Abstract
Eukaryotic development relies on dynamic cell shape changes and segregation of fate determinants to achieve coordinated compartmentalization at larger scale. Studies in invertebrates have identified polarity programmes essential for morphogenesis; however, less is known about their contribution to adult tissue maintenance. While polarity‐dependent fate decisions in mammals utilize molecular machineries similar to invertebrates, the hierarchies and effectors can differ widely. Recent studies in epithelial systems disclosed an intriguing interplay of polarity proteins, adhesion molecules and mechanochemical pathways in tissue organization. Based on major advances in biophysics, genome editing, high‐resolution imaging and mathematical modelling, the cell polarity field has evolved to a remarkably multidisciplinary ground. Here, we review emerging concepts how polarity and cell fate are coupled, with emphasis on tissue‐scale mechanisms, mechanobiology and mammalian models. Recent findings on the role of polarity signalling for tissue mechanics, micro‐environmental functions and fate choices in health and disease will be summarized.
Keywords: cell fate, cell mechanics, cell polarity, epidermis, polarity proteins
Subject Categories: Cell Adhesion, Polarity & Cytoskeleton; Development & Differentiation
This review summarises the recent insights into the interplay of polarity proteins, cell mechanics and fate decisions in development, homeostasis and disease context with a focus on mammalian model systems.
Introduction
Cell polarity refers to the asymmetric shape and uneven distribution of cellular constituents, such as organelles and macromolecules. In epithelial tissues, polarization governs major processes, e.g. the formation of intercellular adhesions responsible for tissue cohesion, the definition of the apical and basal domains and the segregation of fate determinants (Kemphues, 2000; Goldstein & Macara, 2007; St Johnston & Ahringer, 2010; Nance & Zallen, 2011; Campanale et al, 2017; Riga et al, 2020). Epithelial polarity is also reflected in polarized localization of cytoskeleton components, gradients of soluble molecules or asymmetric distribution of adhesive structures. In invertebrates, conserved polarity proteins of the Crumbs, Par and Scribble polarity complexes (Fig 1A and B) are essential for the establishment of apico‐basal polarity and have emerged as important molecules for cell fate decisions (Motegi et al, 2020). Links between cell polarity networks and fate specification have been firmly demonstrated in the context of asymmetric cell division, where polarity proteins and the spindle orientation machinery collaborate to drive segregation of fate determinants (Inaba & Yamashita, 2012). Intriguingly, recent findings from epithelial systems have disclosed an unexpected, tight interplay of polarity proteins, adhesion molecules and mechanochemical pathways in tissue organization. Below, we discuss the importance of core apico‐basal polarity networks and mechanics in driving fate decisions at tissue scale.
Figure 1. Conserved polarity regulators: molecular overview.
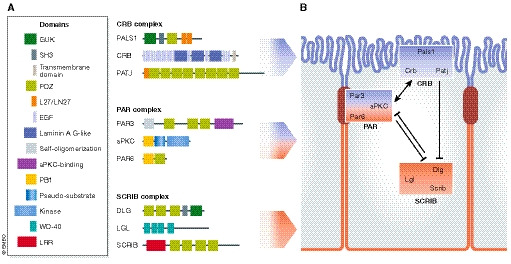
(A) Apical–basal polarity complexes in mammals. There are three main conserved polarity complexes in mammals, the apical Crumbs, the apico‐junctional Par and the basolateral Scribble complex. Together, they mediate the establishment of apico‐basal polarity. The Crumbs complex is composed of Pals1 and Pals1‐associated tight junction (PATJ) homologue proteins. The Par complex consists of partitioning‐defective 3 (Par3), partitioning‐defective 6 (Par6) and atypical kinase C (aPKC). The Scribble complex includes lethal giant larvae (Lgl), discs large (Dlg) and Scribble (Scrib) proteins. These polarity complexes consist of several scaffold proteins containing protein–protein interaction domains (e.g. PDZ) mediating the binding of different polarity proteins across the apical and basolateral domains. The kinases aPKC and Par1 (not shown) play an important role by promoting the mutual exclusion of proteins from the apical or basolateral domains (see Box 1 for details). (B) Establishment of epithelial apico‐basal polarity. The three polarity complexes, Crumbs, Par and Scribble complexes, distribute across the apical and the basolateral domain. During formation of epithelial intercellular junctions, Par3A localizes to the future tight junctions, which is required for the apical targeting of aPKC and Par6. Crumbs3 recruits Pals1, which in turns recruits Par6. When aPKC phosphorylates Par3, Par3 dissociates from aPKC/Par6. Upon Cdc42‐mediated Par6 activation, aPKC can phosphorylate Lgl1/2 and Par1 (not shown), excluding them from the apical domain. Conversely, the Ser/Thr kinase Par1b at the basolateral site phosphorylates Par3, preventing its diffusion to the basal domain.
Tissue challenges during development and homeostasis: Intrinsic and extrinsic regulation of cell fate by polarity signalling
Exploring cell polarity across taxa: learning from different systems
Research in the field of cell polarity is characterized by its wide range of model systems utilized, spanning fungi, plants, invertebrate and vertebrate animal models. Prominent studies in lower organisms have revealed intimate links between control of cell polarity and cell fate. Among these, a foundation in the field of cell polarity was the identification of partitioning‐defective Par genes, which, when mutated, cause defects in the first, asymmetric cell division of the Caenorhabditis elegans zygote (Kemphues et al, 1988) (Fig 2A). Since then, this system has served as a key screening platform to reveal factors required for asymmetric segregation of molecules in dividing cells (Rose & Gonczy, 2014). In Drosophila melanogaster, detailed genetic studies in various developing tissues and organs have clarified mechanisms of epithelial polarization, targeted RNA and protein transport, and oriented cell divisions. The neuroepithelium, for instance, hosts remarkably consistent events of cell division, with stem cells engaging polarity proteins to divide asymmetrically to give rise to two daughter cells of different fate (Wodarz, 2005; Homem & Knoblich, 2012) (Fig 2B). Prompted by these findings in invertebrates, the idea of a tight involvement of core polarity components in the control of spindle orientation has also been embraced for higher organisms. Interestingly, however, while some concepts could be extended to mammalian contexts (e.g. similar molecular interactions between polarity and spindle regulators) (Williams & Fuchs, 2013; Venkei & Yamashita, 2018; Rizzelli et al, 2020), the causal relationship between spindle orientation and fate remains a largely open question (Finegan & Bergstralh, 2019; Kotak, 2019; van Leen et al, 2020) (Fig 2C). Mechanisms of mammalian cell polarization have been intensively studied in cell cultures, e.g. those of monolayered epithelia and primary cultures of neurons. These systems unravelled roles of polarity proteins in neuronal specification (Nishimura et al, 2004; Hapak et al, 2018) and epithelial adhesion and barrier function (Iden & Collard, 2008; Roignot et al, 2013). Notably, however, polarity genes have widely diversified from invertebrates to mammals (Assémat et al, 2008) (Box 1), posing questions of functional redundancy of related genes. Though up‐and‐coming, there is still a significant gap of knowledge regarding in vivo functions of polarity regulators in mammals. Interestingly, as outlined below, recent insights on this came from interdisciplinary studies on cell polarity and mechanobiology, recognizing the joint contribution of these entities in steering cell fate.
Figure 2. Coordination of cell polarity and cell fate determination: examples.
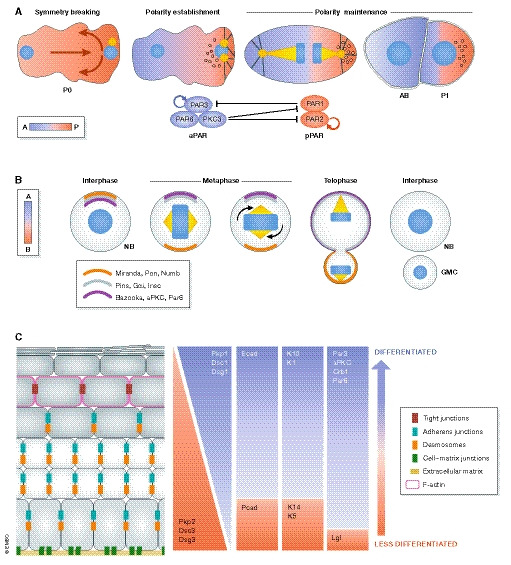
(A) The sperm entry site determines the anterior–posterior axis and gives rise to the first polarized cell of the Caenorhabditis elegans embryo (P0) (Nance & Zallen, 2011). Initially, PAR3, PAR6 and PKC3 (the aPKC homologue in C. elegans) are localized across the plasma membrane; however, a symmetry breaking event induces their anterior localization, hence referred to as the anterior polarity proteins (aPAR). PAR1 and PAR2 are evenly distributed within the cytoplasm and upon symmetry breaking are concentrated at the posterior pole, thus collectively referred to as the posterior polarity proteins (pPAR). The phosphorylation of pPAR proteins by PKC3 results in the establishment of the zygote a‐p polarity. aPARs are under constant inhibition by pPARs, i.e. PAR1 phosphorylates PAR3 and removes it from the cortex. The basis for this sorting process is the existence of actomyosin flow directed to the anterior part of the embryo, passively transporting the aPar proteins. After sorting, the actomyosin flow finally diminishes (in a Par‐dependent manner), which culminates in two domains with distinct Par polarity (polarity establishment) (Goehring et al, 2011; Gross et al, 2019). P0 cells eventually undergo an asymmetric cell division that produces a blastomere (AB) and a germline cell (P1). The two daughter cells will not only inherit different polarity proteins but also fate determinant molecules and structures like P‐granules, RNA multi‐phase condensates that become restricted to the posterior side and are segregated to the P1 germline cell (Goldstein & Macara, 2007; Hoege & Hyman, 2013; Rose & Gonczy, 2014; Illukkumbura et al, 2020). (B) The neuroblast (NB), the Drosophila melanogaster neural stem cell, sustains its self‐renewal capacity as a result of an asymmetric cell division (ACD). The two daughter cells display different fates and morphologies: a large pluripotent cell and a smaller one, committed to differentiation, called the ganglion mother cell (GMC). NBs are apico‐basally polarized and exhibit asymmetry of polarity proteins. Baz (Par3), aPKC and Par6 are enriched apically and restrict the localization of basal fate determinants. This is achieved by aPKC‐mediated phosphorylation of Numb and its scaffold protein Miranda, which displaces both proteins from the apical site to the basal cortex. Subsequently, the polarity and the spindle orientation machineries jointly mediate the segregation of apical and basal fate determinants. Inscuteable (Insc) binds Baz and Pins (LGN in mammals), which in turn connect Gαi to microtubules together with Mud (NuMA in mammals), a dynein‐binding protein. Together, they promote spindle reorientation along the apical–basal axis and segregation of fate determinants (Prehoda, 2009). (C) The murine epidermis displays apico‐basal polarization with differential expression of polarity proteins across the epidermal layers (Simpson et al, 2011; Niessen et al, 2012; Niessen et al, 2013; Ali et al, 2016). Likewise, cell adhesion and cytoskeletal components distribute asymmetrically within the epidermis. The basal layer expresses both P‐ and E‐cadherin, while in the suprabasal layers, E‐cadherin dominates. Similarly, desmosomal components distribute across the apico‐basal axis: the basal layer expresses desmocollin 2/3, desmoglein 3 and plakophilin 2, whereas desmocollin 1, desmoglein 1 and plakophilin 1 are largely confined to the suprabasal layers. Tight junctions are restricted to the last viable layer, the stratum granulosum (SG2) and participate in the epidermal barrier. Likewise, the epidermal cytoskeleton exhibits apico‐basal polarization. Intermediate filaments vary in their set‐up across the epidermis, with specific expression of keratin K5/K14 heterodimers in the basal layer and K1/K10 dimers in suprabasal layers. Actin is enriched in the SG2 layer, associated with the formation of a junctional actomyosin ring (Simpson et al, 2011; Baroni et al, 2012). Intracellular organelles such as the centrosome, Golgi apparatus and cilia are also apico‐basally polarized in the skin epidermis (Muroyama & Lechler, 2012). The skin also manifests planar cell polarization of hair follicles across the anterior–posterior and proximal–distal axis (Devenport, 2014).
Box 1: Mammalian polarity proteins and complexes.
The apical Crumbs complex
Mammals express three homologues of the apical transmembrane protein Crumbs (Crb), (Crb1–3), composed of a short cytoplasmic tail and a large extracellular region (not present in Crb3, resulting in a substantially smaller size than Crb1 and Crb2). The cytoplasmic tail contains a FERM‐binding motif and a PDZ‐binding motif. The extracellular region contains multiple (up to 29) EGF‐like and 3 Laminin A G‐domain‐like repeats.
PATJ is a scaffold protein, composed of a L27C domain, which mediates the interaction with Pals1, and multiple PDZ domain‐containing proteins via its PDZ domain PATJ can interact with Par6. There is an additional mammalian homologue, MUPP1, which contains a L27 at the N‐terminus and several PDZ domains.
Pals1 (Stardust homologue) is an adaptor protein of 77 kDa, member of the membrane‐associated guanylate kinase (MAGUK) family. Pals1 contains two L27C/N‐binding domains that mediate binding to Lin7 and Patj, respectively. Additionally, Pals1 has a PDZ domain by which interacts with Crb1 and Crb3, a SH3 domain, and a GUK domain. In mammals, Pals1 is also known as MPP5.
The apico‐junctional Par complex
Par3 (Bazooka homologue) is a scaffold protein with several splice variants (180, 150 and 100 kDa). Mammals exhibit two Par3 genes: Pard3a and Pard3b. Par3 has three conserved regions, CR1, CR2 and CR3, respectively. The CR1 region (or N‐terminal domain, NTD) is responsible for self‐association via a PB1‐like domain. The CR2 region contains three consecutive PDZ domains, which mediate binding to Par6, JAM‐A and nectin 1. This region is also involved in phosphoinositide (PIP2/PIP3) association. The Par3A CR3 region comprises the aPKC‐binding site, whereas Par3B has been reported not to bind to aPKC isoforms. The C‐terminus of Par3 can bind to Kif3a. Par3A is target of different kinases (ERK2, LIMK; Par1, PP1) and phosphatases, with aPKC‐mediated phosphorylation at S827 regulating the stability of the aPKC‐Par3 interaction.
aPKC is a serine/threonine kinase and member of the larger PKC kinase family. Mammals express two related isoforms: aPKCiota (in humans; lambda in mice) and aPKCzeta, as well as an N‐terminally truncated form, PKMzeta. aPKCs contain a PB1 domain (mediating interaction with the PB1 domain of Par6), a C1 domain (comprising a pseudosubstrate sequence involved in self‐inhibition) and the C‐terminal kinase domain. aPKC differs from classical and novel PKC kinases by being calcium insensitive since it is lacking the C2 domain.
Par6 is an adaptor protein. Three Par6 genes have been identified in mammals: Pard6a, Pard6b and Pard6g. Pard6 is composed of a PB1 domain, interacting with aPKC, a semi‐Crib‐motif by which interacts with Cdc42/ Rac1 and a single PDZ domain that mediates binding to Par3A and Crumbs3.
The basolateral Scribble complex
Scrib (Scribble homologue) is a cytoplasmic LAP family protein of 175 kDa. It contains sixteen LRR domains, which are necessary for its basolateral targeting, two LAP domains by which Scrib interacts with Lgl, a linker region, and four PDZ domains The PDZ3 and 4 are involved in ZO2 binding.
Dlg (discs large homologue) is a scaffold protein of the membrane‐associated guanylate kinase (MAGUK) family. There are two splice variants (alpha and beta) that differ in their N‐terminus: the first contains a palmitoylation site, whereas the latter includes an L27 domain responsible for self‐association and binding to MPP proteins. In mammals, there are five disc large genes (Dlg1–5) but Dlg1 is most closely related to the D. melanogaster Dlg. Dlg1 is a protein of approximately 120 kDa and contains three PDZ domains by which it binds to GluR1, APC and PTEN. This is followed by an SH3 domain, which enables Dlg1 to interact with the Lin2 serine/threonine kinase (CASK). The Dlg1 C‐terminus harbours a GUK domain.
Lgl (lethal giant larvae homologue) is an adaptor protein. Two Lgl genes have been identified in mammals: Llgl1 and Llgl2. Lgl is composed of several WD40 domains. There are other two very related proteins to Lgl in mammals: the syntaxin‐binding protein 5 (Lgl3) and syntaxin‐binding protein‐like (Lgl4).
Interplay of polarity and mechanics in different tissues
Cell and tissue behaviour were long perceived as the result of genetic and biochemical interactions. Meanwhile, also material properties of tissues are considered to be important for tissue function and behaviour (Xi et al, 2019). Cells can sense mechanical forces (mechanosensation), react to and transmit them (mechanotransduction), and the physical and biochemical pathways co‐exist and are interdependent (Hannezo & Heisenberg, 2019; Xi et al, 2019). Contributing to this recent paradigm change was the realization that cell adhesion and the cytoskeleton are instrumental for sensing and transducing forces, and that morphogenetic processes obey certain mechanical principles known from material science and Newtonian physics (Chen et al, 2018). The actomyosin cytoskeleton is fundamental for this, as it is the main source of contractile stresses that are applied via either cell–cell or cell–matrix adhesions (Ladoux et al, 2015; Ladoux & Mège, 2017; Kechagia et al, 2019). While the presence of polarity proteins at these sites of adhesion precedes the existence of an anisotropic contractile network, the coordination of polarity with adhesion molecules ultimately defines the polarity domains, junction maturation and cellular fate.
Polarity, mitotic spindle orientation and mechanical cues in non‐mammalian systems
The cell division plane is typically established by spindle positioning right before chromosome segregation. Several cues can contribute to spindle orientation that are either of cell‐intrinsic origin, such as centrosome and spindle anchoring position via the action of molecular motors, or of extrinsic origin, like exposure to a differential micro‐environment, mechanical stimuli inducing elongation (e.g. stretch), or adhesion asymmetry (Finegan & Bergstralh, 2019). So far it has been difficult to dissect the various contributions that guide spindle orientation, especially at a tissue‐scale level. Moreover, the exact consequences of spindle orientation for cell fate, morphogenesis and homeostasis appear to be highly context‐dependent (Wodarz, 2005; da Silva & Vincent, 2007; Cabernard & Doe, 2009; Lu & Johnston, 2013; Finegan et al, 2019; Loyer & Januschke, 2020). The fly embryonic neuroepithelium represents a long‐standing model in which division orientation is seamlessly coupled to fate determination (Fig 2B). Apical polarity cues such as Bazooka (Baz, in mammals termed Par3) or aPKC are essential for the establishment of apico‐basal asymmetry in neuroblasts (NBs) and for spindle positioning. Baz/aPKC, via their interactions with the spindle regulators Pins and Mud, sequester astral microtubules, thereby retaining the centrosome apically and orienting the spindle (St Johnston & Ahringer, 2010; Inaba & Yamashita, 2012; Venkei & Yamashita, 2018). Additionally, division orientation can be modulated by changes in external factors and tissue mechanics (Fig 3A–C). External cues provided by the last‐born NB, for example, help orient the spindle of subsequent divisions and stabilize NB‐cortex glia interactions, thereby promoting stress resistance of the stem cell pool (Loyer & Januschke, 2018). Micro‐environmental guidance of spindle orientation is also evident when developing tissues undergo large deformations: D. melanogaster embryo segmentation is characterized by extension of the germ band, a process that requires coordination of cell intercalation, junction or cortical tension remodelling (mediated by Baz/Par3 and Myosin II) and oriented cell divisions. During germ band segmentation, supracellular mechanical boundaries are created, with a defined actomyosin‐based boundary cable that “traps” the spindle pole and hence orients cell divisions perpendicular to tension‐bearing mechanical boundaries (Scarpa et al, 2018) (Fig 4A).
Figure 3. Analogies in symmetry breaking and self‐organization between early embryogenesis and epithelial tissues.
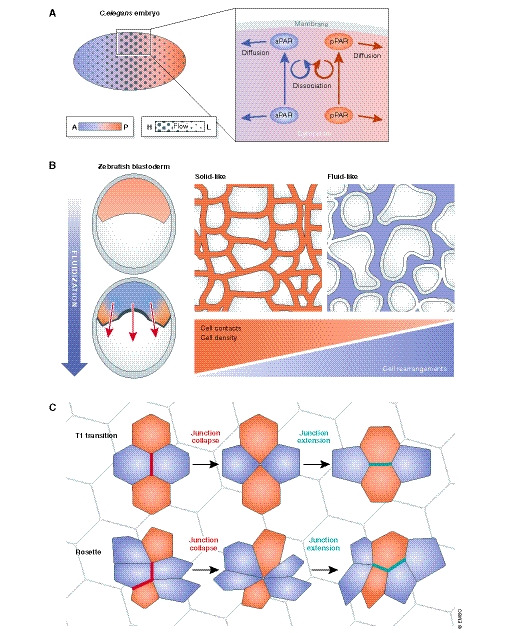
(A) In the Caenorhabditis elegans one cell embryo, establishment of anterior and posterior polarity domains is achieved by antagonistic interactions between the polarity components of both domains and contributions by anterior‐directed cortical actomyosin flow. The shear stress generated by the actomyosin flow acts on the cytoplasm and leads to the passive transport of cytoplasmic components (Illukkumbura et al, 2020). PAR network interactions maintain a polarized state, following the segregation of the aPAR to the anterior site, while the pPAR remains posterior. Polarization of the embryo is therefore the result of continuous diffusion of PAR molecules between areas where they can associate with each other and areas promoting their dissociation. This phenomenon can be described by a reaction–diffusion model, in which the PAR components transit between the cytoplasm and a membrane‐associated state where they laterally diffuse until being detached by an antagonist (Goehring et al, 2011; Rodriguez et al, 2017; Gross et al, 2019). This type of intracellular self‐organization forms the basis of various polarization events in small systems (Ganguly et al, 2012; Niwayama et al, 2016; Stückemann et al, 2017). In epithelial tissues, self‐organization manifests at a supracellular level and can drive cell sorting, tissue organization and pattern formation (Kondo & Miura, 2010; Shyer et al, 2015, 2017). (B) Tissue fluidity as an organizational principle in morphogenesis. Tissue morphogenesis results from the combination of forces driving tissue movements (e.g. pressure, collective cell movements) and the material properties of the tissue (viscoelasticity). Symmetry breaking events will initiate body axis formation (e.g. anterior–posterior, dorsal–ventral) and progressive polarization. Tissues with different viscoelastic properties will respond differentially to external forces. Despite apparent high viscosity of tissues, if a rheological threshold is reached (“yield stress”), they will undergo a “solid” to “fluid” transition and will behave like fluids. Tissue fluidity depends on cell rearrangements such as cell intercalations and turn‐over of junctional complexes. Anterior–posterior gradients of the yield stress have been implicated in body axis elongation (Mongera et al, 2018) and in gastrulation (Petridou et al, 2019) of the zebrafish. Fluidization in tissues due to mitosis or apoptotic events is thought to affect tissue viscoelasticity (Ranft et al, 2010) and tissue compression (Krajnc et al, 2018). In mammalian epithelia, state transitions have been implicated in the pathophysiology of asthma (Park et al, 2015) and fate decisions (Miroshnikova et al, 2018). (C) Intercalation is the process in which cells actively exchange their neighbours. There are two main types of planar intercalations that occur during morphogenesis: T1 transitions and rosettes. Both types require that actomyosin exerts force (i.e. cortical tension) on adherens junctions, thereby defining the junctional length (i.e. elongation or shrinking of the cell contact). Cells constantly fluctuate their cell–cell adhesions as a result of actomyosin dynamics, in order to delay or speed up cell rearrangements and consequently the neighbour exchange rate (David et al, 2014). Neighbour exchange is an essential behaviour of tissues and contributes to many processes such as tissue bending (Mason et al, 2013; Shook et al, 2018), elongation (Lienkamp et al, 2012) and wound healing, but can also modulate tissue fluidity (Rauzi et al, 2015; Curran et al, 2017; Tetley et al, 2019). There are several constraints that dictate an optimal cell arrangement. Cells must maintain their function (e.g. barrier performance), sustain their growth‐to‐differentiation balance (i.e. self‐renewal) and be able to dynamically interact with their neighbours (Tetley & Mao, 2018).
Figure 4. Intrinsic and extrinsic cues in spindle orientation and fate decisions.
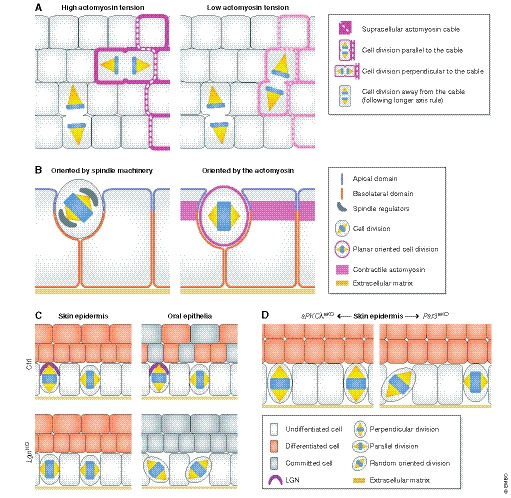
(A) In the Drosophila melanogaster germ band, spindle orientation of boundary cells is guided by the topology and anisotropy of the tensile actomyosin network, whereas inner cells not facing the boundary divide along their longer axis (Scarpa et al, 2018). (B) In the D. melanogaster follicular epithelium, spindle orientation is not coupled to tissue elongation, challenging the tissue context dependency of these mechanisms. Inhibition of mitosis in follicular cells does not affect tissue extension but impairs the optimal packing of the cells. Interestingly, in this context Pins/Mud are necessary for the apico‐basal positioning of the spindle (left) but not for its planar positioning (right) (Finegan et al, 2019). (C) In mammals, deletion of the spindle regulator LGN differentially affects mitotic spindle orientation, perhaps based on its apical vs. lateral localization in different epithelia (Byrd et al, 2016; Lough et al, 2019). In the mouse skin epidermis, LGN loss elicits mostly planar cell divisions, and in the oral tongue epithelium, it randomizes the spindle orientation. In these epithelia, LGN deficiency reduces differentiation or stratification, whereas LGN is not required for embryonic hair follicle development (Byrd et al, 2016). See text for details. (D) In the adult murine epidermis, aPKCλ deletion results in increased perpendicular divisions and epidermal differentiation (Niessen et al, 2013). Contrarily, epidermal Par3 deletion results in more planar and random divisions and increased differentiation (Ali et al, 2016).
Cells engage actomyosin and adherens junctions (AJs) when dividing planar to the tissue elongation axis (Fig 4B), whereby cortical tension couples the spindle orientation machinery to actual spindle positioning (Lam et al, 2020). When the actomyosin network is disturbed or when AJs are disrupted, cells fail to orient their spindle along the planar axis (Finegan et al, 2019). Stretching Xenopus laevis embryonic animal caps further showed that, similar to reports in D. melanogaster pupal notum (Bosveld et al, 2016), spindle positioning largely follows the positions of tricellular junctions (which are polarized according to the axis of the principal local stress) and depends on cadherins and polarization of the spindle machinery (i.e. LGN) to tricellular junctions. In this system, mechanical stress induced mitotic entry in a myosin‐dependent manner rather than directly affecting spindle orientation (Nestor‐Bergmann et al, 2019). Together, these reports emphasize that spindle orientation results from the interplay between cell shape, mechanics and spindle machinery but that the individual contributions of any of these processes vary from tissue to tissue.
Polarity, mitotic spindle orientation and mechanical cues in mammalian systems
Above studies focused mostly on lower organisms, but how about the control of spindle orientation in mammals? There, the level of complexity is raised by gene diversification (and hence often redundancy of mammalian isoforms) and differential requirements during morphogenesis and homeostasis. An intriguing example for this is the role of LGN in spindle orientation. LGN localization is cell‐type specific (Morin et al, 2007; Konno et al, 2008; Shitamukai et al, 2011; Matsuzaki & Shitamukai, 2015), and in the murine oral cavity, LGN shows distinct distributions depending on the type of oral epithelium. It localizes apically in the palate, buccogingival and ventral tongue epithelia, promoting perpendicular divisions and stratification, but in the dorsal tongue, LGN resides basally or laterally, promoting tissue expansion via planar cell divisions (Byrd et al, 2016) (Fig 4C). This suggests an exciting possibility that fate can be steered towards self‐renewal or differentiation depending on the polarization of LGN. Moreover, LGN inactivation impaired the formation of filiform papillae, a ventral tongue appendage. In contrast, disrupting LGN in the embryonic epidermis did not affect the formation of hair follicles (Byrd et al, 2016), main epidermal appendages analogous to the papillae. Thus, spindle regulators have distinct functions in different tissues, supporting the idea that some of these genetically conserved players perform different tasks depending on the respective epithelial framework. Further, in the skin epidermis LGN participates in a tension‐dependent correction mechanism of spindle orientation. During telophase, LGN collaborates with AJ tension sensitive proteins, such as α‐catenin and afadin, to perpendicularly orient spindles (Lough et al, 2019). This mechanism suggests an extra layer of control over spindle orientation, where the spindle machinery itself cooperates with mechanoresponsive cortical elements to position the spindle.
Gene inactivation studies in mammalian epidermis further revealed intriguing and in part non‐overlapping functions of apical polarity proteins in this barrier‐forming epithelium: whereas epidermal aPKCλ knock‐out mice showed more perpendicular spindle orientation (Niessen et al, 2013), epidermal Par3 deletion resulted in random and planar spindle orientation in different epidermal compartments (Ali et al, 2016), albeit both mutations led to increased differentiation (Niessen et al, 2013; Ali et al, 2016) (Fig 4D). This presents an example in mammalian epithelia where spindle orientation is seemingly not coupled to fate determination. Interestingly, NuMA‐mutant mice showed increased planar cell divisions and an inability to differentiate (Seldin et al, 2016), thus implicating NuMA‐mediated spindle orientation in fate determination during epidermal homeostasis. These findings therefore emphasize that cell shape, cell polarity signals and tissue mechanics can steer cell fate, whereby the relation of spindle orientation with fate is highly context‐dependent.
Coupling of polarity, cytoskeleton and tension anisotropy during early lineage commitment in mammalian embryos
The previous section highlighted that tension anisotropy can serve as an important polarizing cue and is able to control spindle orientation. Does this concept also apply to directional movement of cells? Can differences in tension define the asymmetric positioning of cells? Indeed, for example in the early mouse blastocyst, the inner cell mass (ICM) is established by translocation of non‐polarized cells to the centre of the embryo, clearly separating from the trophectoderm, which is composed of polarized cells. Several polarity proteins are apically enriched in trophectoderm cells, and loss‐of‐function experiments suggested a role for Par3 (Plusa et al, 2005), Par6B (Alarcon, 2010; Hirate et al, 2013; Lim et al, 2020) and aPKC (Plusa et al, 2005; Hirate et al, 2013; Maître et al, 2016) isoforms in lineage allocation. For long, spindle orientation was considered the major mechanism of asymmetric cell division during ICM allocation. However, recent findings challenged this view. Samarage et al (2015) reported that apical constriction driven by heterogeneity in cortical tension – rather than spindle orientation – was responsible for ICM rearrangement. The first embryo cell divisions are randomly oriented, whereas between the 8‐ and 16‐cell stage, following their own division or that of their neighbours, some embryonic cells constrict their apical site and translocate to the embryo's centre. The apical constriction process relies on polarized myosin II enrichment around constricting cells. Remarkably, cell–cell adhesion seems dispensable for the constriction process, but cortical tension in the neighbouring cells does affect the inner cell movement (Samarage et al, 2015). Recently, the asymmetric inheritance of keratins (K) was reported as a very early mechanism directing the lineage segregation in mouse embryos. The stochastic expression of K8 and K18 filaments in a subset of cells at the 8‐cell embryo stage establishes an intercellular polarity, with one daughter cell inheriting an apical pool of poorly diffusing keratin polymers, while the other cell will remain largely keratin‐free. The apical, outside K8/K18‐positive cells will give rise to the trophectoderm, while the K8/K18‐negative cells will contribute to the ICM. Disrupting keratin networks by K8/K18 knock‐down reduced apical PARD6B and PKCζ and prevented trophectoderm specification. Vice versa, PARD6B depletion impaired keratin polarization at the apical site and instead promoted the symmetrical inheritance of keratins. This indicates that the apical localization of keratins and classical polarity proteins creates a polymer‐based polarity memory as an early event during lineage specification (Lim et al, 2020). Interestingly, also alterations in embryo size by means of blastocoel volume oscillations and concomitant changes in trophectoderm cortical tension contribute to asymmetric fate determination in mouse blastocysts (Chan et al, 2019). Together, these data emphasize the role of polarity, cytoskeleton and tension anisotropy for early lineage commitment in mammalian embryos.
Integrating cell and tissue polarity, mechanics and fate decisions: Insights from mammalian skin
Mammalian epidermal polarity and mechanics during morphogenesis
The mammalian skin epidermis is a prime example of an apico‐basally polarized, multi‐layered tissue (see Fig 2C). Polarity proteins are expressed throughout the epidermis, albeit with layer‐specific localization patterns (Niessen et al, 2012). For example, aPKC and Par3 in the basal layer can be detected both in the cytoplasm and diffusely at peripheral membranes, whereas within the stratum granulosum II (SG2) they show distinct localization to tight junctions (TJs). The differential localization suggests distinct functions and signalling events in basal vs. suprabasal epidermal layers (Niessen et al, 2012). A central question in the field of skin biology is how cell division of stem/progenitor cells in the basal layer is coupled to differentiated fate of suprabasal epidermal cells to ensure self‐renewal and skin barrier function. Spindle orientation has been implicated in this process, coordinating epidermal expansion and stratification during morphogenesis (Poulson & Lechler, 2010; Williams et al, 2011). Moreover, as the case in invertebrate systems, epidermal cells appear to engage different polarity proteins to orient mitotic spindles (Williams et al, 2014). Yet, despite causing alterations in spindle orientation during epidermal morphogenesis, ablation of Par3 or aPKC isoforms did not prevent early epidermal stratification (Niessen et al, 2013; Williams et al, 2014; Ali et al, 2016), opening the possibility that alternative mechanisms to spindle orientation play a role in epidermal morphogenesis and stratification. Interestingly, evidence emerges that polarization of the actomyosin tension and of cell–cell adhesion contributes to coupling of self‐renewal and differentiation of progenitors. Miroshnikova et al (2018) proposed a model based on the concept that proliferation in the basal layer results in epithelial jamming (for state transitions, see Fig 3B). The compressive forces generated polarization of cell shapes and stresses, reducing locally the cortical tension and increasing cell–cell adhesion via E‐cadherin. Interestingly, the delamination rate was higher in close proximity to cell divisions, thus sustaining the average cell density (Miroshnikova et al, 2018) (Fig 5A). Another recent study utilized 3D organotypic keratinocyte cultures to show that the actin nucleator Dia1 promotes epithelial jamming in the basal layer. Dia1 is predominantly expressed in basal keratinocytes where it is thought to enhance lateral cell–cell adhesions and hence columnar cell shape (preprint: Harmon et al, 2021). Distinct expression of actin modulators thereby may help instruct keratinocyte shape anisotropy across stratified epidermal tissues.
Figure 5. Compartmentalized epidermal cell–cell adhesion molecules and tissue mechanics in skin epidermal morphogenesis and fate.
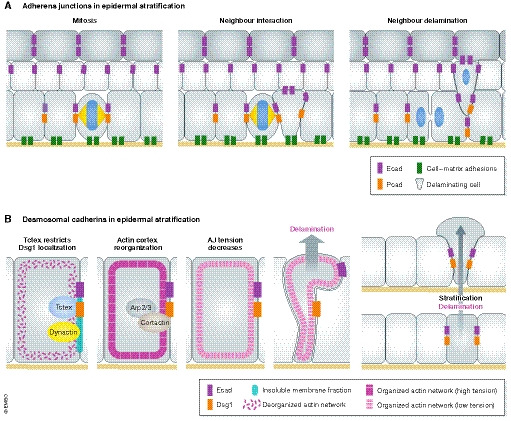
(A) Cellular interactions in developing epidermis: a mitotic division compresses its direct neighbours. The compressed cell changes its cadherin‐based adhesion profile, weakens its substrate adhesions and delaminates (Miroshnikova et al, 2018). (B) Desmoglein 1 promotes actin cortex reorganization via Arp2/3 and cortactin, which decreases cortical tension. A softer actin cortex promotes keratinocyte delamination (Nekrasova et al, 2018). See text for details.
The coordination of cell–cell junctions with cell mechanics is particularly evident during apico‐basal polarization of intercellular junctions. E‐cadherin, albeit ubiquitously expressed, has been shown to couple localized actomyosin contractility at AJs with spatiotemporal control of EGF receptor activity to restrict TJs to the upper SG2 layer (Rübsam et al, 2017). These data thus implicate this classical cadherin in apico‐basal polarization of junctional tension and barrier function. Interestingly, also other cell–cell adhesion molecules seem to contribute to tension anisotropy across epidermal layers. For example, Dsg1 is a desmosomal cadherin that shows a polarized distribution across the tissue axis, with increasing levels from basal to suprabasal layers. When Dsg1 expression is initiated in basal epidermal layers, this triggers actin reorganization, leading to reduced cortical tension at AJs. Following the drop of tension at AJs, basal cells delaminate to the suprabasal layer. This switch might be mediated by a molecular crosstalk between desmosomal and molecular motors (Dsg1, Tctex1/dynein cargo adaptor and actin/actin‐modulating proteins) (Nekrasova et al, 2018) (Fig 5B). Remarkably, ectopic expression of Dsg1 in simple epithelial cells is sufficient to form a multi‐layered epithelium (Nekrasova et al, 2018), implicating Dsg1 in key events mediating the stratification of epithelia.
Patterned cell rearrangements are also important during the morphogenesis of epidermal appendages such as hair follicles. The planar polarization of hair follicles relies on supracellular movements and coordination of the epidermal epithelium with the dermal condensate (DC). Placode polarization is associated with radial movements and planar cell polarity (PCP) control of Rho and myosin II activity. Sonic hedgehog (Shh) signalling instructs progenitor fates together with the PCP machinery, inducing cell rearrangements and neighbour exchanges (mainly by junctional remodelling and cell intercalation) in an actomyosin‐dependent manner. These supracellular movements also reposition progenitors along the A–P axis and displace the DC, leading to asymmetric DC signalling and cell fate asymmetry (Cetera et al, 2018). Moreover, hair follicle fate specification was recently shown to depend on mechanical tension in suprabasal epidermal layers, with high contractility of differentiated keratinocytes counteracting the commitment of basal layer cells towards hair follicle fate (Ning et al, 2021), further highlighting links between tissue polarity, mechanics and fate.
Mammalian epidermal polarity and mechanics in tissue homeostasis
Similar to this emerging picture in the developing epidermis, it becomes more and more evident that polarity, adhesion and mechanical forces also shape epidermal homeostasis in the adult organism, though not necessarily through control of spindle orientation. Mesa et al (2018) imaged and tracked basal stem/progenitor cells in the adult ear epidermis in vivo and found that neighbouring cells were more likely to acquire opposite fates. When one cell differentiated, a cell in its vicinity was subsequently more likely to divide than a non‐direct neighbour, thereby coordinating basal cell delamination and differentiation with the expansion of neighbouring cells (Mesa et al, 2018). The differentiation event is thought to provide space in the basal epidermal layer, allowing neighbouring stem/progenitor cells to progress through the cell cycle and thus coupling differential cell fate independent of spindle orientation (Fig 6A) (Mesa et al, 2018). Of note, this contrasts the findings of Miroshnikova et al (2018) in embryonic epidermis, where mitosis precedes differentiation (Miroshnikova et al, 2018), suggesting context dependency (e.g. cell density and proliferation rate). Next to such neighbour interactions, apico‐basal polarity proteins were recently implicated in control of epidermal differentiation in adult epidermis, albeit through mechanical control of mitotic accuracy rather than spindle orientation. Epidermal deletion of Par3 elicits DNA damage accumulation and p53 expression in the hair follicle stem cell compartment and throughout the epidermis (Dias Gomes et al, 2019). Intriguingly, the DNA damage in Par3‐deficient epidermal keratinocytes was a consequence of altered mechanical properties including reduced actomyosin activity, causing insufficient mitotic rounding, low mitotic fidelity and increased genome instability. Reconstituting RhoA activity or directly enhancing myosin activity was sufficient to rescue mitotic fidelity and to prevent DNA damage‐dependent p53 induction (Fig 6B). Additionally, in an in vitro stratification model the altered viscoelastic properties biased Par3 KO cells towards suprabasal layers, which could be reverted by enhancing actomyosin contractility (Dias Gomes et al, 2019). Together, these different studies showed how coordinated cellular behaviours, polarity proteins and cell mechanics contribute to the homeostasis of stratified epithelia.
Figure 6. Epidermal fate decisions and tissue mechanics in adult epidermis.
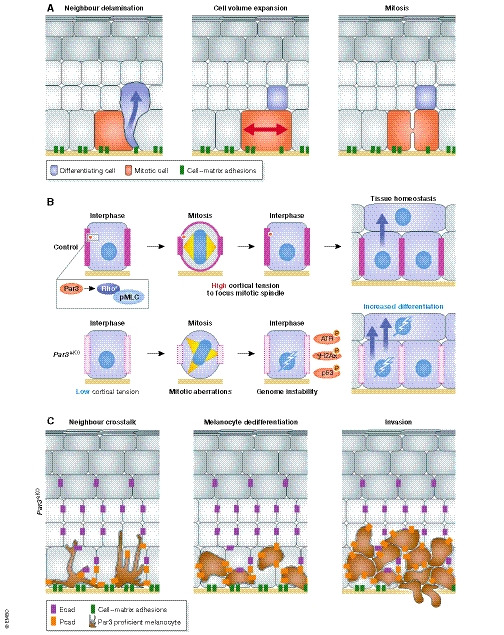
(A) In adult ear epidermis, differentiation of a basal layer keratinocyte changes the aspect ratio of the neighbour cell, now compelled to fill the gap. Cell elongation triggers mitotic division, which equilibrates cell numbers (Mesa et al, 2018). (B) Epidermal Par3 acts upstream of Rho/actomyosin contractility to promote intrinsic force generation and mitotic rounding, thereby maintaining mitotic accuracy and cellular fitness at the genomic level. Loss of Par3 alters keratinocyte mechanics resulting in mitotic infidelity and accumulation of DNA damage and p53, which in turn fuels differentiation (Dias Gomes et al, 2019). (C) The loss of Par3 in keratinocytes leads to upregulation of surface P‐cadherin in keratinocytes. Altered cadherin engagement in keratinocyte–melanocyte interactions promotes melanocyte proliferation and phenotypic switch, providing a niche for melanocyte transformation (Mescher et al, 2017). See text for details.
Next to intraepithelial mechanisms, the skin epithelium can also impinge on the fate of neighbouring tissue‐resident cell types such as epidermal melanocytes. Par3 inactivation in surrounding keratinocytes, in addition to the mechanical changes mentioned above, induced melanocyte dedifferentiation, altered motility and increased melanoma formation and invasion. The loss of Par3 in keratinocytes led to upregulation of the basal layer cadherin P‐cadherin, which was required and sufficient to mediate melanocyte proliferation and phenotype switch (Mescher et al, 2017) (Fig 6C). This study thus demonstrates that polarity networks in the epithelial micro‐environment can modulate the fate of adjacent cell types through direct cell–cell adhesion. Furthermore, these findings raise the question if polarity protein‐mediated cellular mechanics contribute to tissue‐scale instruction of fate determination in the epidermis and perhaps other epithelia.
Tissue‐level decisions in regeneration, degeneration and cancer
Polarization and mechanics in wound‐induced regeneration
Epithelial tissues occasionally face acute damage, for instance during wounding. In such situation, the epithelial barrier is compromised, putting the organism at risk due to an increased exposure to environmental factors. Therefore, the ability to repair the tissue and regain homeostasis is of fundamental importance for survival; however, the regenerative capacity varies immensely among species. Some organisms can regenerate seamlessly even large parts of their body (e.g. hydra and planarians), while others have limited regenerative potential that further declines with age (e.g. humans). Cell mechanics and the actomyosin cytoskeleton are fundamental to wound closure and tissue repair in most epithelia (Guzmán‐Herrera & Mao, 2020), yet the exact mechanisms governing directed wound closure in vivo are only beginning to unfold. Here, we focus on the tissue‐scale aspects of wound healing from a polarity perspective.
In the D. melanogaster imaginal disc, reduction of junctional tension (e.g. by lowering myosin activity) increases cell intercalation events and tissue fluidization, which in turn accelerates wound closure (Tetley et al, 2019). Interestingly, wounds display a supracellular actomyosin network characterized by anisotropic myosin distribution, heterogeneous actin density and actin cables of varying thickness among different cell–cell contacts. This supracellular actin and myosin heterogeneity partially stems from the wound topology itself and leads to stochastic actomyosin contractions. Myosin activity further relies on the polarization of tension‐mediated strain‐activated ion channels (SAICs). Thus, wounding induces strain, which activates SAICs that in turn cause stochastic actomyosin contraction, a process that may foster stress dissipation (Zulueta‐Coarasa & Fernandez‐Gonzalez, 2018). Moreover, injured cells produce mitochondrial reactive oxidative species (ROS), which elicit polarization signals leading to actin and myosin anisotropy. Src kinase acts as a ROS sensor and mediates the trafficking and removal of E‐cadherin by endocytosis, thereby “weakening” cellular junctions, which is required for localized actomyosin remodelling (Hunter et al, 2018). Together, these results depict a mechanism by which damaged cells induce a tissue‐scale polarity response in order to heal epithelial wounds.
Importantly, there is also increasing evidence for a role of polarity signalling in mammalian wound healing. Following injury, the shape and polarity of cells at or close to the wound changes significantly (Paladini et al, 1996; Aragona et al, 2017). A range of in vitro studies indeed suggested a role of mammalian polarity proteins for efficient epithelial migration and wound closure (Iden et al, 2012; Trepat & Sahai, 2018). Such functions could be confirmed in part in murine models of tissue repair: the embryonic epidermis of Scribble mutant mice, for instance, shows defective wound healing, likely due to an inability to recruit Cdc42 and Rac1 to the cell edge (Dow et al, 2007). Moreover, aPKCλ, but not the aPKCζ isoform, has recently been implicated in polarizing the wound edge of keratinocytes in vitro and in efficient closure of large skin wounds in vivo, although by so far unknown mechanisms (Noguchi et al, 2019). Meanwhile, single‐cell transcriptomics revealed complex heterogeneity, high plasticity and fate changes during epidermal wound healing (Joost et al, 2018; Haensel et al, 2020; Phan et al, 2020). Longitudinal imaging of skin reepithelization after wounding further revealed a dynamic tissue‐scale programme that steers efficient wound healing: keratinocytes at the wound edge migrate directionally towards the wound centre, a process facilitated by Rac1. In the adjacent mixed zone, cells both migrate and proliferate, orienting their division axis towards the wound during collective movement. Such coordination of proliferation, division orientation and polarized migration promotes the local expansion of the epithelium, culminating in its repair (Park et al, 2017).
Altered cell polarity in tissue dysfunction and cancer
Next to tissue regeneration, changes in cell and tissue polarity also occur during other processes of disrupted homeostasis, such as tissue degeneration and tumorigenesis (Mescher & Iden, 2015). With increasing organismal age, alterations of biochemical and mechanical properties are associated with stem cell depletion (Matsumura et al, 2016; Liu et al, 2019), niche dysfunction (Segel et al, 2019) and dysplasia (Wolfenson et al, 2019).
Polarity in loss of tissue fitness and ageing
How do tissues maintain homeostasis following stochastic mutations or damage? Quality control mechanisms exist that evaluate and feedback cell fitness, resulting in maintenance or removal of damaged cells from a tissue. Next to immune surveillance mechanisms, a well‐studied process governing quality control is cell competition. The basic principle behind it is that cells in a high fitness state cause the elimination of unfit cells. The cell polarity field contributed to the formation of cell competition concepts, with many studies reporting how aberrant polarity signalling triggered such events in lower organisms and cell culture (Merino et al, 2016; Vishwakarma & Piddini, 2020). Mutations for Scribble and Dlg in fly epithelial cells resulted in their elimination via JNK and TNF signalling (Igaki et al, 2009; Yamamoto et al, 2017; Caria et al, 2018). Related mechanisms may also play a role in mammals. In MDCK cells, depletion of Scribble sensitized cells to crowding. Scribble knock‐down cells, when crowded by their wild‐type neighbours, were eliminated by cell death. This hypersensitivity to cell–cell contact was mediated by a ROCK‐p38‐p53 axis upstream of cell death, directly connecting alterations in mechanical properties, sensibility to crowding and upregulation of low‐fitness markers (Wagstaff et al, 2016). This, together with the finding that loss of Par3 in epidermal cells leads to DNA damage‐driven p53 upregulation and increased differentiation (Dias Gomes et al, 2019), emphasizes intriguing links between different polarity networks and p53‐mediated cell fitness, with the outcome of p53 action (i.e. apoptosis vs differentiation) likely depending on the specific epithelial traits.
Moreover, cell fitness has been linked to cell division orientation. Hematopoietic stem cells (HSCs), for instance, divide asymmetrically to generate progenitors that undergo either myeloid or lymphoid fate to give rise to all cells of the hematopoietic system, ranging from megakaryocytes to B lymphocytes. Although HSCs do not display a clear morphological polarity, they show asymmetry at the level of the microtubule cytoskeleton and activity of small Rho GTPases, such as Cdc42. Interestingly, cell division orientation of HSCs depends on Cdc42 activity, and aged HSCs exhibit reduced polarization, associated with lower Cdc42 activity and increased symmetric division. Comparing the epigenetic landscape of young vs old HSCs and their transcriptome revealed distinct profiles. Daughter cells of young HSCs, which maintained Cdc42 activity and frequently divided asymmetrically, showed differential allocation of epigenetic histone marks for open chromatin, correlating with stem cell potential. Progeny of old, symmetrically dividing HSCs instead presented with lower self‐renewal capability and reduced potential to generate differentiated cells of the lymphoid lineage, implicating links between cell polarity and control of epigenetic features (Fig 7A) (Florian et al, 2018). Similar connections between loss of regenerative potential and altered cell division polarity have been reported in the retina (Quinn et al, 2018; Kraut & Knust, 2019; Kujawski et al, 2019) and muscle tissue (Dumont et al, 2015).
Figure 7. Polarity, tissue degeneration, EMT and cancer.
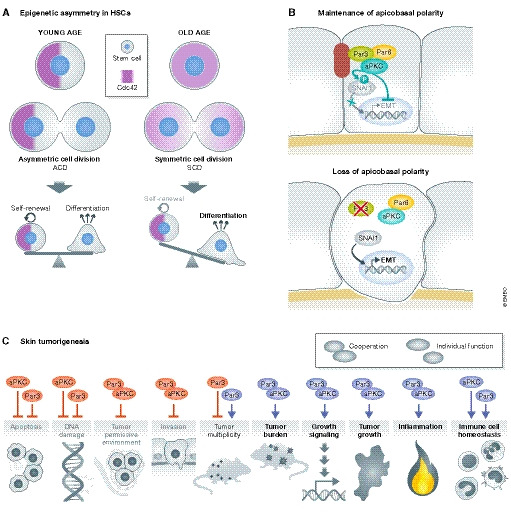
(A) Asymmetric cell division of hematopoietic stem cells (HSCs) includes the polarized segregation of Cdc42, which is important for the maintenance of stemness and is associated with polarized signatures of epigenetic marks. An age‐related shift towards symmetric cell division (SCD) in HSCs diminishes self‐renewal capacity (Florian et al, 2018). (B) Core Par complex polarity proteins ensure cyto‐architecture and prevent epithelial‐to‐mesenchymal transition (EMT). The kinase aPKC phosphorylates the transcription factor SNAI1, promoting its degradation and preventing EMT (Jung et al, 2019). (C) Cooperative and independent in vivo functions of Par3 and aPKCλ in skin tumorigenesis based on oncogenic Ras‐driven mouse tumour models (Iden et al, 2012; Vorhagen et al, 2018).
EMT, early oncogenic lesions and hijacking of cell architecture
Epithelial–mesenchymal transition (EMT) is an important process in development but is also associated with cancer cell invasion. Through complex cellular reprogramming, epithelial cells acquire key mesenchymal, migratory characteristics as a result of activation of distinct transcription factors (such as Snail, Twist and Zeb), ECM remodelling and loss of apico‐basal polarity, which culminates in alterations of cell–cell adhesion and of the cytoskeleton (Moreno‐Bueno et al, 2008; Godde et al, 2010; Lamouille et al, 2014). aPKCλ and Par6 have been reported to participate in EMT in small cell lung cancer (Regala et al, 2005; Gunaratne et al, 2013), whereas Par3 loss promotes tumour cell invasion in the mammary gland (McCaffrey et al, 2012; Xue et al, 2012) and skin cancers (Iden et al, 2012) in vivo. The role of Par3 complex proteins in EMT was recently further supported in a 3D model of primary mammary epithelial cells. When cultivated at 3D conditions that allowed full apico‐basal polarization, Par3 and aPKC localized to TJs. When Snai1 was induced at these conditions, aPKC phosphorylated Snai1, targeting it for degradation. Instead, when apico‐basal polarity was not pronounced, as the case in 2D cultures of mammary cells, or upon loss of Par3, aPKC could not phosphorylate Snai1, leading to stabilization and nuclear translocation of Snai1, followed by expression of EMT‐related genes (Jung et al, 2019) (Fig 7B). These data implicate polarity signalling in the maintenance of cyto‐architecture and in preventing EMT.
Intriguingly, cancer cells can also control cyto‐architecture and cell mechanics by hijacking actomyosin signalling for optimal cell division. Activating RasV12 in mammary cells is sufficient to enhance mitotic rounding, which increases the ability to divide under confinement, thereby perhaps providing an advantage to mutant cells when dividing in stiff environments (Matthews et al, 2020). Likewise, induction of EMT was recently shown to alter cell mechanics in a cell cycle‐dependent manner, with a mitosis‐specific enhancement of cortical mechanics and rounding (Hosseini et al, 2020). Oncogene‐mediated shaping of the cytoskeleton may also instruct the overall tumour tissue architecture beyond the cellular scale. Messal et al, (2019) found that the growth direction of neoplastic lesions in tubular single‐layered epithelia is in part determined by the lumen's physical properties (Messal et al, 2019). Lesions in small ducts mostly grew outwards, while those in large ducts predominantly grew inwards, with the growth direction correlating with epithelial curvature and mechanical changes in transformed cells: ductal epithelial cells usually showed apically polarized F‐actin and pMLC2 (phospho‐myosin light chain II), whereas upon KRas‐induced transformation, this asymmetry was lost and pMLC2 was redistributed, resulting in altered mechanical properties (Messal et al, 2019). Another recent study in murine multi‐layered epidermis explored the significance of tumour architectures typically observed in non‐invasive basal cell carcinoma (BCC) and more invasive squamous cell carcinoma (SCC). Fiore et al, (2020) found that oncogenic mutations in Shh signalling (common in BCCs) differentially affected the mechanical properties of early tumours and their micro‐environments when compared to mutations in the Ras signalling pathway (common in SCCs). Ectopic activation of Shh signalling resulted in softer basement membranes and promoted BCC‐like bud‐type overgrowths. HRas mutations instead were associated with higher basement membrane stiffness and predominantly led to SCC‐like tissue folding and invasiveness. Notably, these two types of lesions differed in their levels of actomyosin tension in suprabasal layers, at interfaces to healthy cells (being high at bud but lower at fold borders), and in their ability to remodel the basement membrane (Fiore et al, 2020). Together, these studies reinforce the importance of controlling cytoskeletal polarization and distinct architectures at both cellular and tissue levels and underline that mechanical properties of tissues can fundamentally affect dysplastic outcome.
Polarity signalling, cancer models and tailored therapeutics
The majority of cancers arise from epithelial cells, and the various stages of tumorigenesis are often accompanied by dramatic changes in cell and tissue architecture, posing the question whether polarity networks also play functional roles in malignancies. Loss‐of‐function studies in D. melanogaster provided clear evidence for a tumour‐suppressive role of polarity proteins (Bilder, 2004; Elsum et al, 2012; Khursheed & Bashyam, 2014; Mescher & Iden, 2015). Meanwhile, various studies in mouse models and organoids also unravelled a tight connection between (altered) polarity signalling and oncogenic signalling in mammals (Mescher & Iden, 2015). Against initial predictions from fly models, however, mammalian polarity proteins, such as aPKC, Par3 and Lgl2, were shown to act as both pro‐oncogenes and tumour suppressor genes (Iden et al, 2012; Garg et al, 2014; Marques et al, 2016; Vorhagen et al, 2018; Saito et al, 2019). In a Ras‐driven two‐stage skin cancer model, Par3 and aPKC collaborate in promoting skin tumorigenesis through ERK, Akt and Stat signalling, while also possessing independent functions: Par3 is a tumour suppressor in certain skin tumours (e.g. keratoacanthoma) (Iden et al, 2012; Vorhagen et al, 2018), whereas loss of aPKC causes a stronger induction of apoptosis in keratinocytes than Par3 inactivation (Vorhagen et al, 2018) (Fig 7C). Similarly as in flies, Scribble acts as a tumour suppressor in this skin cancer model (Pearson et al, 2015), although its subcellular localization seems important in counteracting some but not all properties of cancer cells (Stephens et al, 2018), highlighting the complexity of polarity protein functions in mammalian cancer. Comprehensive reviews on polarity proteins and cancer can be found here (Saito et al, 2018; Stephens et al, 2018; Reina‐Campos et al, 2019; Fomicheva et al, 2020). Notably, also genetic disruption of spindle orientation (via expression of a NuMA mutant) was recently shown to cooperate with oncogenic KRas in causing strong skin tissue overgrowth (Morrow et al, 2019), further implicating regulated cell division orientation in cancer.
Clearly, polarity networks can be altered or hijacked in different types of human cancers. Studies on cell polarity signalling in the context of cancer were fuelled by the development of several animal models for human cancers. The Lkb1 KO mouse represents a model for Peutz–Jeghers syndrome (Miyoshi et al, 2002), Par3 epidermal knock‐out combined with carcinogen‐induced HRas mutations is a model for keratoacanthoma (Iden et al, 2012; Vorhagen et al, 2018), and D. melanogaster Hippo or Scribble mutants can be used to mimic tissue overgrowth for drug screening (Humbert et al, 2008; Elsum et al, 2012; Snigdha et al, 2019). There is growing evidence supporting the potential use of inhibitors against polarity kinases such as aPKC (Butler et al, 2015), LKB1 (Par4) (Momcilovic & Shackelford, 2015; Ciccarese et al, 2019) or MARK1 (Par1c) (Levy et al, 2013; Voura et al, 2019). For example, in BCC, the most common skin cancer, inhibition of aPKCλ is arising as coadjuvant for conventional therapy (Mirza et al, 2017). BCCs in advanced stage often utilize the Hedgehog pathway (Hh) to maintain their growth and survival, and inhibition of Smoothened (a transmembrane Hh signalling component) presents a clinically validated strategy (Oro, 1997; Mirza et al, 2017). Downstream of Smoothened, the Gli transcription factors of the Hh pathway are deacetylated by HDAC1/2, which regulates their chromatin association and consequently Gli‐dependent transcription (Canettieri et al, 2010; Coni et al, 2013). Targeting HDAC1/2 has been useful in some cancers but its dose‐related cytotoxic effects impede its use for BCC. aPKCι phosphorylates Gli1 to promote the Gli1‐HDAC1/2 association (Mirza et al, 2019), and a small molecule aPKC inhibitor PSI, when combined with lowered doses of HDAC inhibitors, prevents BCC proliferation in vitro and in patient‐derived explants (Mirza et al, 2017). Interestingly, Gli1 either associates with the nuclear lamina or chromatin in an aPKC‐dependent manner, thereby changing “nuclear polarization” and modulating signal amplification in BCCs (Mirza et al, 2019). Thus, inhibiting aPKC might represent a strategy to treat Smoothened inhibitor‐resistant BCCs. In summary, targeting polarity signalling – and in particular polarity kinases – has promising anti‐cancer potential.
Conclusions & perspectives
Polarity‐regulated cell fate decisions underlie tissue formation and maintenance. Although the core polarity machineries are well conserved from invertebrates to mammals, there are clear differences how these molecules exert their functions with respect to cell fate. Findings from diverse model systems remarkably illustrate that, next to the apico‐basal axis, polarity also manifests at many other levels, such as adhesion asymmetries or the anisotropy of forces. Moreover, it becomes increasingly clear that spindle orientation is not the sole mechanism how mother cells segregate fate determinants. Regarding self‐renewal, it is exciting that distinct intrinsic and extrinsic parameters are essential to maintain tissue stem cell pools and their niches. Additionally, polarity proteins regulate stem/progenitor cell behaviour and modulate how they interact with their neighbours, keeping tissues in check. Ultimately, mammalian tissues show a considerable tolerance to loss of cyto‐architecture, steering self‐renewal and differentiation according to their needs to maintain overall homeostasis. During ageing or disease, these properties decline, rendering tissues more susceptible to dysplasia.
In the future, to better understand mechanisms that safeguard tissue integrity it will be crucial to further map the details and dynamics of tissue polarity networks. Spurred by techniques such as single‐cell RNA sequencing, it will be possible to trace cell lineages and to deconstruct and reconstruct polarity networks during differentiation. Additionally, despite great advances, our current molecular view of the core polarity complexes is still incomplete and rather static. Some polarity complexes – either because they are transient or underrepresented – might be very difficult to detect experimentally. Novel approaches including single‐cell biochemistry, endogenous gene tagging and super‐resolution imaging might unveil new dynamics of the different polarity complexes and amend our current views on how polarity proteins cooperate with cytoskeletal, mechanochemical and signalling entities in development, health and disease.
Author contributions
MDG drafted the text and figures with inputs from SI. Both authors revised and finalized the manuscript for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We thank all members of the Iden laboratory for stimulating discussions and Sina Knapp for critical reading of the review draft. This work was supported by Saarland University, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grants SPP1782‐ID79/2‐1 and SPP1782‐ID79/2‐2 and Projektnummer 73111208 – SFB 829, A10), Excellence Initiative of the German federal and state governments (CECAD Cologne) and Center for Molecular Medicine Cologne (CMMC). Open Access funding enabled and organized by ProjektDEAL.
The EMBO Journal (2021) 40: e106787.
References
- Alarcon VB (2010) Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo. Biol Reprod 83: 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali NJA, Dias Gomes M, Bauer R, Brodesser S, Niemann C, Iden S (2016) Essential role of polarity protein Par3 for epidermal homeostasis through regulation of barrier function, keratinocyte differentiation, and stem cell maintenance. J Invest Dermatol 136: 2406–2416 [DOI] [PubMed] [Google Scholar]
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascré G, Simons BD, Blanpain C (2017) Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun 8: 14684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assémat E, Bazellières E, Pallesi‐Pocachard E, Le Bivic A, Massey‐Harroche D (2008) Polarity complex proteins. Biochim Biophys Acta 1778: 614–630 [DOI] [PubMed] [Google Scholar]
- Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, Wolf R (2012) Structure and function of the epidermis related to barrier properties. Clin Dermatol 30: 257–262 [DOI] [PubMed] [Google Scholar]
- Bilder D (2004) Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev 18: 1909–1925 [DOI] [PubMed] [Google Scholar]
- Bosveld F, Markova O, Guirao B, Martin C, Wang Z, Pierre A, Balakireva M, Gaugue I, Ainslie A, Christophorou N et al (2016) Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis. Nature 530: 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AM, Scotti Buzhardt ML, Erdogan E, Li S, Inman KS, Fields AP, Murray NR (2015) A small molecule inhibitor of atypical protein kinase C signaling inhibits pancreatic cancer cell transformed growth and invasion. Oncotarget 6: 15297–15310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd KM, Lough KJ, Patel JH, Descovich CP, Curtis TA, Williams SE (2016) LGN plays distinct roles in oral epithelial stratification, filiform papilla morphogenesis and hair follicle development. Development 143: 2803–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabernard C, Doe CQ (2009) Apical/basal spindle orientation is required for neuroblast homeostasis and neuronal differentiation in Drosophila . Dev Cell 17: 134–141 [DOI] [PubMed] [Google Scholar]
- Campanale JP, Sun TY, Montell DJ (2017) Development and dynamics of cell polarity at a glance. J Cell Sci 130: 1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, Pietrosanti L, De Smaele E, Ferretti E, Miele E et al (2010) Histone deacetylase and Cullin3–RENKCTD11 ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol 12: 132–142 [DOI] [PubMed] [Google Scholar]
- Caria S, Magtoto CM, Samiei T, Portela M, Lim KYB, How JY, Stewart BZ, Humbert PO, Richardson HE, Kvansakul M (2018) Drosophila melanogaster guk‐holder interacts with the scribbled PDZ1 domain and regulates epithelial development with scribbled and discs large. J Biol Chem 293: 4519–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Leybova L, Joyce B, Devenport D (2018) Counter‐rotational cell flows drive morphological and cell fate asymmetries in mammalian hair follicles. Nat Cell Biol 20: 541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CJ, Costanzo M, Ruiz‐Herrero T, Mönke G, Petrie RJ, Bergert M, Diz‐Muñoz A, Mahadevan L, Hiiragi T (2019) Hydraulic control of mammalian embryo size and cell fate. Nature 571: 112–116 [DOI] [PubMed] [Google Scholar]
- Chen T, Saw TB, Mège R‐M, Ladoux B (2018) Mechanical forces in cell monolayers. J Cell Sci 131: jcs218156 [DOI] [PubMed] [Google Scholar]
- Ciccarese F, Zulato E, Indraccolo S (2019) LKB1/AMPK pathway and drug response in cancer: a therapeutic perspective. Oxid Med Cell Longev 2019: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coni S, Antonucci L, D'Amico D, Di Magno L, Infante P, De Smaele E, Giannini G, Di Marcotullio L, Screpanti I, Gulino A et al (2013) Gli2 acetylation at lysine 757 regulates hedgehog‐dependent transcriptional output by preventing its promoter occupancy. PLoS One 8: e65718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S, Strandkvist C, Bathmann J, de Gennes M, Kabla A, Salbreux G, Baum B (2017) Myosin II controls junction fluctuations to guide epithelial tissue ordering. Dev Cell 43: 480–492.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Luu O, Damm EW, Wen JWH, Nagel M, Winklbauer R (2014) Tissue cohesion and the mechanics of cell rearrangement. Development 141: 3672–3682 [DOI] [PubMed] [Google Scholar]
- Devenport D (2014) The cell biology of planar cell polarity. J Cell Biol 207: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias Gomes M, Letzian S, Saynisch M, Iden S (2019) Polarity signaling ensures epidermal homeostasis by coupling cellular mechanics and genomic integrity. Nat Commun 10: 3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Peterson AS, Jane SM, Russell SM, Humbert PO (2007) The tumour‐suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26: 2272–2282 [DOI] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, Von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA (2015) Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 21: 1455–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsum I, Yates L, Humbert PO, Richardson HE (2012) The Scribble‐Dlg‐Lgl polarity module in development and cancer: from flies to man. Essays Biochem 53: 141–168 [DOI] [PubMed] [Google Scholar]
- Finegan TM, Bergstralh DT (2019) Division orientation: disentangling shape and mechanical forces. Cell Cycle 18: 1187–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegan TM, Na D, Cammarota C, Skeeters AV, Nádasi TJ, Dawney NS, Fletcher AG, Oakes PW, Bergstralh DT (2019) Tissue tension and not interphase cell shape determines cell division orientation in the Drosophila follicular epithelium. EMBO J 38: e100072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore VF, Krajnc M, Quiroz FG, Levorse J, Pasolli HA, Shvartsman SY, Fuchs E (2020) Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature 585: 433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian MC, Klose M, Sacma M, Jablanovic J, Knudson L, Nattamai KJ, Marka G, Vollmer A, Soller K, Sakk V et al (2018) Aging alters the epigenetic asymmetry of HSC division. PLOS Biol 16: e2003389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomicheva M, Tross EM, Macara IG (2020) Polarity proteins in oncogenesis. Curr Opin Cell Biol 62: 26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Williams LS, Palacios IM, Goldstein RE (2012) Cytoplasmic streaming in Drosophila oocytes varies with kinesin activity and correlates with the microtubule cytoskeleton architecture. Proc Natl Acad Sci USA 109: 15109–15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG (2014) Protein kinase C and cancer: what we know and what we do not. Oncogene 33: 5225–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde NJ, Galea RC, Elsum IA, Humbert PO (2010) Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions. J Mammary Gland Biol Neoplasia 15: 149–168 [DOI] [PubMed] [Google Scholar]
- Goehring NW, Hoege C, Grill SW, Hyman AA (2011) PAR proteins diffuse freely across the anterior‐posterior boundary in polarized C. elegans embryos. J Cell Biol 193: 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Macara IG (2007) The PAR proteins: fundamental players in animal cell polarization. Dev Cell 13: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P, Kumar KV, Goehring NW, Bois JS, Hoege C, Jülicher F, Grill SW (2019) Guiding self‐organized pattern formation in cell polarity establishment. Nat Phys 15: 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratne A, Thai BL, Di Guglielmo GM (2013) Atypical protein kinase C phosphorylates Par6 and facilitates transforming growth factor ‐induced epithelial‐to‐mesenchymal transition. Mol Cell Biol 33: 874–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán‐Herrera A, Mao Y (2020) Polarity during tissue repair, a multiscale problem. Curr Opin Cell Biol 62: 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel D, Jin S, Sun P, Cinco R, Dragan M, Nguyen Q, Cang Z, Gong Y, Vu R, MacLean AL et al (2020) Defining epidermal basal cell states during skin homeostasis and wound healing using single‐cell transcriptomics. Cell Rep 30: 3932–3947.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannezo E, Heisenberg CP (2019) Mechanochemical feedback loops in development and disease. Cell 178: 12–25 [DOI] [PubMed] [Google Scholar]
- Hapak SM, Rothlin CV, Ghosh S (2018) PAR3–PAR6–atypical PKC polarity complex proteins in neuronal polarization. Cell Mol Life Sci 75: 2735–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon RM, Devany J, Gardel ML (2021) Dia1 coordinates differentiation and cell sorting in a stratified epithelium. bioRxiv 10.1101/2021.01.04.425231 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirate Y, Hirahara S, Inoue K‐I, Suzuki A, Alarcon V, Akimoto K, Hirai T, Hara T, Adachi M, Chida K et al (2013) Polarity‐dependent distribution of angiomotin localizes hippo signaling in preimplantation embryos. Curr Biol 23: 1181–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C, Hyman AA (2013) Principles of PAR polarity in Caenorhabditis elegans embryos. Nat Rev Mol Cell Biol 14: 315–322 [DOI] [PubMed] [Google Scholar]
- Homem CCF, Knoblich JA (2012) Drosophila neuroblasts: a model for stem cell biology. Development 139: 4297–4310 [DOI] [PubMed] [Google Scholar]
- Hosseini K, Taubenberger A, Werner C, Fischer‐Friedrich E (2020) EMT‐induced cell‐mechanical changes enhance mitotic rounding strength. Adv Sci 7: 2001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE (2008) Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27: 6888–6907 [DOI] [PubMed] [Google Scholar]
- Hunter MV, Willoughby PM, Bruce AEE, Fernandez‐Gonzalez R (2018) Oxidative stress orchestrates cell polarity to promote embryonic wound healing. Dev Cell 47: 377–387.e4 [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG (2008) Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859 [DOI] [PubMed] [Google Scholar]
- Iden S, van Riel WE, Schäfer R, Song J‐Y, Hirose T, Ohno S, Collard JG (2012) Tumor type‐dependent function of the Par3 polarity protein in skin tumorigenesis. Cancer Cell 22: 389–403 [DOI] [PubMed] [Google Scholar]
- Igaki T, Pastor‐Pareja JC, Aonuma H, Miura M, Xu T (2009) Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila . Dev Cell 16: 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illukkumbura R, Bland T, Goehring NW (2020) Patterning and polarization of cells by intracellular flows. Curr Opin Cell Biol 62: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M, Yamashita YM (2012) Asymmetric stem cell division: precision for robustness. Cell Stem Cell 11: 461–469 [DOI] [PubMed] [Google Scholar]
- Joost S, Jacob T, Sun X, Annusver K, La Manno G, Sur I, Kasper M (2018) Single‐cell transcriptomics of traced epidermal and hair follicle stem cells reveals rapid adaptations during wound healing. Cell Rep 25: 585–597.e7 [DOI] [PubMed] [Google Scholar]
- Jung H‐Y, Fattet L, Tsai JH, Kajimoto T, Chang Q, Newton AC, Yang J (2019) Apical–basal polarity inhibits epithelial–mesenchymal transition and tumour metastasis by PAR‐complex‐mediated SNAI1 degradation. Nat Cell Biol 21: 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechagia JZ, Ivaska J, Roca‐Cusachs P (2019) Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol 20: 457–473 [DOI] [PubMed] [Google Scholar]
- Kemphues K (2000) PARsing embryonic polarity. Cell 101: 345–348 [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS (1988) Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52: 311–320 [DOI] [PubMed] [Google Scholar]
- Khursheed M, Bashyam MD (2014) Apico‐basal polarity complex and cancer. J Biosci 39: 145–155 [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F (2008) Neuroepithelial progenitors undergo LGN‐dependent planar divisions to maintain self‐renewability during mammalian neurogenesis. Nat Cell Biol 10: 93–101 [DOI] [PubMed] [Google Scholar]
- Kondo S, Miura T (2010) Reaction‐diffusion model as a framework for understanding biological pattern formation. Science 329: 1616–1620 [DOI] [PubMed] [Google Scholar]
- Kotak S (2019) Mechanisms of spindle positioning: lessons from worms and mammalian cells. Biomolecules 9: 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnc M, Dasgupta S, Ziherl P, Prost J (2018) Fluidization of epithelial sheets by active cell rearrangements. Phys Rev E 98: 022409 [DOI] [PubMed] [Google Scholar]
- Kraut RS, Knust E (2019) Changes in endolysosomal organization define a pre‐degenerative state in the crumbs mutant Drosophila retina. PLoS One 14: e0220220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski S, Sonawane M, Knust E (2019) penner/lgl2 is required for the integrity of the photoreceptor layer in the zebrafish retina. Biol Open 8: bio041830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoux B, Nelson WJ, Yan J, Mège RM (2015) The mechanotransduction machinery at work at adherens junctions. Integr Biol 7: 1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoux B, Mège RM (2017) Mechanobiology of collective cell behaviours. Nat Rev Mol Cell Biol 18: 743–757 [DOI] [PubMed] [Google Scholar]
- Lam MSY, Lisica A, Ramkumar N, Hunter G, Mao Y, Charras G, Baum B (2020) Isotropic myosin‐generated tissue tension is required for the dynamic orientation of the mitotic spindle. Mol Biol Cell 31: 1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leen EV, di Pietro F, Bellaïche Y (2020) Oriented cell divisions in epithelia: from force generation to force anisotropy by tension, shape and vertices. Curr Opin Cell Biol 62: 9–16 [DOI] [PubMed] [Google Scholar]
- Levy A, Newman M, Brownlees J, Harding D, Osborne J, Lewis S, Gillen K, Hall G, Mpamhanga C, Merritt A et al (2013) Identification of potent inhibitors of microtubule affinity regulating kinase for inhibition of tau hyperphosphorylation. Mol Neurodegener 8: P28 [Google Scholar]
- Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G (2012) Vertebrate kidney tubules elongate using a planar cell polarity‐dependent, rosette‐based mechanism of convergent extension. Nat Genet 44: 1382–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HYG, Alvarez YD, Gasnier M, Wang Y, Tetlak P, Bissiere S, Wang H, Biro M, Plachta N (2020) Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature 585: 404–409 [DOI] [PubMed] [Google Scholar]
- Liu N, Matsumura H, Kato T, Ichinose S, Takada A, Namiki T, Asakawa K, Morinaga H, Mohri Y, De Arcangelis A et al (2019) Stem cell competition orchestrates skin homeostasis and ageing. Nature 568: 344–350 [DOI] [PubMed] [Google Scholar]
- Lough KJ, Byrd KM, Descovich CP, Spitzer DC, Bergman AJ, Beaudoin GM, Reichardt LF, Williams SE (2019) Telophase correction refines division orientation in stratified epithelia. Elife 8: e49249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyer N, Januschke J (2018) The last‐born daughter cell contributes to division orientation of Drosophila larval neuroblasts. Nat Commun 9: 3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyer N, Januschke J (2020) Where does asymmetry come from? Illustrating principles of polarity and asymmetry establishment in Drosophila neuroblasts. Curr Opin Cell Biol 62: 70–77 [DOI] [PubMed] [Google Scholar]
- Lu MS, Johnston CA (2013) Molecular pathways regulating mitotic spindle orientation in animal cells. Development 140: 1843–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maître JL, Turlier H, Illukkumbura R, Eismann B, Niwayama R, Nédélec F, Hiiragi T (2016) Asymmetric division of contractile domains couples cell positioning and fate specification. Nature 536: 344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques E, Englund Ji, Tervonen Ta, Virkunen E, Laakso M, Myllynen M, Mäkelä A, Ahvenainen M, Lepikhova T, Monni O et al (2016) Par6G suppresses cell proliferation and is targeted by loss‐of‐function mutations in multiple cancers. Oncogene 35: 1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason FM, Tworoger M, Martin AC (2013) Apical domain polarization localizes actin‐myosin activity to drive ratchet‐like apical constriction. Nat Cell Biol 15: 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M, Kurata S, Hoeijmakers J, Nishimura EK (2016) Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science (80‐) 351: aad4395 [DOI] [PubMed] [Google Scholar]
- Matsuzaki F, Shitamukai A (2015) Cell division modes and cleavage planes of neural progenitors during mammalian cortical development. Cold Spring Harb Perspect Biol 7: a015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HK, Ganguli S, Plak K, Taubenberger AV, Win Z, Williamson M, Piel M, Guck J, Baum B (2020) Oncogenic signaling alters cell shape and mechanics to facilitate cell division under confinement. Dev Cell 52: 563–573.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Montalbano JA, Mihai C, Macara IG (2012) Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22: 601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino MM, Levayer R, Moreno E (2016) Survival of the fittest: essential roles of cell competition in development, aging, and cancer. Trends Cell Biol 26: 776–788 [DOI] [PubMed] [Google Scholar]
- Mesa KR, Kawaguchi K, Cockburn K, Gonzalez D, Boucher J, Xin T, Klein AM, Greco V (2018) Homeostatic epidermal stem cell self‐renewal is driven by local differentiation. Cell Stem Cell 23: 677–686.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Iden S (2015) Par proteins in tumor formation and progression. In Cell Polarity 2, Ebnet K (ed.), pp 145–165. Cham: Springer International Publishing; [Google Scholar]
- Mescher M, Jeong P, Knapp SK, Rübsam M, Saynisch M, Kranen M, Landsberg J, Schlaak M, Mauch C, Tüting T et al (2017) The epidermal polarity protein Par3 is a non–cell autonomous suppressor of malignant melanoma. J Exp Med 214: 339–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messal HA, Alt S, Ferreira RMM, Gribben C, Wang VMY, Cotoi CG, Salbreux G, Behrens A (2019) Tissue curvature and apicobasal mechanical tension imbalance instruct cancer morphogenesis. Nature 566: 126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnikova YA, Le HQ, Schneider D, Thalheim T, Rübsam M, Bremicker N, Polleux J, Kamprad N, Tarantola M, Wang I et al (2018) Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat Cell Biol 20: 69–80 [DOI] [PubMed] [Google Scholar]
- Mirza AN, Fry MA, Urman NM, Atwood SX, Roffey J, Ott GR, Chen B, Lee A, Brown AS, Aasi SZ et al (2017) Combined inhibition of atypical PKC and histone deacetylase 1 is cooperative in basal cell carcinoma treatment. JCI Insight 2: e97071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza AN, McKellar SA, Urman NM, Brown AS, Hollmig T, Aasi SZ, Oro AE (2019) LAP2 proteins chaperone GLI1 movement between the lamina and chromatin to regulate transcription. Cell 176: 198–212.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Nakau M, Ishikawa T, Seldin MF, Oshima M, Taketo MM (2002) Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res 62: 2261–2266 [PubMed] [Google Scholar]
- Momcilovic M, Shackelford DB (2015) Targeting LKB1 in cancer‐exposing and exploiting vulnerabilities. Br J Cancer 113: 574–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, Serwane F, Lucio AA, Giammona J, Campàs O (2018) A fluid‐to‐solid jamming transition underlies vertebrate body axis elongation. Nature 561: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Bueno G, Portillo F, Cano A (2008) Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 27: 6958–6969 [DOI] [PubMed] [Google Scholar]
- Morin X, Jaouen F, Durbec P (2007) Control of planar divisions by the G‐protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci 10: 1440–1448 [DOI] [PubMed] [Google Scholar]
- Morrow A, Underwood J, Seldin L, Hinnant T, Lechler T (2019) Regulated spindle orientation buffers tissue growth in the epidermis. Elife 8: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F, Plachta N, Viasnoff V (2020) Novel approaches to link apicobasal polarity to cell fate specification. Curr Opin Cell Biol 62: 78–85 [DOI] [PubMed] [Google Scholar]
- Muroyama A, Lechler T (2012) Polarity and stratification of the epidermis. Semin Cell Dev Biol 23: 890–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Zallen JA (2011) Elaborating polarity: PAR proteins and the cytoskeleton. Development 138: 799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasova O, Harmon RM, Broussard JA, Koetsier JL, Godsel LM, Fitz GN, Gardel ML, Green KJ (2018) Desmosomal cadherin association with Tctex‐1 and cortactin‐Arp2/3 drives perijunctional actin polymerization to promote keratinocyte delamination. Nat Commun 9: 1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor‐Bergmann A, Stooke‐Vaughan GA, Goddard GK, Starborg T, Jensen OE, Woolner S (2019) Decoupling the roles of cell shape and mechanical stress in orienting and cueing epithelial mitosis. Cell Rep 26: 2088–2100.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen MT, Iden S, Niessen CM (2012) The in vivo function of mammalian cell and tissue polarity regulators ‐ how to shape and maintain the epidermal barrier. J Cell Sci 125: 3501–3510 [DOI] [PubMed] [Google Scholar]
- Niessen MT, Scott J, Zielinski JG, Vorhagen S, Sotiropoulou PA, Blanpain C, Leitges M, Niessen CM (2013) aPKCλ controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol 202: 887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning W, Muroyama A, Li H, Lechler T (2021) Differentiated daughter cells regulate stem cell proliferation and fate through intra‐tissue tension. Cell Stem Cell 28: 436–452.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K (2004) Role of the PAR‐3‐KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol 6: 328–334 [DOI] [PubMed] [Google Scholar]
- Niwayama R, Nagao H, Kitajima TS, Hufnagel L, Shinohara K, Higuchi T, Ishikawa T, Kimura A (2016) Bayesian inference of forces causing cytoplasmic streaming in Caenorhabditis elegans embryos and mouse oocytes. PLoS One 11: e0159917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi N, Hirose T, Suzuki T, Kagaya M, Chida K, Ohno S, Manabe M, Osada SI (2019) Atypical protein kinase C isoforms differentially regulate directional keratinocyte migration during wound healing. J Dermatol Sci 93: 101–108 [DOI] [PubMed] [Google Scholar]
- Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Scott MP (1997) Basal cell carcinomas in mice overexpressing sonic hedgehog. Science (80‐) 276: 817–821 [DOI] [PubMed] [Google Scholar]
- Paladini RD, Takahashi K, Bravo NS, Coulombe PA (1996) Onset of re‐epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol 132: 381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JA, Kim JH, Bi D, Mitchel JA, Qazvini NT, Tantisira K, Park CY, McGill M, Kim SH, Gweon B et al (2015) Unjamming and cell shape in the asthmatic airway epithelium. Nat Mater 14: 1040–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Gonzalez DG, Guirao B, Boucher JD, Cockburn K, Marsh ED, Mesa KR, Brown S, Rompolas P, Haberman AM et al (2017) Tissue‐scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat Cell Biol 19: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HB, McGlinn E, Phesse TJ, Schlüter H, Srikumar A, Gödde NJ, Woelwer CB, Ryan A, Phillips WA, Ernst M et al (2015) The polarity protein Scrib mediates epidermal development and exerts a tumor suppressive function during skin carcinogenesis. Mol Cancer 14: 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou NI, Grigolon S, Salbreux G, Hannezo E, Heisenberg C‐P (2019) Fluidization‐mediated tissue spreading by mitotic cell rounding and non‐canonical Wnt signalling. Nat Cell Biol 21: 169–178 [DOI] [PubMed] [Google Scholar]
- Phan QM, Fine GM, Salz L, Herrera GG, Wildman B, Driskell IM, Driskell RR (2020) Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. Elife 9: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusa B, Frankenberg S, Chalmers A, Hadjantonakis AK, Moore CA, Papalopulu N, Papaioannou VE, Glover DM, Zernicka‐Goetz M (2005) Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J Cell Sci 118: 505–515 [DOI] [PubMed] [Google Scholar]
- Poulson ND, Lechler T (2010) Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol 191: 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehoda KE (2009) Polarization of Drosophila neuroblasts during asymmetric division. Cold Spring Harb Perspect Biol 1: a001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PM, Alves CH, Klooster J, Wijnholds J (2018) CRB2 in immature photoreceptors determines the superior‐inferior symmetry of the developing retina to maintain retinal structure and function. Hum Mol Genet 27: 3137–3153 [DOI] [PubMed] [Google Scholar]
- Ranft J, Basan M, Elgeti J, Joanny JF, Prost J, Jülicher F (2010) Fluidization of tissues by cell division and apoptosis. Proc Natl Acad Sci USA 107: 20863–20868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzi M, Krzic U, Saunders TE, Krajnc M, Ziherl P, Hufnagel L, Leptin M (2015) Embryo‐scale tissue mechanics during Drosophila gastrulation movements. Nat Commun 6: 8677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, Fields AP (2005) Atypical protein kinase Cι is an oncogene in human non‐small cell lung cancer. Cancer Res 65: 8905–8911 [DOI] [PubMed] [Google Scholar]
- Reina‐Campos M, Diaz‐Meco MT, Moscat J (2019) The dual roles of the atypical protein kinase Cs in cancer. Cancer Cell 36: 218–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga A, Castiglioni VG, Boxem M (2020) New insights into apical‐basal polarization in epithelia. Curr Opin Cell Biol 62: 1–8 [DOI] [PubMed] [Google Scholar]
- Rizzelli F, Malabarba MG, Sigismund S, Mapelli M (2020) The crosstalk between microtubules, actin and membranes shapes cell division. Open Biol 10: 190314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Peglion F, Martin J, Hubatsch L, Reich J, Hirani N, Gubieda AG, Roffey J, Fernandes AR, St Johnston D et al (2017) aPKC Cycles between functionally distinct PAR protein assemblies to drive cell polarity. Dev Cell 42: 400–415.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roignot J, Peng X, Mostov K (2013) Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol 5: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L, Gonczy P (2014) Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook 1–43 [DOI] [PubMed] [Google Scholar]
- Rübsam M, Mertz AF, Kubo A, Marg S, Jüngst C, Goranci‐Buzhala G, Schauss AC, Horsley V, Dufresne ER, Moser M et al (2017) E‐cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat Commun 8: 1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Desai RR, Muthuswamy SK (2018) Reinterpreting polarity and cancer: the changing landscape from tumor suppression to tumor promotion. Biochim Biophys Acta ‐ Rev Cancer 1869: 103–116 [DOI] [PubMed] [Google Scholar]
- Saito Y, Li L, Coyaud E, Luna A, Sander C, Raught B, Asara JM, Brown M, Muthuswamy SK (2019) LLGL2 rescues nutrient stress by promoting leucine uptake in ER + breast cancer. Nature 569: 275–279 [DOI] [PubMed] [Google Scholar]
- Samarage CR, White MD, Álvarez YD, Fierro‐González JC, Henon Y, Jesudason EC, Bissiere S, Fouras A, Plachta N (2015) Cortical tension allocates the first inner cells of the mammalian embryo. Dev Cell 34: 435–447 [DOI] [PubMed] [Google Scholar]
- Scarpa E, Finet C, Blanchard GB, Sanson B (2018) Actomyosin‐driven tension at compartmental boundaries orients cell division independently of cell geometry in vivo . Dev Cell 47: 727–740.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA et al (2019) Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573: 130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin L, Muroyama A, Lechler T (2016) NuMa‐microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. Elife 5: e12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F (2011) Oblique radial glial divisions in the developing mouse neocortex induce self‐renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci 31: 3683–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook DR, Kasprowicz EM, Davidson LA, Keller R (2018) Large, long range tensile forces drive convergence during Xenopus blastopore closure and body axis elongation. Elife 7: e26944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyer AE, Huycke TR, Mahadevan L, Tabin CJ, Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ (2015) Bending gradients: how the intestinal stem cell article bending gradients : how the intestinal stem cell gets its home. Cell 161: 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyer AE, Rodrigues AR, Schroeder GG, Kassianidou E, Kumar S, Harland RM (2017) Emergent cellular self‐organization and mechanosensation initiate follicle pattern in the avian skin. Science 357: 811–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva SM, Vincent JP (2007) Oriented cell divisions in the extending germband of Drosophila . Development 134: 3049–3054 [DOI] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ (2011) Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol 12: 565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha K, Gangwani KS, Lapalikar GV, Singh A, Kango‐Singh M (2019) Hippo signaling in cancer: lessons from Drosophila Models. Front Cell Dev Biol 7: 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J (2010) Cell polarity in eggs and epithelia: parallels and diversity. Cell 141: 757–774 [DOI] [PubMed] [Google Scholar]
- Stephens R, Lim K, Portela M, Kvansakul M, Humbert PO, Richardson HE (2018) The scribble cell polarity module in the regulation of cell signaling in tissue development and tumorigenesis. J Mol Biol 430: 3585–3612 [DOI] [PubMed] [Google Scholar]
- Stückemann T, Cleland JP, Werner S, Thi‐Kim VuH, Bayersdorf R, Liu SY, Friedrich B, Jülicher F, Rink JC (2017) Antagonistic self‐organizing patterning systems control maintenance and regeneration of the anteroposterior axis in planarians. Dev Cell 40: 248–263.e4 [DOI] [PubMed] [Google Scholar]
- Tetley RJ, Mao Y (2018) The same but different: cell intercalation as a driver of tissue deformation and fluidity. Philos Trans R Soc B Biol Sci 373: 20170328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley RJ, Staddon MF, Heller D, Hoppe A, Banerjee S, Mao Y (2019) Tissue fluidity promotes epithelial wound healing. Nat Phys 15: 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X, Sahai E (2018) Mesoscale physical principles of collective cell organization. Nat Phys 14: 671–682 [Google Scholar]
- Venkei ZG, Yamashita YM (2018) Emerging mechanisms of asymmetric stem cell division. J Cell Biol 217: 3785–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma M, Piddini E (2020) Outcompeting cancer. Nat Rev Cancer 20: 187–198 [DOI] [PubMed] [Google Scholar]
- Vorhagen S, Kleefisch D, Persa O‐D, Graband A, Schwickert A, Saynisch M, Leitges M, Niessen CM, Iden S (2018) Shared and independent functions of aPKCλ and Par3 in skin tumorigenesis. Oncogene 37: 5136–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voura M, Khan P, Thysiadis S, Katsamakas S, Queen A, Hasan GM, Ali S, Sarli V, Hassan MI (2019) Probing the inhibition of microtubule affinity regulating kinase 4 by N‐substituted acridones. Sci Rep 9: 1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff L, Goschorska M, Kozyrska K, Duclos G, Kucinski I, Chessel A, Hampton‐O’Neil L, Bradshaw CR, Allen GE, Rawlins EL et al (2016) Mechanical cell competition kills cells via induction of lethal p53 levels. Nat Commun 7: 11373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, Fuchs E (2011) Asymmetric cell divisions promote Notch‐dependent epidermal differentiation. Nature 470: 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Fuchs E (2013) Oriented divisions, fate decisions. Curr Opin Cell Biol 25: 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Ratliff LA, Postiglione MP, Knoblich JA, Fuchs E (2014) Par3–mInsc and Gαi3 cooperate to promote oriented epidermal cell divisions through LGN. Nat Cell Biol 16: 758–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A (2005) Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr Opin Cell Biol 17: 475–481 [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Yang B, Sheetz MP (2019) Steps in mechanotransduction pathways that control cell morphology. Annu Rev Physiol 81: 585–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Saw TB, Delacour D, Lim CT, Ladoux B (2019) Material approaches to active tissue mechanics. Nat Rev Mater 4: 23–44 [Google Scholar]
- Xue B, Krishnamurthy K, Allred DC, Muthuswamy SK (2012) Loss of Par3 promotes breast cancer metastasis by compromising cell‐cell cohesion. Nat Cell Biol 15: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Ohsawa S, Kunimasa K, Igaki T (2017) The ligand Sas and its receptor PTP10D drive tumour‐suppressive cell competition. Nature 542: 246–250 [DOI] [PubMed] [Google Scholar]
- Zulueta‐Coarasa T, Fernandez‐Gonzalez R (2018) Dynamic force patterns promote collective cell movements during embryonic wound repair. Nat Phys 14: 750–758 [Google Scholar]


