Figure 1.
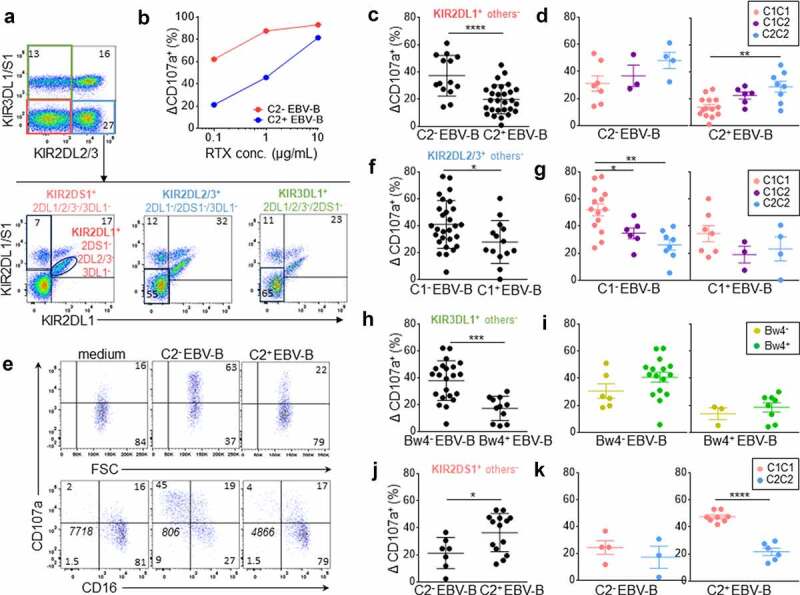
Inhibitory KIR-HLA interaction brings rituximab-dependent educated KIR+ NK cell degranulation down to the degranulation baseline of uneducated counterpart. (a) Representative density plots illustrating the strategy to target three NK cell subsets expressing either KIR2DL1+ others− (KIR2DS1−/2DL2/3−/3DL1−), KIR2DL2/3+ others− (KIR2DL1/S1−/3DL1+) or KIR3DL1+ others− (KIR2DL1/2/3−2DS1−) and one NK cell subset expressing only KIR2DS1+ (KIR2DL1−/2DL2/3−/3DL1−) from PBMC as illustrated for a representative donor expressing KIR2DL1, KIR2DS1, KIR2DL3 and KIR3DL1. A first mAb combination led to exclude KIR3DL1/S1+ and KIR2DL2/3+ NK cells (red gate) and a second mAb combination led to select either KIR2DL1+ 2DS1− NK cells or KIR2DS1+ 2DL1+ NK cells. Similarly, a first mAb combination led to select KIR3DL1+ NK cells and to exclude KIR2DL2/3+ NK cells (green gate) and a second mAb combination to exclude KIR2DL1+ and KIR2DS1+ NK cells. To target KIR2DL2/3+ others−, the first combination led to target KIR2DL2/3+ and exclude KIR3DL1/S1+ NK cells and the second one led to exclude KIR2DL1+ and KIR2DS1+ NK cells. (b) Determination of the optimal rituximab (RTX) concentration to observe inhibiting KIR2DL1-C2 interaction on NK cell ADCC. RTX-dependent KIR2DL1+ others− NK cell degranulation (Δ CD107a+) frequency against C2− and C2+ EBV-B cell lines for a representative blood donor determined by flow cytometry at different RTX concentrations (0.1, 1, and 10 µg/mL) using 1:1 PBMC:EBV-B cell line ratio. (c) RTX dependent KIR2DL1+ others− NK cell degranulation (Δ CD107a+) frequency against C2− (n = 14) and C2+ (Bw4−, n = 14 and Bw4+, n = 14) EBV-B cell lines for all blood donors determined by flow cytometry. Specific CD107a expression is reported as ΔCD107a calculating the difference of CD107a+ NK cell frequency of the test (EBV-B + RTX) and of the NK alone (medium). (d) Frequencies of RTX-dependent KIR2DL1+ others− NK cell degranulation (Δ CD107a+) against one C2− EBV-B cell line for C1C1 (n = 7), C1C2 (n = 3) and C2C2 (n = 4) blood donors and two C2+ (Bw4− and Bw4+) EBV-B cell lines for C1C1 (n = 14), C1C2 (n = 6) and C2C2 (n = 8) blood donors. (e) Representative density plots of RTX-dependent KIR2DL1+ others− NK cell degranulation (CD107a) against C2− and C2+ EBV-B cell lines. CD16 expression is evaluated on NK cells. The frequencies are indicated on the density plot and the mean fluorescent intensity of CD16 on NK cells is indicated in italic. (f) RTX-dependent KIR2DL2/3+ others− NK cell degranulation (Δ CD107a+) frequency against C1− (Bw4−, n = 14 and Bw4+, n = 14) and C1+ (n = 14) EBV-B cell lines for all blood donors. (g) RTX-dependent KIR2DL2/3+ others− NK cell degranulation against C1− EBV-B cell lines for C1C1 (n = 14), C1C2 (n = 6) and C2C2 (n = 8) blood donors and against C1+ EBV-B cell lines for C1C1 (n = 7), C1C2 (n = 3) and C2C2 (n = 4) blood donors. (h) RTX-dependent KIR3DL1+ others− NK cell degranulation against Bw4− (Bw4−, n = 11 and Bw4+, n = 11) and Bw4+ (n = 11) EBV-B cell lines for all blood donors. (i) RTX-dependent KIR3DL1+ others− NK cell degranulation against Bw4− EBV-B cell lines for Bw4− (n = 6) and Bw4+ (n = 16) blood donors and against Bw4+ EBV-B cell lines for Bw4− (n = 3) and Bw4+ (n = 8) blood donors. (j) RTX-dependent KIR2DS1+ others− NK cell degranulation (% CD107a+) against C2− (n = 7) and C2+ (Bw4−, n = 7 and Bw4+, n = 7) EBV-B cell lines for all blood donors. (k) RTX-dependent KIR2DS1+ others− NK cell degranulation against 2 C2+ (Bw4− and Bw4+) EBV-B cell lines for C1C1 (n = 8) and C2C2 (n = 6) blood donors and against one C2− EBV-B cell line for C1C1 (n = 4) and C2C2 (n = 3) blood donors. *p < .05, **p < .01, ***p < .001, ****p < .0001
