ABSTRACT
Almost all life activities of plants are accompanied by electrophysiological information. Plant’s electrical parameters are considered to be the fastest response to environment. In this study, the theoretically intrinsic relationships between the clamping force and leaf resistance (R) and inductive reactance (XL) were revealed as 3-parameter exponential decay based on bioenergetics for the first time. The intrinsic resistance (IR), capacitive reactance (IXc), inductive reactance (IXL), impedance (IZ), and capacitance (IC) in plant leaves were successfully monitored. The nutrient flux per unit area (UNF), nutrient transfer rate (NTR) and nutrient transport capacity (NTC) in plants based on IR, IXc, IXL, IZ and IC were defined to reflect nutrient transport characteristics. The results indicate that IXc and IXL could be used to manifest the relative composition characteristics of cell membrane proteins, and are inversely proportional to the amount of surface and binding proteins that induce membrane Xc and XL in plant leaves, respectively. UNF, NTR or NTC exhibited good correlations with crude protein or crude ash, and accurately revealed the nutrient transport strategies of tested plants and their diversity. This study highlights that plant’s electrophysiological information could effectively manifest the composition and nutrient transport characteristics of membrane proteins in plant cells.
KEYWORDS: Electrophysiological information, bioenergetics, membrane protein composition, nutrient transport
1. Introduction
Almost all life activities in plants, including the metabolism of substance and energy, development, stress resistance and signal transduction, involve charge separation, electron movement, proton and dielectric transport, etc.1–4 The electrical signals (variation, action and system potential) in plants can directly or indirectly induce the changes of various physiological processes, including gene expression, photosynthesis, respiration, transpiration, phytohormon production, substance flow, energy metabolism and plant growth, etc.4,5 And the interactions between electrical activity and physiological processes in plants can be analyzed by simulation.6–8 Moreover, these responses are considered to increase plant resistance to stress factors, for example, the localized burning induced a variation potential that decreased photosynthesis parameters and increased photosystem II damage of pea leaves, in turn increased resistance of photosystem I to heating.9–12 The changes of structure, composition and ion permeability in plant cells by exogenous stressors will inevitably lead to significant changes in electrical signals.13–18
Therefore, plant’s electrical parameters are considered to be the fastest response to environmental stimulus such as drought, heat stimulation, cold stimulation, salt stimulation, diseases and insect pests, exogenous force.10,12,14,15,19–23 Many reports indicated that the impedance (Z) and capacitance (C) of plants have been used to evaluate plant’s physiological status.24–29 For instance, in our previous studies, Zhang et al. demonstrated that the electrophysiological properties of the plants could reflect their ability to resist drought and define leaf tensity based on physiological capacitance to represent plant drought resistance.29 And Javed et al. evaluated the irrigation effects of the diluted salted water using leaf tensity of plants.26 As well as Xing et al. used leaf tensity to rapidly determine water requirement information in Brassica napus L. and predict re-watering time of Orychophragmus violaceus L .28,30
Previously, a traditional approach, the electrical parameters in plants are measured by the insertion of two electrodes into the stem or leaf.31,32 However, this method is unstable and difficult to manipulate, and the acquired electrical signals lack representativeness, reproducibility and comparability because of needling injury, as well as different environments, users and other factors. Moreover, the intrinsic or spontaneous electrical parameters in plants are not detected by the needling method. In our previous reports, plant’s electrical parameters under specific clamping force have been successfully obtained using parallel-plate capacitor.26,28–30 Although this method can overcome the above defects, it also cannot detect the intrinsic real-time electrical parameters in plants. Guo et al. reported the capacitance (C) values of maize leaves increased with clamping forces, while this intrinsic mechanism or relationship between clamping force and the electrophysiological information of plants was not revealed.33 Thus, it is of great practical significance to clarify the intrinsic mechanism between clamping forces and electrophysiological parameters and provide a rapid, accurate and real-time technique for monitoring the physiological state of plant leaves.
The electrical properties of plant cells are derived from the cell membrane with a double electric layer. Membrane lipids and proteins, the main compositions of cell membrane, can be regarded as insulating layer, have a high electrical resistivity, enabling the plant cell to store electric charge.18,34 Generally, a mesophyll cell can be regarded as a concentric sphere capacitor with both inductor and resistor function, and many aligned mesophyll cells make up the leaf capacitor.2,23,35 The ions, ion groups and electric dipoles in mesophyll cells are electrolytes of leaf capacitor and most related to electrophysiological information.23,36 The electrophysiological information of plant leaves varied with the ions, ion groups and electric dipole concentrations in plant cells. Different clamping forces which can be regarded as different exogenous stimuli inevitably lead to the changes of the ion, ion group and electric dipole concentrations in plant leaves, which causes the changes of electrophysiological information of plants. In our previous study, the theoretically intrinsic relationships between the clamping force and leaf Z or capacitive reactance (Xc) and C were revealed as 3-parameter exponential decay or linear models for the first time, respectively.23 And the novel intracellular water use indices based on plant’s electrophysiological parameters accurately revealed the life strategies of intracellular water metabolism in plant leaves. Xing et al. found that leaf Z which obtained by using the above intrinsic relationship provides more reliable information of plant water status compared with water potential, and defined leaf water dissipation rate based on leaf Z .22
Cells are the site of all biochemical reactions, and cell membrane side is an important barrier to ensure a stable environment inside the cell. It has been estimated that 15 ~ 30% of the nuclear gene encoded proteins are involved in nutrient transport on the cell membrane, and the energy used by cells in nutrient transport up to two-thirds of the total energy consumed by cells.17 The nutrient transport capacity of cells is most closely related to the type and quantity of surface and binding proteins in cell membrane; thus, the composition and content of membrane protein can indirectly reflect the nutrient transport capacity of cells. Protein detection methods of biological samples include conventional, electrochemical, molecular biology, electrophoresis and mass spectrometry methods.37 However, the detection of membrane proteins is limited to single cell or single proteins, and the existing protein detection technology is difficult to accurately evaluate the composition characteristic of cell membrane protein.37,38 Moreover, the nutrient transport capacity ultimately affects the nutrient use efficiency of plants, and the most commonly used method of plant nutrient use evaluation is the ratio of total nutrient in plants to total input nutrient.39,40 However, this nutrient use efficiency also does not directly reflect the nutrient transport capacity. To the best of our knowledge, the composition and nutrient transport characteristics of membrane protein has rarely been reported.
The fully expanded leaves, which account for a high proportion of plant biomass, determine and reflect the plant nutrient metabolism. Since the concentration of electrolytes in cells (ions, ion groups and electric dipoles) in leaf cells is directly affected by the nutrient metabolism in plant leaves, and then it is accompanied by vigorously electrical activities. In this study, it was first clarified and constructed the intrinsic mechanisms and physical models between clamping forces and leaf resistance (R), inductive reactance (XL). Subsequently, the intrinsic electrophysiological parameters in plant leaves were monitored through these mechanism equations. And then the nutrient flux per unit area (UNF), nutrient transfer rate (NTR) and nutrient transport capacity (NTC) in plant leaves in the light of the intrinsic electrophysiological parameters were defined to evaluate the nutrient transport strategies of various tested plants. This study aims to clarify the intrinsic mechanisms among the electrophysiological information in plants and cell membrane proteins, and provide a novel, feasible technique for real-time monitoring plant nutrient transport.
2. Materials and methods
2.1. Experimental materials
The two Broussonetia papyrifera grown in the agricultural and moderate rocky desertification soil in Puding county, Guizhou Province (26°37′ N, 105°77′ E). Rhus chinensis Mill. and Toona sinensis grown in the moderate rocky desertification soil in Puding county, and Ipomoea batatas (L.) Lam. and Senecio scandens Buch.-Ham. ex D. grown in the cultivated soil in Puding county. Solanum tuberosum L. and Capsicum annuum L. were grown in the potted agricultural soil of Guizhou vocational college of agriculture in Qingzhen county, Guizhou Province (26°58′ N, 106°43′ E). The average annual temperature, sunshine hour and precipitation in Puding and Qingzhen counties were 15.1°C and 14.1°C, 1164.9 and 1128.2 hours and 1378.2 and 1180.9 mm, respectively. The growth age, habitat information, measurement conditions and sampling weather of all tested plants are shown in Table 1. The fully expanded leaves of fresh branch as experimental materials were measured. First, the fully expanded leaves were taken from the third, fourth, and fifth leaf positions of each branch, and the fresh leaves were immediately soaked in water for 30 min. Then, the water on the surface of the leaves was removed. Three branches of each plant were measured. The tested leaves were sampled and measured at 8 ~ 10 a.m. on sunny days, and the measurement temperature was room temperature (25.0 ± 2.0°C).
Table 1.
Growth age, habitat information, measuring condition and sampling weather of all tested plants
| Plants | Places | Age (Year) | Habitats | Soil properties |
Measurement conditions |
Sampling weather | |||
|---|---|---|---|---|---|---|---|---|---|
| pH | Organic matter content (g/kg) | Soil moisture content (%) | Time | Temperature (°C) |
|||||
| B. papyrifera 1 | Puding county | 1 | AS | 6.27 ± 0.03 | 4.35 ± 0.65 | 18.46 ± 0.02 | 2018.08.25 a.m. | 25.0 ± 2.0 | Sunny |
| B. papyrifera 2 | 1 | MRDS | 6.85 ± 0.03 | 3.58 ± 0.33 | 15.51 ± 0.02 | ||||
| T. sinensis | 3 | MRDS | 6.67 ± 0.03 | 3.65 ± 0.05 | 15.73 ± 0.19 | 2018.08.26 a.m. | |||
| R. chinensis | 3 | MRDS | 6.81 ± 0.03 | 3.64 ± 0.27 | 16.13 ± 0.15 | 2018.08.24 a.m. | |||
| S. scandens | 1 | MRDS | 6.44 ± 0.02 | 4.98 ± 0.34 | 19.17 ± 0.21 | 2018.08.27 a.m. | |||
| I. batatas | 1 | AS | 6.31 ± 0.01 | 4.63 ± 0.21 | 18.53 ± 0.42 | 2018.08.26 a.m. | |||
| S. tuberosum | Qingzhen county | 1 | PAS | 6.32 ± 0.05 | 4.82 ± 0.53 | 19.65 ± 0.21 | 2018.08.15 a.m. | ||
| C. annuum | 1 | PAS | 6.34 ± 0.07 | 4.86 ± 0.31 | 19.72 ± 0.13 | ||||
Note: AS: agricultural soil, MRDS: moderately rocky desertification soil, PAS: potted agricultural soil.
2.2. Leaf electrophysiological parameters and crude ash measurement
The fully expanded leaves from the third, fourth, and fifth leaf positions of three branches in plants were measured. The leaf electrophysiological parameters were measured using a LCR-6300 tester (Gwinstek, Taiwan, China) with a frequency and voltage of 3 kHz and 1.5 V, respectively, as described by our previous studies.22,23,29 Every mesophyll cell can be regarded as a concentric sphere capacitor, many aligned mesophyll cells make up the leaf capacitor, the parallel connection modes of LCR is thus applied. Firstly, the leaf was put between the two electrodes of a self-made parallel-plate capacitor with a diameter of 7 mm, which the experimental setup and a schematic diagram of the parallel-plate capacitor were used as described by Zhang et al.23 And then leaf C, Z and R at different clamping forces were continuously collected by adding the same quality iron blocks, and recorded 11–13 data each clamping force. Finally, leaf Xc and XL were, respectively, obtained according to formula (1) and (2):
| (1) |
| (2) |
where Xc = capacitive reactance, π = 3.1416, f = frequency, C = physiological capacitance, XL = inductive reactance, Z = impedance, R = resistance.
Three tested leaves of each branch were rinsed with distilled water, dried in the shade, smashed and mixed for the determination of crude ash and protein. Crude ash and protein of samples were determined as described by Rayees et al. with slight modifications.41 5.00 g of the sample was placed in a crucible and then the content of crude ash was measured by incinerating the sample in muffle furnace at 500 ± 25.0 C for 6 hours. For crude protein determination, 2.00 g of the sample was digested using copper catalyst and sulfuric acid, and then titrated by 0.1 mol/L hydrochloric acid for nitrogen measurement after the distillation of ammonia. The content of crude protein was calculated by multiplying the conversion coefficient 6.25 by the percentage of nitrogen according to Kjeldhal method.
2.3. Intrinsic mechanism relationships of clamping force (F) and leaf R, Xc and XL
Mesophyll cell can be regarded as a concentric sphere capacitor with both inductor and resistor functions.23 The simplified equivalent circuit of mesophyll cell is displayed in Figure 1. The ions, ion groups and electric dipoles in mesophyll cells were used as electrolytes, and a parallel-plate capacitor sensor could be formed by placing the leaf between the two plates of the parallel-plate capacitor. The leaf R, Xc and XL varied with the ions, ion groups and electric dipole concentrations in the plant leaves, and different clamping forces which can be regarded as different exogenous stimuli inevitably lead to the changes of the ion, ion group and electric dipole concentrations in plant leaves.
Figure 1.
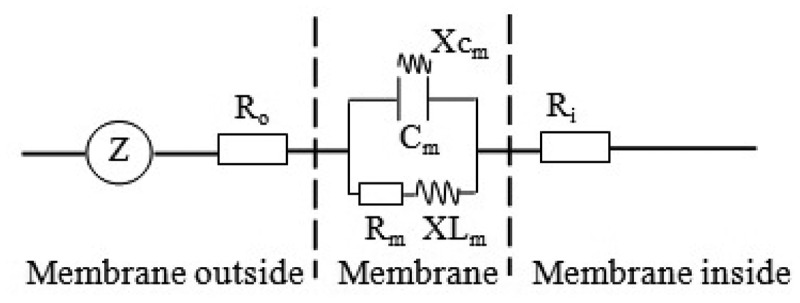
Simplified equivalent circuit of cells. Z = impedance, Cm = capacitance of membrane, Rm = resistance of membrane, Xcm = capacitive reactance of membrane, XLm = inductive reactance of membrane, Ro = resistance outside membrane, Ri = resistance inside membrane
The concentration of the electrolytes determines R inside and outside the cell membrane. External stimuli change the membrane permeability of the electrolytes and affect their inside and outside concentration of the cell membrane.23 Under different clamping forces, the membrane permeability of the electrolytes that respond to R in the plant cell membrane changed. According to the bioenergetics, the Nernst equation can be used to quantitatively describe the potential of electrolytes inside and outside the cell membrane.23 Thus, the concentration differences in the electrolytes that respond to R inside and outside the cell membrane obey the Nernst equation and can be expressed as follows:
| (3) |
where E = the electromotive force (V), E0 = the standard electromotive force (V) which is the potential of electrolytes inside and outside cell membrane under standard (or resting) state, R0 = the gas constant (8.314570 J K−1 mol−1), T = the thermodynamic temperature (K), Ci = the concentration of the electrolytes that respond to R inside the cell membrane (mol L−1), Co = the concentration of the electrolytes that respond to R outside the cell membrane (mol L−1), F0 = Faraday constant (96485 C mol−1), and nR = the number of transferred electrolytes (mol).
A mesophyll cell can be regarded as a concentric sphere capacitor. When leaf cell container is subjected to clamping force, the pressure work (W) done by clamping force can be expressed as follows:
| (4) |
where P = the pressure intensity on the leaf cells (Pa), V = the cell volume (m3).
Different clamping forces inevitably lead to the changes of the ion, ion group and electric dipole concentrations in leaf cells, and the electromotive force of the leaf cell capacitor change accordingly. Thus, the pressure work done by clamping force on leaf cells can be converted into the internal energy of the electromotive force, and they have a direct relationship, W = a E, that is:
| (5) |
where P = the pressure intensity on the leaf cells (Pa), a = the energy conversion coefficient of the electromotive force, and V = the cell volume (m3). , where F = the clamping force (N) and S = the effective area of the electrode plate (m2). F can be calculated by the gravity formula:
| (6) |
where M = the iron block mass (kg), m = the mass of the plastic rod and the plate electrode (kg), and g = 9.8 N/kg.
For mesophyll cells, the sum of Co and Ci is certain. Ci is directly proportional to the conductivity of the electrolytes that respond to R, and the conductivity is the reciprocal of R. Hence, can be expressed as , where f0 = the ratio coefficient of the conversion between Ci and R (that is, R caused by unit Ci), and CT = Co ± Ci. Therefore, formula (5) was transformed into formula (7):
| (7) |
Formula (7) was rewritten:
| (8) |
and
| (9) |
Formula (9) takes the exponents of both sides:
| (10) |
Further:
| (11) |
Because d, formula (11) was transformed into:
| (12) |
For the same leaf tested in the same environment, the d, a, E0,R0,T, nR, F0, CT, and f0 of formula (12) are constant. Let =, k1 =, b1 =, and the intrinsic mechanism relationships of leaf R and F was:
| (13) |
where y0, k1 and b1 are model parameters.
When F = 0, the intrinsic resistance (IR) of the plant leaves could be obtained:
| (14) |
Similar to R, the intrinsic mechanism relationships of leaf Xc and F were revealed as in our previous study :23
| (15) |
where p0, k2 and b2 are model parameters.
When F = 0, the intrinsic capacitive reactance (IXc) of plant leaves could be calculated as:
| (16) |
Similar to R, the intrinsic mechanism relationships of leaf XL and F are revealed as (Additional file 1):
| (17) |
where q0, k3 and b3 are model parameters.
When F = 0, the intrinsic inductive reactance (IXL) of plant leaves could be calculated as:
| (18) |
Thus, the intrinsic impedance (IZ) and intrinsic capacitance (IC) of plant leaves were, respectively, obtained according to formula (19) and (20):
| (19) |
| (20) |
where π = 3.1416, f = frequency.
2.4. Determination of the nutrient transport parameters
Plant cells have the electrical properties of low capacitance and high resistance, it could be assumed that electrical cells were connected in parallel manner, and many aligned mesophyll cells make up the leaf capacitor. The IR of the leaf cells in plant is calculated according to formula (21):
| (21) |
It is assumed that the inside and outside membrane resistance of each cell is equal; then, IR1, IR2, IR3, … IRn can represent intrinsic resistance of each unit cell membrane. It is assumed that the intrinsic resistance of each cell membrane is equal, that is IR1 = IR2 = IR3 = … = IRn = IR0. Thus, the IR of the plant leaves was obtained:
| (22) |
Due to membrane resistance being most closely related to proteins and lipids of cell membrane, then n can be characterized as the relative amount of proteins and lipids that induce membrane R in plant leaves.
Similarly, the IXc of the leaf cells in plant was obtained:
| (23) |
Due to membrane capacitive resistance being most closely related to surface proteins of cell membrane, then IXc or p can be characterized as the relative amount of surface proteins that induce membrane capacitive resistance in plant leaves. Clearly, IXc is inversely proportional to p. The lower IXc, the more surface proteins that induce membrane Xc in plant leaves.
Similarly, the IXL of the leaf cells in plant was obtained:
| (24) |
Due to membrane inductive resistance is most closely related to binding proteins of cell membrane, then IXL or q can be characterized as the relative amount of binding proteins that induce membrane inductive resistance in plant leaves. Similarly, IXL is inversely proportional to q. The lower IXL, the more binding proteins that induce membrane XL in plant leaves.
The cell membrane proteins are most closely related to the nutrient transport; thus, the relative nutrient flux per unit area (UNF) could be represented by formula (25):
| (25) |
Moreover, in our previous report, the intracellular water transfer rate of plant leaves was defined and applied.23 Since nutrients are soluble in water, the water transfer rate and the nutrient transfer rate (NTR) are conceptually similar and assigned the same value; thus, it can be calculated by formula (26):
| (26) |
Therefore, the nutrient transport capacity (NTC) is UNF multiplied by NTR:
| (27) |
2.5. Data analyses
Data were shown as the means ± standard deviation (SD) (n = 9). The data were analyzed using Statistical Package for the Social Sciences (SPSS 18.0) software (SPSS Inc., Chicago, IL, USA). The difference significances between-group means were treated statistically by one-way analysis of variance (ANOVA). A correlation matrix of the study was based on Pearson’s correlation coefficients.
3. Results
3.1. Verification of intrinsic mechanism relationships
The fitting equation parameters of between clamping force and leaf R, Xc, and XL of B. papyrifera grown in agricultural and moderately rocky desertification soils are shown in Table 2. Figure 2 randomly lists the fitting curves for 1–4 leaf of B. papyrifera in agricultural soil. The correlation coefficients (R2) of the fitting equations of R-F, Xc-F, and XL-F for nine leaves of B. papyrifera grown in agricultural and moderately rocky desertification soils were 0.9044 ~ 0.9929, 0.9033 ~ 0.9910 and 0.9085 ~ 0.9895, and 0.9722 ~ 0.9976, 0.9910 ~ 0.9986 and 0.9862 ~ 0.9976, respectively. Moreover, all the P values of the fitting equation parameters were lower than 0.0001. These results show that the relationships of between clamping force and leaf R, Xc, and XL display good correlations, and highlight that the intrinsic mechanism relationships of those are authentic existence.
Table 2.
The fitting equation parameters of B. papyrifera in two habitats
| B. papyrifera | Branch-leaf | R-F |
Xc-F |
XL-F |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| y0/k1/b1 | R2 | p< | p0/k2/b2 | R2 | p< | q0/k2/b2 | R2 | p< | ||
| AS-Bp | 1–3 | 0.08/0.18/0.29 | 0.9692 | 0.0001 | 0.05/0.45/0.26 | 0.9302 | 0.0001 | 0.11/0.39/0.27 | 0.9450 | 0.0001 |
| 1–4 | 0.05/0.23/0.37 | 0.9815 | 0.0001 | 0.05/0.23/0.48 | 0.9789 | 0.0001 | 0.08/0.39/0.42 | 0.9790 | 0.0001 | |
| 1–5 | 0.08/0.07/0.39 | 0.9920 | 0.0001 | 0.06/0.33/0.86 | 0.9826 | 0.0001 | 0.13/0.35/0.55 | 0.9794 | 0.0001 | |
| 2–3 | 0.06/0.20/0.50 | 0.9515 | 0.0001 | 0.13/0.38/0.39 | 0.9617 | 0.0001 | 0.10/0.36/0.78 | 0.9372 | 0.0001 | |
| 2–4 | 0.06/0.23/1.23 | 0.9044 | 0.0001 | 0.05/0.38/1.45 | 0.9033 | 0.0001 | 0.09/0.52/1.35 | 0.9085 | 0.0001 | |
| 2–5 | 0.08/0.10/0.48 | 0.9897 | 0.0001 | 0.07/0.32/0.60 | 0.9802 | 0.0001 | 0.13/0.39/0.55 | 0.9486 | 0.0001 | |
| 3–3 | 0.09/0.12/0.33 | 0.9584 | 0.0001 | 0.10/0.39/0.21 | 0.9589 | 0.0001 | 0.17/0.36/0.24 | 0.9607 | 0.0001 | |
| 3–4 | 0.07/0.20/0.83 | 0.9864 | 0.0001 | 0.14/0.38/0.93 | 0.9910 | 0.0001 | 0.12/0.37/0.67 | 0.9815 | 0.0001 | |
| 3–5 | 0.14/0.23/0.60 | 0.9929 | 0.0001 | 0.15/0.35/0.92 | 0.9873 | 0.0001 | 0.15/0.35/0.77 | 0.9895 | 0.0001 | |
| MRDS-Bp | 1–3 | 2.94/38.07/0.60 | 0.9952 | 0.0001 | 2.95/4.83/0.20 | 0.9968 | 0.0001 | 5.93/38.75/0.58 | 0.9955 | 0.0001 |
| 1–4 | 3.05/31.34/0.93 | 0.9722 | 0.0001 | 3.53/3.62/0.44 | 0.9912 | 0.0001 | 5.99/30.86/0.88 | 0.9862 | 0.0001 | |
| 1–5 | 4.42/34.92/0.75 | 0.9936 | 0.0001 | 3.65/3.56/0.32 | 0.9968 | 0.0001 | 7.18/35.14/0.71 | 0.9939 | 0.0001 | |
| 2–3 | 1.06/29.00/0.46 | 0.9936 | 0.0001 | 2.95/4.83/0.20 | 0.9968 | 0.0001 | 2.83/29.96/0.41 | 0.9911 | 0.0001 | |
| 2–4 | 1.76/43.49/0.92 | 0.9976 | 0.0001 | 2.34/5.61/0.35 | 0.9932 | 0.0001 | 3.99/42.87/0.84 | 0.9968 | 0.0001 | |
| 2–5 | 4.36/30.58/0.37 | 0.9944 | 0.0001 | 3.10/3.94/0.13 | 0.9936 | 0.0001 | 7.29/31.44/0.37 | 0.9944 | 0.0001 | |
| 3–3 | 4.36/35.92/0.54 | 0.9974 | 0.0001 | 3.59/4.04/0.22 | 0.9986 | 0.0001 | 7.30/36.80/0.52 | 0.9976 | 0.0001 | |
| 3–4 | 2.38/23.44/0.47 | 0.9940 | 0.0001 | 2.98/3.96/0.20 | 0.9910 | 0.0001 | 5.16/24.31/0.45 | 0.9942 | 0.0001 | |
| 3–5 | 8.23/30.93/0.38 | 0.9956 | 0.0001 | 4.41/3.10/0.23 | 0.9962 | 0.0001 | 11.14/32.08/0.37 | 0.9958 | 0.0001 | |
Figure 2.
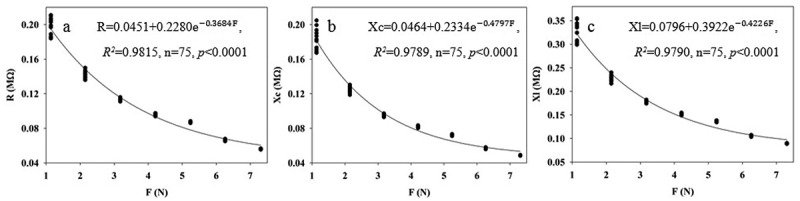
Fitting equations of the relationship between R (a), Xc (b), XL (c) of the fourth expanded leaf of the first branch of B. papyrifera grown in the agricultural soils and champing force (f)
AS-Bp: B. papyrifera grown in the agricultural soil, MRDS-Bp: B. papyrifera grown in the moderate rocky desertification soil, the same as below.
3.2. Electrophysiological information and nutrient transport of B. papyrifera grow in two habitats
The intrinsic electrophysiological information and the nutrient transport capacity of B. papyrifera in two habitats were successfully monitored using the corresponding equation parameters. As shown in Table 3, the leaf IR, IXc, IXL and IZ of B. papyrifera in the agricultural soil are significantly (p < .01) lower than those of that in the moderately rocky desertification soil, and had higher (p < .01) IC. Theoretically, the lower IXc and IXL, the more surface and binding proteins that induce, respectively, membrane Xc and XL in plant leaves. Actually, crude protein of B. papyrifera in the agricultural soil is significantly (p < .01) higher than those of that in the moderately rocky desertification soil (Figure 3(a)), which is in good agreement with IXc and IXL. Moreover, for the same plant, the leaf IXc is lower than IXL which shows that binding proteins are more than surface proteins. As displayed in Table 3, the leaf of B. papyrifera in the agricultural soil had lower (p < .01) UNF compared with that in the moderately rocky desertification soil, but its NTR is higher (p < .01) which supports it with a higher (p < .01) NTC. As shown in Figure 3(a), crude ash of B. papyrifera in the agricultural soil is significantly (p < .01) higher than those of that in the moderately rocky desertification soil, which is highly consistent with NTC.
Table 3.
The electrophysiological and nutrient transport parameters of B. papyrifera in two habitats
| Plants | IR (MΩ) | IXc (MΩ) | IXL (MΩ) | IZ (MΩ) | IC (pF) | UNF | NTR | NTC |
|---|---|---|---|---|---|---|---|---|
| AS-Bp | 0.25 ± 0.06 bB | 0.46 ± 0.06 bB | 0.51 ± 0.04 bB | 0.24 ± 0.07 bB | 118.01 ± 15.60 aA | 1.05 ± 0.24 bB | 48.80 ± 17.12 aA | 48.60 ± 9.77 aA |
| MRDS-Bp | 36.69 ± 6.01 aA | 7.44 ± 0.37 aA | 39.89 ± 5.87 aA | 7.32 ± 0.39 aA | 7.14 ± 0.36 bB | 5.84 ± 0.70 aA | 0.37 ± 0.03 bB | 2.13 ± 0.24 bB |
aValues indicate the mean ± SD, n = 9. Small letters indicate significant differences at 5% level (p < 0.05), and capital letters indicate significant differences at 1% level (p < 0.01).
Figure 3.
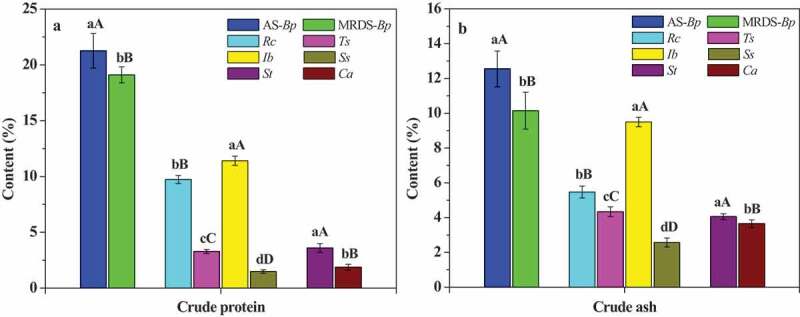
Crude protein (a) and crude ash (b) of six plants. Rc: R. chinensis, Ts: T. sinensis, Ib: I. batatas, Ss: S. scandens, St: S. tuberosum, Ca: C. annuum
3.3. Correlation of IR, IXc, IXL, UNF, NTR, NTC, crude protein and crude ash
The Pearson correlation coefficients for the relationship of IR, IXc, IXL, UNF, NTR, NTC, crude protein and crude ash are shown in Table 4. IR is significantly correlated with UNF. IXc is significantly correlated with NTR. IXL of B. papyrifera in the moderately rocky desertification soil is significantly correlated with UNF and NTR. IXc and IXL are significantly negatively correlated with crude protein, which is in accordance with the lower IXc and IXL, the more surface and binding proteins that induce, respectively, membrane Xc and XL in plant leaves. UNF and NTC are significantly correlated with crude protein. NTR of B. papyrifera in the moderately rocky desertification soil is significantly correlated with crude ash. NTC of B. papyrifera in the agricultural soil is significantly correlated with crude ash. These results demonstrate that IR, IXc, IXL, UNF, NTR or NTC exhibit good correlations with crude protein and crude ash.
Table 4.
Correlation between the electrophysiological and nutrient transport parameters of B. papyrifera.
| Parameters | AS-Bp |
MRDS-Bp |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UNF | NTR | NTC | Crude protein |
Crude ash |
UNF | NTR | NTC | Crude protein |
Crude ash |
|
| IR | 0.96a | −0.83a | −0.38 | −0.50 | −0.26 | 0.96a | −0.63 | 0.59* | −0.25 | 0.55* |
| IXC | 0.23 | −0.77* | −.99a | −0.69* | −0.55* | 0.38 | −1.00a | −0.28 | −0.71* | 0.72* |
| IXL | −0.01 | 0.05 | 0.14 | −0.65* | −0.24 | 0.96a | −0.60* | 0.62 | −0.50* | 0.53* |
| UNF | −0.77* | −0.14 | 0.75* | −0.04 | −0.41 | 0.78* | −0.65* | 0.40 | ||
| NTR | 0.70* | −0.18 | 0.35 | 0.24 | 0.24 | −0.72* | ||||
| NTC | 0.86a | 0.69* | −0.56* | −0.06 | ||||||
aCorrelation is significant at the 0.01 level, *Correlation is significant at the 0.05 level (2-tailed).
3.4. Electrophysiological information and nutrient transport of the herbaceous and woody plants
As illustrated in Table 5, the IR, IXc, IXL, IZ and IC of different plants are obviously different, the IXc is lower than IXL in same plant. As shown in Table 4 and Figure 3, the UNF, NTC, crude protein and crude ash of R. chinensis are significantly (p < .01) higher than those of T. sinensis, and its NTR is significantly (p < .01) lower. The NTR, NTC, crude protein and crude ash of I. batatas were significantly (p < .01) higher than those of S. scandens, while UNF is more low.
Table 5.
The electrophysiological and nutrient transport parameters of four plants
| Plants | IR (MΩ) | IXc (MΩ) | IXL (MΩ) | IZ (MΩ) | IC (pF) | UNF | NTR | NTC |
|---|---|---|---|---|---|---|---|---|
| Rc | 6.70 ± 0.74 bB | 1.63 ± 0.13 bcB | 1.81 ± 0.09 cB | 4.86 ± 1.22 bB | 32.84 ± 2.88 aA | 7.86 ± 1.14 aA | 1.25 ± 0.34 bB | 9.87 ± 3.37 aA |
| Ts | 3.10 ± 0.66 cC | 2.24 ± 0.41 aA | 3.03 ± 0.32 aA | 2.32 ± 0.61 cC | 24.44 ± 4.60 bA | 2.43 ± 0.43 cC | 2.34 ± 0.95 aA | 5.49 ± 1.71 bB |
| Ib | 5.67 ± 0.72 bB | 1.94 ± 0.34 bA | 2.12 ± 0.45 bB | 4.72 ± 0.92 bB | 28.16 ± 5.40 aA | 5.73 ± 0.96 bB | 1.17 ± 0.31 bB | 6.73 ± 2.34 bB |
| Ss | 12.17 ± 0.46 aA | 2.75 ± 0.41 aA | 2.86 ± 0.45 aA | 10.62 ± 1.73 aA | 19.76 ± 3.29 cB | 8.87 ± 1.38 aA | 0.43 ± 0.09 cC | 3.84 ± 1.14 cC |
Small letters indicate significant differences at 5% level (p < 0.05), and capital letters indicate significant differences at 1% level (p < 0.01).
3.5. Electrophysiological information and nutrient transport of S. tuberosum and C. annuum
As shown in Table 6, the leaf IR, IXc, IXL and IZ of S. tuberosum are significantly (p < .01) lower than those of C. annuum in the same growth habitat, while IC is higher (p < .01). And IXc is lower than IXL in the same plant. Crude protein and crude ash of S. tuberosum are significant (p < .01) higher than that of C. annuum (Figure 3(a, b)). S. tuberosum has lower (p < .01) UNF compared with C. annuum, while its NTR and NTC are higher (p < .01).
Table 6.
The electrophysiological and nutrient transport parameters of S. tuberosum and C. annuum.
| Plants | IR (MΩ) | IXc (MΩ) | IXL (MΩ) | IZ (MΩ) | IC (pF) | UNF | NTR | NTC |
|---|---|---|---|---|---|---|---|---|
| St | 0.31 ± 0.01 bB | 0.28 ± 0.05 bB | 0.46 ± 0.04 bB | 0.22 ± 0.03 bB | 193.73 ± 37.20 aA | 1.78 ± 0.20 bB | 66.64 ± 17.31 aA | 121.47 ± 47.99 aA |
| Ca | 4.07 ± 1.99 aA | 1.61 ± 0.29 aA | 4.94 ± 1.97 aA | 1.47 ± 0.28 aA | 34.16 ± 7.08 bB | 3.31 ± 0.98 aA | 4.16 ± 1.34 bB | 13.49 ± 4.67 bB |
Small letters indicate significant differences at 5% level (p < 0.05), and capital letters indicate significant differences at 1% level (p < 0.01).
4. Discussion
Almost all life activities in plants involve charge separation, electron movement, proton and dielectric transport, etc.1–4 In mesophyll cells, cells and organelles are both surrounded by the cell membrane composed of 50% lipids, 40% proteins and 2 ~ 10% sugars.34 Membrane lipids and membrane proteins can be regarded as insulating layer, have a high electrical resistivity, enabling the plant cell to store electric charge.18 Surface (or peripheral) proteins account for 20 ~ 30% of membrane proteins, bind to lipids on both sides of the membrane with charged amino acids or groups, and binding (or intrinsic) proteins account for 70 ~ 80% of membrane proteins, bind to lipids through hydrophobic hydroxyl groups in the membrane.34 Surface proteins affect the capacitive reactance and capacitance, while binding proteins affect the inductive reactance and inductance. Therefore, the mesophyll cells can be regarded as a concentric sphere capacitor with both inductor and resistor function, and the ions, ion groups and electric dipoles are equivalent to electrolytes of capacitor.4,23,35,36
When plant leaves are subjected to clamping force stimuli (or environmental stresses), the cell membrane permeability of leaves changes instantly, and then the concentrations of the ion, ion group and electric dipole inevitably change, resulting in the changes of the leaf R, Xc and XL. As a major discovery in plant electrophysiology, the theoretically intrinsic relationships between the clamping force and leaf Z or Xc was revealed as three-parameter exponential decay model based on Nernst equation in our previous study.23 Nernst equation can quantitatively describe the potential formed by ions between systems A and B, and it can theoretically also be used to quantitatively describe the diffusion potential of the electrolytes inside and outside the cell membrane. Based on this fact, the R or XL = y of the theoretically intrinsic relationships between clamping force and leaf R or XL also was revealed for the first time. The results in this study showed that the relationships between clamping force and leaf R, Xc, and XL displayed good correlations, and highlight that the aforementioned intrinsic mechanism is authentic existence. Generally, the intrinsic real-time electrophysiological information in plants are not detectable.21 In this study, the IR, IXc, IXL, IZ and IC of plant leaves were successfully obtained via the theoretically intrinsic relationships between clamping force and leaf electrophysiological parameters, which overcome the poor representativeness, stability and reproducibility of the traditional needing approach.
Currently, the detection of membrane proteins is limited to single cell or single proteins, and the existing protein detection technology hardly evaluate the composition characteristics of cell membrane protein.37,38 The results in this study showed that IXc and IXL were significantly negatively correlated with crude protein. It supported that IXc and IXL could be used to manifest the relative composition of surface and binding proteins in cell membrane, that was, the lower IXc and IXL, the more surface and binding proteins that induce, respectively, membrane Xc and XL in plant leaves. This is closely related to the fact that the high content of membrane proteins promoted the nutrient elements to pass through cell membrane more smoothly and orderly, thus made the cell membrane resistivity lower.42 In this study, plant with high crude proteins had relatively lower IR, IXc, IXL, IZ and higher IC, which strongly supported the feasibility of using IXc and IXL to characterize the composition characteristics of membrane proteins. This study found that a phenomenon was common in all tested plants, that was, the IXc was lower than IXL in same plant. This result perfectly proves the life fact that binding proteins are more than surface proteins on cell membrane.4,14,34
In this study, the results showed that UNF, NTR or NTC exhibited good correlations with crude protein or crude ash, which supported that they could reflect the nutrient metabolism of plants. Due to the poor nutritional environments, plants in rocky desertification soils are more vulnerable to low nutrient stress than those in cultivated soils.26,30,43 The results showed that B. papyrifera in the agricultural soil had lower IR, IXc, IXL, IZ, UNF and higher IC, NTR, NTC, crude ash, crude protein as compared to that in the moderately rocky desertification soil. B. papyrifera in the agricultural soil grow well under the high nutrient (or crude ash) conditions, and its cell membrane proteins (crude protein) were relatively much and nutrients are efficiently transport which supported it higher NTR and NTC. B. papyrifera in the moderately rocky desertification soil had higher UNF which supported its tolerance to low nutrient stress and adaptation to harsh environments. NTC was significantly positively correlated to crude protein and crude ash of B. papyrifera in the agricultural soil, and negatively correlated to those of B. papyrifera in the moderately rocky desertification soil. NTR was significantly negatively correlated to crude ash of B. papyrifera in the moderately rocky desertification soil, and non-significantly positively correlated to that of B. papyrifera in the agricultural soil. This also indicated that the same plant has different nutrient metabolism strategies in different habitats. The UNF, NTC, crude protein and crude ash of R. chinensis are higher than those of T. sinensis, but its NTR is lower. The NTR, NTC, crude protein and crude ash of I. batatas were higher than those of S. scandens, while UNF is more low. The results showed that the higher NTC in same species plants, the higher crude protein and crude ash, as well as the nutrient transport of plants has diversity. The IR, IXc, IXL, IZ, UNF of S. tuberosum are lower than those of C. annuum, and its IC, NTR, NTC, crude ash, crude protein are higher. The results showed that S. tuberosum with high membrane protein (crude protein) and nutrient (crude ash) contents promote the efficient transport and utilization of nutrients by its membrane proteins, which made it had higher NTR and NTC.
These results obviously showed that the nutrient transport of plants had diversity, and four nutrient transport strategies in the tested plants were found, which are (1) low UNF, high NTR, high NTC (AS-Bp, Ib, St), (2) high UNF, low NTR, low NTC (MRDS-Bp, Ss, Ca), (3) high UNF, low NTR, high NTC (Rc), (4) low UNF, high NTR, low NTC (Ts). Previously, the monitoring of the transport capacity of plant nutrients has rarely been reported. Innovatively, UNF, NTR and NTC defined based on IR, IXc, IXL, IZ and IC for the first time in this study commendably reflected the nutrient transport strategies in various tested plant and their diversity, and could monitor the nutrient transport status of plants in real time. Additionally, the novel nutrient parameters were obtained by the intrinsic electrophysiological information in plants, which had good authenticity, stability, comparability and reproducibility. These nutrient transport indices also strongly supported the feasibility of using IXc and IXL to characterize the composition characteristics of cell membrane proteins. This study highlights that IR, IXc and IXL of plant’s electrophysiological information could effectively manifest the composition and nutrient transport characteristics of membrane protein in plant cells.
5. Conclusion
The present work provided a novel method based on plant’s electrophysiological information for accurately manifest the composition and nutrient transport characteristics of membrane protein in plant cells. The theoretically intrinsic relationships among the leaf R, XL and clamping force were revealed on the basis of Nernst equation for the first time, and the leaf IR, IXc, IXL, IZ and IC of the intrinsic electrophysiological parameters in plants were monitored via these relationships. IXc and IXL perfectly characterize the relatively composition characteristic of cell membrane proteins which induce membrane Xc and XL. UNF, NTR and NTC were firstly defined based on IR, IXc, IXL, IZ and IC which accurately revealed and reflected the nutrient transport strategies in tested plants.
Supplementary Material
Acknowledgments
We thank the Support Plan Projects of Science and Technology Department of Guizhou Province [No. (2021)YB453], the National Natural Science Foundation of China (No. U1612441-2), the Science and technology innovation talent project of Guizhou Province [No. (2016)5672], and the scientific and technological achievement transformation project of Guizhou Province [No. (2017)4124] for supporting this research.
Funding Statement
This work was supported by the Support Plan Projects of Science and Technology Department of Guizhou Province [No. (2021)YB453], the National Natural Science Foundation of China (No. U1612441-2), the Science and technology innovation talent project of Guizhou Province [No. (2016)5672], and the scientific and technological achievement transformation project of Guizhou Province [No. (2017)4124].
Abbreviations
- C
capacitance
- Z
impedance
- R
resistance
- Xc
capacitive reactance
- XL
inductive reactance
- IR
intrinsic resistance
- IXc
intrinsic capacitive reactance
- IXL
intrinsic inductive reactance
- IZ
intrinsic impedance
- IC
intrinsic capacitance
- UNF
the nutrient flux per unit area
- NTR
nutrient transfer rate
- NTC
nutrient transport capacity
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Authors’ contributions
YYW constructed conception. YYW and CZ designed research. CZ, YS, LF and HTL performed research. CZ and DX analyzed data. CZ and YYW wrote the paper. All authors read and approved the final manuscript.
References
- 1.Fromm J, Lautner S.. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2010;30(3):1–10. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 2.Volkov AG. 2006. Plant electrophysiology: theory and methods. Springer. [Google Scholar]
- 3.Szechyńska-Hebda M, Lewandowska M, Karpiński S.. Electrical signaling, photosynthesis and systemic acquired acclimation. Front Physiol. 2017;8:684. doi: 10.3389/fphys.2017.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukhov V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth Res. 2016;130(1–3):373–387. doi: 10.1007/s11120-016-0270-x. [DOI] [PubMed] [Google Scholar]
- 5.Sukhov V, Sukhova E, Vodeneev V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog Biophys Mol Bio. 2019;146:63–84. doi: 10.1016/j.pbiomolbio.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Sukhov V, Vodeneev V. A mathematical model of action potential in cells of vascular plants. J Membrane Biol. 2009;232(1–3):59–67. doi: 10.1007/s00232-009-9218-9. [DOI] [PubMed] [Google Scholar]
- 7.Sukhov V, Nerush V, Orlova L, Vodeneev V. Simulation of action potential propagation in plants. J Theor Biol. 2011;291:47–55. doi: 10.1016/j.jtbi.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Sukhov V, Akinchits E, Katicheva L, Vodeneev V. Simulation of variation potential in higher plant cells. J Membrane Biol. 2013;246(4):287–296. doi: 10.1007/s00232-013-9529-8. [DOI] [PubMed] [Google Scholar]
- 9.Sukhov V, Surova L, Sherstneva O, Vodeneev V. Influence of variation potential on resistance of the photosynthetic machinery to heating in pea. Physiol Plantarum. 2014;152(4):773–783. doi: 10.1111/ppl.12208. [DOI] [PubMed] [Google Scholar]
- 10.Sukhov V, Surova L, Sherstneva O, Bushueva A, Vodeneev V. Variation potential induces decreased PSI damage and increased PSII damage under high external temperatures in pea. Funct Plant Biol. 2015;42(8):727–736. doi: 10.1071/FP15052. [DOI] [PubMed] [Google Scholar]
- 11.Surova L, Sherstneva O, Vodeneev V, Sukhov V. Variation potential propagation decreases heat-related damage of pea photosystem I by 2 different pathways. Plant Signal Behav. 2016;11(3):E1145334. doi: 10.1080/15592324.2016.1145334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukhov V, Gaspirovich V, Mysyagin S, Vodeneev V. High-temperature tolerance of photosynthesis can be linked to local electrical responses in leaves of pea. Funct Front Physiol. 2017;8:763. doi: 10.3389/fphys.2017.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi WG, Hilleary R, Swanson SJ, Kim SH, Gilroy S. Rapid, long-distance electrical and calcium signaling in plants. Annu Rev Plant Biol. 2016;67(1):287–307. doi: 10.1146/annurev-arplant-043015-112130. [DOI] [PubMed] [Google Scholar]
- 14.Favre P, Greppin H, Agosti RD. Accession-dependent action potentials in Arabidopsis. J Plant Physiol. 2011;168(7):653–660. doi: 10.1016/j.jplph.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Gil PM, Gurovich L, Schaffer B, Alcayaga J, Rey S, Iturriaga R. Root to leaf electrical signaling in avocado in response to light and soil water content. J Plant Physiol. 2008;165(10):1070–1078. doi: 10.1016/j.jplph.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Hedrich R, Salvador-Recatala V, Dreyer I. Electrical wiring and long-distance plant communication. Trends Plant Sci. 2016;21(5):376–387. doi: 10.1016/j.tplants.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen CT, Kurenda A, Stolz S, Chetelat A, Farmer EE. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc Natl Acad Sci U S A. 2018;115(40):10178–10183. doi: 10.1073/pnas.1807049115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan X, Wang Z, Huang L, Wang C, Hou R, Xu Z, Qiao X. Research progress on electrical signals in higher plants. Progr Nat Sci Mater Int. 2009;19(5):531–541. doi: 10.1016/j.pnsc.2008.08.009. [DOI] [Google Scholar]
- 19.Gallé A, Lautner S, Flexas J, Fromm J. Environmental stimuli and physiological responses: the current view on electrical signaling. Environ Exp Bot. 2015;114:15–21. doi: 10.1016/j.envexpbot.2014.06.013. [DOI] [Google Scholar]
- 20.Macedo FCO, Dziubinska H, Trebacz ORF, Moral RA. Action potentials in abscisic acid-deficient tomato mutant generated spontaneously and evoked by electrical stimulation. Acta Physiol Plant. 2015;37. [Google Scholar]
- 21.Wang ZY, Qin XH, Li JH, Fan LF, Zhou Q, Wang YQ, Zhao X, Xie CJ, Wang ZY, Huang L. Highly reproducible periodic electrical potential changes associated with salt tolerance in wheat plants. Environ Exp Bot. 2019;160:120–130. doi: 10.1016/j.envexpbot.2019.01.014. [DOI] [Google Scholar]
- 22.Xing DK, Chen L, Wu YY, Zwiazek JJ. Leaf physiological impedance and elasticity modulus in Orychophragmus violaceus seedlings subjected to repeated osmotic stress. Sci Hortic. 2021;276:109763. doi: 10.1016/j.scienta.2020.109763. [DOI] [Google Scholar]
- 23.Zhang C, Su Y WYY, Dk X, Dai Y, Wu YS, Fang L. A plant’s electrical parameters indicate its physiological state: a study of intracellular water metabolism. Plants. 2020;9(10):1256. doi: 10.3390/plants9101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harker FR, Dunlop J. Electrical impedance studies of nectarines during coolstorage and fruit ripening. Postharvest Biol Tec. 1994;4(1–2):125–134. doi: 10.1016/0925-5214(94)90014-0. [DOI] [Google Scholar]
- 25.Ibba P, Falco A, Abera BD, Cantarella G, Petti L, Lugli P. Bio-impedance and circuit parameters: an analysis for tracking fruit ripening. Postharvest Biol Tec. 2020;159:110978. doi: 10.1016/j.postharvbio.2019.110978. [DOI] [Google Scholar]
- 26.Javed Q, Wu YY, Xing DK, Azeem A, Ullah I, Zaman M. Re-watering: an effective measure to recover growth and photosynthetic characteristics in salt-stressed Brassica napus L. Chil J Agr Res. 2017;77(1):78–86. doi: 10.4067/S0718-58392017000100010. [DOI] [Google Scholar]
- 27.Á K, Hlaváčová Z, Vozáry E, Staroňová L. Relationship between moisture content and electrical impedance of carrot slices during drying. Int Agrophys. 2015;29(1):61–66. doi: 10.1515/intag-2015-0013. [DOI] [Google Scholar]
- 28.Xing DK, Xu XJ, Wu YY, Liu YJ, Wu YS, Ni JH, Azeem A. Leaf tensity: a method for rapid determination of water requirement information in Brassica napus L. J Plant Interact. 2018;13(1):380–387. doi: 10.1080/17429145.2018.1478006. [DOI] [Google Scholar]
- 29.Zhang MM, Wu YY, Xing DK, Zhao K, Yu R. Rapid measurement of drought resistance in plants based on electrophysiological properties. Transactions of the ASABE. 2015;58:1441–1446. [Google Scholar]
- 30.Xing DK, Chen XL, Wu YY, Xu XJ, Chen Q, Li L, Zhang C. Rapid prediction of the re-watering time point of Orychophragmus violaceus L. based on the online monitoring of electrophysiological indexes. Sci Hortic. 2019;256:108642. doi: 10.1016/j.scienta.2019.108642. [DOI] [Google Scholar]
- 31.Chen Y, Zhao DJ, Wang ZY, Wang ZY, Tang G, Huang L. Plant electrical signal classification based on waveform similarity. Algorithms. 2016;9(4):1–23. doi: 10.3390/a9040070. [DOI] [Google Scholar]
- 32.Zhao DJ, Wang ZY, Li J, Wen X, Liu A, Wang XD, Hou RF, Wang C, Huang L. Recording extracellular signals in plants: a modeling and experimental study. Math Comput Model. 2013;58(3–4):556–563. doi: 10.1016/j.mcm.2011.10.065. [DOI] [Google Scholar]
- 33.Guo WC, Liu DX, Zhou CC, Han WT. Non-destructive moisture detector for plant leaves based on capacitance. Trans Chin Soc Agric Mach. 2014;45(10):287–293. in Chinese [Google Scholar]
- 34.Hopkins WG, Huner NPA. Introduction to plant physiology. 3rd ed. US, New York: John Wiley & Sons Inc; 2004. p. 27.New York: John Wiley & Sons Inc; 2004. p. 27. [Google Scholar]
- 35.Buckley DJ, Lefebvre M, Meijer EGM, Brown DCW. A signal generator for electrofusion of plant protoplasts. Comput Electron Agr. 1990;5(2):179–185. doi: 10.1016/0168-1699(90)90032-K. [DOI] [Google Scholar]
- 36.Philip N. Biological physics: energy, information life. New York, USA: Freeman and Company; 2003. 413–448. [Google Scholar]
- 37.Zhang AM, Wang R, Xie H, Xie XH, Shi YQ, Jia ZP, Sun K. Summarization on the methodology study of protein detection. Letters in Biotechnology. 2011;22(1):130–134. in Chinese [Google Scholar]
- 38.Li L, Wang Q, Feng J, Tong LL, Tang B. Highly sensitive and homogeneous detection of membrane protein on a single living cell by aptamer and nicking enzyme assisted signal amplification based on microfluidic droplets. Anal Chem. 2014;86(10):5101–5107. doi: 10.1021/ac500881p. [DOI] [PubMed] [Google Scholar]
- 39.Borges BMMN, Strauss M, Camelo PA, Sohi SP, Franco HCJ. Re-use of sugarcane residue as a novel biochar fertiliser – increased phosphorus use efficiency and plant yield. J Clean Prod. 2020;262:121406. doi: 10.1016/j.jclepro.2020.121406. [DOI] [Google Scholar]
- 40.Geng YJ, Chen L, Yang C, Jiao DY, Zhang YH, Cai ZQ. Dry-season deficit irrigation increases agricultural water use efficiency at the expense of yield and agronomic nutrient use efficiency of Sacha Inchi plants in a tropical humid monsoon area. Ind Crops Prod. 2017;109:570–578. doi: 10.1016/j.indcrop.2017.09.022. [DOI] [Google Scholar]
- 41.Rayees B, Dorcus M, Chitra S. Nutritional composition and oil fatty acids of Indian winter melon Benincasa hispida (Thunb.) seeds. Int Food Res J. 2013;20:1151–1155. [Google Scholar]
- 42.Glenn M, Thompson RG, Piene H. Stem electrical capacitance and resistance measurements as related to total foliar biomass fir trees. Can J For Res. 1987;17(9):1071–1074. doi: 10.1139/x87-164. [DOI] [Google Scholar]
- 43.Wu YY, Xing DK, Hang HT, Zhao K. Principles and technology of determination on plant’ adaptation to Karst environment. Beijing: Science Press; 2019. 191. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


