Abstract
Most stroke survivors have very low levels of cardiovascular fitness, which limits mobility and leads to further physical deconditioning, increased sedentary behavior, and heightened risk of recurrent stroke. Although clinical guidelines recommend that aerobic exercise be a part of routine stroke rehabilitation, clinical uptake has been suboptimal. In 2013, an international group of stroke rehabilitation experts developed a user-friendly set of recommendations to guide screening and prescription—the Aerobic Exercise Recommendations to Optimize Best Practices in Care after Stroke (AEROBICS 2013). The objective of this project was to update AEROBICS 2013 using the highest quality of evidence currently available. The first step was to conduct a comprehensive review of literature from 2012 to 2018 related to aerobic exercise poststroke. A working group of the original consensus panel members drafted revisions based on synthesis. An iterative process was used to achieve agreement among all panel members. Final revisions included: (1) addition of 115 new references to replace or augment those in the original AEROBICS document, (2) rewording of the original recommendations and supporting material, and (3) addition of 2 new recommendations regarding prescription. The quality of evidence from which these recommendations were derived ranged from low to high. The AEROBICS 2019 Update should make it easier for clinicians to screen for, and prescribe, aerobic exercise in stroke rehabilitation. Clinical implementation will not only help to narrow the gap between evidence and practice but also reduce current variability and uncertainty regarding the role of aerobic exercise in recovery after stroke.
Stroke is the second most common cause of death and third leading cause of disability worldwide.1 Its long-term effects can be pernicious, resulting in a vicious cycle of declines in cardiovascular fitness, mobility, and functional autonomy, leading to a profoundly sedentary lifestyle,2 very low levels of cardiovascular fitness,3 and increased risk of cardiovascular disease and recurrent stroke.4 Aerobic exercise can help break this relentless cycle by improving aerobic capacity,5 walking ability,5 vascular health,6 and quality of life of stroke survivors.7
Current clinical guidelines recommend cardiovascular training be incorporated into routine stroke rehabilitation but lack specific screening and exercise prescription protocols.8 , 9 Often stroke rehabilitation does not challenge the cardiovascular system.2 , 10 , 11 Some clinicians report lack of confidence in decision-making regarding safe, effective applications of aerobic training for neurological patients.12–14 This gap between evidence and practice means that individuals poststroke may be deprived of an intervention with demonstrated potency to improve physical and mental functions.
In 2011, a group of Canadian and American stroke researchers convened to discuss strategies to remove barriers to clinical implementation of aerobic training after stroke or transient ischemic attack (TIA). One outcome was the compilation of 18 recommendations on pre-participation screening and prescription, written with the proviso that the recommendations be reviewed periodically. This paper provides an overview of the Aerobic Exercise Recommendations to Optimize Best Practices In Care after Stroke (AEROBICS) 2019 Update—a summary of recommendation development and presentation of the recommendations with brief rationale for each. Details are available at https://www.strokengine.ca/wp-content/uploads/2019/03/AEROBICS-2019-last-revised-March.pdf.
Development and Review of the Recommendations
Development of the original AEROBICS Recommendations was guided by the Appraisal of Guidelines Research and Evaluation Consortium.15 An iterative, pragmatic approach was followed—proposing clinically feasible responses to clinically meaningful questions, vetted by a consensus panel, international external reviewers, and stroke survivors (Figure). Rooted in a comprehensive literature synthesis, the recommendations were drafted by an interprofessional consensus panel comprised of Canadian and American experts from physical therapy, stroke neurology, cardiology, psychology, and health policy. Each recommendation was assigned a level of evidence (LOE) adapted from the classification by Guyatt16 (Table). Feedback from expert reviewers and stroke survivors was integrated into the recommendations. The final document consisted of 18 recommendations (7 about screening for safe participation and 11 about prescribing aerobic exercise) with supporting material for each recommendation (underlying rationale, system implications, performance measures, and summary of evidence). The principal target audiences were health professionals and health administrators involved in stroke rehabilitation.
Figure.
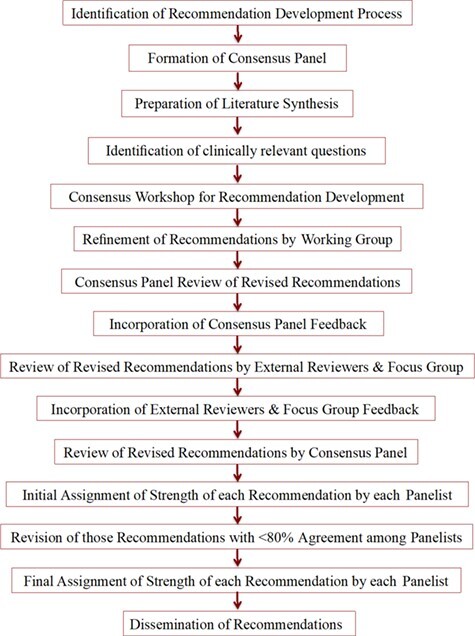
Steps in the development of Aerobic Exercise Recommendations to Optimize Best Practices in Care after Stroke (AEROBICS 2013).
Table.
| Strength of Evidence | Description |
|---|---|
| A | Evidence from RCTs or meta-analyses of RCTs |
| B | Single RCT or well-designed observational study with strong evidence; or well-designed cohort or case-control analytic study; or multiple time series or dramatic results of uncontrolled experiment |
| C | At least 1 well-designed, nonexperimental descriptive study (eg, comparative studies, correlation studies, case studies) or expert committee reports, opinions, and/or experience of respected authorities, including consensus from development and/or reviewer groups |
a RCT = randomized controlled trial.
The first step in updating the AEROBICS document was to conduct a knowledge synthesis of research from 2012 to 2018 related to aerobic exercise poststroke using several databases (PubMed, CINAHL, PEDro, Cochrane Central Register of Controlled Trials). A working group of original consensus panel members drafted revisions to the AEROBICS document based on synthesis. An iterative process was used to achieve agreement among the whole consensus panel. Final revisions included (1) addition of 113 new references to replace or augment those in the original document, (2) rewording of several of the original recommendations and supporting material, and (3) addition of 2 new recommendations regarding prescription (2.11 and 2.12). Each of the 20 recommendations is presented below together with LOE and brief rationale.
Preparticipation Screening for Aerobic Training After Stroke or TIA
Who Should Be Screened for Possible Participation in Aerobic Training After Stroke or TIA?
Recommendation
All patients following a cerebrovascular event (stroke or TIA) should be considered for possible participation in aerobic exercise interventions (LOE = A).
Rationale
Given the wide range of benefits of aerobic exercise for individuals poststroke, there is little justification for not considering implementing aerobic exercise in most cases. Neither age17 nor stroke severity5 should be regarded as barriers to exercise.
When Should an Individual Poststroke or TIA Be Screened for Possible Participation in Aerobic Training?
Recommendation
Screening for aerobic training should be initiated after a cerebrovascular event (stroke or TIA) when the patient is medically stable. To ensure continuity of appropriate interventions, screening should be repeated at transition points along the continuum of care based on changing neuromotor and cardiopulmonary capacities to participate in aerobic training (LOE = B).
Rationale
There are no compelling reasons to delay screening for participation in aerobic training once the individual is medically stable, typically within the early subacute phase. Patients early after stroke are at risk of the rapid decreases in aerobic capacity documented in nondisabled populations following prolonged inactivity.18 , 19
Who Should Determine if an Individual Poststroke or TIA Is Ready to Begin Aerobic Training?
Recommendation
Preparticipation evaluation for aerobic training after stroke or TIA should be provided by appropriately qualified health care professionals, consistent with their scope of practice and practice setting (LOE = B).
Rationale
Because stroke survivors present with a diversity of sensory-motor impairments, neurocognitive deficits, and comorbidities, trained health care professionals should determine safety and suitability for aerobic exercise testing and training.
What Information Is Needed to Determine if an Individual Poststroke or TIA Is Ready to Begin Aerobic Training?
Recommendation
Before engaging in aerobic training, all individuals poststroke or TIA must undergo a screening assessment to identify medical conditions that require special consideration or constitute a contraindication to exercise. Information to support screening should include:
General information: demographics, medical history, medications, cardiac history, seizure history, diabetes control, lifestyle habits
Assessment of contraindications to exercise testing and training
Evaluation of motor function, mobility, balance, swallowing, cognition (ability to follow simple instructions), and communication (verbal and nonverbal comprehension, ability to express pain or distress) (LOE = A)
Rationale
Prescription of an effective and individualized exercise program should be done only after careful clinical evaluation, including identification of health conditions that could be adversely affected by aerobic exercise. Screening also informs goal setting, exercise prescription, and requirements for supervision required. However, the process should not be so onerous as to be a significant barrier to participation.
When Is an Exercise Stress Test Indicated in Pre-Participation Screening for Aerobic Training After Stroke or TIA?
Recommendation
Whenever feasible, a symptom-limited or submaximal exercise stress test should be a component of pre-participation screening for aerobic training after stroke or TIA. However, if the planned aerobic intervention is to be conducted at the lower end of exercise intensities (eg, <50% of predicted heart rate reserve), a submaximal test may be an option (LOE = C).
Rationale
A screening exercise stress test assesses the safety of physical exertion, evaluates cardiorespiratory fitness, and facilitates prescription of a safe, effective, and individualized aerobic exercise program. However, recognizing that low-intensity exercise has lower risk of an abnormal response than high-intensity exercise17 and that mandating a pre-participation stress test for all cases imposes a significant clinical barrier to clinical implementation of aerobic training, a submaximal field test may substitute for an exercise stress test in cases when the planned exercise intensity is low. Nonetheless, care must be taken to ensure that low-intensity exercise is not prescribed simply to circumvent the need for a stress test.
How Should Exercise Testing, as a Component of the Pre-Participation Screen for Aerobic Training After Stroke or TIA, Be Conducted?
Recommendation
Symptom-limited or submaximal stress tests with ECG monitoring involve an adequate warm-up and cool-down and are administered by appropriately trained and experienced health care professionals with direct access to physician support and an external defibrillator. The participant should be on usual medications, avoid any strenuous activity for 24 hours prior to testing, and avoid a heavy meal, caffeine, or nicotine within 2 to 3 hours of testing.
Submaximal exercise tests involve walking, stepping, or cycling at a workload consistent with the planned intensity of the training program. Alternatively, submaximal field tests (eg, 6-Minute Walk Test or Shuttle Walk Test) can be used, which usually entail walking a predefined time or distance (LOE = C).
Rationale
Selection of the test protocol depends on the planned exercise intensity, availability of appropriate equipment and personnel, and the population to be tested. Because symptom-limited tests require exercising to volitional fatigue, medical supervision is advised, particularly for high-risk individuals with symptomatic or known cardiac, pulmonary, or metabolic disease.17 The optimal duration is 8–12 minutes, with testing progression using small workload increments.20 Single or multi-stage submaximal tests can be used for individuals unable to perform the vigorous-intensity exercise due to mobility or cardiovascular limitations, particularly if low-intensity training is planned.17 Submaximal field tests consist of stepping or walking a predefined time or distance.17 For all exercise tests, a 3- to 5-minute warm-up at a metabolic rate about twice the resting level is recommended to prevent premature and excessive local muscle fatigue,17 and a 3- to 5-minute cool-down is recommended to prevent pooling of blood in the peripheral vasculature and a subsequent drop in diastolic blood pressure.
What Should Be Monitored During a Screening Exercise Test?
Recommendation
During screening exercise tests, clinical signs and symptoms, heart rate, blood pressure, and rating of perceived exertion should be monitored before, during, and after termination of the test until baseline values have been approximated. If an exercise stress test is used, continuous monitoring of electrocardiography should also be conducted (LOE = A).
Rationale
Careful monitoring of the individual’s response to exercise testing is essential to ensure safety of the test, determine test termination, assess cardiovascular fitness, and plan intervention.9 During incremental exercise testing, diastolic blood pressure should either remain relatively constant or decrease,17 whereas a decrease or lack of increase in systolic blood pressure may indicate myocardial ischemia, left ventricular dysfunction, or chronotropic insufficiency.21 Rating of perceived exertion on the 0 to 10 or 6 to 20 scale informs the subjective effort expended during an exercise test.22 Twelve-lead ECG monitoring is recommended during stress tests because many individuals poststroke are at risk of or have cardiovascular disease.23
Prescription of Aerobic Exercise Interventions After Stroke or TIA
How Does Aerobic Training Fit Into the Overall Program of Stroke Rehabilitation?
Recommendation
Aerobic training should be incorporated into a comprehensive, interprofessional program of stroke rehabilitation, vascular risk reduction, and secondary stroke prevention. Aerobic training is part of an overall exercise program that may also include, but is not limited to, muscle strengthening and task-oriented training of motor control, balance, gait, and functional use of the upper extremity. Physical activity designed to maintain cardiovascular fitness is an important aspect of community reintegration after stroke (LOE = C).
Rationale
Optimization of recovery after stroke or TIA usually requires a comprehensive plan of complex interventions involving a number of health care providers. Often the metabolic demands of standard rehabilitation activities (eg, progressive task-oriented exercises and mobility training) are not of sufficient intensity to elicit an aerobic training effect.2 , 10 , 11 Thus, it is imperative that the intensity of exercise be monitored to ensure that an adequate intensity has actually been achieved.
Where Should Aerobic Exercise Interventions Be Conducted?
Recommendation
Aerobic exercise programs can be administered in a variety of barrier-free and accessible settings: hospital, outpatient clinics, community, and home. Training of high-risk individuals must be done in a setting with immediate access to external defibrillation and emergency medical response. For lower-risk individuals, home-based aerobic exercise programs may be a safe and effective option (LOE = C).
Rationale
Selection of setting should be based on accessibility, medical status, functional status, social support, and access to care.24 Although the home setting may be the most accessible and convenient, it might be unsafe due to lack of availability to emergency medical equipment and trained personnel, and, for higher-functioning individuals, restrictive because of the absence of appropriate training equipment.24
Who Should Design and Supervise the Aerobic Exercise Intervention?
Recommendation
The aerobic exercise program should be designed by appropriately qualified health care professionals, such as physical therapists or cardiac rehabilitation specialists. The level of supervision is determined by the health care professional based on the individual participant’s health condition. High-risk individuals require constant supervision, whereas low-risk individuals with demonstrated ability to exercise safely and effectively may require only intermittent supervision. Supervision may be provided by a qualified health care professional or an exercise instructor who has been trained by the health care professional (LOE = C).
Rationale
Well-trained exercise/clinical professionals should supervise exercise programs for all individuals who are at high risk for cardiovascular disease or have a chronic disease or health condition.17 Exercise class instructors trained and supervised by health care professionals have successfully conducted low-intensity exercise programs in community settings for older adults poststroke.25
What Format (Individual, Group) Should Be Used for Aerobic Training After Stroke or TIA?
Recommendation
Aerobic exercise interventions can be conducted in either an individual (one-on-one) or group format, with the ratio of participant to supervising personnel determined by the severity of the participant’s neurological and cardiac status as well as the planned exercise intensity and mode of training (LOE = B).
Rationale
Individualized exercise programs are indicated early in the recovery process for people with moderate to severe stroke to ensure safety and effectiveness by adapting the program according to the individual’s presentation and closely monitoring the individual’s response to exercise. When the person is capable of more independent participation without the need for 1-on-1 monitoring, a group format should be considered. This format may also be used for individuals early after minor stroke or TIA.
What Mode of Exercise Should Be Used for Aerobic Training After Stroke or TIA?
Recommendation
Any mode of exercise that activates a large muscle mass for a prolonged period can be used to induce an aerobic training effect (LOE = B).
Rationale
Mode of training should be based on poststroke impairments, comorbidities (eg, arthritis, obesity, seizure history, dementia), participant’s preference, fitness levels, stroke severity, time since stroke, availability, and treatment goals.26 Upper- and lower-body ergometers, cycle ergometers, treadmills (including underwater and robot-assisted treadmills), arm ergometers, and seated steppers have been recommended as training modalities.23 Preliminary evidence suggests that metabolic stress induced by this over ground walking may be inadequate to achieve an aerobic training effect.27
How Long Should an Aerobic Training Program Be Conducted?
Recommendation
A minimum of 8 weeks of aerobic exercise is recommended to achieve a clinically meaningful training effect. However, physical activity should be sustained indefinitely to maintain health benefits (LOE = B).
Rationale
The minimum time to make the long-term physiological and behavioral adaptations to exercise varies from person to person. In general, 8 weeks of aerobic exercise is considered the minimum for these adaptive responses to occur.28 However, because exercise-mediated benefits are lost after 4 to 6 weeks of detraining,29 individuals poststroke should engage in long-term physical activity as part of their daily lives.
How Frequently Should Aerobic Training Sessions Be Conducted?
Recommendation
Structured aerobic exercise should be conducted a minimum of 3 d/wk. On the other days of the week, participants are encouraged to engage in lighter forms of physical activity (LOE = B).
Rationale
Benefits derived from aerobic training are dose dependent, with dose determined by the interaction of frequency, duration, and intensity.30 It is total volume of aerobic exercise that is important for attaining and maintaining cardiorespiratory fitness. Evidence is emerging of the benefits of supplementing an exercise program with nonstructured physical activity (eg, brisk walking) on nonexercise days.
How Long Should Each Aerobic Training Session Last?
Recommendation
Aerobic exercise sessions of >20 minutes are recommended, depending on exercise frequency and intensity. Warm-up and cool-down periods of 3 to 5 minutes are also advised. A gradual progression in the duration may be required, starting with bouts of 5 minutes or less and alternating intervals of rest or lower-intensity exercise (LOE = B).
Rationale
A systematic review of exercise trials concluded that exercise durations >20 minutes were sufficient to achieve benefits for stroke survivors.5 For individuals who are very deconditioned or have substantial motor impairments, exercise may be delivered in “bouts” of 5 minutes or less, with rest periods or lower-intensity activity between bouts.31
What Intensity of Aerobic Exercise Should Be Used?
Recommendation
Intensity of aerobic exercise must be determined on an individual basis, depending on responses to exercise testing, health status (neurologic status, cardiac, and other comorbidities), and planned exercise frequency and duration. Percentage of heart rate reserve (HRR) is often used to establish the target training intensity. Other markers of intensity, such as percentage of maximal heart rate (% HRmax) and rating of perceived exertion (RPE), can be used, particularly when heart rate is compromised by medication (LOE = B).
Light intensity: <40% HRR or <64% HRmax or RPE0–10 < 4 or RPE6–20 < 12
Moderate intensity: 40–60% HRR or 64–76% HRmax or RPE0–10 of 4–5 or RPE6–20 12–13
Vigorous exercise: >60% HRR or >76% HRmax or RPE0–10 >6 or RPE6–20 > 14
Rationale
Establishing and monitoring exercise intensity dictates the level of metabolic stress of the exercise, safeguards against adverse responses to inappropriately stressful exercise, and is the most critical factor in ensuring an adequate dosage to elicit a training effect. The intensity depends on baseline fitness level, neurologic involvement, cardiac status, presence of comorbidities, and goals of the program. Mounting evidence suggests that higher training intensities, including high-intensity interval training, elicit greater improvements in cardiopulmonary fitness.32–34 However, safety and feasibility of attaining a particular training intensity must also be taken into account. The American College of Sports Medicine17 recommends that inactive individuals “start low and go slow.”
What Should Be Monitored During Aerobic Exercise Training?
Recommendation
Ongoing observation of the general response to exercise, frequent heart rate monitoring, and periodic blood pressure and rating of perceived exertion monitoring are recommended for safety purposes and assurance that the participant is exercising at the planned intensity (LOE = A).
Rationale
Careful monitoring is critical to ensure safety and optimize effectiveness of aerobic training.
How Should Aerobic Exercise Progress During a Training Program?
Recommendation
Aerobic exercise should progress on an individual basis, with gradual progression of frequency, duration, and intensity to minimize muscle soreness, fatigue, and injury. Duration should be increased by 5 to 10 minutes every 1 to 2 weeks for the first 4 to 6 weeks and intensity by 5% to 10% of heart rate reserve every 1 to 4 weeks, depending on fitness, health status, training responses, and exercise goals. Changes in blood pressure, heart rate, and rating of perceived exertion in response to the increased exercise dose should be monitored (LOE = B).
Rationale
Gradual progression of session frequency, duration, and intensity provides the metabolic challenge needed to induce a training effect without jeopardizing participant safety.17 The majority of poststroke training studies reviewed followed the recommendation of American College of Sports Medicine to progress exercise training sessions for inactive individuals by increasing duration 5 to 10 minutes every 1 to 2 weeks for the first 4 to 6 weeks or as tolerated.17 Gradual increases in intensity can be achieved during training sessions by systematically manipulating parameters such as speed, revolutions per minute, incline, and extent of balance support.35
What Clinical Outcome Measures Should Be Used to Monitor Effects of Aerobic Training?
Recommendation
Outcomes aligned with participant-oriented goals and anticipated benefits of aerobic training should be assessed periodically to monitor change over time, progress intervention, and transition to other settings or physical activities. The assessment should include measures of cardiovascular endurance/functional capacity (eg, 6-Minute Walk Test, Shuttle Walk Test, heart rate at a fixed submaximal workload, walking speed, daily step counts), cardiovascular health (eg, blood pressure, blood lipids, fasting plasma glucose, waist circumference, medication adherence, tobacco use), and other relevant domains (eg, goal attainment, cognition, emotional well-being, exercise self-efficacy, quality of sleep, quality of life) (LOE = B).
Rationale
Periodic assessment should be aligned with anticipated outcomes, acknowledging that the highest probability of improvement is generally seen in those functions most directly affected by aerobic training5 (eg, improvements in exercise capacity, fatigue resistance, vascular health, mobility).
What Strategies Are Used to Encourage Long-Term Participant Engagement in Aerobic Training After Stroke or TIA?
Recommendation
An individualized plan, endorsed by the health care team, should be implemented to gradually transition from structured, clinical aerobic training to less structured, more self-directed physical activity at home or in the community. Multiple strategies should be used to deal with specific barriers related to health care providers, the environment, and the participant (LOE = B).
Rationale
Evidence suggests that the benefits of aerobic exercise are not sustained in the long term because participants either decrease or stop participating.36 Numerous psychological, cognitive, emotional, environmental, and personal factors influence engagement in physical activity.37 , 38 Strategies aligned with theories of behavioral change (eg, counseling and information,39 behavioral change techniques, social support and education,40 and motivational counseling through videotapes41) enhance self-efficacy and long-term engagement in exercise and physical activity.42
Conclusion
Aerobic training is recognized as a key component of stroke rehabilitation but continues to be used sporadically in clinical settings. These updated recommendations, supported by the highest quality of evidence available, should make it easier for clinicians to screen for and prescribe aerobic exercise in their practice. Clinical implementation will not only help to narrow the gap between evidence and practice but also reduce current variability and uncertainty regarding the role of aerobic exercise in recovery after stroke.
Contributor Information
Marilyn MacKay-Lyons, School of Physiotherapy, Dalhousie University, 5869 University Avenue, Halifax, NS B3H 3J5, Canada; and Department of Physical Medicine and Rehabilitation, QEII Health Sciences Centre, Halifax, Nova Scotia, Canada.
Sandra A Billinger, Physical Therapy and Rehab Science, University of Kansas Medical Center, Kansas City, Kansas.
Janice J Eng, Department of Physical Therapy, University of British Columbia, Vancouver, British Columbia, Canada.
Alex Dromerick, Department of Neurology, Pasquerilla Healthcare Center, Washington, DC.
Nicholas Giacomantonio, Department of Cardiology, QEII Health Sciences Centre, Halifax, Nova Scotia, Canada.
Charlene Hafer-Macko, Department of Neurology, University of Maryland School of Medicine, Baltimore, Maryland.
Richard Macko, Department Neurology, VA Maryland Health Care System, Baltimore, Maryland.
Emily Nguyen, Trinity College Dublin, Dublin, Ireland.
Peter Prior, Department of Psychology, St. Joseph's Health Care London, London, Ontario, Canada.
Neville Suskin, Department of Cardiology, Western University, London, Ontario, Canada.
Ada Tang, School of Rehabilitation Science, McMaster University, Hamilton, Ontario, Canada.
Marianne Thornton, Champlain Regional Stroke Network, Ottawa, Ontario, Canada.
Karen Unsworth, Department of Cardiac Rehabilitation, St. Joseph's Health Care London, London, Ontario, Canada.
Author Contributions
Concept/idea/research design: M. MacKay-Lyons, A. Dromerick, N. Suskin, M. Thornton, K. Unsworth
Writing: M. MacKay-Lyons, S.A. Billinger, J.J. Eng, A. Dromerick, N. Giacomantonio, C. Hafer-Macko, R. Macko, E. Nguyen,P. Prior, A. Tang, M. Thornton
Data collection: C. Hafer-Macko, R. Macko, E. Nguyen, P. Prior
Data analysis: C. Hafer-Macko, E. Nguyen, A. Tang
Project management: M. MacKay-Lyons
Fund procurement: M. MacKay-Lyons
Providing institutional liaisons: M. MacKay-Lyons, R. Macko
Consultation (including review of manuscript before submitting): M. MacKay-Lyons, S.A. Billinger, J.J. Eng, A. Dromerick,N. Giacomantonio, R. Macko, E. Nguyen, P. Prior, N. Suskin, A. Tang, M. Thornton, K. Unsworth
Funding
Development and update of the AEROBICS recommendations were funded by the Canadian Partnership for Stroke Recovery and the Canadian Institutes of Health Research.
Role of the Funding Source
Development and update of the AEROBICS recommendations were funded by the Canadian Partnership for Stroke Recovery and the Canadian Institutes of Health Research. The funders played no role in the design or conduct of the guideline update.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest. M. MacKay-Lyons reports a grant paid to her institution, R. Macko reports a grant paid to his institution on early exercise after stroke and its effects on fitness, function, and metabolism. The papers from this NIH/NICHD grant are not yet written and so are not included in this manuscript. The remaining authors report no conflicts of interest.
References
- 1. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–448. [DOI] [PubMed] [Google Scholar]
- 2. Barrett M, Snow JC, Kirkland MC, et al. Excessive sedentary time during in-patient stroke rehabilitation. Top Stroke Rehabil. 2018;25:366–374. [DOI] [PubMed] [Google Scholar]
- 3. Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. Int J Stroke. 2012;7:499–510. [DOI] [PubMed] [Google Scholar]
- 4. Ivey FM, Hafer-Macko MC, Macko RF. Exercise training for cardiometabolic adaptation after stroke. J Cardiopulm Rehabil Prevent. 2008;28:2–11. [DOI] [PubMed] [Google Scholar]
- 5. Saunders DH, Sanderson M, Hayes S, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2016;3:CD003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Isabella NT, Shkredova DA, Richardson JA, Tang A. Effects of exercise on cardiovascular risk factors following stroke or transient ischemic attack: a systematic review and meta-analysis. Clin Rehabil. 2017;31:1561–1572. [DOI] [PubMed] [Google Scholar]
- 7. Chen MD, Rimmer JH. Effects of exercise on quality of life in stroke survivors: a meta-analysis. Stroke. 2011;42:832–837. [DOI] [PubMed] [Google Scholar]
- 8. Hebert D, Lindsay P, Kirton A, et al. Canadian stroke best practice recommendations: rehabilitation stroke care update 2015. Int J Stroke. 2016;11:459–484. [DOI] [PubMed] [Google Scholar]
- 9. Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. [DOI] [PubMed] [Google Scholar]
- 10. MacKay-Lyons M, Makrides L. Cardiovascular stress during stroke rehabilitation: is the intensity adequate to induce a training effect? Arch Phys Med Rehabil. 2002;83:1378–1383. [DOI] [PubMed] [Google Scholar]
- 11. Kuys S, Brauer SG, Ada L. Routine physiotherapy does not induce a cardiorespiratory training effect post-stroke, regardless of walking ability. Physiother Res Int. 2006;11:219–227. [DOI] [PubMed] [Google Scholar]
- 12. Bayley M, Hurdowar A, Richards C, et al. Barriers to implementation of stroke rehabilitation evidence: findings from a multi-site pilot project. Disabil Rehabil. 2012;34:1633–1638. [DOI] [PubMed] [Google Scholar]
- 13. Doyle L, Mackay-Lyons M. Utilization of aerobic exercise in adult neurological rehabilitation by physical therapists in Canada. J Neurol Phys Ther. 2013;37:20–26. [DOI] [PubMed] [Google Scholar]
- 14. Boyne P, Billinger S, MacKay-Lyons M, Barney B, Khoury J, Dunning K. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of US physical therapists. J Neurol Phys Ther. 2017;41:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brouwers MC, Kho ME, Browman GP, et al. Agree II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guyatt GH, Cook DJ, Jaeschke R, Paulker SG, Schhünermann HJ. Grades of recommendation for antithrombotic agents: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:123S–131S. [DOI] [PubMed] [Google Scholar]
- 17. American College of Sports Medicine . ACSM’s guidelines for exercise testing and prescription. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2017. [Google Scholar]
- 18. Convertino V, Hung J, Goldwater D, DeBusk RF. Cardiovascular responses to exercise in middle-aged men after 10 days of bedrest. Circulation. 1982;65:134–140. [DOI] [PubMed] [Google Scholar]
- 19. Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–V78. [PubMed] [Google Scholar]
- 20. Wenger NK. Exercise testing and training of the elderly coronary patient. Chest. 1992;101:309S–311S. [DOI] [PubMed] [Google Scholar]
- 21. Connuck D. The role of exercise stress testing in pediatric patients with heart disease. Prog Ped Cardiol. 2005;20:45–52. [Google Scholar]
- 22. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 23. Moore GE, Durstine JL, Painter PL. ACSM's exercise management for persons with chronic diseases and disabilities. In: Human Kinetics. 4th ed. 2016.
- 24. Duncan P, Zorowitz R, Bates B, et al. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36:100–143. [DOI] [PubMed] [Google Scholar]
- 25. Stuart M, Benvenuti F, Macko R, et al. Community-based adaptive physical activity program for chronic stroke: feasibility, safety, and efficacy of the Empoli model. Neurorehab Neural Repair. 2009;23:726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ivey FM, Hafer-Macko CE, Macko RF. Exercise rehabilitation after stroke. NeuroRx. 2006;3:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prajapati S, Mansfield A, Gage W, Brooks D, McIlroy W. Cardiovascular responses associated with daily walking in subacute stroke. Stroke Res Treat. 2013;2013:612458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ivey FM, Hafer-Macko CE, Macko RF. Task-oriented treadmill exercise training in chronic hemiparetic stroke. J Rehabil Res Dev. 2008;45:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mujika I, Padilla S. Detraining: loss of training-induced physiological and performance adaptations. Part II: long term insufficient training stimulus. Sports Med. 2000;30:145–154. [DOI] [PubMed] [Google Scholar]
- 30. Fletcher GF. Current status of cardiac rehabilitation. In: Starke RD, ed. Am Family Physician. Dallas, TX: American Academy of Family Physicians; 1998. [PubMed] [Google Scholar]
- 31. Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. [DOI] [PubMed] [Google Scholar]
- 32. Noel M, Jobin J, Marcoux A, Poirier P, Dagenais GR, Bogaty P. Can prolonged exercise-induced myocardial ischaemia be innocuous? Europ Heart J. 2007;28:1559–1565. [DOI] [PubMed] [Google Scholar]
- 33. Boyne P, Welge J, Kissela B, Dunning K. Factors influencing the efficacy of aerobic exercise for improving fitness and walking capacity after stroke: a meta-analysis with meta-regression. Arch Phys Med Rehabil. 2017;98:581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crozier J, Roig M, Eng JJ, et al. High-intensity interval training after stroke: an opportunity to promote functional, cardiovascular and neuroplastic health. Neurorehab Neural Repair. 2018;32:543–556. [DOI] [PubMed] [Google Scholar]
- 35. Marsden DL, Dunn A, Callister R, McElduff P, Levi CR, Spratt NJ. A home- and community-based physical activity program can improve the cardiorespiratory fitness and walking capacity of stroke survivors. J Stroke Cerebrovasc Dis. 2016;25:2386–2398. [DOI] [PubMed] [Google Scholar]
- 36. Mead G, Greig C, Cunningham I, et al. Stroke: a randomized trial of exercise or relaxation. J Am Geriatr Soc. 2007;55:892–899. [DOI] [PubMed] [Google Scholar]
- 37. Bauman A, Sallis J, Dzewaltowski D, Owen N. Toward a better understanding of the influences on physical activity: the role of determinants, correlates, causal variables, mediators, moderators, and confounders. Am J Prevent Med. 2002;23:5–14. [DOI] [PubMed] [Google Scholar]
- 38. Morris JH, Williams B. Discussion - optimising long-term participation in physical activities after stroke: exploring new ways of working for physiotherapists. Physiotherapy. 2009;95:227–233. [DOI] [PubMed] [Google Scholar]
- 39. van der Ploeg H, Streppel K, van der Beek A, et al. Counselling increases physical activity behaviour nine weeks after rehabilitation. Br J Sports Med. 2006;40:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Froehlich-Grobe K, White G. Promoting physical activity among women with mobility impairments: a randomized controlled trial to assess a home- and community-based intervention. Arch Phys Med Rehabil. 2004;85:640–648. [DOI] [PubMed] [Google Scholar]
- 41. Jette A, Lachman M, Giorgetti M, et al. Exerciseーit's never too late: the strong-for-life program. Am J Public Health. 1999;89:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dobkin BH. Behavioral self-management strategies for practice and exercise should be included in neurologic rehabilitation trials and care. Curr Opin Neurol. 2016;29:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]


