiology and Disease Informatics (NIVEDI) has identified 13 priority economically important livestock diseases including zoonotic disease like anthrax. The NADRES captures past disease incidence patterns and the source of data is from all over the country collected through All India Coordinated Research Projec on Animal Disease Monitoring and Surveillance (AICRP on ADMAS) centres and also through Department of Animal Husbandry and Veterinary Services of all the States on monthly basis11.
Fig. 2.
National Animal Disease Referral Expert System: The disease outbreak data collected from animal disease monitoring and surveillance centres, validated before feeding in to data base and then modelled to provide disease forewarning two months in advance. Source: Reproduced with permission from Ref 11.
The NADRES provides monthly livestock disease forewarning at district level which is published in the form of a monthly bulletin to alert the animal husbandry departments, both at the National/State level, to take appropriate control measures11. The disease prediction is categorized on the probability values ranging from 0 to 1. Based on the probability values, the risk of disease occurrence is depicted as very high risk (P=0.81-1.0), high risk (P=0.61-0.80), moderate risk (P=0.41-0.60), low risk (P=0.21-0.40), very low risk (P=0.0-0.20) and no risk (P=0.0)11. Spatial analysis of disease data has been incorporated in NADRES to produce risk maps, hotspot maps and disease maps.
Disease investigation
The activities related to research component of disease surveillance, diagnosis, outbreak investigations and vaccine development for livestock diseases are primarily taken care by research institutes of the ICAR under the aegis of the Department of Agricultural Research and Education, Ministry of Agriculture and Farmers Welfare, Government of India, as well as veterinary colleges under State agricultural and veterinary universities. Additionally, some of the States have animal health and veterinary biological institutes that caters the need of disease investigation and vaccine production. The research institutes of ICAR, viz. NIVEDI, National Institute of High Security Animal Diseases (NIHSAD), Indian Veterinary Research Institute (IVRI), National Research Center on Equines and National Center for Veterinary Type Cultures, are involved in disease surveillance, diagnosis and research on livestock diseases. Through the ICAR-funded AICRP, Disease-specific Network Projects and Outreach programmes namely AICRP on ADMAS, AICRP on FMD, All India Network on Gastrointestinal Parasitism, All India Network Programme on Bluetongue, All India Network on Neonatal Mortality in Farm Animals, Network project on Veterinary Type Culture Collection, Consortium Research Platform on Vaccines and Diagnostics, Outreach programme on Ethnoveterinary Medicine and Outreach programme on Zoonotic Diseases including Surveillance are conducted.
Surveillance activities for major zoonotic diseases and antimicrobial resistance
Brucellosis: Brucellosis is a contagious disease of animals with economic and public health significance. The disease is reported from most of the domestic animals, marine animals and humans as well. In animals the disease is characterized by reproductive disorder and abortion. In India nearly 80 million rural households are engaged in milk production and 75 per cent of milch animals are owned by small and marginal farmers12. The close association of humans with livestock provides greater probability of brucellosis transmission from animals to humans. Considering the huge burden of this disease on both animal and humans, the Government of India has launched a National Animal Disease Control Programme for brucellosis. One of the objectives under this Programme is regular seromonitoring and serosurveillance of the animal population. The components of bovine brucellosis surveillance include detection of brucellosis in domestic bovine, estimating the magnitude of brucellosis infection (i.e., prevalence), measuring progress towards regulatory goals, providing metrics to aid in evaluating compliance with Programme standards, giving stakeholders and decision-makers timely and relevant actionable information13.
As a part of brucellosis surveillance in bovines, ICAR-NIVEDI in collaboration with the Department of Animal Husbandry and Veterinary Services, Government of Karnataka, has screened 64,818 bulk milk samples from 30 districts using milk ring test in July 2012 and March 201314. In addition, seroprevalence of brucellosis in large ruminants was studied through a large scale serosurvey by screening 12,054 serum samples (cattle - 9236 and buffaloes - 2818) from 15 States of India by protein G indirect ELISA. It was found that the true prevalence of brucellosis in cattle and buffaloes were 8.3 and 3.6 per cent, respectively15. Brucellosis in small ruminants is caused mainly by Brucella melitensis. To understand the seroprevalence and spatial distribution of Brucella in small ruminants, a nationwide seroscreening for brucellosis was conducted during 2017-2018. Using indirect ELISA, the overall apparent and true prevalence of brucellosis was 7.45 per cent [95% confidence interval (CI): 7.13-7.79] and 3.79 per cent (95% CI: 3.44-4.17), respectively, in small ruminants16.
Further, as a part of inter-sectoral collaboration, ICAR-NIVEDI in collaboration with National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, medical colleges and department of Animal Husbandry and Veterinary Services is involved in screening of human samples suspected for brucellosis. Active surveillance among risk group is necessary to estimate the disease burden, and currently, national-level surveillance among the human is lacking, while it is done in bovine population. Limited targeted surveillance was carried out in high-risk human population. In one of the studies conducted in Karnataka, 1050 samples from occupationally exposed individuals were screened for brucellosis and the estimated seropositivity was 7.04 per cent17.
Leptospirosis
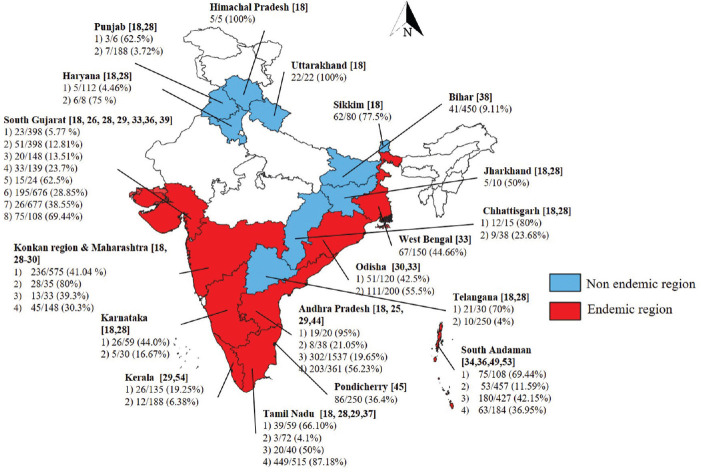
Leptospirosis is one of the major zoonotic diseases caused by Leptospira spp. However, leptospirosis is still underdiagnosed and under-reported due to lack of information and awareness. Leptospirosis causes economic loss to livestock husbandry due to reproductive losses, reduced production and treatment costs. The leptospirosis situation in India is a cause of concern, and it is endemic in all southern States and in coastal States such as Gujarat, Maharashtra, including Andaman and Nicobar Islands of India, where high prevalence was recorded both in animals and humans18. In India, a comprehensive study on the prevalence of leptospirosis in cattle covering large geographical locations is lacking, except a few isolated location-specific reports. The bovine leptospirosis has been reported from 19 States/Union Territories of India with overall sero-prevalence of 30.8 per cent19 (Fig. 3). In one of the studies among human cases with pyrexia of unknown origin, the seropositivity of leptospirosis was found to be 38 per cent. The major Leptospira serovars prevalent were Australis, Bankinang, Tarassovi, Ictirohaemorrhagiae and Pomona (unpublished data).
Fig. 3.
Seroprevalence of bovine (cattle and buffalo) leptospirosis in different States/UTs of India in the last decade depicted State-wise using QGIS software (Version 2.18.0, QGIS.org, http://www.qgis.org). Source: Refs 18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54.
Considering the public health significance of leptospirosis, the Government of India during the 12th five-year plan launched a Programme for Prevention and Control of Leptospirosis in the endemic States, viz. Gujarat, Kerala, Tamil Nadu, Maharashtra and Karnataka, and UT of Andaman and Nicobar Islands. The National Centre for Disease Control (NCDC) has been designated as the nodal agency for implementation of the Programme. The major objective of the Programme is to reduce the morbidity and mortality due to leptospirosis in humans through strengthening of surveillance, capacity building in terms of workforce and diagnostic laboratories, creating awareness and strengthening of the inter-sectoral collaboration.
Japanese encephalitis: Japanese encephalitis virus (JEV) causes severe encephalitis syndrome in humans and abortion in pigs. Japanese encephalitis is prevalent in South Asia, South East Asia and Asia Pacific region. In the year 2015, an estimated 1,00,308 cases and 25,125 deaths occurred due to JEV globally55. Since its first report, JEV has expanded its geographical distribution and presently JE is endemic in 303 districts of 24 States in India with 375 million people at risk of JE56. India experiences outbreaks of JE every year. In 2005, a huge outbreak of JE struck the country with 1700 casualties, mostly children57. The Operational Guidelines - National Programme for Prevention and Control of JE/Acute Encephalitis Syndrome, Ministry of Health and Family Welfare, have described that in addition to human clinical surveillance and entomological surveillance, veterinary surveillance of pigs is an important component to monitor the JEV activity in the locality and also for early warning of impending human JE outbreaks58. From the large-scale serosurveillance studies, it was evident that the prevalence of anti-JEV IgG and anti-JEV IgM in pigs from different parts of India was 32.22 and 16.12 per cent, respectively59,60. The role of pigs as an amplifying host is explored limiting only to detection of antibodies/viral RNA in pigs in endemic areas as a part of public health measure to check for imminent human outbreaks.
Kyasanur forest disease (KFD): Kyasanur forest disease (KFD) is a zoonotic disease unique to Indian subcontinent. The disease though reported first time from Karnataka, has spread to neighbouring States such as Maharashtra, Kerala, Goa and Tamil Nadu in the last few years, suggesting the changing disease ecology. The current system of disease surveillance involves sentinel surveillance for monkey deaths during risk period which triggers the human surveillance in endemic areas61. The changing disease pattern warrants a detailed inter-sectoral study to identify the role of vector, amplifying host and factors associated with forest ecosystem. During the KFD surveillance between 1956 and 2020 (till March), a total of 1484 monkey autopsies were conducted, of which 390 were found positive for KFD infection in Karnataka State61.
Anthrax: Anthrax is an important bacterial disease with high zoonotic significance. A One Health approach in investigation, surveillance and disease risk assessment is critical for effective disease control in humans. In this regard, institutes like ICAR-NIVEDI have taken major initiative in collaboration with CDC, India and established anthrax surveillance and reporting system in anthrax endemic regions of Odisha, Jharkhand and Karnataka States62. The standard protocol for anthrax surveillance under One Health programme was developed based on which many joint investigations of suspected anthrax outbreaks were carried out in endemic districts of Karnataka and Odisha. The diagnostic support was extended to animal health sector in endemic States and hundreds of samples were screened. Various inter-sectoral meetings, orientation cum technical seminars and anthrax outbreak investigation workshops were organized at State and district levels to improve the field and laboratory capacity of the staff working in both the sectors in endemic States. A long-term trend analysis (2001-2020) for anthrax using NADRES data in livestock showed that there is continuous occurrence of anthrax outbreaks, suggesting the need for effective vaccination to prevent this disease in India63.
Porcine cysticercosis: Taenia solium cysticercosis is one of the most important zoonotic parasitic diseases having public health importance, especially in developing countries. Cysticercosis is prevalent in virtually all States of India, the only possible exception being Jammu and Kashmir64. Cysticercosis is highly prevalent in the northern States of Bihar, Odisha, Uttar Pradesh and Punjab. The seroprevalence of porcine cysticercosis was reported to be 11.6 per cent in Tamil Nadu65. The reported overall prevalence of cysticercosis in pigs was 0.88 per cent by post-mortem examination and 0.9 per cent by PCR assay, whereas the prevalence of taeniasis in humans was reported as 3.15 per cent by ELISA and 2.04 per cent by PCR66. In humans, the prevalence of neurocysticercosis (NCC) as a cause of active epilepsy was estimated to be one per 1000 population in India67. It has been demonstrated that the human NCC-associated active epilepsy results in loss of 2.10 million disability-adjusted life years per annum in India68. The existing crisis in the management of cysticercosis at the interface of human and pigs in India can be effectively addressed by systematic surveillance of pigs to identify the hotspots of infection. Timely detection of infection in pigs and effective intervention will help to prevent the transmission of infection to human beings. Since the data available on the prevalence of disease in India are scattered, national-level surveillance of porcine cysticercosis is required.
Rabies: Rabies is an acute, progressive and fatal zoonotic disease with serious public health and economic consequences in India. In India, dogs are responsible for about 97 per cent of human rabies, followed by cats (2%), jackals, mongoose and others (1%)69. The disease is mainly transmitted by the bite of a rabid dog. The Integrated Disease Surveillance Programme (IDSP), under the Ministry of Health and Family Welfare has reported 74 lakhs animal bites in the year 2018 when compared to 42 lakhs in 2012. There were 593 suspected human rabies deaths reported by 30 of the 36 States and UTs during 201769.
The National Action Plan for Rabies Elimination (NAP-RE) (dog mediated) in India provides a broad framework for combating rabies with a vision to reduce human deaths due to dog mediated rabies to zero by 2030. Under NAP-RE, animal sector strategy includes vaccination of at least 70 per cent of the dog population in a defined geographical area annually for three consecutive years69. The surveillance of rabies in dogs includes clinical and laboratory surveillance.
Highly pathogenic avian influenza: Avian influenza infection in poultry and other avian species is caused by influenza A viruses. Avian influenza viruses have been classified into subtypes based on haemagglutinins (HA) and neuraminidase (NA) proteins. At least 16 types of HA and nine types of NA have been identified in avian influenza virus in birds70. Among the subtypes, H5N1 is a highly pathogenic subtype that poses serious threat to poultry industry with public health importance and has pandemic potential71. Because of antigenic shift and antigenic drift, the new avian influenza subtypes may emerge that could result in serious consequences72. The country witnessed the first high pathogenic avian influenza H5N1 outbreak in poultry in 200673. Thereafter, several avian influenza outbreaks in poultry have been reported from different parts of the country. Since the disease has zoonotic potential and serious economic consequences, the DAHD, Government of India, has prepared an action plan and revised from time to time to guide the State Governments for prevention, control and containment of avian influenza in the country. The revised Action Plan for Prevention, Control and Containment of Avian Influenza, 2021, has provided surveillance plan for avian influenza with the aim of early warning, detection and to take containment measure73. The surveillance for avian influenza includes the screening of both domestic poultry and migratory birds. The Action Plan provides the necessary details for clinical, viral and serological surveillance. The samples collected during the surveillance process are screened at RDDLs, whereas ICAR – NIHSAD, Bhopal, acts as national referral laboratory for avian influenza.
Antimicrobial resistance (AMR): AMR is a global challenge, which brought international bodies such as World Health Organization, World Organization for Animal Health and Food and Agriculture Organization (FAO) to jointly address the factors responsible for emergence of AMR across the globe. Bacteria produce metabolites such as endotoxins and lipopolysaccharides, leading to infections for which most antibiotic remain ineffective74. Global AMR Surveillance System Report in 2020 revealed that the major clinically relevant antibiotic-resistant species were Acinetobacter spp., Candida auris, Clostridioides difficile, Escherichia coli, Enterococcus spp., Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella spp., Shigella spp., Streptococcus pneumoniae and Neisseria gonorrhoeae75. The spread of antibiotic resistance is also determined by geographical and climatic condition, policies and socio-economic status76. Surveillance is essential for informing policies and interventions, including stewardship and infection prevention and control. It is the basis for monitoring the emergence and spread of AMR and for evaluating the effectiveness of local, national and global strategies to mitigate AMR. To understand the pattern of resistance for each bacterium, AMR profile needs to be studied in detail through next-generation technologies to reach to any consensus for developing strategies to fight against this menace.
In India with the cooperation of the FAO and USAID, the ICAR prepared a network, viz. Indian Network of Fisheries and Animal AMR in 2018. This national network includes 15 ICAR institutions and three State Agriculture Universities with 20 centres (nine centres from fisheries and 11 from the livestock sector). The network is aimed at detecting the AMR in different production systems and identifying the spread of resistant bacterial strains and genes and trends through a structured surveillance programme77.
Inter-sectoral coordination (ISC) for prevention and control of zoonotic diseases
Zoonotic diseases cause considerable morbidity and mortality in humans globally. It is interesting to know that more than 75 per cent of emerging and re-emerging diseases are zoonotic and the inter-sectoral–coordinated approach involving all relevant sectors (medical, veterinary and wildlife departments) with ‘One Health Vision’ is the need of the hour for effective surveillance, prevention and control of existing zoonotic diseases and newly evolving zoonotic threats in human beings. Keeping this in view, the Department of Health and Family Welfare, Ministry of Health and Family Welfare, Government of India launched a scheme ‘Strengthening of Inter-sectoral Coordination for Prevention and Control of Zoonotic Diseases’ during 2012 [12th five-year plan (2012-2017)] to strengthen inter-sectoral coordination between the sectors for prevention and control of zoonotic diseases of public health importance and the Programme is being run under the umbrella scheme of NCDC, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India78. The objectives of the Programme include establishing inter-sectoral coordination, communication between different stakeholders, laboratory capacity building, awareness and capacity building for effective prevention and control of zoonotic diseases.
Integrated disease surveillance programme (IDSP): The IDSP under the NCDC, Ministry of Health and Family Welfare, Government of India was established to enable laboratory-based surveillance of epidemic-prone diseases in the country. The main objectives of the IDSP are to monitor disease trends, detect the disease outbreaks and to respond immediately to contain the disease outbreaks56. The IDSP works at three levels with Centre, State and district surveillance units. The Central surveillance unit is located in NCDC, Delhi, the respective States/UT headquarters will have the State surveillance units (SSU) and the district surveillance units (DSU) at districts.
The IDSP carries out surveillance for several diseases including JE, anthrax and leptospirosis79. Considering the need for application of knowledge of veterinary sector in prevention and control of zoonotic diseases and to enhance inter-sectoral coordination having a veterinarian in the surveillance units was felt necessary. Hence, several States have included veterinarians as a team member of rapid response teams for zoonotic disease investigation80.
The IDSP has network of district and State -level laboratories for early identification of epidemic-prone diseases. However, several ICAR institutes, viz. IVRI, NIHSAD, NIVEDI, NRCE and other veterinary institutions/universities are also involved in diagnosis of zoonotic diseases. To quote some examples, the NIVEDI, Bengaluru is a south regional coordinator under the ISC for prevention and control of zoonotic diseases, a programme under the NCDC, Delhi, and is providing diagnostic service, laboratory capacity building and human resource development for human leptospirosis to IDSP units in Karnataka and other States10. To strengthen the surveillance of re-emerging zoonotic diseases such as glanders, NRCE, Hisar, and Central Military Veterinary Laboratory (CMVL), Meerut, play a significant role by acting as reference laboratories identified by the Ministry of Health and Family Welfare, Government of India for the diagnosis of glanders in humans81. Further, to institutionalize the integration of research and development with One Health approach, the Joint Task Force of ICMR-ICAR initiative on zoonoses is a positive step82. In addition, NCDC and IVRI organize joint orientation training courses for medical and veterinary officers83. This underscores the need to integrate the disease surveillance, data sharing, human resource, laboratory capacity between public health and veterinary sectors for effective prevention and control of zoonotic diseases, thereby achieving the larger goal of One Health.
Regional cooperation in transboundary animal disease surveillance: The transboundary animal diseases (TADs) are a major threat to livestock and humans. These diseases have potential to cause large-scale damage, staking the food security of the country and region, thereby crippling the nation's and region's economy significantly. The economic loss may be direct in the form of mortality and morbidity in affected population or indirect due to required counterepizootic measures. Additionally, the loss in trade and probable zoonotic transmission pose great risk to the nation. To strengthen the regional co-operation with the neighbouring countries, India had undertaken laboratory capacity building and human resource development for diagnosis of TADs in collaboration with international agencies viz., South Asian Association for Regional Cooperation (SAARC), Association of Southeast Asian Nations (ASEAN) and United States Centers for Disease Control and Prevention (CDC). Several training programmes were conducted by animal health institutes including ICAR-NIVEDI as part of regional cooperation62.
Conclusion
The disease surveillance is an important activity that provides the basis for knowing the disease burden in a country for follow up actions to control, prevent and eventually to eradicate the disease. Although large but resource-limited countries like India have system for animal disease surveillance and monitoring, yet it is limited to a few diseases. The endemic and emerging zoonotic diseases reported nationally need special attention across the sectors. A joint effort to utilize the existing surveillance system both in animal and human sectors and plan to establish the new system for emerging zoonotic disease surveillance are need of the hour. It is time to take One Health approach and work on to delineate the modalities to face such situations to protect the health of livestock and human.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Department of Animal Husbandry and Dairying (DAHD). 20th Livestock Census-2019, All India Report 2019. New Delhi: Ministry of Fisheries, Animal Husbandry and Dairying; 2019. [Google Scholar]
- 2.Department of Animal Husbandry and Dairying (DAHD). Annual Report 2019-2020. New Delhi: Ministry of Fisheries, Animal Husbandry and Dairying; 2020. [Google Scholar]
- 3.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–9. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11:1842–7. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FAO/OIE/WHO. Taking a multisectoral, one health approach: A tripartite guide to addressing zoonotic diseases in countries. World Health Organization. 2019 [Google Scholar]
- 6.World Organisation for Animal Health (OIE). Animal health surveillance. In: Terrestrial Animal Health Code, 2019. Ch. 1.4. Paris, France: World Organization for Animal Health; 2019. [Google Scholar]
- 7.Department of Animal Husbandry and Dairying (DAHD). Basic Animal Husbandry Statistics 2019. New Delhi: Ministry of Fisheries, Animal Husbandry and Dairying; 2019. [Google Scholar]
- 8.Department of Animal Husbandry and Dairying. Ministry of Fisheries, Animal Husbandry and Dairying, Government of India. [accessed on March 12, 2021]. Available from: http://dahd.nic.in/
- 9.Department of Agricultural Research and Education. Ministry of Agriculture and Formers Welfare, Government of India. [accessed on March 12, 2021]. Available from: http://dare.nic.in/
- 10.National Institute of Veterinary Epidemiology and Disease Informatics (NIVEDI). Annual Report 2019. Bengaluru, Karnataka, India: National Animal Disease Referral Expert System (NADRES); 2019. [Google Scholar]
- 11.Suresh KP, Hemadri D, Patil SS, Krishnamoorthy P, Jacob SS, Shome BR. Livestock Disease Forewarning Monthly Bulletin- April 2021. Vol. 9. Bengaluru: ICAR-NIVEDI; 2021. pp. 1–117. [Google Scholar]
- 12.Department of Animal Husbandry and Dairying (DAHD). Annual Report 2018-2019. New Delhi: Ministry of Fisheries, Animal Husbandry and Dairying; 2020. [Google Scholar]
- 13.National Bovine Brucellosis Surveillance Plan. Veterinary Services Centers for Epidemiology and Animal Health National Surveillance Unit. USA: Fort Collins; 2012. [Google Scholar]
- 14.Shome R, Nagalingam M, Shome , BR , Padmashree BS, Misri J, Kamal A, et al. Surveillance of bovine brucellosis using milk ring test under National Control Program on brucellosis in Karnataka, India. Indian J Anim Sci. 2015;85:1077–80. [Google Scholar]
- 15.Shome R, Triveni K, Swati S, Ranjitha S, Krithiga N, Shome BR, et al. Spatial seroprevalence of bovine brucellosis in India – A large random sampling survey. Comp Immunol Microbiol Infect Dis. 2019;65:124–7. doi: 10.1016/j.cimid.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Shome R, Kalleshamurthy T, Rathore Y, Ramanjinappa KD, Skariah S, Nagaraj C, et al. Spatial sero-prevalence of brucellosis in small ruminants of India: Nationwide crosssectional study for the year 2017-2018. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13871. doi: 10.1111/tbed.13871. [DOI] [PubMed] [Google Scholar]
- 17.Shome R, Kalleshamurthy T, Shankaranarayana PB, Giribattanvar P, Chandrashekar N, Mohandoss N, et al. Prevalence and risk factors of brucellosis among veterinary health care professionals. Pathog Glob Health. 2017;111:234–9. doi: 10.1080/20477724.2017.1345366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balamurugan V, Alamuri A, Bharathkumar K, Patil SS, Govindaraj GN, Nagalingam M, et al. Prevalence of Leptospira serogroup-specific antibodies in cattle associated with reproductive problems in endemic States of India. Trop Anim Health Prod. 2018;50:1131–8. doi: 10.1007/s11250-018-1540-8. [DOI] [PubMed] [Google Scholar]
- 19.Balamurugan V, Thirumalesh SR, Veena S, Alamuri A, Nagalingam M, Sridevi R, et al. Investigation on the distribution of Leptospira serovars and its prevalence in bovine in Konkan region, Maharashtra, India. Adv Anim Vet Sci. 2016;4:19–26. [Google Scholar]
- 20.Alamuri A, Thirumalesh SR, Kumari SS, Kumar KV, Roy P, Balamurugan V. Seroprevalence and distribution of serogroup-specific pathogenic Leptospira antibodies in cattle and buffaloes in the State of Andhra Pradesh, India. Vet World. 2019;12:1212–7. doi: 10.14202/vetworld.2019.1212-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alamuri A, Veena S, Vinod Kumar K, Kalyani H, Rahman H, Shome BR, et al. Changing trend in the prevalence and emergence of Leptospira serogroup-specific antibodies in livestock in Gujarat, India. Proc Natl Acad Sci India Sect B Biol Sci. 2020;90:1145–51. [Google Scholar]
- 22.Balakrishnan G. Involvement of Leptospira serovars with different clinical conditions of leptospirosis in cattle. Biomed Pharmacol J. 2014;7:125–8. [Google Scholar]
- 23.Balakrishnan G, Govindarajan R, Parimal R, Gopu P, Jayakumar V, Manohar BM. Diagnosis of leptospiral mastitis in a cow by polymerase chain reaction. Tamilnadu J Vet Anim Sci. 2009;5:75–6. [Google Scholar]
- 24.Balakrishnan G, Parimal Roy J, Kirubaharan J, Meenambigai TV, Chandran ND. Phylogenetic analysis of bovine Leptospira isolates. Indian J Vet Sci Biotechnol. 2015;10:36–9. [Google Scholar]
- 25.Balakrishnan G, Roy P, Govindarajan R, Ramaswamy V, Murali Manohar B. Bovine leptospirosis in Andhra Pradesh. Indian Vet J. 2011;88:140–1. [Google Scholar]
- 26.Balakrishnan G, Roy P, Govindarajan R, Ramaswamy V, Murali Manohar B. Seroepidemiological studies on leptospirosis among bovines in an organized farm. Int J Agro Vet Med Sci. 2011b;5:511. [Google Scholar]
- 27.Balakrishnan G, Roy P, Govindarajan R, Ramaswamy V, Murali Manohar B. Seroepidemiological studies on leptospirosis among bovines in an organized farm. Int J Agro Vet Med Sci. 2011c;10:87–8. [Google Scholar]
- 28.Balamurugan V, Alamuri A, Veena S, Bharathkumar K, Patil SS, Govindaraj G, et al. Investigation on the prevalence of Leptospira serovar Hardjo in organised cattle dairy farms of India. Indian J Anim Sci. 2016a;86:1145–7. [Google Scholar]
- 29.Balamurugan V, Thirumalesh SR, Sridevi R, Govindaraj G, Nagalingam M, Hemadri D, et al. Microscopic agglutination test analysis identifies prevalence of intermediate species serovars in ruminants in endemic States of India. Proc Natl Acad Sci India Sect B Biol Sci. 2016;86:469–75. [Google Scholar]
- 30.Balamurugan V, Thirumalesh SR, Sridevi R, Mohandoss N, Govindaraj G, Hemadri D, et al. Seroprevalence of bovine leptospirosis in Odisha, India. World J Vet Sci. 2013;1:1–7. [Google Scholar]
- 31.Balamurugan V, Thirumalesh SR, Veena S, Alamuri A, Nagalingam M, Sridevi R, et al. Investigation on the distribution of Leptospira serovars and its prevalence in bovine in Konkan region Maharashtra, India. Adv Anim Vet Sci. 2016c;4:19–26. [Google Scholar]
- 32.Balamurugan V, Veena S, Thirumalesh SR, Alamuri A, Sridevi R, Sengupta PP, et al. Distribution of serogroup specific antibodies against leptospirosis in livestock in Odisha. Indian J Anim Sci. 2017;87:546–51. [Google Scholar]
- 33.Behera SK, Sabarinath T, Kumar A, Das SC, Palai TK, Patra D, et al. Seroprevalence of leptospirosis among suspected cattle in eastern part of India: A comparative study between rLipL32ELISA and MAT. Iran J Vet Res. 2014;15:285–9. [Google Scholar]
- 34.Lall C, Kumar KV, Raj RV, Sugunan AP, Sunish IP, Sharma S, et al. Trend in the seroprevalence of leptospirosis among cattle and goat populations of South Andaman. Indian J Vet Res. 2017;26:37–40. [Google Scholar]
- 35.Deneke Y, Sabarinath T, Gogia N, Lalsiamthara J, Viswas KN, Chaudhuri P. Evaluation of recombinant LigB antigen-based indirect ELISA and latex agglutination test for the serodiagnosis of bovine leptospirosis in India. Mol Cell Probes. 2014;28:141–6. doi: 10.1016/j.mcp.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Mitra J, Chowdhury S, Pattanayak S. Seroprevalence of bovine leptospirosis in South Andaman Islands, India. Explor Anim Med Res. 2015;5:96–101. [Google Scholar]
- 37.Natarajaseenivasan K, Vedhagiri K, Sivabalan V, Prabagaran SG, Sukumar S, Artiushin SC, et al. Seroprevalence of Leptospira borgpetersenii serovar javanica infection among dairy cattle, rats and humans in the Cauvery river valley of Southern India. Southeast Asian J Trop Med Public Health. 2011;42:679–86. [PubMed] [Google Scholar]
- 38.Pandian SJ, Ray PK, Chandran PC, Kumar M. Seroprevalence of Brucella abortus and Leptospira Hardjo in cattle. Vet World. 2015;8:217–20. doi: 10.14202/vetworld.2015.217-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panwala T, Mulla S. Seroprevalence of the cattle leptospirosis in South Gujarat region of India. IOSR J Agric Vet Sci. 2015;8:8–11. [Google Scholar]
- 40.Panwala TH. Epidemiological study on human, cattle and rodent leptospirosis in South Gujarat region of India. Ann Pathol Lab Med. 2017;4:A476–81. [Google Scholar]
- 41.Patel JM, Vihol PD, Raval JK, Patel KM, Chaudhari NF, Rathod PH, et al. Seroprevalence of leptospirosis in clinically ailing bovine. J Anim Res. 2015;5:31. [Google Scholar]
- 42.Patel JM, Prasad MC, Vihol PD, Kalyani IH, Prajapati MG. Seroprevalence of Leptospira Hardjo in Cattle of Gujarat, India. Int J Curr Microbiol Appl Sci. 2017;6:1304–10. [Google Scholar]
- 43.Patel JM, Vihol PD, Dabas VS, Prasad MC, Patel JH, Chaudhari CF, et al. Seroepidemiological study of leptospirosis in buffaloes of South Gujarat, India. Buffalo Bull. 2016;35:383–8. [Google Scholar]
- 44.Prameela RD, Sreenivasulu D, Vijayachari P, Nataraj Seenivasan N. Seroepidemiology of leptospirosis in Andhra Pradesh. Arch Clin Microbiol. 2013;4:1–10. [Google Scholar]
- 45.Rajan B, Kumar S, Pillai RM, Antony PX, Mukhopadhyay HK, Balakrishnan S, et al. Comparative study on serodiagnosis of bovine leptospirosis by micro agglutination test (MAT) and indirect ELISA. Int J Curr Microbiol Appl Sci. 2017;6:1551–8. [Google Scholar]
- 46.Sachan N, Nautiyal B, Chaudhary PT, Singh VP, Agarwal RK. Seroprevalence of leptospirosis in the animals in Rohilkhand region of Uttar Pradesh, India. Int J Agro Vet Med Sci. 2013;6:361. [Google Scholar]
- 47.Sehgal SC, Sugunan AP, Vijayachari P. Outbreak of leptospirosis after the cyclone in Orissa. Natl Med J India. 2002;15:22–3. [PubMed] [Google Scholar]
- 48.Senthilkumar TM, Subathra M, Ramadass P, Ramaswamy V. Serodiagnosis of bovine leptospirosis by IgG-enzyme-linked immunosorbent assay and latex agglutination test. Trop Anim Health Prod. 2010;42:217–22. doi: 10.1007/s11250-009-9409-5. [DOI] [PubMed] [Google Scholar]
- 49.Sharma S, Vijayachari P, Sugunan AP, Roy S, Natarajaseenivasan K. Seroprevalence and carrier status for leptospirosis in cattle and goats in Andaman Island, India. J Vet Sci Technol. 2014;5:5. [Google Scholar]
- 50.Sharma S, Vijayachari P, Sugunan AP, Sehgal SC. Leptospiral carrier State and seroprevalence among animal population – A cross-sectional sample survey in Andaman and Nicobar Islands. Epidemiol Infect. 2003;131:985–9. doi: 10.1017/s095026880300880x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soman M, Jayaprakasanand V, Mini M. Seroprevalence of leptospirosis in human beings and animals in Central and North Kerala. IOSR J Agric Vet Sci. 2014;7:38–41. [Google Scholar]
- 52.Rao GS, Surendran NS. Incidence and serotypes of bovine Leptospira in Andhra Pradesh. Indian Vet J. 1970;47:296–8. [PubMed] [Google Scholar]
- 53.Sunder J, Sujatha T, Kundu A, Kundu MS. Carrier status and seroprevalence of leptospirosis in cattle of South Andaman. Indian J Anim Res. 2017;52:140–3. [Google Scholar]
- 54.Tresamol VP, Anju Antony M, Mini KV, Joseph S. Seroprevalence of leptospirosis among cattle in and around Thrissur District, Kerela. Int J Livest Res. 2017;7:45–48. [Google Scholar]
- 55.Quan TM, Thao TTN, Duy NM, Nhat TM, Clapham H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000-2015. Elife. 2020;9:e51027. doi: 10.7554/eLife.51027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ministry of Health and Family Welfare, (MOHFW). Annual Report 2020-21. Delhi: Department of Health and Family Welfare, Ministry of Health and Family Welfare, Government of India; 2021. [Google Scholar]
- 57.Kulkarni R, Sapkal GN, Kaushal H, Mourya DT. Japanese encephalitis: A brief review on Indian perspectives. Open Virol J. 2018;12:121–30. doi: 10.2174/1874357901812010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ministry of Health and Family Welfare (MOHFW). Operational Guidelines National Programme for Prevention and Control of Japanese Encephalitis/Acute Encephalitis Syndrome. Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2014. [Google Scholar]
- 59.Dhanze H, Bhilegaonkar KN, Rawat S, Chethan Kumar HB, Kumar A, Gulati BR, et al. Development of recombinant nonstructural 1 protein based indirect enzyme linked immunosorbent assay for sero-surveillance of Japanese encephalitis in swine. J Virol Methods. 2019;272:113705. doi: 10.1016/j.jviromet.2019.113705. [DOI] [PubMed] [Google Scholar]
- 60.Dhanze H, Kumar MS, Singh V, Gupta M, Bhilegaonkar KN, Kumar A, et al. Detection of recent infection of Japanese encephalitis virus in swine population using IgM ELISA: A suitable sentinel to predict infection in humans. J Immunol Methods. 2020;486:112848. doi: 10.1016/j.jim.2020.112848. [DOI] [PubMed] [Google Scholar]
- 61.Directorate of Health and Family Welfare Services (DHFWS). Operational Manual Kyasanur Forest Disease. Bengaluru, Karnataka: Directorate of Health and Family Welfare Services, Government of Karnataka; 2020. [Google Scholar]
- 62.ICAR-National Institute of Veterinary Epidemiology and Disease Informatics. Annual Report 2018-19. [accessed on March 12, 2021]. Avaialble from: https://nivedi.res.in/pdf/Reports/Annual%20Report%202018-19.pdf .
- 63.NADRES v2: Redefining livestovk disease forewarning. Disease trends. [accessed on March 10, 2021]. Available from: https://www.nivedi.res.in/Nadres_v2/disease_trends.php .
- 64.Rajashekhar V. Epidemiology of Taenia solium taeniasis/cysticercosis in India and Nepal. Southeast Asian J Trop Med Public Health. 2004;35:247–51. [Google Scholar]
- 65.Mohan VR, Tharmalingam J, Muliyil J, Oommen A, Dorny P, Vercruysse J, et al. Prevalence of porcine cysticercosis in Vellore, South India. Trans R Soc Trop Med Hyg. 2013;107:62–4. doi: 10.1093/trstmh/trs003. [DOI] [PubMed] [Google Scholar]
- 66.Vaidya V, Paturkar A, Zende R, Gatne M, Dighe D, Waghmare R, et al. Scenario of porcine cysticercosis and human taeniasis in Maharashtra State, India. Turk J Vet Anim Sci. 2018;42:353–8. [Google Scholar]
- 67.Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135–9. doi: 10.1212/01.wnl.0000249113.11824.64. [DOI] [PubMed] [Google Scholar]
- 68.Singh BB, Khatkar MS, Gill JP, Dhand NK. Estimation of the health and economic burden of neurocysticercosis in India. Acta Trop. 2017;165:161–9. doi: 10.1016/j.actatropica.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Ministry of Health and Family Welfare, (MOHFW). Draft National Action Plan for Eliminating Dog Mediated. Rabies from India. National Centre for Disease Control, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. 2020 [Google Scholar]
- 70.World Organization for Animal Health. Avian influenza (infection with avian influenza viruses). Ch. 3.3.4. Paris France: OIE Terrestrial Manual; 2018. [Google Scholar]
- 71.World Health Organization. Avian influenza: Assessing the pandemic threat. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 72.Peiris Malik JS, de Jong MD, Guan Y. Avian influenza virus (H5N1): A threat to human health. Clin Microbiol Rev. 2007;20:243–67. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Department of Animal Husbandry and Dairying (DAHD). Action Plan for Prevention, Control & Containment of Avian Influenza (Revised – 2021) New Delhi: Department of Animal Husbandry and Dairying, Ministry of Fisheries, Animal Husbandry and Dairying, Government of India; 2021. [Google Scholar]
- 74.Baron S. Medical microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch; 1996. [PubMed] [Google Scholar]
- 75.World Health Oragization. Global antimicrobial resistance and use surveillance system (GLASS) report: early implementation 2020. Geneva: WHO; 2020. [Google Scholar]
- 76.Wellington EM, Boxall AB, Cross P, Feil EJ, Gaze WH, Hawkey PM, et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect Dis. 2013;13:155–65. doi: 10.1016/S1473-3099(12)70317-1. [DOI] [PubMed] [Google Scholar]
- 77.Rathore G, Lal KK, Bhatia R, Jena JK. INFAAR – A research platform for accelerating laboratory-based surveillance of antimicrobial resistance in fisheries and aquaculture in India. Curr Sci. 2020;119:1884–5. [Google Scholar]
- 78.National Center for Disease Control. Program for strengthening inter-sectoral Cordination for prevention and control of Zoonotic diseases. Operational guidelines for Regional coordinators. [accessed on March 10, 2021]. Available from: https://ncdc.gov.in/WriteReadData/l892s/17851707131573187386.pdf .
- 79.Integrated Disease Surveillance Program. Disease Wise Major Outbreak Reported by the State (2008-2020) [accessed on March 10, 2021]. Available from https://www.idsp.nic.in/outbreak_d/Home.html .
- 80.National Center for Disease Control. Training Manual for Veterinary Consultants under IDSP. Delhi: National Centre for Disease Control, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2016. [Google Scholar]
- 81.CD Alert. Glanders: A public health concern. Delhi: National Centre for Disease Control; 2017. [Google Scholar]
- 82.Indian Council of Medical Research. Annual Report 2015-16. New Delhi: ICMR, Department of Health Research, Ministry of Health and Family Welfare; 2016. [Google Scholar]
- 83.Integrated Disease Surveillance Programme (IDSP). Joint Monitoring Mission Report. Integrated Disease Surveillance Programme 2015. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2015. [Google Scholar]