Abstract
Background
For patients with COVID-19 who undergo emergency endotracheal intubation, data are limited regarding the practice, outcomes, and complications of this procedure.
Research Question
For patients with COVID-19 requiring emergency endotracheal intubation, how do the procedural techniques, the incidence of first-pass success, and the complications associated with the procedure compare with intubations of critically ill patients before the COVID-19 pandemic?
Study Design and Methods
We conducted a retrospective study of adult patients with COVID-19 at Montefiore Medical Center who underwent first-time endotracheal intubation by critical care physicians between July 19, 2019, and May 1, 2020. The first COVID-19 patient was admitted to our institution on March 11, 2020; patients admitted before this date are designated the prepandemic cohort. Descriptive statistics were used to compare groups. A Fisher exact test was used to compare categorical variables. For continuous variables, a two-tailed Student t test was used for parametric variables or a Wilcoxon rank-sum test was used for nonparametric variables.
Results
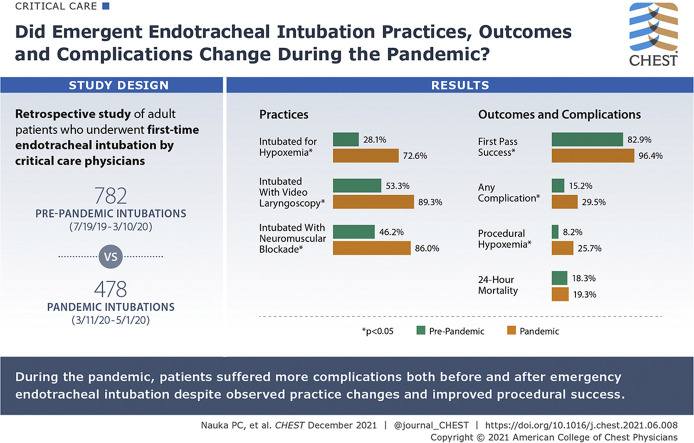
One thousand two hundred sixty intubations met inclusion criteria (782 prepandemic cohort, 478 pandemic cohort). Patients during the pandemic were more likely to be intubated for hypoxemic respiratory failure (72.6% vs 28.1%; P < .01). During the pandemic, operators were more likely to use video laryngoscopy (89.4% vs 53.3%; P < .01) and neuromuscular blocking agents (86.0% vs 46.2%; P < .01). First-pass success was higher during the pandemic period (94.6% vs 82.9%; P < .01). The rate of associated complications was higher during the pandemic (29.5% vs 15.2%; P < .01), a finding driven by a higher rate of hypoxemia during or immediately after the procedure (25.7% vs 8.2%; P < .01).
Interpretation
Video laryngoscopy and neuromuscular blockade were used increasingly during the COVID-19 pandemic. Despite a higher rate of first-pass success during the pandemic, the incidence of complications associated with the procedure was higher.
Key Words: airway management, intratracheal, intubation, mechanical ventilation
Abbreviations: CLG, Clinical Looking Glass; IQR, interquartile range; Spo2, oxygen saturation
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 1993
Patients with COVID-19 often experience severe hypoxemic respiratory failure requiring emergent endotracheal intubation.1,2 Because of the severity of illness seen in patients with COVID-19 and the potential for disease transmission, guidelines have been published to standardize intubation practices.3, 4, 5, 6, 7, 8 Based on these guidelines, our institution adopted a hospital-wide policy advocating for increased use of neuromuscular blocking agents and video laryngoscopy to improve first-pass success while minimizing risk to personnel.
Despite the publication of protocols, a paucity of evidence is available describing the way in which the COVID-19 pandemic changed the practice of emergency airway management, its outcomes, and its associated complications. Early case series from outside of the United States reported that patients with COVID-19 requiring emergent endotracheal intubation frequently experience high rates of complications, including severe hypoxemia.9, 10, 11, 12 These reports consist of limited cohorts of patients intubated mostly by senior clinicians, the findings of which may not be generalizable to what is being experienced within the United States inside of academic medical centers. A multinational, observational cohort study described 4,476 episodes of emergency endotracheal intubation with association between procedural first-pass success and provider- and location-specific variables. These data did not include patient-level characteristics or comparison with prepandemic intubations.13
To determine to what extent the onset of the COVID-19 pandemic altered emergency airway management, we performed a retrospective, observational study comparing procedural practices, outcomes, and complications before the COVID-19 pandemic with those outcomes during the pandemic.
Methods
Setting
This retrospective cohort study was approved by the institutional review board at the Albert Einstein College of Medicine, which also issued a waiver of informed consent (Identifier: 2019-10752). We examined patients intubated at the three campuses of Montefiore Medical Center located in the Bronx, New York, between July 19, 2019, and May 1, 2020.
Patients were eligible if they underwent intubation performed outside of the operating room or ED. Intubations were excluded if age was younger than 18 years, intubation records were missing, or a repeat intubation during study period was performed on a patient already included in the study.
The patient cohort was split into two groups. The pandemic group included patients undergoing intubation between March 11, 2020, until the cohort collection on May 1, 2020. March 11, 2020, was the first date a patient with confirmed COVID-19 was admitted to our institution. The second group served as a historical control and included all patients undergoing intubation between July 19, 2019 and March 10, 2020.
Hospital Characteristics Before and During the COVID-19 Pandemic
Before the COVID-19 pandemic, our institution consisted of 1,491 beds, including 106 ICU beds spanning eight units. Before the pandemic, all ICUs were staffed by the critical care medicine service 24 h/day. During the pandemic surge in April 2020, the total number of hospital beds expanded to 1,628 and the number of ICU beds increased to 306. At the height of the surge on April 12, 1,127 patients with COVID-19 were admitted.
Airway Management Teams
Emergency endotracheal intubations that occur outside the operating room or ED are handled by the critical care service. On two campuses, a dedicated critical care consultation service manages intubations on non-ICU wards. The third campus has an ICU team that responds to airway emergencies in addition to their unit responsibilities.
Postgraduate year 4 and beyond critical care fellows perform most intubations. Airway management training for fellows consists of a 2-day simulation course. In addition to available standard intubation trays, the critical care team carries an airway management bag consisting of sedatives, neuromuscular blocking agents, vasoactive drugs, and airway adjuncts including a video laryngoscope (either GlideScope GLVTM, GlideScope GoTM, or GlideScope TitaniumTM; Verathon, Inc.).
Variables
Clinical Looking Glass (CLG; Clinical Analytics) is a tool developed by our institution to help researchers extract data from electronic medical records.14 This tool is well validated and has been used in numerous other investigations.15 CLG was used to extract intubation notes, demographic and clinical characteristics, and COVID-19 polymerase chain reaction testing results (Xpert Xpress SARS-CoV-2 assay; Cepheid) (e-Appendix 1).
Demographic and patient characteristics, including age, sex, BMI, race, history of OSA within the prior 5 years, ethnicity, socioeconomic status, and hospital location, were extracted using CLG. We also determined Charlson Comorbidity Index and laboratory-based acute physiology score. Pao 2 to Fio 2 ratio immediately after intubation and the presence of a vasopressor order between 72 h before and 1 h after intubation also were extracted using CLG.16 , 17 In the case that Pao 2 was not available, we estimated the value based on oxygen saturation.18 We also manually extracted the last available peripheral oxygen saturation (Spo 2) and Fio 2 before intubation from the electronic medical record.
An intubation attempt was defined as any insertion of a laryngoscope into the oropharynx. Preoxygenation was defined as use of any oxygen delivery method before induction of sedatives or paralytics, and apneic oxygenation was defined as passive flow of oxygen typically via nasal cannula or high-flow nasal cannula during the intubation attempt. Nasal bilevel positive pressure ventilation was not used for apneic oxygenation in this cohort.
Outcomes
Our primary outcome of interest was the rate of first-pass success of the procedure. First-pass success is reported easily, previously was found to correlate with a lower frequency of adverse procedural outcomes, and often is used as the primary outcome of airway management investigations.15 , 19, 20, 21 We also reported the use of paralytics and video laryngoscopy before and after the onset of the pandemic. To describe the impact of the pandemic on intubation-associated complications, we reported severe hypoxemia (peripheral saturation < 80%), hypotension (systolic BP < 70 mm Hg), esophageal intubation, witnessed aspiration of gastric contents, and difficult intubation (need for more than two attempts at laryngoscopy). An intubation-associated complication was defined as occurring after administration of procedural pharmacotherapy or, in the case of no pharmacotherapy, first laryngoscope blade insertion and up until 5 min after the final intubation attempt. Other outcomes of interest include difficult intubation and mortality in the 24 h immediately after the procedure.
Statistical Analysis
Demographic and patient characteristics between the pandemic and historical control groups were examined using standard descriptive statistics. The Fisher exact test was used to compare categorical variables. A two-tailed Student t test for parametric variables or a Wilcoxon rank-sum test for nonparametric variables was used for continuous variables.
We performed an exploratory multivariate logistic regression to compare the effect of intubating under pandemic conditions on first-pass success, periprocedural complications, and 24-h mortality (e-Appendix 2). Covariables were selected based on prior literature and clinical experience among patients with COVID-19.15 , 22, 23, 24, 25, 26, 27, 28, 29, 30 We also performed a sensitivity analysis to examine the robustness of our model. Given that airway management during cardiac arrest is a unique circumstance, we redefined our cohort to exclude all intubations performed for this indication. Statistical analysis was performed using Stata version 16.1 software (StataCorp LLC).
Results
Study Population
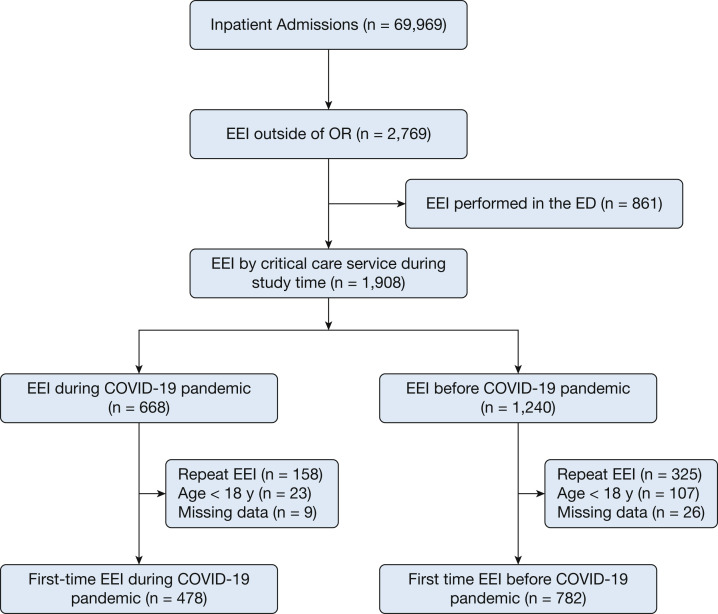
During the study period, our institution had 69,969 inpatient admissions (Fig 1 ). A total of 2,769 patients underwent emergent endotracheal intubation outside of the operating room, of which 861 were performed in the ED and subsequently were excluded from our analysis. During the study period, 1,908 intubations performed by critical care personnel. Six hundred forty-eight patients were excluded because of repeat intubation, pediatric age, or missing outcome variables. The final cohorts consisted of 478 patients (37.9%) during the pandemic and 782 patients (62.1%) before the pandemic.
Figure 1.
Flow diagram showing patients included in the cohort. EEI = emergent endotracheal intubation; OR = operating room.
Patient Characteristics
Patients undergoing intubation during the pandemic were younger (pandemic mean ± SD, 63.7 ± 13.7 years vs prepandemic mean ± SD, 64.7 ± 14.7 years; P = .01), had a higher BMI (pandemic median, 29.4 kg/m2 [interquartile range (IQR), 25.4-34.6 kg/m2] vs prepandemic median, 27.1 kg/m2 [IQR, 22.7-32.0 kg/m2]; P < .01), a lower Charlson Comorbidity Index (pandemic median, 2 [IQR, 0-5] vs prepandemic median, 5 [IQR, 3-7]; P < .01), and a lower admission laboratory-based acute physiology score (pandemic mean ± SD, 79.7 ± 29.8 vs prepandemic mean ± SD, 89.3 ± 34.3; P < .01) (Table 1 ). No significant differences were found between the two groups with regard to sex, history of OSA, or socioeconomic status.
Table 1.
Baseline Characteristics of Study Cohort
| Variable | Total (N = 1,260) | Pandemic Period (n = 478) | Before the Pandemic Period (n = 782) | P Value |
|---|---|---|---|---|
| Age, y | 64.7 ± 14.7 | 63.7 ± 13.7 | 65.3 ± 15.2 | .01 |
| Male sex | 738 (58.6) | 294 (61.5) | 444 (56.8) | .10 |
| BMI, kg/m2a | 28.0 (23.5-33.0) | 29.4 (25.4-34.6) | 27.1 (22.7-32.0) | < .01 |
| OSA history | 176 (14.0) | 62 (13.0) | 114 (14.6) | .43 |
| Race | .17 | |||
| Black | 450 (35.7) | 170 (35.6) | 280 (35.8) | |
| White | 153 (12.2) | 48 (10.0) | 105 (13.4) | |
| Other/not specified | 657 (52.1) | 260 (54.4) | 397 (50.8) | |
| SES scoreb | –2.27 (–5.79 to –1.04) | –2.12 (–5.21 to –1.05) | –2.34 (–5.93 to –0.94) | .43 |
| LAPS | 85.6 ± 33.0 | 79.7 ± 29.8 | 89.3 ± 34.3 | < .01 |
| Charlson Comorbidity Index | 4 (2-7) | 2 (0-5) | 5 (3-7) | < .01 |
| COVID-19 testing resultsc | < .01 | |||
| Positive | 396 (31.4) | 394 (82.4) | 2 (0.3) | |
| Negative | 62 (4.9) | 52 (10.9) | 10 (1.3) | |
| Not performed | 802 (63.7) | 32 (6.7) | 770 (98.4) | |
| Campus | < .01 | |||
| Moses | 661 (52.5) | 226 (47.3) | 435 (55.6) | |
| Wakefield | 161 (12.8) | 71 (14.9) | 90 (11.5) | |
| Einstein | 416 (33.0) | 166 (34.7) | 250 (31.0) | |
| Children’s Hospital at Montefiored | 22 (1.7) | 15 (3.1) | 7 (0.9) | |
| Hospital location of intubation | < .01 | |||
| Floor | 760 (62.5) | 359 (82.5) | 401 (51.3) | |
| ICU | 424 (34.8) | 73 (18.8) | 351 (44.9) | |
| Othere | 33 (2.7) | 3 (0.7) | 30 (3.9) | |
| Peripheral Spo2 before intubationf | 96 (92-99) | 94 (90-97) | 97 (94-100) | < .01 |
| Spo2 to Fio2 ratio before intubationf | 245 (99-357) | 98 (92-190) | 313 (192-448) | < .01 |
| Pao2 to Fio2 ratio after intubationg | < .01 | |||
| < 100 | 186 (14.8) | 100 (20.9) | 86 (11.0) | |
| 100-200 | 239 (19.0) | 79 (16.5) | 160 (20.5) | |
| 200-300 | 121 (9.6) | 24 (5.0) | 97 (12.4) | |
| > 300 | 714 (56.7) | 275 (57.5) | 439 (56.1) | |
| Use of vasopressorsh | 665 (52.8) | 175 (36.6) | 490 (62.7) | < .01 |
Data are presented as No. (%), mean ± SD, or median (interquartile range), unless otherwise indicated. LAPS = laboratory-based acute physiology score; SES = socioeconomic status; Spo2 = oxygen saturation.
Data missing for 40 patients.
Calculated using census data regarding wealth and income and represented as the number of SDs from the mean SES score for New York State. SES data were missing for 270 patients.
Patients could undergo COVID-19 testing within the prepandemic category if they were admitted before March 11, 2020, and remained hospitalized past this date.
Patients admitted to the Children’s Hospital at Montefiore older than 18 years were eligible for inclusion if they underwent emergent endotracheal intubation by critical care personnel. During pandemic conditions, units within Children’s Hospital were used to accommodate an increased volume of hospitalized patients.
Nontraditional locations included cardiac catheterization and interventional radiology suites. Location data were not available for 43 patients. Two patients were admitted to the critical care service and intubated in the ED by critical care providers, and so were counted in the “Other” category.
Last recorded Spo2 value and corresponding Fio2 before intubation were extracted. Peripheral saturations were not available for 40 patients.
Calculated from first Fio2 and first Pao2 after emergent endotracheal intubation or estimated from Spo2 to Fio2 ratio if Pao2 was unavailable.
Considered positive if occurring 72 h before 1 h after emergent endotracheal intubation.
A significant difference regarding the indication for intubation was driven by a higher incidence of hypoxemic respiratory failure during the pandemic (pandemic, 72.6% vs prepandemic, 28.1%). Correspondingly, patients during the pandemic showed lower peripheral saturations (94% vs 97%; P < .01) and Spo 2 to Fio 2 ratios (98 vs 313; P < .01) immediately before intubation. Patients during the pandemic were more likely to experience severe hypoxemic respiratory failure (Pao 2 to Fio 2 ratio after intubation, < 100; 20.9% vs 11.0%) after intubation (Table 2 ). The incidence of peri-intubation shock was lower during the pandemic period (36.6% vs 62.7%; P < .01). Patients during the pandemic were more likely to be intubated outside of an ICU (81.2% vs 55.1%; P < .01).
Table 2.
Periprocedural Characteristics of Emergent Endotracheal Intubation Performed Before and During the Pandemic
| Variable | Total (N = 1,260) | Pandemic (n = 478) | Before the Pandemic (n = 782) | P Value |
|---|---|---|---|---|
| Indicationa | < .01 | |||
| Hypoxemia | 567 (45.0) | 347 (72.6) | 220 (28.1) | |
| Airway protection | 286 (22.7) | 60 (12.6) | 226 (28.9) | |
| Cardiac arrest | 135 (10.7) | 18 (3.8) | 117 (15.0) | |
| Hypercarbia | 50 (4.0) | 9 (1.9) | 41 (5.2) | |
| Hemodynamic instability | 121 (9.6) | 9 (1.9) | 1112 (14.3) | |
| Other | 101 (8.0) | 35 (7.3) | 66 (8.4) | |
| CL gradeb | < .01 | |||
| 1 | 1,029 (82.7) | 430 (90.9) | 599 (77.6) | |
| 2a | 157 (12.6) | 34 (7.2) | 123 (15.9) | |
| 2b | 41 (3.3) | 4 (0.9) | 37 (4.8) | |
| 3 | 16 (1.3) | 3 (0.6) | 13 (1.7) | |
| 4 | 2 (0.2) | 2 (0.4) | 0 (0) | |
| Any preoxygenation | 1,208 (95.9) | 450 (94.1) | 758 (96.9) | .02 |
| BVM | 434 (34.4) | 37 (7.7) | 397 (50.8) | |
| NRB | 341 (27.0) | 240 (50.2) | 101 (12.9) | |
| NRB + BVM | 35 (2.8) | 3 (0.6) | 32 (4.1) | |
| NRB + HFNC | 52 (4.1) | 48 (10.0) | 4 (0.5) | |
| NRB + NC | 23 (1.8) | 12 (2.5) | 11 (1.4) | |
| HFNC | 134 (10.6) | 67 (14.0) | 67 (8.6) | |
| NC | 58 (4.6) | 14 (2.9) | 44 (5.6) | |
| NIV | 103 (8.2) | 14 (2.9) | 89 (11.4) | |
| None | 52 (4.1) | 28 (5.9) | 24 (3.1) | |
| Other | 28 (2.2) | 15 (3.1) | 13 (1.7) | |
| Any apneic oxygenation | 278 (22.1) | 115 (24.1) | 163 (20.8) | .18 |
| HFNC | 149 (11.8) | 79 (16.5) | 70 (9.0) | |
| NC | 129 (10.2) | 36 (7.5) | 93 (11.9) | |
| None | 982 (77.9) | 363 (75.9) | 619 (79.1) | |
| Any sedation | 1,039 (82.5) | 431 (90.2) | 608 (77.8) | < .01 |
| Etomidate | 844 (67.0) | 345 (72.2) | 499 (63.8) | |
| Propofol | 481 (38.2) | 216 (45.2) | 265 (33.9) | |
| Ketamine | 9 (0.7) | 0 (0) | 9 (1.2) | |
| Fentanyl | 17 (1.4) | 6 (1.3) | 11 (1.4) | |
| Midazolam | 24 (1.9) | 11 (2.3) | 13 (1.7) | |
| Any paralytics | 772 (61.3) | 411 (86.0) | 361 (46.2) | < .01 |
| Succinylcholine | 426 (33.8) | 280 (58.6) | 146 (18.7) | |
| Rocuronium | 132 (10.5) | 43 (9.0) | 89 (11.4) | |
| Vecuronium | 225 (17.9) | 98 (20.5) | 127 (16.1) | |
| Use of video laryngoscopy | 844 (67.0) | 427 (89.3) | 417 (53.3) | < .01 |
| Use of bougie | 48 (5.5) | 19 (6.6) | 29 (5.0) | .35 |
| Nonattending operator | 1,089 (86.4) | 402 (84.1) | 687 (87.9) | .06 |
| Anesthesia operator | 29 (2.3) | 10 (2.1) | 19 (2.4) | .85 |
Data are presented as No. (%), unless otherwise indicated. BVM = bag-valve mask; CL = Cormack-Lehane; HFNC = high-flow nasal cannula; NC = regular nasal cannula; NIV = noninvasive ventilation; NRB = nonrebreather mask.
Indication for intubation was missing for 17 patients.
C-L grade was not recorded for 15 patients.
Emergent Endotracheal Intubation Characteristics
The use of preoxygenation was lower during the pandemic (94.1% vs 96.9%; P = .02), driven largely by a notable drop in the use of bag mask ventilation for preoxygenation (7.7% vs 50.8%) (Table 2). No significant difference was found regarding receipt of apneic oxygenation (24.1% vs 20.8%; P = .18). Use of a neuromuscular blocking agent during intubation was more likely during the pandemic period (86.0% vs 46.2%; P < .01), as was use of a sedative agent (90.2% vs 77.8%; P < .01). Video laryngoscopy was used more commonly during the pandemic period (89.3% vs 53.3%; P < .01). A Cormack-Lehane grade 1 view of the vocal cords was more likely to be achieved during the pandemic period (90.9% vs 77.6%; P < .01).
Emergent Endotracheal Intubation Outcomes
First-pass success occurred in 461 pandemic group patients (96.4%) and 648 patients intubated before the pandemic (82.9%) (P < .01) (Table 3 , Figs 2, 3 ). The incidence of difficult intubation was lower in the pandemic group (1.0% vs 3.6%; P < .01).
Table 3.
Intubation Outcome Measures Before and During the Pandemic
| Variable | Total (N = 1,260) | Pandemic (n = 478) | Before the Pandemic (n = 782) | P Value |
|---|---|---|---|---|
| First-pass success | 1,109 (88.0) | 461 (96.4) | 648 (82.9) | < .01 |
| Difficult intubation | 33 (2.6) | 5 (1.0) | 28 (3.6) | < .01 |
| Any complication | 260 (20.6) | 141 (29.5) | 119 (15.2) | < .01 |
| Procedural hypoxemia | 187 (14.8) | 123 (25.7) | 64 (8.2) | < .01 |
| Hypotension | 84 (6.7) | 31 (6.5) | 53 (6.8) | .91 |
| Gastric aspiration | 48 (3.8) | 6 (1.3) | 42 (5.4) | < .01 |
| Esophageal intubation | 13 (1.0) | 1 (0.2) | 12 (1.5) | .02 |
| 24-h mortality | 235 (18.7) | 92 (19.3) | 143 (18.3) | .71 |
Data are presented as No. (%), unless otherwise indicated.
Figure 2.
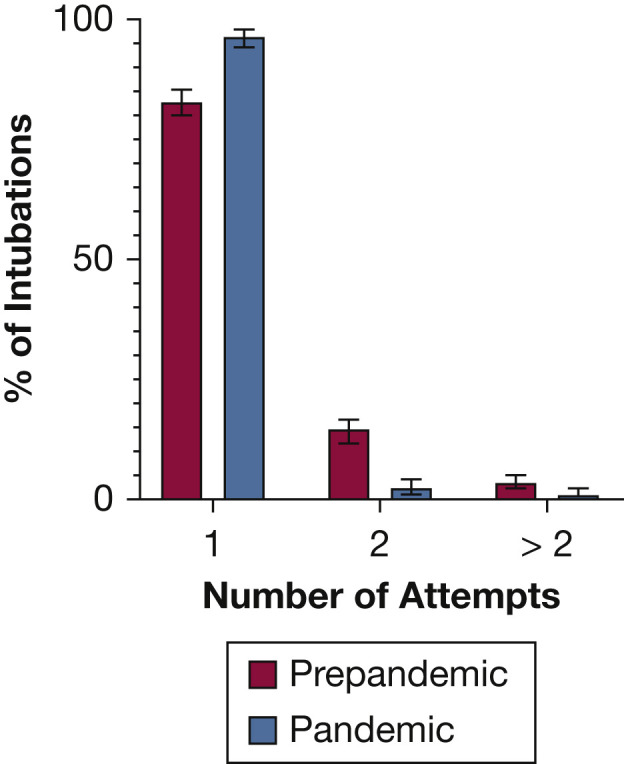
Bar graph showing the number of intubation attempts before the pandemic and during pandemic period.
Figure 3.
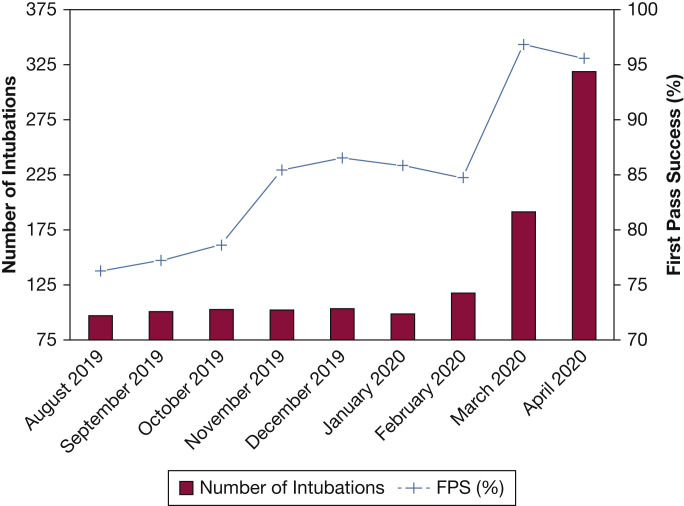
Graph showing intubation volume and FPS rates over study period. Study enrollment began on July 19, 2019. Given the lack of complete data from July 2019, only data from August 1, 2019, onward is depicted. FPS = first-pass success.
Emergent Endotracheal Intubation Complications
The pandemic cohort had higher incidence of any periprocedural adverse event (29.5% vs 15.2%; P < .01) largely driven by a higher rate of severe hypoxemia during the procedure (25.7% vs 8.2%; P < .01). Intubation during the pandemic was associated with lower occurrence of gastric aspiration (1.3% vs 5.4%; P < .01) and witnessed esophageal intubation (0.2% vs 1.5%; P = .02). No difference was found in periprocedural hypotension between the two cohorts (6.5% vs 6.8%; P = .91). The 24-h mortality rate was similar compared with the prepandemic period (19.3% vs 18.3%; P = .71).
Exploratory Regression Model
The pandemic period was associated with significantly increased odds of first-pass success in our adjusted multivariate model (adjusted OR, 4.91; 95% CI, 2.65-9.11; P < .01) (e-Table 1). The odds of any periprocedural complication were higher in the pandemic group (adjusted OR, 2.21; 95% CI, 1.51-3.04; P < .01). The 24-h mortality rate did not differ between the groups (adjusted OR, 1.26; 95% CI, 0.86-1.84; P = .23). Removal of patients requiring intubation in the setting of a cardiac arrest did not alter models for first-pass success or periprocedural complication; however, 24-h mortality was higher in the pandemic group (adjusted OR, 2.48; 95% CI, 1.59-3.88; P < .01).
Discussion
We demonstrated that at our institution, the use of neuromuscular blocking agents and video laryngoscopy dramatically increased during the pandemic. The procedural outcome of first-pass success of emergent endotracheal intubation also was higher during this period. Despite these changes to practice and procedural success, complications associated with emergent endotracheal intubation increased during the COVID-19 pandemic, an observation largely driven by an increased incidence of severe hypoxemia during the procedure.
With regard to the practice of emergency endotracheal intubation at our institution, our data reflect the impact of local and external guidance that aimed to optimize both the safety of the patient and the clinical team.5 , 31 To maximize first-pass success and infection control, full neuromuscular blockade and video laryngoscopy were advocated for all intubations performed on patients with COVID-19 (e-Figs 1, 2). After the onset of the pandemic, the use of these interventions increased dramatically as compared with the prepandemic periods in this data set and in our prior work dating back to 2016.32
Before the COVID-19 pandemic, our rate of first-pass success mirrored rates reported recently by the International Observational Study to Understand the Impact and Best Practices of Airway Management in Critically Ill Patients (INTUBE) study group.33 The first potential factors driving the observed increase in first-pass success during the course of our study include the changes to intubation practice discussed. Both neuromuscular blockade30 and video laryngoscopy demonstrate a potential to improve the first-pass success of airway management procedures for the critically ill,34 particularly when the intubation operator is a trainee.35
The dramatic improvement in first-pass success rates during the COVID-19 pandemic raises the question of whether the combination of video laryngoscopy and neuromuscular blockade should be adopted more widely in other institutions for the endotracheal intubation of critically ill patients. Although the changes mostly were put into place as a response to the COVID-19 pandemic, this could be an opportunity to improve care for patients with and without COVID-19 on a permanent basis. Although our results indeed are thought provoking, we emphasize that they represent an association found through an observational and retrospective study design. Further prospective and randomized studies are needed that examine the combination of video laryngoscopy and neuromuscular blockade during airway management in the critically ill.
Inclusion of the covariables measuring use of video laryngoscopy and neuromuscular blockade in our exploratory adjusted model did not account fully for the dramatic improvement in procedural success noted during the pandemic. Additional explanations pertain to the timing of the academic year and the volume of procedures being performed. Most of the intubations in this study were performed by trainees, who have the potential to be increasingly skilled as the academic year progresses.36 Consistent with this hypothesis is that first-pass success trended upward through most months of the year preceding the onset of the pandemic (Fig 3). Additionally, the dramatic increase in the number of intubations with the onset of the pandemic may have resulted in an accelerated progression toward expertise.
Alternatively, it was demonstrated recently that as the number of intubations specifically performed on patients with COVID-19 by a specific operator increases, so does procedural first-pass success.13 This may relate to the need for acclimation to specific procedural stressors, such as the need for enhanced provider personal protective equipment and the potential for rapid oxygenation desaturation. The number of procedures performed before and during this study period by each individual trainee or senior clinician was not available in our data set, and so remains a limitation of our observations. Additionally, our inability to account fully for the dramatic improvement in first-pass success may be attributed to failure to identify and measure alternative confounding patient or procedure-related factors.
The last important finding of our investigation pertains to the increased rate of observed procedural complications after the onset of the pandemic. This finding largely was driven by an increased rate of procedural severe hypoxemia during the pandemic. Given that the number of attempts required for intubation previously was associated with increasing adverse events,37 the discordance between procedural complications and improved first-pass success was surprising.
Several factors could account for this finding. Despite lower baseline comorbidity and severity of illness scores, most of the patients being intubated during the pandemic experienced significantly worse hypoxemic respiratory failure as measured by Pao 2 to Fio 2 ratio immediately after intubation and Spo 2 to Fio 2 ratio before intubation. Additionally, our data show that at our institution, bag-valve mask ventilation was used much less commonly for preoxygenation during the pandemic and could have contributed to worsening hypoxemia that was unable to be corrected before laryngoscope blade insertion.38 We integrated the last recorded Spo 2 to Fio 2 ratio into our adjusted model to account for this finding and found that this did not alter the results.
Although a substantial number of guidelines have been published, published data are limited regarding the actual circumstances and outcomes of emergency endotracheal intubations since the onset of the COVID-19 pandemic.11 , 13 , 39, 40, 41 The prior published literature consists of a case series of 202 patients intubated at two hospitals in Wuhan, China,9 and findings from the multinational Intubate COVID group.33 These studies demonstrated similar use of neuromuscular blockade, high use of video laryngoscopy, and high rates of first-pass success.
Our study adds to the above limited literature regarding the airway management of patients with COVID-19 in that it consists of patient-level information regarding demographics, severity of illness, procedural techniques, and complications not previously reported in the literature. To our knowledge, this is the first study that has compared the practice and outcomes of emergency intubations performed during the pandemic with the same procedure performed before the onset of the pandemic. This is also the first report of patients with COVID-19 regarding the success and safety of intubations performed predominantly by trainees.9, 10, 11, 12 Our decision to exclude subsequent intubations on the same patient eliminates the introduction of bias.
The main limitation of our investigation is its retrospective design. Intubation procedure data that are self-reported by the operator are at risk of bias (e-Fig 3).42 To evaluate the impact of preintubation hypoxemia on the observed outcomes, we manually extracted last available peripheral saturation with calculation of Spo 2 to Fio 2 ratio before intubation. These data may be prone to the limitations of retrospective chart review, although the findings of lower saturations during the pandemic were consistent with expectations. Integration of these data into our exploratory models did not change the results substantially. A second limitation pertains to the discordant months in the academic year between the pandemic period group and the comparator group. A third limitation of our work is the lack of availability of potential confounders pertaining to some variables. Details pertaining to certain anatomic predictors of a difficult airway (Mallampati score, reduced mobility of the cervical spine, and limiting mouth opening < 3 cm) were not available for our analysis.22 We were able to report other previously described predictors of a difficult airway, including prior diagnosis of OSA, severe hypoxemia before intubation, and intubation by a nonanesthesiologist.
Interpretation
We showed that compared with intubations before the pandemic, airway management during the COVID-19 pandemic was associated with changes in practice, procedural success, and complications. Institutional guidelines resulted in near universal adoption of video laryngoscopy and neuromuscular blockade. Despite a higher rate of first-pass success, patients were more likely to experience peri-intubation adverse events, a finding dominated by increased rates of severe hypoxemia during and immediately after the procedure. The dramatic improvement in first-pass success rates after the escalation in use of video laryngoscopy and neuromuscular blockade raises interesting questions regarding whether this combination should be considered more widely for the intubation of critically ill patients. We showed that with appropriate guidelines and supervision, critical care trainees can achieve outcomes similar to those of experienced operators in published cohorts of patients with COVID-19. Although advances have been made recently regarding the treatment of patients with COVID-19, future investigation is needed to improve further the safety of emergency airway management for patients with COVID-19.
Take-home Points.
Study Questions: Did emergent endotracheal intubation practices, outcomes, and complications change during the COVID-19 pandemic as compared with the prepandemic period?
Results: As compared with before the COVID-19 pandemic, use of video laryngoscopy and neuromuscular blocking agents increased, although patients were more likely to experience postintubation procedural complications, a finding driven by postintubation hypoxemia.
Interpretation: During the COVID-19 pandemic, patients experienced more profound hypoxemia both before and after emergency endotracheal intubation, despite observed practice changes and improved procedural success.
Acknowledgments
Author contributions: D. G. F. had full access to all the data in the study and is the guarantor for the integrity of the data and the accuracy of the data analysis. P. C. N. and D. G. F. contributed to study conception and study design. P. C. N., D. G. F., J.-T. C., A. L. S., and L. A. E. contributed substantially to data acquisition, data analysis and interpretation, and writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Additional information: The e-Appendixes, e-Figures, and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Chand S., Kapoor S., Orsi D., et al. COVID-19-associated critical illness-report of the first 300 patients admitted to intensive care units at a New York City medical center. J Intensive Care Med. 2020;35(10):963–970. doi: 10.1177/0885066620946692. [DOI] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewster D.J., Chrimes N., Do T.B., et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020;212(10):472–481. doi: 10.5694/mja2.50598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75(6):785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67(5):568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissman D.N., de Perio M.A., Radonovich L.J., Jr. COVID-19 and risks posed to personnel during endotracheal intubation. JAMA. 2020;323(20):2027–2028. doi: 10.1001/jama.2020.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo M.Z., Huang Y.G., Ma W.H., et al. Expert recommendations for tracheal intubation in critically ill patients with novel coronavirus disease 2019. Chin Med Sci J. 2020;35(2):105–109. doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao W., Wang T., Jiang B., et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125(1):e28–e37. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Li J., Zhou M., Chen Z. Summary of 20 tracheal intubation by anesthesiologists for patients with severe COVID-19 pneumonia: retrospective case series. J Anesth. 2020;34(4):599–606. doi: 10.1007/s00540-020-02778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad I., Jeyarajah J., Nair G., et al. A prospective, observational, cohort study of airway management of patients with COVID-19 by specialist tracheal intubation teams. Can J Anaesth. 2021;68(2):196–203. doi: 10.1007/s12630-020-01804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi A., Sokhi J., Lockie C., Ward P.A. Emergency tracheal intubation in patients with COVID-19: experience from a UK centre. Anesthesiol Res Pract. 2020;2020:8816729. doi: 10.1155/2020/8816729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong D.J.N., El-Boghdadly K., Owen R., et al. Emergency airway management in patients with COVID-19: a prospective international multicenter cohort study. Anesthesiology. 2021;135(2):292–303. doi: 10.1097/ALN.0000000000003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellin E., Fletcher D.D., Geberer N., Islam S., Srivastava N. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med. 2010;85(8):1362–1368. doi: 10.1097/ACM.0b013e3181df0f3b. [DOI] [PubMed] [Google Scholar]
- 15.Fein D.G., Mastroianni F., Murphy C.G., et al. Impact of a critical care specialist intervention on first pass success for emergency airway management outside the ICU. J Intensive Care Med. 2021;36(1):80–88. doi: 10.1177/0885066619886816. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Escobar G.J., Greene J.D., Scheirer P., Gardner M.N., Draper D., Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 18.Rice T.W., Wheeler A.P., Bernard G.R., Hayden D.L., Schoenfeld D.A., Ware L.B. Comparison of the Sp02/FI02 ratio and the Pa02/FI02 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 19.Griesdale D.E., Bosma T.L., Kurth T., Isac G., Chittock D.R. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008;34(10):1835–1842. doi: 10.1007/s00134-008-1205-6. [DOI] [PubMed] [Google Scholar]
- 20.Mort T.C. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99(2):607–613. doi: 10.1213/01.ANE.0000122825.04923.15. table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Sakles J.C., Patanwala A.E., Mosier J.M., Dicken J.M. Comparison of video laryngoscopy to direct laryngoscopy for intubation of patients with difficult airway characteristics in the emergency department. Intern Emerg Med. 2014;9(1):93–98. doi: 10.1007/s11739-013-0995-x. [DOI] [PubMed] [Google Scholar]
- 22.De Jong A., Molinari N., Terzi N., et al. Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med. 2013;187(8):832–839. doi: 10.1164/rccm.201210-1851OC. [DOI] [PubMed] [Google Scholar]
- 23.Leong S.M., Tiwari A., Chung F., Wong D.T. Obstructive sleep apnea as a risk factor associated with difficult airway management—a narrative review. J Clin Anesth. 2018;45:63–68. doi: 10.1016/j.jclinane.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Driver B.E., Prekker M.E., Klein L.R., et al. Effect of use of a bougie vs endotracheal tube and stylet on first-attempt intubation success among patients with difficult airways undergoing emergency intubation: a randomized clinical trial. JAMA. 2018;319(21):2179–2189. doi: 10.1001/jama.2018.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch-Allen A.J., Ayas N., Mountain S., Dodek P., Peets A., Griesdale D.E. Influence of residency training on multiple attempts at endotracheal intubation. Can J Anaesth. 2010;57(9):823–829. doi: 10.1007/s12630-010-9345-x. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt U.H., Kumwilaisak K., Bittner E., George E., Hess D. Effects of supervision by attending anesthesiologists on complications of emergency tracheal intubation. Anesthesiology. 2008;109(6):973–977. doi: 10.1097/ALN.0b013e31818ddb90. [DOI] [PubMed] [Google Scholar]
- 27.Combes X., Andriamifidy L., Dufresne E., et al. Comparison of two induction regimens using or not using muscle relaxant: impact on postoperative upper airway discomfort. Br J Anaesth. 2007;99(2):276–281. doi: 10.1093/bja/aem147. [DOI] [PubMed] [Google Scholar]
- 28.Lundstrom L.H., Duez C.H.V., Norskov A.K., et al. Effects of avoidance or use of neuromuscular blocking agents on outcomes in tracheal intubation: a Cochrane systematic review. Br J Anaesth. 2018;120(6):1381–1393. doi: 10.1016/j.bja.2017.11.106. [DOI] [PubMed] [Google Scholar]
- 29.Lundstrom L.H., Moller A.M., Rosenstock C., Astrup G., Wetterslev J. High body mass index is a weak predictor for difficult and failed tracheal intubation: a cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anesthesia Database. Anesthesiology. 2009;110(2):266–274. doi: 10.1097/ALN.0b013e318194cac8. [DOI] [PubMed] [Google Scholar]
- 30.Mosier J.M., Sakles J.C., Stolz U., et al. Neuromuscular blockade improves first-attempt success for intubation in the intensive care unit. A propensity matched analysis. Ann Am Thorac Soc. 2015;12(5):734–741. doi: 10.1513/AnnalsATS.201411-517OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keene A.B., Shiloh A.L., Eisen L., et al. Critical care surge during the COVID-19 pandemic: implementation and feedback from frontline providers. J Intensive Care Med. 2021;36(2):233–240. doi: 10.1177/0885066620973175. [DOI] [PubMed] [Google Scholar]
- 32.Fein D.G., Mastroianni F., Murphy C.G., et al. Impact of a critical care specialist intervention on first pass success for emergency airway management outside the ICU. J Intensive Care Med. 2021;36(1):80–88. doi: 10.1177/0885066619886816. [DOI] [PubMed] [Google Scholar]
- 33.Russotto V., Myatra S.N., Laffey J.G., et al. Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325(12):1164–1172. doi: 10.1001/jama.2021.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverberg M.J., Li N., Acquah S.O., Kory P.D. Comparison of video laryngoscopy versus direct laryngoscopy during urgent endotracheal intubation: a randomized controlled trial. Crit Care Med. 2015;43(3):636–641. doi: 10.1097/CCM.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 35.Arulkumaran N., Lowe J., Ions R., Mendoza M., Bennett V., Dunser M.W. Videolaryngoscopy versus direct laryngoscopy for emergency orotracheal intubation outside the operating room: a systematic review and meta-analysis. Br J Anaesth. 2018;120(4):712–724. doi: 10.1016/j.bja.2017.12.041. [DOI] [PubMed] [Google Scholar]
- 36.Buis M.L., Maissan I.M., Hoeks S.E., Klimek M., Stolker R.J. Defining the learning curve for endotracheal intubation using direct laryngoscopy: a systematic review. Resuscitation. 2016;99:63–71. doi: 10.1016/j.resuscitation.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Sakles J.C., Chiu S., Mosier J., Walker C., Stolz U. The importance of first pass success when performing orotracheal intubation in the emergency department. Acad Emerg Med. 2013;20(1):71–78. doi: 10.1111/acem.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casey J.D., Janz D.R., Russell D.W., et al. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med. 2019;380(9):811–821. doi: 10.1056/NEJMoa1812405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T.A., Lai K.H., Chang H.T. Impact of a severe acute respiratory syndrome outbreak in the emergency department: an experience in Taiwan. Emerg Med J. 2004;21(6):660–662. doi: 10.1136/emj.2003.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng P.W., Wong D.T., Bevan D., Gardam M. Infection control and anesthesia: lessons learned from the Toronto SARS outbreak. Can J Anaesth. 2003;50(10):989–997. doi: 10.1007/BF03018361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho P.L., Tang X.P., Seto W.H. SARS: hospital infection control and admission strategies. Respirology. 2003;8(suppl 1):S41–S45. doi: 10.1046/j.1440-1843.2003.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinderknecht A.S., Dyas J.R., Kerrey B.T., Geis G.L., Ho M.H., Mittiga M.R. Studying the safety and performance of rapid sequence intubation: data collection method matters. Acad Emerg Med. 2017;24(4):411–421. doi: 10.1111/acem.13145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.