Abstract
Aim:
This study investigated our Enzymelinks, COX-2-10aa-mPGES-1 and COX-2-10aa-PGIS, as cellular cross-screening targets for quick identification of lead compounds to inhibit inflammatory PGE2 biosynthesis while maintaining prostacyclin synthesis.
Methods:
We integrated virtual and wet cross-screening using Enzymelinks to rapidly identify lead compounds from a large compound library.
Results:
From 380,000 compounds virtually cross-screened with the Enzymelinks, 1576 compounds were identified and used for wet cross-screening using HEK293 cells that overexpressed individual Enzymelinks as targets. The top 15 lead compounds that inhibited mPGES-1 activity were identified. The top compound that specifically inhibited inflammatory PGE2 biosynthesis alone without affecting COX-2 coupled to PGI2 synthase (PGIS) for PGI2 biosynthesis was obtained.
Conclusion:
Enzymelink technology could advance cyclooxygenase pathway-targeted drug discovery to a significant degree.
Keywords: : COX-2, cyclooxygenase-2, Enzymelink, inhibitors, microsomal prostaglandin E2 synthase-1, mPGES-1, PGIS, prostacyclin synthase
Currently, non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenase (COX), are the most common and effective drugs used to treat inflammation, pain and fever. Their anti-inflammatory effects result from reducing production of pro-inflammatory prostaglandin E2 (PGE2) synthesized by inducible COX-2 coupled to inducible microsomal PGE2 synthase-1 (mPGES-1) [1–4]. However, COX-1 and -2 are upstream enzymes in the arachidonic acid (AA) metabolism pathway, which is also coupled to other downstream synthases to produce a variety of prostanoids, such as thromboxane A2 (TXA2) for platelet aggregation, prostacyclin (PGI2) for vascular protection [5,6] and noninflammatory/basal level PGE2 for gastrointestinal protection. NSAID inhibition of upstream COX could cause harmful side effects, such as excessive bleeding and GI and cardiovascular insults [1–6]. Development of drugs to specifically eliminate pro-inflammatory PGE2 while maintaining biosynthesis of PGI2, basal PGE2 and other prostanoids has been attempted for decades. However, specific PGE2 inhibitors are currently still not available on the market [4].
Three PGE2 synthases, including inducible mPGES-1, non-inducible mPGES-2 and cytosolic PGE2 synthase (cPGES), have been identified and characterized [3,4,7–9] (Figure 1A). mPGES-1 is the inducible enzyme involved in producing pro-inflammatory PGE2 through coordination with inducible COX-2 by accepting COX-2-produced unstable PGH2 as a substrate [3,4,9]. Recently, it has been reported that mPGES-1 could also couple with COX-1 to produce inflammatory PGE2 in a transgenic mouse study [10]. Because COX-1 and COX-2-produced PGH2 is also a substrate for PGI2 synthase (PGIS) to produce vascularly protective PGI2, currently used COX-2 inhibitors have a significant side effect of promoting heart disease through inhibiting PGI2 biosynthesis [1]. We recently identified that inflammatory COX-2 and mPGES-1 have a complex-like configuration anchored to the endoplasmic reticulum (ER) membrane, which enables effective catalyzation of AA to pro-inflammatory PGE2 under pathogenic stimulation [9]. This clearly shows that establishing a system that mimics endogenous inflammatory PGE2 biosynthesis by inducible COX-2 coupling with inflammatory mPGES-1 without affecting COX-2 coupling with downstream PGIS is a key step toward finding specific inflammatory PGE2 inhibitors. To meet this goal, we have successfully created a set of single-chain (SC) Enzymelinks in which COX-2 is covalently linked to mPGES-1 (COX-2-10aa-mPGES-1 [9,11]) or PGIS (COX-2-10aa-PGIS [12,13]). Due to the shorter distances between the catalytic domains of COX-2 and mPGES-1 or PGIS, the two Enzymelinks have a kinetic advantage for converting the initial substrate, AA, to pro-inflammatory PGE2 or vascularly protective PGI2, respectively, via triple catalytic reactions within a single polypeptide chain [9,11,12]. These successful Enzymelinks have enabled intentional, specific PGE2 and PGI2 biosyntheses [9,14].
Figure 1. . Construction of 3D models for Enzymelinks.
(A) SC-COX-2-10aa-mPGES-1 and (B) SC-COX-2-10aa-mPGES-1. The structures show unique triple-catalytic reactions within their single polypeptide chains. The structural models were constructed by Molecular Operating Environment software using high-resolution 3D crystal structures for COX-2, mPGES-1 and PGIS. Three catalytic pockets (for AA, PGG2 and PGH2) within each single polypeptide chain of the Enzymelinks are shown in green circles. The potential compounds competing with AA, PGG2 and PGH2 sites to inhibit PGE2 or PGI2 biosyntheses are shown using different symbols.
PGIS: PGI2 synthase.
Several lead compounds that inhibit mPGES-1, identified by screening assays using unstable prostaglandin H2 (PGH2) as the substrate and overexpressed cellular mPGES-1 as the target, have been reported [15–25]. However, it is hard to exclude the possibilities of nonspecific inhibition and cross-inhibition of PGIS among these previously reported compounds. Firstly, the substrate, PGH2, used for screening is unstable and partially degrades into nonspecific products. Secondly, degraded PGH2 and its side products have very similar chemical structures to that of PGE2, which may interfere with the PGE2 immunoassay used for drug screening. Finally, due to mPGES-1 and PGIS sharing and competing for PGH2 as their substrates, the lead compounds that competed with PGH2 and bound to the substrate pocket of mPGES-1 may also bind to other downstream synthases, such as PGIS. In particular, a low micromole concentration of AA-produced PGH2 by COX-2 is satisfactory for both mPGES-1 and PGIS to biosynthesize PGE2 and PGI2. This suggests that PGIS has a PGH2 binding affinity similar to that of mPGES-1 [11–13]. To solve these issues, in this study, we have established a cross-screening assay using novel Enzymelinks COX-2-10aa-mPGES-1 and COX-2-10aa-PGIS as targets and stable AA as the substrate to rapidly and accurately measure the pro-inflammatory PGE2 produced by inducible COX-2 coupled to mPGES-1 and PGI2 biosynthesis by the COX-2 coupled to PGIS. This study has demonstrated the superior use of Enzymelinks for cross-screening lead compounds targeting the COX pathway.
Methods and materials
Materials
HEK293 cell lines were purchased from ATCC (VA, USA). [3H]-PGH2 and [14C]-AA were purchased from Amersham Pharmacia Biotech (NJ, USA). A 30,000 drug-like (low cytotoxicity) compound library was purchase from ChemBridge Corporation (CA, USA).
Designs of Enzymelinks
SC-COX-2-10aa-mPGES-1 and SC-COX2-10aa-PGIS. The software packages of Sybyl and Molecular Operating Environment (MOE) were used to construct the 3D structural models of SC-COX-2-10aa-mPGES-1 and SC-COX-2-PGIS based on data described previously [10–12].
Virtual screening and ligand docking
The compounds containing benzene structure from PubChem (NCBI) compound database were generated and then filtered by Lipinski's rules (molecular weight [Mw] <500, log p < 5, hydrogen-bond donors <5 and hydrogen-bond acceptors <10). Docking of the compound libraries with SC-COX-2-10aa-mPGES-1 and SC-COX-2-10aa-PGIS was performed using Sybyl-X 2.1 Surflex-Dock and MOE software packages.
Construction of cDNA plasmids and stale expression of Enzymelinks in HEK293
cDNA construction and stable expression of the recombinant SC-COX-2-10aa-mPGES-1 and SC-COX-2-PGIS were performed as previously described [10–12].
HPLC scintillation profiling assay
Enzyme activity was determined by monitoring metabolites of [14C]-AA or [3H]-PGH2 by HEK293 cells expressing SC-COX-2-mPGES-1 or SC-COX-2-10aa-PGIS. The enzymatic reaction in the presence or absence of the individual compound was initiated by adding [14C]-AA or [3H]-PGH2 to the harvested cells in a total reaction volume of 0.1 mL. After incubation for 5 min the reaction was terminated by adding 0.2 ml of buffer A (H2O containing 35% acetonitrile and 0.1% acetic acid). After centrifugation (at 13,000 rpm for 10 min), the supernatant was loaded onto a C18 column (4.6 × 250 mm, using buffer A with a gradient from 35 to 100% of acetonitrile for 40 min) and connected to a flow scintillation analyzer (Packard 150TR) collecting the full metabolite profile of the [14C]-AA or [3H]-PGH2.
High Through-put Screening (HTS)
HEK cells cultured on a 96-well plate (50 μL/well) were incubated with an individual compound (50–100 μM) and substrate AA (0.5 μM) for 10 min at 37°C in a humidified 5% CO2 incubator. The medium of the cultured cells containing the produced PGE2 were transferred to another 96-well plate and subjected to competitive chemiluminescent immunoassay (CLIA) to quantify the amount of PGE2 produced. For CLIA, 96-well plates were coated with a bovine serum albumin (BSA)-PGE2 conjugate. The collected cell medium and the anti-PGE2 antibody were pre-incubated and added to the plates. The remaining free anti-PGE2 antibodies bound to BSA-PGE2 conjugate. Subsequently, the plates were washed with PBS three times. Anti-mouse horseradish peroxidase (HRP) secondary antibody was used to identify the antibodies captured on the plates. A total of 60 plates were screened by this cell-based HTS (Robotic HTS station, Beckman, IN, USA). First, 100 μM of the total 1596 compounds were processed for CLIA. Then, 1 and 10 μM of the top 15 highest relative light unit compounds were further processed, respectively.
Results
Construction of 3D structural models for SC-COX-2-mPGES-1 and SC-COX-2-10aa-PGIS
To conduct a large-scale virtual cross-screening, the 3D structure models of our Enzymelinks, SC-COX-2-10aa-mPGES-1 (Figure 1A) and SC-COX-2-PGIS [11–13] (Figure 1B), were constructed by covalently linking COX-2 with mPGES-1 or PGIS using high-resolution x-ray structures of COX-2 [26]. Protein Data Bank identifier (PDB ID): 4PH9, resolution: 1.81 Å), PGIS [27]. PDB ID: 3B6H, resolution: 1.62 Å) and mPGES-1 [28]. PDB ID: 4YL1, resolution 1.41 Å) through a known transmembrane helical linker (10aa, Figure 1) [11–13]. The three catalytic sites of the Enzymelinks able to continuously catalyze AA into PGG2, then PGH2 and the final product PGE2 (A) or PGI2 (B) are shown in Figure 1. In addition, the putative three types of inhibitors binding to the three catalytic sites of each Enzymelink are also shown using different shapes.
Large-scale virtual cross-screening for identification of lead compounds that specifically inhibit mPGES-1 using the combination of Enzymelinks COX-2-10aa-mPGES-1 & COX-2-10aa-PGIS as targets
After filtering PubChem database using Lipinski's rule, a 380,000 benzene-containing compound library was created for virtual screening. Cross-screening was performed in three steps. First, each individual compound was docked with a 3D model of SC-COX-2-10aa-mPGES-1 using the auto-docking softwares Sybyl program (TRIPOS, MO, USA) and Molecular Operating Environment (MEO, Montreal, Canada). Second, the identified compounds that demonstrated binding to the SC-COX-2-10aa-mPGES-1 were further cross-docked with COX-2-10aa-PGIS as targets. Finally, the identified compounds from step 2 were docked with the SC-COX-2-10aa-mPGES-1 again, and 19 compounds with top scores (-log10[Kd]) binding to mPGES-1 but not COX-2 and PGIS were identified (Figure 2).
Figure 2. . Virtual cross-screening using SC-COX-2-10aa-mPGES-1 and SC-COX-2-10aa-PGIS as targets.

Three steps of docking screenings: (A) First, 388,000 compounds were docked with SC-COX-2-10aa-mPGES-1. Then, the compounds bound to SC-COX-2-10aa-mPGES-1 were further docked with SC-COX-2-10aa-PGIS (B). Third, the compounds bound to SC-COX-2-10aa-mPGES-1, but not SC-COX-2-10aa-PGIS were docked with SC-COX-2-10aa-mPES-1 again.
Identification of the drug-like chemical library from the virtual screening for further cellular cross-screening
The 19 compounds identified by virtual cross-screening as specifically targeting mPGES-1 were categorized by functional groups, including imidazone, imidazolidine, oxazolidine, acetate, propanoate, benzensulfonamide, amide, sulfamide and triazole (Figure 3). Using this chemical information, we narrowed down 1596 drug-like compounds from our 30,000 synthetic chemical compound library obtained from the ChemBridge Corporation (CA, USA) through 3D Quantitative Structure and Activity Relationship (3D-QSAR) analysis using the Sybyl and MEO programs. That these 1596 compounds bound to COX-2-10aa-mPGES-1 but not COX-2-10aa-PGIS was also confirmed using the third step of virtual cross-docking as described earlier (Figure 3).
Figure 3. . Selecting synthetic compounds for wet screening.
Based on the functional groups of the 19 compounds identified from virtual screening, 1596 synthetic compounds with similar functional groups were identified from a 30,000 drug-like chemical library (ChemBridge Corporation).
Establishing an mPGES-1 assay system using stable AA versus unstable PGH2 as a substrate to increase assay stability and screening accuracy
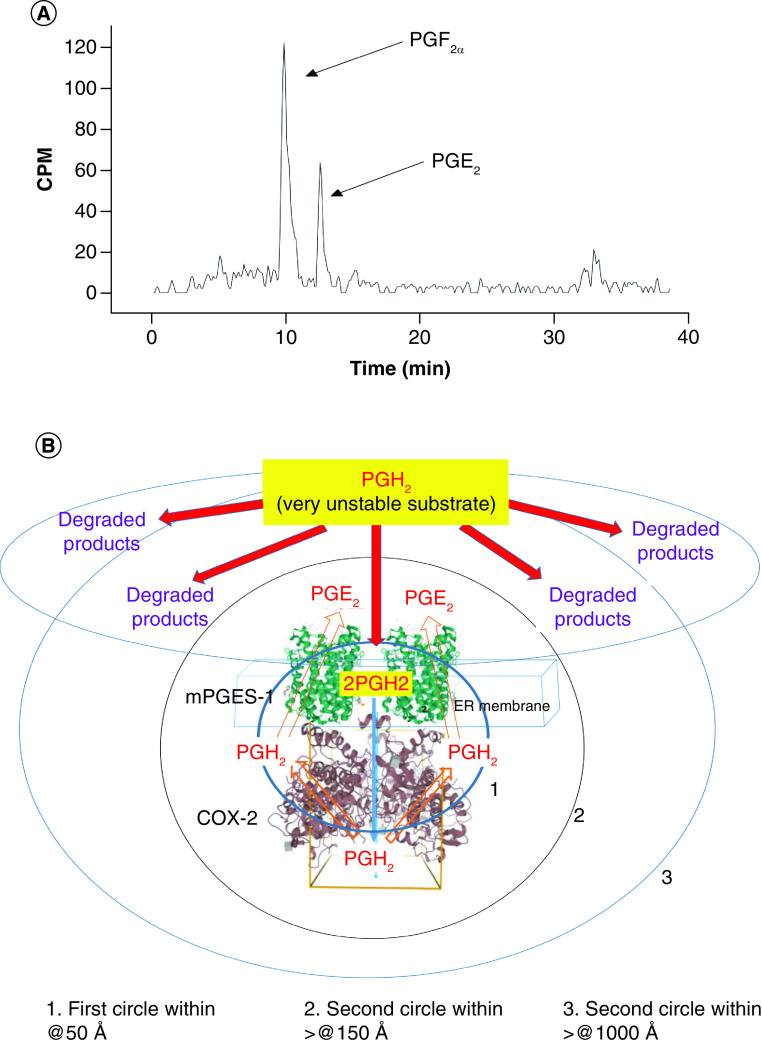
As described earlier, PGH2 is a COX-produced mediator notably unstable in the assay solution and cellular environment and could be quickly degraded into side products, such as PGF2α, that are structurally similar to PGE2. Thus, these degraded side products could partially cross-react with an anti-PGE2 antibody, which is used in PGE2 immunoassay. To show the disadvantages of using PGH2 for compound screening, which could increase likelihood of high background and false positives, a typical Enzymelink assay using unstable commercial PGH2 as substrate was analyzed (Figure 4A). Using a real-time HPLC scintillation analyzer applying [3H]-PGH2 (1 μM) as a substrate to HEK-COX-2-10aa-mPGES-1 cells, a large portion of added [3H]-PGH2 was degraded into [3H]-PGF2α and others (~65%), whereas a smaller portion of [3H]-PGH2 was converted to [3H]-PGE2 (~35%, Figure 4A). A schematic presentation of the diffusion and degradation of the unstable PGH2 as a substrate for SC-COX-2-10aa-mPGES-1 used in the Figure 4A assay is shown in Figure 4B. PGH2 added directly into the assay solution diffuses into its environment (using schematic scales of 50 Å, 150 Å and >1000 Å as models). Because of the unstable chemical property of PGH2 in the cellular environment, a large portion of the PGH2 is degraded into side products, and only a small portion of PGH2 is diffused and presented to the substrate site of the Enzymelink to be isomerized into end product PGE2 (Figure 4B). This indicates the importance of replacing unstable PGH2 with a stable substrate to improve the screening assay accuracy.
Figure 4. . Disadvantages of using unstable PGH2 as a substrate.
(A) Metabolite profile analysis using [3H]-PGH2 as substrate for SC-COX-2-10aa-mPGES-1. First, 1 μM of [3H]-PGH2 was added to the suspension of 0.1 mg of HEK293 cells stably expressing SC-COX-2-10aa-mPGES-1. After a 5-min reaction, the sample was centrifuged, and the supernatant was applied to C18-HPLC scintillation analyzer. The metabolites from the [3H]-PGH2 were plotted in a real-time mode [10–12]. (B) A schematic presentation of the diffusion and degradation for the unstable PGH2 as substrate for SC-COX-2-10aa-mPGES-1. Only a small portion of the added PGH2 could be converted into the end product, PGE2 within the center of SC-COX-2-10aa-mPGES-1 due to PGH2‘s diffusion and unstable properties during the progress of the assay.
Using stable AA as a substrate for PGE2 biosynthesis
Using the same real-time HPLC scintillation analyzer, our study showed that after substituting unstable [3H]-PGH2 with stable [14C]-AA (1 μM) as a substrate, almost >95% of the added [14C]-AA was converted to [14C]-PGE2 (Figure 5A). These superior assay stability and high yield are resulted from the continuous three steps of chain reactions within a single polypeptide chain by the Enzymelink, COX-2-10aa-mPGES-1, in which the Enzymelink catalyzed stable [14C]-AA to [14C]-PGG2 to [14C]-PGH2 (by the COX-2 domain) and from [14C-PGH2] to [14C]-PGE2 (instantly by the mPGES-1 domain. A schematic representation of the experimental substrate diffusion of the stable AA used in the assay is shown using the similar three scales for diffusion distances: 50, >150 and >1000 Å (Figure 5B). A limited amount of the stable substrate AA was presented to the Enzymelink COX-2-10aa-mPGES-1 through concentration-based diffusion (Figure 5B). With increasing conversion of AA to PGE2 by the Enzymelink, the stable AA in the surrounding should also move to the catalytic site of the Enzymelink to be continuously converted into PGG2, then PGH2 and finally to PGE2 until completion of the three reactions and use of most of the AA (Figure 5B). In this case, the unstable mediator PGH2 synthesized by the COX domain was immediately diffused (with 50-Å distance) into the substrate binding site of the mPGES-1 domain of the Enzymelink molecule to be isomorized into the end product PGE2 (Figure 4A). Under these assay conditions, the possibility of PGH2 escaping from the Enzyemlink molecule and degrading into side products was able to be eliminated (Figure 5A).
Figure 5. . Advantages of using stable AA as a substrate.
(A) Metabolite profile analysis using the very stable [14C]-AA as substrate for SC-COX-2-10aa-mPGES-1. First, 0.5 μM of [14C]-AA was added to the suspension of 0.1 mg of microsomes purified from the HEK293 cells stably expressing SC-COX-2-10aa-mPGES-1. After a 5-min reaction, the sample was centrifuged, and the supernatant was applied to C18-HPLC scintillation analyzer. The metabolites from the [14C]-AA were separated and plotted in real-time mode [10–12]. (B) A schematic presentation of the diffusion and degradation for the stable AA as substrate for SC-COX-2-10aa-mPGES-1. Almost all the stable AA added could be converted into the end product, PGE2, through its concentration-based diffusion and stable properties during the progress of assay.
The first step of cellular high-throughput screening using COX-2-10aa-mPGES-1 & stable AA
Using the selected 1596 drug-like compounds, AA as a stable substrate and COX-2-10aa-mPGES-1 cell line as the target, cell-based HTS was conducted (4788 assays, n = 3). Using competitive enzyme immunoassay, higher PGE2 production should result in fewer light units. In contrast, lower PGE2 production should result in greater light units (Figure 6A). Ninety-six of the 1596 compounds inhibiting COX-2-10aa-mPGES-1 to produce inflammatory PGE2 were identified (data not shown). This approximately 6% hit rate is almost seven times higher than that of previous screenings, which used unstable PGH2 as a substrate and mPGES-1 cell as the target [25].
Figure 6. . Cell-based drug screening.
(A) Cell-based high-throughput screening for 1596 compounds. The individual compound (with a final concentration of 100 μM) and the stable substrate AA (0.5 μM) were mixed and added into the 96-well plate coated with HEK293 cells stably expressing SC-COX-2-10aa-mPGES-1 for 10 min. The generated PGE2 was measured by ELISA kit through competitive immunoassay. The higher PGE2 production indicates stronger inhibitory effects by the added compounds. The results were presented using mean and SD (M = 23.1, SD1 = 4.7, SD2 = 9.5 and SD3 = 14.2). The top 15 lead compounds able to significantly (>SD3) inhibit PGE2 production by SC-COX-2-10aa-mPGES-1 were grouped and labeled. (B) Dose-response curves. For comparison of the inhibitory effects of the top lead compounds on PGE2 production by HEK293 cells stably expressing SC-COX-2-10aa-mPGES-1, the identified 15 compounds (1 μM, 10 μM and 100 μM) were further analyzed by the dose response assay using the same method as described earlier. NS-398 (COX-2 inhibitor) was used as a positive control (PS).
The second step of cellular HTS using COX-2-10aa-PGIS cell line & stable AA
The 96 compounds identified in the first test that inhibited PGE2 biosynthesis by COX-2-10aa-mPGES-1 were subjected to cross-screening using stable AA as the substrate and COX-2-10aa-PGIS as a target. The inhibitory effect of the compounds on PGI2 biosynthesis by COX-2-10aa-PGIS was used as an indication of cross-binding to COX-2 and PGIS. Thus, any compounds with overlapping cross-inhibition of COX-2-10aa-mPGS-1 and COX-2-10aa-PGIS were removed from the pool. This step excluded the compounds with potential side effects similar to regular NSAIDs, which could reduce PGI2 biosynthesis by inhibiting the formation of PGIS substrate, PGH2 from COX-2. As a result, 15 compounds that inhibited COX-2-10-mPGES-1 but not COX-2 and PGIS remained (Figure 6A).
Validation of the top hits by dose response study using enzyme immunoassay
The top 15 compounds that specifically inhibited mPGES-1 were further validated by a dose-response study using PGE2 enzyme immunoassay. All compounds dose-dependently inhibited the production of inflammatory PGE2 to different degrees by the HEK-COX-2-10aa-mPGES-1 (Figure 6B), with compound 10 being the most active (Figure 6B). The upstream COX-2 inhibitor NS-398 was used as a positive control (Figure 6B).
[14C]-AA metabolite profile analysis for the best compound
A highly specific [14C]-AA metabolite profile analysis was used to further validate the accuracy of the cross-screening results using enzyme immunoassay. The COX-2-10aa-mPGES-1 cells were incubated with [14C]-AA as the stable substrate in the presence of the individual compounds identified earlier. The metabolites produced by the conversion of [14C]-AA during the assay were separated by a C18-HPLC column and detected by a scintillation analyzer using real-time mode. [14C]-AA metabolite profiles with and without addition of compound 10 are shown in Figure 7. PGE2 production was reduced by more than 90% (@25 CPM) by compound 10 (Figure 7B). In contrast, in the absence of compound 10, PGE2 production was not inhibited (@290 CPM, Figure 7A).
Figure 7. . [14C]-AA metabolite-profile analysis for the best compound inhibiting PGE2 production by SC-COX-2-10aa-mPGES-1.
After incubation of compound 10 (50 μM) plus substrate AA (0.5 μM) with the HEK293 cells expressing SC-COX-2-10aa-mPGES-1 (0.5 mg) for 5 min, the metabolites from [14C]-AA were analyzed by the HPLC scintillation analyzer using real-time mode. (A) DMSO (a solvent for the compounds as a negative control) and (B) in the presence of compound 10.
Cross-screening using SC-COX-2-10aa-PGIS as a target to eliminate the compounds inhibiting PGI2 biosynthesis by COX-2 coupled to PGIS
A major side effect of currently available NSAIDs, especially COX-2 inhibitors, is vascular vulnerability from reduced COX-2 coupling to PGIS for PGI2 biosynthesis. PGI2 biosynthesis by COX-2-10aa-PGIS in the presence and absence of a COX-2 inhibitor was compared (Figure 8). The COX-2 inhibitor (NS-398) completely wiped out AA metabolization to the vascular protector PGI2 (Figure 8, A-NS398) compared to that of the control reagent (Figure 8A). Inhibition of PGI2 production is known to increase risk of cardiovascular diseases. Thus, if our cross-Enzymelink screening exhibits superior ability to identify the specific lead compound for inhibition of inflammatory PGE2 biosynthesis, it should exclusively inhibit COX-2 coupled to mPGES-1 without affecting COX-2 coupled to PGIS. Two of the top lead compounds, 10 and 12, were studied for cross-inhibition of PGI2 biosynthesis via COX-2-10aa-PGIS. Up to 1 mM, compound 10 did not show any inhibition of PGI2 biosynthesis by COX-2-coupled-to-PGIS (SC-COX-2-10aa-PGIS) (Figure 8, compound 10). Addition of 0.1 mM compound 11 did not show inhibition, but addition of 1 mM resulted in 40% inhibition of PGI2 production by COX-2-10aa-PGIS (Figure 8, compound 11). Thus, compound 10 was the most specific lead identified.
Figure 8. . Cross-screening to eliminate the compounds with cross-inhibitory PGI2 biosynthesis.
The cells were treated with NS-398 (100 μM, positive control), DMSO (negative control), compound 10 and compound 11. For compounds 10 and 11, 0.01 mM (red line) and 1 mM (black line) of the compounds were used to determine their effect on PGI2 biosynthesis by SC-COX-2-10aa-PGIS.
Discussion
PGE2 produced by inducible COX-2 coupled with mPGES-1 is directly related to the processes of inflammation and related diseases, such as cancer [29–34]. For decades since the discovery of COX-2 and mPGES-1, screening for inhibitors that reduce inflammatory PGE2 biosynthesis has been assayed by separately targeting upstream COX-2 and downstream mPGES-1. COX-2 inhibitors have been well developed, but their side effects caused by shutting down upstream COX-2 production of PGH2 affects all downstream syntheses of other prostanoids. Notably, decreased production of PGI2 has become a major issue as a significant promoter of heart diseases. Recently, lead compounds that inhibit mPGES-1 activity have been identified by several groups [20–25]. However, full characterization of their specificities on affecting PGI2 biosynthesis have not yet been settled. Another method using co-expressed COX-2 and mPGES-1 for determination of inflammatory PGE2 has also been reported. However, the compounds identified from the co-expression system could potentially bind to COX-2 rather than mPGES-1. The similar natures of the substrate/inhibitor binding pockets of the other COX-downstream syntheses sharing/cross-binding with that of mPGES-1 could result in as-of-yet fully identified inhibitory mPGES-1 lead compounds undesirably inhibiting other COX-downstream enzymes, such as PGIS. The previous methods highlight the difficulty of attaining high-specificity mPGES-1 inhibitors without affecting parallel downstream synthase activity, such as PGIS. The current study aimed to address these issues. A stable substrate is the basis for securing an enzyme assay reproducibility and accuracy. This study has demonstrated significant advantages of developing a more reliable and accurate enzyme assay for high specific lead compound screening. For example, the data described in Figure 3 clearly shows that using AA as a stable substrate represents a significant advance in higher specificity, reliability and effectively limited interference from endogenous non-inflammatory prostanoid biosynthesis. In drug discovery history, advances in drug target identification and characterization have served as the foundation for improvements in drug screening and discovery. Here, the cross-Enzymelink screening allows for rapid distinction of inhibition of COX coupled with either mPGES-1 or PGIS. From the data described in Figure 7, compound 10 is the top specific lead compound for exclusive inhibition of COX-2 coupled to mPGES-1 producing PGE2, absent cross-inhibition of COX-2 coupled to PGIS producing PGI2. In other words, compound 10 is a promising specific lead mPGES-1 inhibitor with great potential for development into a next-generation novel anti-inflammatory drug with greatly reduced heart disease risk. Thus, our reliable biosynthesis assay is a key to attaining inhibitory lead compounds that specifically target inflammatory PGE2 synthesized by COX-2 coupled to mPGES-1. It should be noted that the study is focused on finding the lead compounds of mPGES-1 inhibitors to eliminate the side effects of COX-2 inhibitor reducing PGI2 biosynthesis. Because of the lack of specific COX-1 inhibitors in clinical use and medically approved mPGES-1 inhibitors, we used COX-2 inhibitor as a positive control.
Using traditional methods, the cost of large-scale drug screening for mPGES-1 lead compounds is high because results require large quantities of expensive recombinant membrane proteins, unstable PGH2 and immunoassay kits. This study is the first instance of integrating virtual and wet screening, and single and cross-screening together for identification of specific lead compounds targeting inflammatory PGE2 biosynthesis with a low cost profile and short time horizon. For example, notably, the cost of the stable AA is approximately a mere 1–5% of that of synthetic PGH2.
In general, the established approach described in this study could be applied to develop screening of specific inhibitory lead compounds targeting other COX-downstream synthases as well. For example, an additional Enzymelink, COX-1-10aa-TXAS [13], was recently engineered, produced and characterized. Cross-screening using Enzymelinks COX-1-10aa-PGIS and COX-1-10aa-TXAS will be able to identify specific inhibitory lead compounds targeting TXAS to reduce thrombotic TXA2 production without inhibiting PGIS production of the counter-molecule, PGI2. Thus, the study presented here has significant impact for advancement of specific bioassays and drug screening within the field of prostanoid biosynthesis through the COX pathway. It should be indicated that current study was focused on establishing Enzymelink approach for drug screening and discovery. Further characterization of the identified individual lead compounds will be part of our future studies.
Conclusion
The study has demonstrated the superior use of Enzymelinks for drug screening and identification of the lead compounds that inhibit mPGES-1 while maintaining cellular prostacyclin-synthase activity. These findings suggest that our Enzymelink technology could be applied to advance drug discovery targeting additional COX-downstream synthases.
Future perspective
The Enzymelink approach reported here for cross-target drug screening has made available a new method for advancing future drug screening. For example, our next work with the Enzymelinks COX-1-10aa-PGIS and COX-1-10aa-TXAS cross-screening drug library is identifying lead compounds able to downregulate thrombotic TXA2 biosynthesis while simultaneously upregulating vascular-protective PGI2 biosynthesis to advance heart disease treatment. Lead compound 10 identified in this study will be further characterized for anti-inflammatory effects on inhibiting inflammatory factor-induced mPGES-1 expression in cell lines and in primary cultured cells. The functional groups of the identified compound 10 will be further modified and optimized to enhance therapeutic efficacy. The therapeutic effects will be further analyzed in cellular and animal models.
Summary points.
A set of Enzymelinks, COX-2-10aa-mPGES-1 and COX-2-10aa-PGIS were established and the first successfully used for cross-target drug screening.
The Enzymelink-based approach has advanced current drug screening, rapidly identifying lead compounds.
The top 15 lead compounds inhibiting mPGES-1 activity were rapidly identified by the combination of virtual and wet high-throughput screening using Enzymelinks as cross-targets.
The top compound that specifically inhibited inflammatory mPGES-1-based PGE2 biosynthesis alone without affecting COX-2 coupled to PGIS for PGI2 biosynthesis was obtained.
Footnotes
Author contributions
DT Ruan, H Akasaka, N Hong and R Lu performed the computational and other experiments, analyzed data and prepared the initial figures. K-H Ruan designed the experiments. DT Ruan made and presented the figures. DT Ruan and K-H Ruan wrote, edited and revised the manuscript.
Financial & competing interests disclosure
This work was supported by National Institutes of Health grants RO1 HL56712 and HL79389 to K-H Ruan and American Heart Association grants 10GRNT4470042 and 14GRNT20380687 to K-H Ruan. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Vane JR. Back to an aspirin a day? Science 296(5567), 474–475 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294(5548), 1871–1875 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Murakami M, Naraba H, Tanioka T et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 275(42), 32783–32792 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Makoto M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 68-69, 383–399 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Ruan KH. Advance in understanding the biosynthesis of prostacyclin and thromboxane A2 in the endoplasmic reticulum membrane via the cyclooxygenase pathway. Mini. Rev. Med. Chem. 4(6), 639–647 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Dogné JM, de Leval X, Hanson J et al. New developments on thromboxane and prostacyclin modulators part I: thromboxane modulators. Curr. Med. Chem. 11(10), 1223–1241 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Murakami PM, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 68-69, 383–399 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, Komoto J, Watanabe K et al. Crystal structure and possible catalytic mechanism of microsomal prostaglandin E synthase type 2 (mPGES-2). J. Mol. Biol. 348(5), 1163–1176 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Hironari A, So SP, Ruan KH. Relationship of the topological distances and activities between mPGES-1 and COX-2 versus COX-1: implications of the different post-translational endoplasmic reticulum organizations of COX-1 and COX-2. Biochemistry 54(23), 3707–3715 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Xu C, Huo X et al. The cyclooxygenase-1/mPGES-1/endothelial prostaglandin EP4 receptor pathway constrains myocardial ischemia-reperfusion injury. Nat. Commun. 10, 1888 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan KH, Cervantes V, So SP. Engineering of a novel hybrid enzyme: an anti-inflammatory drug target with triple catalytic activities directly converting arachidonic acid into the inflammatory prostaglandin E2. Protein Eng. Des. Sel. 22(12), 733–740 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan KH, Deng H, So SP. Engineering of a protein with cyclooxygenase and prostacyclin synthase activities that converts arachidonic acid to prostacyclin. Biochemistry 45(47), 14003–14011 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Ruan K-H, So S-P, Cervantes V et al. An active triple-catalytic hybrid enzyme engineered by linking cyclo-oxygenase isoform-1 to prostacyclin synthase that can constantly biosynthesize prostacyclin, the vascular protector. FEBS J. 275(23), 5820–5829 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Ann. Rev. Pharmacol. Toxicol. 38(January), 97–120; (1998). [DOI] [PubMed] [Google Scholar]
- 15.Ding K, Zhou Z, Hou S et al. Structure-based discovery of mPGES-1 inhibitors suitable for preclinical testing in wild-type mice as a new generation of anti-inflammatory drugs. Sci. Rep. 8, 5205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauro G, Cantone V, Potenza M et al. Discovery of 3-hydroxy-3-pyrrolin-2-one-based mPGES-1 inhibitors using a multi-step virtual screening protocol. Medchemcomm. 9(12), 2028–2036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou S, Zhou Z, Ding K et al. DREAM-in-CDM approach and identification of a new generation of anti-inflammatory drugs targeting mPGES-1. Sci. Rep. 10, 10187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozen G, Gomez I, Daci A et al. Inhibition of microsomal PGE synthase-1 reduces human vascular tone by increasing PGI2: a safer alternative to COX-2 inhibition. Br. J. Pharmacol. 174(22), 4087–4098 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Chen Y, Wang Y et al. Inhibition of COX-2, mPGES-1 and CYP4A by isoliquiritigenin blocks the angiogenic Akt signaling in glioma through ceRNA effect of miR-194-5p and lncRNA NEAT1. J. Exp. Clin. Cancer Res. 38, 371 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kats A, Båge T, Georgsson P, Jönsson J et al. Inhibition of microsomal prostaglandin E synthase-1 by aminothiazoles decreases prostaglandin E2 synthesis in vitro and ameliorates experimental periodontitis in vivo. FASEB J. 27(6), 2328–2341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francesco LD, Bruno A, Ricciotti E et al. Pharmacological characterization of the microsomal prostaglandin E2 synthase-1 inhibitor AF3485 in vitro and in vivo. Front Pharmacol. 11, 374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terracciano S, Lauro G, Strocchia M et al. Structural insights for the optimization of dihydropyrimidin-2(1H)-one based mPGES-1 inhibitors. ACS Med. Chem. Lett. 6(2), 187–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding K, Zhou Z, Zhou S et al. Design, synthesis, and discovery of 5-((1,3-diphenyl-1H-pyrazol-4-yl) methylene) pyrimidine-2,4,6(1H,3H,5H)-triones and related derivatives as novel inhibitors of mPGES-1. Bioorg. Med. Chem. Lett. 28(5), 858–862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waltenberger B, Wiechmann KW, Bauer J et al. Pharmacophore modeling and virtual screening for novel acidic inhibitors of microsomal prostaglandin E2 synthase-1 (mPGES-1). J. Med. Chem. 54(9), 3163–3174 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frédéric M, Guiral S, Fortin L-J et al. Mark mPGES-1 inhibitor screening: an automated multistep high-throughput screening assay for the identification of lead inhibitors of the inducible enzyme mPGES-1. J. Biomolec. Screen. 10(6), 599–605 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Orlando BJ, Lucido MJ, Malkowski MG. The structure of ibuprofen bound to cyclooxygenase-2. J. Struct. Biol. 189, 62–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y-C, Chiang C-W, Yeh H-C et al. Structures of prostacyclin synthase and its complexes with substrate analog and inhibitor reveal a ligand-specific heme conformation change. J. Biol. Chem. 283, 2917–2926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luz JG, Antonysamy S, Kuklish SL et al. Crystal structures of mPGES-1 inhibitor complexes form a basis for the rational design of potent analgesic and anti-inflammatory therapeutics. J. Med. Chem. 58, 4727–4737 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Joel S. Fluorine – a vital element in the medicine chest. Pharmaceuticals 26–30 (2005). [Google Scholar]
- 30.Liggett JL, Zhang X, Eling TE, Baek SJ. Anti-tumor activity of non-steroidal anti-inflammatory drugs: cyclooxygenase-independent targets. Cancer Lett. 346(2), 217–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masako N, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Sem. Immunopathol. 35(2), 123–137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masako N, Montrose DC, Clark P, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 68(9), 3251–3259 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Masako N, Gokhale V, Meuillet EJ, Rosenberg DW. MPGES-1 as a target for cancer suppression. a comprehensive invited review ‘phospholipase A2 and lipid mediators’. Biochimie. 92, 660–664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daisuke K, Murakami M, Nakatani Y et al. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J. Biol. Chem. 278(21), 19396–19405 (2003). [DOI] [PubMed] [Google Scholar]