Abstract
Context
COVID-19 is a novel coronavirus that causes a severe infection in the respiratory system. Nigella sativa L. (Ranunculaceae) is an annual flowering plant used traditionally as a natural food supplement and multipurpose medicinal agent.
Objective
The possible beneficial effects of N. sativa, and its constituent, thymoquinone (TQ) on COVID-19 were reviewed.
Methods
The key words including, COVID-19, N. sativa, thymoquinone, antiviral effects, anti-inflammatory and immunomodulatory effects in different databases such as Web of Science (ISI), PubMed, Scopus, and Google Scholar were searched from 1990 up to February 2021.
Results
The current literature review showed that N. sativa and TQ reduced the level of pro-inflammatory mediators including, IL-2, IL-4, IL-6, and IL-12, while enhancing IFN-γ. Nigella sativa and TQ increased the serum levels of IgG1 and IgG2a, and improved pulmonary function tests in restrictive respiratory disorders.
Discussion and conclusions
These preliminary data of molecular docking, animal, and clinical studies propose N. sativa and TQ might have beneficial effects on the treatment or control of COVID-19 due to antiviral, anti-inflammatory and immunomodulatory properties as well as bronchodilatory effects. The efficacy of N. sativa and TQ on infected patients with COVID-19 in randomize clinical trials will be suggested.
Keywords: Immunomodulation, anti-inflammatory, antiviral effects, medicinal plant
Introduction
Nigella sativa L. (Ranunculaceae), or black seed, has been used traditionally as a food additive and spice (Khazdair, Anaeigoudari, Hashemzehi et al. 2019). The use of plants and botanical compounds for immune enhancement has been reported by several recent studies and traditional medicine sources (Roxas and Jurenka 2007). Nigella sativa is among the most commonly used herbal plants practiced in Iranian traditional medicine (Gilani et al. 2004). Nigella sativa is traditionally used for the treatment of various types of disorders including diabetes, cough, fever, eczema, bronchitis, and influenza (Ali and Blunden 2003). Pharmacological effects of N. sativa including the anti-inflammatory, antioxidant (Mohebbatia, Khazdair, Karimia et al. 2017; Bordoni et al. 2019), antimicrobial (Emeka et al. 2015), neuro-protective (Mohebbatia, Khazdairb, Hedayatia et al. 2017; Khazdair, Anaeigoudari, Hashemzehi et al. 2019), and reno-protective properties (Mohebbati et al. 2017) were reported.
COVID-19 is an enveloped virus with a single-stranded RNA genome, and the third known coronavirus after severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS-CoV) (Malik et al. 2020). Infection with COVID-19 leads to severe respiratory disorders and pneumonia-like symptoms in humans (Shanmugaraj et al. 2020). COVID-19 has high transmissibility and infectivity compared with SARS and MERS (Liu et al. 2020). Traditionally, it has been known that some medicinal plants and their products possess immune-regulatory properties. The isolation of plant bioactive components occurred in the nineteenth century (Phillipson 2001; Khazdair, Anaeigoudari, Kianmehr et al. 2019).
It has been reported that about 64% of the world population use herbal remedies for the treatment of various disorders (Farnsworth 2008). Moreover, nearly 50% of synthetic drugs are derived from phytochemicals (Newman and Cragg 2012). Herbs synthesize chemicals as a part of their defence system to combat pathogens; and a considerable number of such compounds are effective anti-infective agents. For example, naturally occurring hydroxylated phenols and flavonoids are effective against infections (Dixon et al. 1983). Alkaloids, as the most common plant-based bioactive metabolites, as well as flavonoids have antifeedant and larvicidal effects (Levin and York 1978).
Natural products and essential oils are well recognized for their antiviral, anti-inflammatory and immuno-modulatory activities (Asif et al. 2020; Kumar et al. 2020). It has been reported that various monoterpenoid phenols obtained from plants including carvacrol have the potential to inhibit the binding of viral spike (S) glycoprotein to the host cell (Kulkarni et al. 2020). Also carvacrol can inhibit ACE2 activity and suggested that it may block the host cell entry of SARS-CoV-2 (Abdelli et al. 2020).
This review tries to explain the traditional and new pharmacological properties of N. sativa and its main ingredient, thymoquinone on COVID-19 induced infection in the respiratory system based on anti-inflammatory effects and antiviral activities.
Methods
Data of the current study were obtained from the most popular scientific databases, Web of Science (ISI), PubMed, Scopus, and Google Scholar by searching keywords: ‘COVID-19’ and ‘Nigella sativa’ or ‘thymoquinone’ or ‘Antivirus effects’ in the title and ‘inflammatory lung diseases’ or ‘immunomodulatory effects’ in the title or abstract. Relevant published articles in the English language up to February 2021 were included. All studies evaluating the effects of N. sativa or thymoquinone on viral diseases, and inflammatory lung diseases were included. Articles with insufficient information and in another language were excluded from the review.
Results
The potential immunomodulatory effects of medicinal herbs
Immunotherapy is characterized as an approach to disease management by producing or enhancing an immune response to a present disorder (Vanderlugt and Miller 2002). Cytokines such as, interleukins (IL), chemokines, interferons (IFN), and tumour necrosis factors (TNF) are small, non-structural proteins, which have multitude effects in various organs (Dinarello 2007). The pro-inflammatory mediators include IL-17, IL-1β, and TNF-α, and anti-inflammatory mediators include, IL-10, and IL-1ra (Su et al. 2012). The pathogenic roles of cytokines including; IL-6, IL-10, IL-17, IL-23, IFN-α and IFN-γ in a heterogenic autoimmune inflammatory disease such as systemic lupus erythematosus (SLE) is shown (Su et al. 2012). The roles of Th2 cytokines such as IL-4 in the pathogenesis of asthma is also reported (Steinke and Borish 2001).
Deregulation of the immune system has been known as the main cause of many diseases; thus, management of immune responses could be a beneficial therapeutic strategy for the treatment of these diseases. Some medicinal plants might affect the functions of the immune system by modulation of the production/release of immune-globulins and cytokines, immune cells activities, and cellular coreceptor expression (Das et al. 2004).
Immune system response to COVID-19
As antigens, viruses stimulate humoral and cellular immune responses. The induction of the immune system response to a virus is mediated by virus-specific T and B cells (Cox and Brokstad 2020). The pattern of antibody production, especially the production of immunoglobulins M and G (IgM and IgG), against SARS-CoV-2 is similar to common acute viral infections (Li et al. 2003). The number of CD4+ and CD8+ T cells as humoral responses significantly reduced in the peripheral blood of infected patients with SARS-CoV2 (Xu et al. 2020). Similarly, reduction of CD4+ and CD8+ T cells in the acute phase of infection with SARS-CoV is also associated with reduction in the number of CD4+ and CD8+ T cells (Fan et al. 2009). Moreover, CD8+ T cells also showed a similar effect in infected mice with MERS-CoV (Zhao et al. 2014).
Cytokine storm is one of the main mechanisms for acute respiratory distress syndrome (ARDS), the systemic inflammatory response resulting from the release of large amounts of pro-inflammatory mediators including IFN-α, IL-1b, IL (6 − 12 − 18 and 33), TNF-α, TGFβ, etc. and chemokines by immune effector cells in SARS-CoV infection (Cameron et al. 2008; Huang et al. 2020). Patients with MERS-CoV infection showed elevated levels of pro-inflammatory mediators in the serum similar to infection with SARS-CoV (Min et al. 2016). The cytokine storm will be started by the immune system to cause ARDS and various organs to failure, which may be lead to death in severe cases infected with SARS-CoV-2 and MERS-CoV (Xu et al. 2020).
Pharmacological effects of N. sativa
Nigella sativa is widely grown in the Mediterranean region, the west of Asia, Middle East, southern Europe, and north Africa (Tembhurne et al. 2014). Nigella sativa seed has been traditionally used for the treatment of fever, infection, chest congestion, inflammation, cough, bronchitis, asthma, chronic headache, dysmenorrhoea, obesity, diabetes, flatulence, and diarrhoea (Durmuskahya and Ozturk 2013; Nasir et al. 2014). The active ingredient of N. sativa seed is mainly thymoquinone (TQ) which showed anti-inflammatory effects by suppression of prostaglandins and leukotrienes as inflammatory mediators (Hajhashemi et al. 2004). Antioxidant and anti-epileptic, as well as anti-Alzheimer’s and anti-Parkinson’s disease effects of N. sativa and TQ, were previously reported (Khazdair 2015).
Antiviral activities of N. sativa and TQ
Antiviral effect of N. sativa oil in murine cytomegalovirus (MCMV) model was investigated by administration of N. sativa oil (100 μg/100 μL, i.p.) to BALB/c mice. Treatment with N. sativa oil significantly reduced the load of virus in spleen and liver 3 days after infection compared to the control group. The antiviral effect of the plant oil accorded with increasing serum level of IFN-γ and increasing numbers of CD4+ helper T cells. Moreover, the titre of virus was undetectable in liver and spleen after 10 days while it was detectable in control mice (Salem and Hossain 2000). The antiviral effect of plant oil is related to the increasing response of CD4 cells. The effects of N. sativa oil on pathogenesis and immune response of H9N2 avian influenza virus (H9N2 AIV) in infected turkeys showed positive results. Turkeys fed with diets containing 2%, 4% and 6% of N. sativa oil significantly decreased mortality rate along with an increased in the body weight of turkeys. Turkeys fed with N. sativa (6%) had significantly lower titre of virus compared to the control group. Moreover, N. sativa increased the mRNA expression of IFN-γ compared to the control group. The enhancement of antibody titre against H9N2 AIV in turkeys fed with N. sativa showed the immune regulatory effects of the plant. Additionally, treatment of (H9N2 AIV) infected turkeys with TQ and curcumin significantly increased expression of IFN-γ and antibody titre against H9N2 AIV in birds. TQ also reduced virus shedding and enhanced immune responses in treated animals that lead to suppress pathogenesis of H9N2 viruses (Umar et al. 2016).
Treatment of a hepatitis C virus (HCV) infected patient, who was not eligible for IFN-α therapy, with capsules of N. sativa oil (450 mg) for 3 months (three times daily), significantly decreased the viral load and also improved oxidative stress due to augmented total antioxidant activity. Moreover, N. sativa oil improved red blood cells (RBC), platelet counts, total protein, and albumin in HCV-infected patient (Barakat et al. 2013).
The beneficial effects of N. sativa oil on immunity in microbial infection could be augmented by Zinc (Zn) supplement. The possible therapeutic effects of N. sativa and Zn supplements to treat COVID-19 was suggested (Rahman 2020).
The potential efficacy of N. sativa oil (500 mg soft-gel capsules) one capsule orally twice daily for 10 days plus standard of care treatment on the outcomes of patients with mild COVID-19 was investigated (Koshak et al. 2020).
The pharmacological properties of N. sativa seed and TQ including immunomodulatory, antioxidant and anti-inflammatory active and their potential therapeutic strategy against COVID‐19 were reviewed (Islam et al. 2020). It has been reported that some natural products such as TQ have high to a moderate binding affinity to the heat Shock Protein A5 (HSPA5) substrate-binding domain β (SBDβ), which reported to be the recognition site for the SARS-CoV-2 spike. This natural compound may be used to reduce the risk of COVID-19 or possibly to treat the disease, especially in high-risk people (Elfiky 2020).
It has been reported that the binding affinity of a N. sativa constituent, dithymoquinone (DTQ), was higher than a positive control (chloroquine), which has the high potential affinity binding at SARS–CoV-2:ACE2 interface. Then, it could be predicted as an inhibitor to disrupt viral-host interactions (Ahmad et al. 2020).
The constituents of N. sativa such as, α-hederin, thymohydroquinone, and TQ have efficiently binding to ACE2 and potential therapeutic effects of these bioactive components to combat COVID-19 in-silico study was suggested (Jakhmola Mani et al. 2020). The affinity of TQ on SARS–Cov-2 E protein and inhibitory effects on E protein ion channel were showed in the molecular docking study (Mohideen 2021). It has been reported that TQ may inhibit SARS–CoV‐2 and interfere with its binding to ACE2 receptors in molecular docking studies (Bouchentouf and Missoum 2020; Sekiou et al. 2020). This can prevent virus entry and replication inside the host cell. The results of these studies demonstrate the potential of TQ on virus machinery as well as virus entry and the replication in the host cells. Antiviral effects of N. sativa and TQ are shown in Table 1.
Table 1.
Antiviral effects of N. sativa and TQ.
| Type of plant Extract | Effective doses | Model of study | Effects | Reference |
|---|---|---|---|---|
| N. Sativa oil | 100 μg/100 μL | MCMV | Inhibited the virus titres in spleen and liver 3 and significantly reduced the viral load in the liver and spleen. Raising IFN-γ serum level and increasing numbers of CD4+ helper T cells | Salem and Hossain (2000) |
| Fed diets of N. Sativa | 2%, 4% and 6% | H9N2 AIV | Significantly increased the body weight and reduced mortality was observed in turkeys. Significantly lower virus titre than those in control group. Moreover, fed diets of N. sativa increased the expression levels of IFN-γ mRNA compared to the control group. | Umar et al. (2016) |
| N. sativa oil | Capsule (450 mg) | HCV patients | Significant decreased the viral load and also improvement of the oxidative stress due to augmented total antioxidant activity. N. sativa oil also improved RBC, platelet counts, total protein and albumin in HCV patients | Barakat et al. (2013) |
| Fed diets of TQ | 5 g/kg | H9N2 AIV | Significantly increased antibody titre against H9N2 and increased gene expression of IFN-γ. TQ also reduced virus shedding and enhanced immune responses in treated animals that lead to suppress pathogenesis of H9N2 viruses | Umar et al. (2016) |
MCMV: murine cytomegalovirus; H9N2 AIV: H9N2 avian influenza virus; HCV: hepatitis C virus.
Immunomodulatory and anti-inflammatory effects of N. sativa and TQ
Nigella sativa
This herb has been used as a safe herbal food against inflammatory diseases including asthma, allergy, and metabolic syndrome due to its immunomodulatory effects and low cytotoxicity (Gholamnezhad et al. 2015). Ethanol extract of N. sativa seed (1000 µg/mL) reduced the secretion of IL-4 in phytohemagglutinin (PHA) and concanavalin A (Con A)-stimulated splenocytes. In addition, N. sativa seed extracts (500 and 1000 µg/mL) decreased IFN-γ secretion in stimulated and non-stimulated splenocytes (Gholamnezhad et al. 2015). The effects of N. sativa seed aqueous extract (1, 10, 50, and 100 µg/mL) on splenocytes proliferation, the function of macrophages in BALB/c mice, and natural killer (NK) activity of C57/BL6 showed significant and dose-dependently elevation of the splenocytes proliferation (Majdalawieh et al. 2010). The pattern of cytokine secretion by splenocytes was changed by the plant extract in favour of Th2 pathway. The aqueous extract of N. sativa seed (50 and 100 µg/mL) significantly enhanced the secretion of IL-4 and IL-10 but suppressed TNF-α, IL-6, and NO by primary macrophages. In addition, the plant extract increased NK cells cytotoxic activity against YAC-1 tumour cells which suggested an antitumor activity for the plant (Majdalawieh et al. 2010).
Nevertheless, cytokines production by splenic mononuclear cells (MNCs) of the treated ovalbumin (OVA)-sensitized mice with N. sativa oil (252 mg, p.o.) were not significantly different from that of the non-treated group. These results indicated that N. sativa oil has no immune regulatory effect on Th1 and Th2 cell responsiveness to allergen stimulation (Büyüköztürk et al. 2005).
Nigella sativa oil exhibits airway anti-inflammatory and immune-regulatory effects which may support its use for treatment of allergic asthma. The eosinophil count in peripheral blood, IgG1 and IgG2a levels, cytokines profile including, IL-2, IL-10, IL-12, and IFN-γ levels, and also inflammatory cells in the lung tissue were significantly decreased by the plant oil in a mouse model of allergic asthma. The plant showed comparable immunomodulatory properties with those of dexamethasone except for its effect on IFN-γ level (Abbas et al. 2004).
Nigella sativa seed ethanolic extract supplementation (200 mg/kg/day, p.o.) in control, moderately trained, and over-trained rats changed the cytokines profile. Immediately after exercise, IL-6, IL-10, and TNF-α were increased while IL-4 was decreased in rats’ serum. Moreover, IFNγ/IL-4 ratio significantly increased in animals treated with the plant extract (Gholamnezhad et al. 2014).
Pre-treatment of OVA-sensitized guinea pigs with N. sativa seed hydro-ethanolic extract (1.25 and 2.50 g/L, p.o.) reduced IL-4 level, but increased the level of IFN-γ and ameliorated almost all lung histological changes in the sensitized animals (Boskabady, Keyhanmanesh et al. 2011). In another experiment, N. sativa hydro-ethanolic extract (0.08 g, p.o.) decreased neutrophil numbers and restored IL-4 and IFN-γ levels in sulphur mustard (40 mg/m3) exposed guinea pigs (Boskabady, Keyhanmanesh et al. 2011; Boskabady, Vahedi et al. 2011).
Administration of N. sativa seeds volatile oil (2.5 µL ≅ 2.10 µg, intramuscular (i.m.), twice a week for 30 days) in rats significantly reduced antibody titre (1280 versus 2560) compared to the control animals. Furthermore, the splenocytes and neutrophils counts were significantly decreased, but peripheral lymphocytes and monocytes were increased in the experimental animals that received N. sativa seeds volatile oil (Islam et al. 2004). Serum protein and total immunoglobulin levels of fish fed with diets containing 1, 2.5 and 5% of N. sativa oil for 21 days were significantly increased. Furthermore, haematocrit level was significantly increased in group fed with N. sativa (5%) compared to the control group (Dorucu et al. 2009).
IL-1β and IL-4 levels were increased following addition of N. sativa seed aqueous extract (1 and 2 µg/mL) to culture medium of non-activated peripheral blood mononuclear cells (PBMC) and allogeneic cells. Whole N. sativa proteins (0.1, 1 or 10 µg/mL) suppressed the production of IL-8 in non-stimulated as well as pokeweed mitogen-activated PBMC cells. Whole soluble N. sativa seed extract (2 µg/mL) also increased TNF-α production. Furthermore, fractionated extract of N. sativa was less effective than whole N. sativa proteins (Haq et al. 1999).
Aqueous N. sativa seed extract (50 µg/mL) suppressed lymphocytes response to all mitogens and allogeneic cells. Also, N. sativa extract (0.5 µg/mL) stimulated lymphocytes response to allogeneic cells. Moreover, below-10-kDa fraction of N. sativa stimulated the production of IL-1β and IL-3 by human lymphocytes without need for any mitogen or other human allogeneic cells. The most marked increase in IL-3 production was noted when N. sativa extract (0.5 µg/mL) was added to lymphocytes culture. However, N. sativa extract did not effect on IL-2 secretion by mitogen-activated lymphocytes (Haq et al. 1995). In autoimmune encephalomyelitis (AE)-induced in Wistar rats, whole N. sativa seed (2.8 g/kg, bw) reduced the expression of transforming growth factor beta 1 (TGF β1) and increased remyelination in the cerebellum (Noor et al. 2015). Oral administration of hydro-ethanolic extract of N. sativa seed (100, 200, 400 mg/kg, i.p.) on LPS (1 mg/kg, i.p.)-induced lung injury in rats, decreased the total and different WBC counts as well as oxidative stress biomarkers in the bronchoalveolar lavage fluid (BALF) and serum. Furthermore, treatment with N. sativa extract dose-dependently reduced TGF-β1, IFN-γ, PGE2, IL-4 levels in the BALF as well as pathological changes in the lung (Mokhtari-Zaer et al. 2020).
In a clinical study, dietary supplementation with N. sativa oil improved the immune response in healthy elderly subjects (Salem 2005). Prostaglandin E2 production was significantly reduced in individuals who received N. sativa oil (750 mg) compared to the placebo treated group (750 mg soybean oil) (Wu et al. 1999).
Administration of N. sativa oil capsules (40–80 mg/kg/day, p.o.) in patients with allergic rhinitis, atopic eczema and asthma significantly reduced the levels of IgE, endogenous cortisol and eosinophil count in plasma and urine compared to their pre-treatment values (Kalus et al. 2003).
Together, the results of different studies indicated that N. sativa influences serum immunoglobulins, antibody titre, eosinophil count, cytokine profiles, and Th1/Th2 balance. Therefore, N. sativa could be applied for the treatment of inflammatory diseases such as allergy and asthma. Immunomodulatory effects of N. sativa are summarized in Table 2.
Table 2.
Anti-inflammatory and immuno-modulatory effects of N. sativa.
| Type of plant extract | Effective doses | Model of study | Effects | Reference |
|---|---|---|---|---|
| Ethanolic extract | 1000 µg/mL | Splenocytes cells |
 IL-4 and IFN-γ IL-4 and IFN-γ |
Gholamnezhad et al. (2015) |
| Aqueous extract | 2 µg/mL | PBMC cells |
 TNF-α, IL-1β and IL-4 TNF-α, IL-1β and IL-4 IL-8 IL-8 |
Haq et al. (1999) |
| 100 µg/mL | Splenocytes cells |
 IL-6, TNF-α, and NO IL-6, TNF-α, and NO IL-4 and IL-10 IL-4 and IL-10 |
Majdalawieh et al. (2010) | |
| 0.5 µg/mL | Human lymphocyte |
 IL-1β and IL-3 IL-1β and IL-3 |
Haq et al. (1995) | |
| Ethanolic extract | 200 mg/kg, p.o. | Rat |
 IL-6, IL-10 and TNFα IL-6, IL-10 and TNFα IL-4 IL-4 |
Gholamnezhad et al. (2014) |
| Hydroethanolic | 2500 mg/L, p.o. | Guinea pigs |
 IL-4, IL-4, IFN-γ IFN-γ |
Boskabady, Keyhanmanesh et al. (2011) |
| 80 mg/L, p.o. | Guinea pigs |
 Neutrophil number and restored the IL-4 and IFN-γ changes Neutrophil number and restored the IL-4 and IFN-γ changes |
Boskabady, Vahedi et al. (2011) | |
| Volatile oil | 2.10 µg, i.m | Rat |
 Splenocytes and neutrophils counts Splenocytes and neutrophils counts Peripheral lymphocytes and monocytes Peripheral lymphocytes and monocytes |
Islam et al. (2004) |
| Aqueous extract | 2.8 g/kg,bw | Rat |
 The expression of transforming growth factor beta 1 (TGF β1) The expression of transforming growth factor beta 1 (TGF β1) |
Noor et al. (2015) |
| Diet of N. sativa | 5% | Rainbow trout |
 Serum protein and total Ig levels Serum protein and total Ig levels |
Dorucu et al. (2009) |
| Supplement of N. sativa oil | 10 mg/kg, p.o. | Human |
 Production of prostaglandin E2 Production of prostaglandin E2
|
Salem (2005) |
| Capsule of N. sativa oil | 40 mg/kg/day, p.o. | Human |
 IgE, eosinophil count, endogenous cortisol in plasma and urine IgE, eosinophil count, endogenous cortisol in plasma and urine |
Kalus et al. (2003) |
IL: interleukins; IFN-γ: interferon gamma; TNF-α: tumour necrosis factors alpha; PBMC: peripheral blood mononuclear cell; TGF β1: transforming growth factor beta 1; Ig: immunoglobulin.
Thymoquinone (TQ)
Treatment of LPS-activated mast cells with TQ (10 μM) restored LPS-induced changes in IL-5 and IL-13 at mRNA and protein levels but did not affect IL-10 production. In addition, TQ inhibited globin transcription factor (GATA) binding at the IL-5 promoter induced by LPS stimulation (El Gazzar 2007). In addition, TQ treatment (1–20 μM) significantly inhibited the release of IL-10, IL-12, and TNF-α from LPS-induced dendritic cells (DCs) and suppressed phosphorylation of pro-survival factors protein kinase B (AKT) and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), but induced caspase-3 and caspase-8 activity in DCs (Xuan et al. 2010). TQ (0–25 μM) also dose-dependently inhibited TNFα-induced nuclear factor-κB (NF-κB) activation via inhibition of NF-κB kinase (IKK) activity, and subsequently suppressed phosphorylation and activation of NF-κBα (IκBα) (Sethi et al. 2008).
The gene expression of NF-κB regulated of anti-apoptotic factors such as inhibitor of apoptosis proteins (IAP)-1, IAP2, XIAP Bcl-2, and Bcl-xL, and angiogenic (MMP-9 and VEGF) gene products were also down regulated in human myeloid leukaemia cell line (KBM-5) by TQ (Sethi et al. 2008). The gene expression of IL-1β, TNF-α, monocyte chemoattractant protein-1 (MCP-1), and cyclooxygenase-2 (COX-2) in pancreatic ductal adenocarcinoma (PDA) cells were significantly reduced by TQ (25–75 μM). TQ also showed an incisory effect on TNF-α mediated of NF-κB activation in placenta-derived cells (PDA) but decreased the transport of NF-κB from the cytosol to the nucleus (Chehl et al. 2009).
The prophylactic effect of TQ (3 mg/kg, i.p.) on the levels of IL-4 and IFN-γ was studied in sensitized guinea pigs and the results showed decreased IL-4 but increased IFN-γ levels (Rana Keyhanmanesh et al. 2014).
Administration of TQ (3 mg/kg, i.p.) decreased the production of leukotriene B4 (LTB4) and leukotriene C4 (LTC4) in the BALF of mice. Furthermore, the levels of IL-4, IL-5, and IL-13 were also significantly decreased while IL-10 was increased when TQ administered before OVA challenge (El Gazzar, El Mezayen, Nicolls et al. 2006). Similarly, TQ (3 mg/kg, i.p.) significantly decreased elevated serum levels of IgE and IgG1 and also inhibited allergen induced lung inflammation and production of mucus by goblet cells. TQ also significantly inhibited IL-4, IL-5, and IL-13 but increased IFN-γ production in the BALF. In addition, a small effect of TQ was observed on the production of IL-4 in OVA-stimulated cultured lung cells. These results indicated the effect of TQ on reduction of airway inflammation (inhibition of eosinophil infiltration into the airways) and Th2 cytokines productions (El Gazzar, El Mezayen, Marecki et al. 2006). Intraperitoneal administration of TQ (5 or 10 mg/kg) 30 min before LPS injection (1 mg/kg i.p.) decreased the levels of IL-6 and TNF-α in treated rats (Bargi et al. 2017). Orally administration of TQ (10, 20, and 40 mg/kg/day, p.o.) for 14 days after Alzheimer’s disease (AD) induction in rats, decreased amyloid-β (Aβ) formation and accumulation, and also reduced the levels of TNF-α and IL-1β. Furthermore, it significantly down regulated the expression of NF-κB and interferon regulatory factor 3 (IRF-3) mRNAs (Abulfadl et al. 2018).
In diabetic rat mothers, TQ supplementation (20 mg/kg, p.o.) during pregnancy and lactation periods restored IL-2 levels and T cells proliferation and saved both circulating and thymus-homing T cells in the rat offspring (Badr et al. 2011). These results indicated an inhibitory effect for TQ on eosinophilia, Th2 cytokines, and allergen-specific antibodies which resulted in reduction of allergen-induced inflammation. These results showed immune-modulatory effect of TQ and suggests its therapeutic value in allergic and immune-deficiency induced disorders.
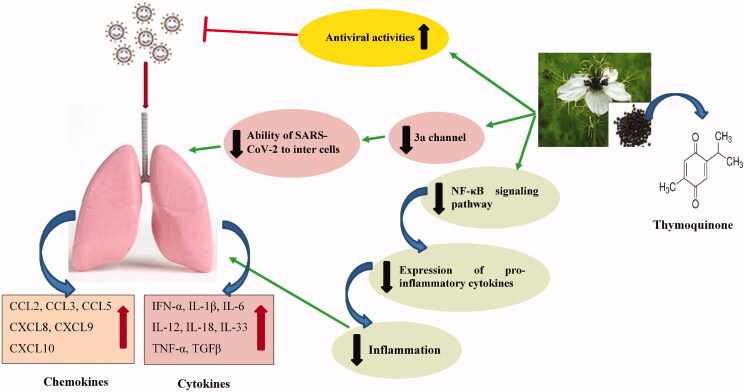
The relaxation of TQ on trachea smooth muscles due to blockade muscarinic and/or β2 agonistic activity on tracheal tissue from guinea pigs has been reported (Bashir et al. 2020). Anti-inflammatory and immunomodulatory effects of N. sativa and TQ are shows in Table 3 and Figure 1.
Table 3.
Anti-inflammatory and immuno-modulatory effects of TQ.
| Bioactive compound | Effective doses | Model of study | Effects | Reference |
|---|---|---|---|---|
| TQ | 10 μM | Mast cells |
 IL-5 and IL-13 mRNA expression IL-5 and IL-13 mRNA expression |
El Gazzar (2007) |
| 20 μM | Dendritic cells |
 IL-10, IL-12, and TNFα IL-10, IL-12, and TNFα  Caspase 3 and caspase 8 Caspase 3 and caspase 8 |
Xuan et al. (2010) | |
| 25 μM | KBM-5 cells |
 NF-κB activation, anti-apoptotic, and angiogenic gen NF-κB activation, anti-apoptotic, and angiogenic gen |
Sethi et al. (2008) | |
| 75 μM | (PDA) cells |
 IL-1β, TNFα, MCP-1, and COX-2 IL-1β, TNFα, MCP-1, and COX-2 |
Chehl et al. (2009) | |
| 3 mg/kg, i.p. | Guinea pigs |
 IL-4 IL-4  IFN-γ IFN-γ |
Rana Keyhanmanesh et al. (2014) | |
| 3 mg/kg, i.p. | Mice |
 LTB4 and LTC4, IL-4, IL-5 and IL-13 LTB4 and LTC4, IL-4, IL-5 and IL-13 IL-10 IL-10 |
El Gazzar, El Mezayen, Marecki et al. (2006) | |
| 3 mg/kg, i.p. | Mice |
 IgE and IgG1, IL-4, IL-5, and IL-13 and IFN-γ IgE and IgG1, IL-4, IL-5, and IL-13 and IFN-γ |
El Gazzar, El Mezayen, Nicolls (2006) | |
| 20 mg/kg, p.o. | Rat |
 IL-2 and T cell proliferation IL-2 and T cell proliferation |
Badr et al. (2011) | |
| 5 or 10 mg/kg, i.p. | Rat |
 IL-6, TNF-α, and NO metabolites IL-6, TNF-α, and NO metabolites |
Bargi et al. (2017) | |
| 10, 20, and 40 mg/kg/day, p.o. | Rat |
 Amyloid-β (Aβ) formation and accumulation, and also decreased TNF-α and IL-1β Amyloid-β (Aβ) formation and accumulation, and also decreased TNF-α and IL-1β |
Abulfadl et al. (2018) |
KBM-5: myeloid leukaemia cell line; PDA: pancreatic ductal adenocarcinoma cells; NF-κB: nuclear factor-κB; MCP-1: monocyte chemoattractant protein-1; COX-2: cyclooxygenase-2; LTB4: leukotriene B4; LTC4: leukotriene C4; NO: nitric oxide.
Figure 1.
The possible anti-inflammatory and immunomodulatory effects of N. sativa and TQ on COVID-19 induced acute respiratory distress syndrome (ARDS).
Effects of N. sativa and TQ on respiratory disorders, the clinical evidences
The prophylactic effect of N. sativa boiled extract was shown in asthmatic patients. Administration hydro-ethanolic extract of N. sativa seed extract (15 mg/kg/day) for a 3-month period improved respiratory symptoms such as chest wheeze, and pulmonary function test (PFT) values in asthmatic patients compared to the placebo treated group. Furthermore, the need for bronchodilator drugs were decreased in N. sativa compared to the placebo-treated patients (Boskabady et al. 2007). The bronchodilatory effect of N. sativa seed hydro-ethanolic extract (50 and 100 mg/kg/day, p.o.) in asthmatic patients in comparison with theophylline (6 mg/kg/day) increased pulmonary function tests (PFTs) values, and specific airway conductance (sGaw) compared to the baseline measurements. However, this effect on the most PFT values was less than that of theophylline (Boskabady et al. 2010). Similarly, a 2-month adjuvant treatment with N. sativa seed boiled extract (187 mg/kg/day, p.o.) in sulphur mustard poisoned patients showed a decline in the use of bronchodilator drugs compared to the baseline. In addition, the respiratory symptoms and PFT values significantly improved with no adverse effects during the study (Boskabady and Farhadi 2008).
The effect of probiotics or combination with N. sativa seed extract (15 mg/kg/day) significantly improved the asthma control test (ACT) score in the patients compared to before intervention (Kardani et al. 2013).
Conclusions
This review article descriptively highlights the possible effects of N. sativa and its major constituent with their underlying mechanism(s) of action on COVID-19. According to our literature survey, N. sativa and TQ have various important properties including, antiviral properties, stimulation of humoral and cellular immune responses, modulation of immune responses, improvement of eosinophil counts and IgE serum levels, reduction of pro-inflammatory cytokine (IL-4, IL-1β, IL-6, TGF-β, and IL-17), and enhancement of anti-inflammatory cytokines, IFN-γ and FOXP3. In addition, N. sativa and TQ showed relaxant effects on tracheal smooth muscle (in vitro) and also improved PFT values in obstructive lung diseases such as asthma. Since, ARDS along with cytokine storm of pro-inflammatory cytokines is the main cause of death among COVID-19 patients and with reference to the anti-inflammatory and immunomodulatory effects of N. sativa and TQ, as well as the protective effects on obstructive lung diseases, this herb may be useful for the treatment of COVID-19. However, clinical studies are required to support drug effectiveness.
Acknowledgement
The authors thank the research council of Birjand University of Medical Sciences.
Funding Statement
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure statement
The authors declared no conflicts of interest in this article.
References
- Abbas AT, Abdel-Aziz MM, Zalata K, Abd A-G-D.. 2004. Effect of dexamethasone and Nigella sativa on peripheral blood eosinophil count, IgG1 and IgG2a, cytokine profiles and lung inflammation in murine model of allergic asthma. Egypt J Immunol. 12:95–102. [PubMed] [Google Scholar]
- Abdelli I, Hassani F, Bekkel Brikci S, Ghalem S.. 2020. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from Western Algeria. J Biomol Struct Dynam. 2020:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulfadl Y, El-Maraghy N, Ahmed AE, Nofal S, Abdel-Mottaleb Y, Badary O.. 2018. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum Exp Toxicol. 37(10):1092–1104. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Abbasi HW, Shahid S, Gul S, Abbasi SW.. 2020. Molecular docking, simulation and MM-PBSA studies of Nigella sativa compounds: a computational quest to identify potential natural antiviral for COVID-19 treatment. J Biomol Struct Dynam. DOI: 10.1080/07391102.2020.1775129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B, Blunden G.. 2003. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 17:299–305. [DOI] [PubMed] [Google Scholar]
- Asif M, Saleem M, Saadullah M, Yaseen HS, Al Zarzour R.. 2020. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 28(5):1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Alwasel S, Ebaid H, Mohany M, Alhazza I.. 2011. Perinatal supplementation with thymoquinone improves diabetic complications and T cell immune responses in rat offspring. Cell Immunol. 267:133–140. [DOI] [PubMed] [Google Scholar]
- Barakat EMF, El Wakeel LM, Hagag RS.. 2013. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J Gastroenterol. 19:2529–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargi R, Asgharzadeh F, Beheshti F, Hosseini M, Sadeghnia HR, Khazaei M.. 2017. The effects of thymoquinone on hippocampal cytokine level, brain oxidative stress status and memory deficits induced by lipopolysaccharide in rats. Cytokine. 96:173–184. [DOI] [PubMed] [Google Scholar]
- Bashir A, Arfat Y, Rasheed M, Iftikhar S, Aziz RS, Rana M, Rashid M.. 2020. Thymoquinone and bronchodilation: the possible mechanism and therapeutic potential of an emerging natural drug in reactive airway disease. Issu Biol Sci Pharm Res. 8:1–19. [Google Scholar]
- Bordoni L, Fedeli D, Nasuti C, Maggi F, Papa F, Wabitsch M, De Caterina R, Gabbianelli R.. 2019. Antioxidant and anti-inflammatory properties of Nigella sativa oil in human pre-adipocytes. Antioxidants. 8(2):12–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady M, Mohsenpoor N, Takaloo L.. 2010. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 17(10):707–713. [DOI] [PubMed] [Google Scholar]
- Boskabady MH, Farhadi J.. 2008. The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: a randomized, double-blind, placebo-controlled trial. J Alternat Complement Med. 14(9):1137–1144. [DOI] [PubMed] [Google Scholar]
- Boskabady MH, Javan H, Sajady M, Rakhshandeh H.. 2007. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 21(5):559–566. [DOI] [PubMed] [Google Scholar]
- Boskabady M-H, Keyhanmanesh R, Khameneh S, Doostdar Y, Khakzad M-R.. 2011. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Univ Sci B. 12(3):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady MH, Vahedi N, Amery S, Khakzad MR.. 2011. The effect of Nigella sativa alone, and in combination with dexamethasone, on tracheal muscle responsiveness and lung inflammation in sulfur mustard exposed guinea pigs. J Ethnopharmacol. 137(2):1028–1034. [DOI] [PubMed] [Google Scholar]
- Bouchentouf S, Missoum N.. 2020. Identification of compounds from Nigella sativa as new potential inhibitors of 2019 novel Coronasvirus (COVID-19): Molecular docking study. Preprints. [Google Scholar]
- Büyüköztürk S, Gelincik A, Özşeker F, Genç S, Şavran FO, Kıran B, Yıllar G, Erden S, Aydın F, Çolakoğlu B, et al. . 2005. Nigella sativa (black seed) oil does not affect the T-helper 1 and T-helper 2 type cytokine production from splenic mononuclear cells in allergen sensitized mice. J Ethnopharmacol. 100:295–298. [DOI] [PubMed] [Google Scholar]
- Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ.. 2008. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 133:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA.. 2009. Anti‐inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB. 11:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RJ, Brokstad KA.. 2020. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat Rev Immunol. 20:581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, Chakrabarty R, Das S.. 2004. Saffron can prevent chemically induced skin carcinogenesis in Swiss albino mice. Asian Pac J Cancer Prev. 5:70–76. [PubMed] [Google Scholar]
- Dinarello CA. 2007. Historical insights into cytokines. Eur J Immunol. 37(S1):S34–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R, Dey P, Lamb C.. 1983. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 55:1–69. [DOI] [PubMed] [Google Scholar]
- Dorucu M, Colak SO, Ispir U, Altinterim B, Celayir Y.. 2009. The effect of black cumin seeds, Nigella sativa, on the immune response of rainbow trout, Oncorhynchus mykiss. Mediter Aquacult J. 2(1):27–33. [Google Scholar]
- Durmuskahya C, Ozturk M.. 2013. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malaysia. 42:1431–1438. [Google Scholar]
- El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC.. 2006. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 6:1135–1142. [DOI] [PubMed] [Google Scholar]
- El Gazzar M, El Mezayen R, Nicolls MR, Marecki JC, Dreskin SC.. 2006. Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochim Biophys Acta. 1760:1088–1095. [DOI] [PubMed] [Google Scholar]
- El Gazzar M. 2007. Thymoquinone suppresses in vitro production of IL-5 and IL-13 by mast cells in response to lipopolysaccharide stimulation. Inflamm Res. 56(8):345–351. [DOI] [PubMed] [Google Scholar]
- Elfiky AA. 2020. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J Biomol Struct Dynam. 39:3194–3203. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeka LB, Emeka PM, Khan TM.. 2015. Antimicrobial activity of Nigella sativa L. seed oil against multi-drug resistant Staphylococcus aureus isolated from diabetic wounds. Pak J Pharm Sci. 28:1985–1990. [PubMed] [Google Scholar]
- Fan Y-Y, Huang Z-T, Li L, Wu M-H, Yu T, Koup RA, Bailer RT, Wu C-Y.. 2009. Characterization of SARS-CoV-specific memory T cells from recovered individuals 4 years after infection. Arch Virol. 154:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth NF. 2008. The role of ethnopharmacology in drug development. Bioact Compound Plant. 735:2–21. [DOI] [PubMed] [Google Scholar]
- Gholamnezhad Z, Boskabady MH, Hosseini M.. 2014. Effect of Nigella sativa on immune response in treadmill exercised rat. BMC Complement Alternat Med. 14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamnezhad Z, Rafatpanah H, Sadeghnia HR, Boskabady MH.. 2015. Immunomodulatory and cytotoxic effects of Nigella sativa and thymoquinone on rat splenocytes. Food Chem Toxicol. 86:72–80. [DOI] [PubMed] [Google Scholar]
- Gilani AuH, Jabeen Q, Asad Ullah Khan M.. 2004. A review of medicinal uses and pharmacological activities of Nigella sativa. Pak J Biol Sci. 7(4):441–445. [Google Scholar]
- Hajhashemi V, Ghannadi A, Jafarabadi H.. 2004. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother Res. 18(3):195–199. [DOI] [PubMed] [Google Scholar]
- Haq A, Abdullatif M, Lobo PI, Khabar KS, Sheth KV, Al-Sedairy ST.. 1995. Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity. Immunopharmacology. 30(2):147–155. [DOI] [PubMed] [Google Scholar]
- Haq A, Lobo PI, Al-Tufail M, Rama NR, Al-Sedairy ST.. 1999. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int J Immunopharmacol. 21(4):283–295. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. . 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Hossain KS, Sarker PP, Ferdous J, Hannan MA, Rahman MM, Chu D-T, Uddin MJ.. 2020. Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure. Phytother Res. 2020:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam N, Begum P, Ahsan T, Huque S, Ahsan M.. 2004. Immunosuppressive and cytotoxic properties of Nigella sativa. Phytother Res. 18(5):395–398. [DOI] [PubMed] [Google Scholar]
- Jakhmola Mani R, Sehgal N, Dogra N, Saxena S, Pande Katare D.. 2020. Deciphering underlying mechanism of Sars-CoV-2 infection in humans and revealing the therapeutic potential of bioactive constituents from Nigella sativa to combat COVID19: in-silico study. J Biomol Struct Dynamic. DOI: 10.1080/07391102.2020.1839560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, Kiesewetter H.. 2003. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 17(10):1209–1214. [DOI] [PubMed] [Google Scholar]
- Kardani AK, Fitri LE, Barlianto W, Olivianto E, Kusuma C.. 2013. The effect of house dust mite immunotherapy, probiotic and Nigella sativa in the number of Th17 cell and asthma control test score. IOSR J Dent Med Sci. 6:37–47. [Google Scholar]
- Khazdair MR. 2015. The protective effects of Nigella sativa and its constituents on induced neurotoxicity. J Toxicol. 2015:1–7. Article ID 841823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazdair MR, Anaeigoudari A, Hashemzehi M, Mohebbati R.. 2019. Neuroprotective potency of some spice herbs, a literature review. J Tradit Complement Med. 9(2):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazdair MR, Anaeigoudari A, Kianmehr M.. 2019. Anti-asthmatic effects of Portulaca oleracea and its constituents, a review. J Pharmacopunct. 22:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshak AE, Koshak EA, Mobeireek AF, Badawi MA, Wali SO, Malibary HM, Atwah AF, Alhamdan MM, Almalki RA, Madani TA, et al. . 2020. Nigella sativa supplementation to treat symptomatic mild COVID-19: a structured summary of a protocol for a randomised, controlled, clinical trial. Trials. 21(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SA, Nagarajan SK, Ramesh V, Palaniyandi V, Selvam SP, Madhavan T.. 2020. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J Mol Struct. 1221:128823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choudhir G, Shukla SK, Sharma M, Tyagi P, Bhushan A, Rathore M.. 2020. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J Biomol Struct Dynam. 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA, York BM.. 1978. The toxicity of plant alkaloids: an ecogeographic perspective. Biochem Syst Ecol. 6(1):61–76. [Google Scholar]
- Li G, Chen X, Xu A.. 2003. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med. 349(5):508–509. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J.. 2020. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 27:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalawieh AF, Hmaidan R, Carr RI.. 2010. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J Ethnopharmacol. 131(2):268–275. [DOI] [PubMed] [Google Scholar]
- Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W.. 2020. Emerging novel coronavirus (2019-nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Quarter. 40:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A, Shin HM, Choi JY, Inn KS, Kim JH, et al. . 2016. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebbati R, Shafei MN, Beheshti F, Soukhtanloo M, Roshan NM, Anaeigoudari A, Parhizgar S, Hosseinian S, Khazdeir MR, Rad AK.. 2017. Mixed hydroalcoholic extracts of Nigella sativa and Curcuma longa improves adriamycin-induced renal injury in rat. Saudi J Kidney Dis Transplant. 28:1270–1281. [DOI] [PubMed] [Google Scholar]
- Mohebbatia R, Khazdair MR, Karimia S, Abbasnezhadd A.. 2017. Hepatoprotective effects of combination hydroalcoholic extracts of Nigella sativa and Curcuma longa on adriamycin-induced oxidative stress in rat. J Rep Pharm Sci. 6:93–102. [Google Scholar]
- Mohebbatia R, Khazdairb MR, Hedayatia M.. 2017. Neuroprotective effects of medicinal plants and their constituents on different induced neurotoxicity methods: a review. J Rep Pharm Sci. 6:34–50. [Google Scholar]
- Mohideen AKS. 2021. Molecular docking analysis of phytochemical thymoquinone as a therapeutic agent on SARS-Cov-2 envelope protein. Biointerface Res Appl Chem. 11:8389–8401. [Google Scholar]
- Mokhtari-Zaer A, Norouzi F, Askari VR, Khazdair MR, Roshan NM, Boskabady M, Hosseini M, Boskabady MH.. 2020. The protective effect of Nigella sativa extract on lung inflammation and oxidative stress induced by lipopolysaccharide in rats. J Ethnopharmacol. 253:112653. [DOI] [PubMed] [Google Scholar]
- Nasir A, Siddiqui M, Mohsin M.. 2014. Therapeutic uses of shoneez (Nigella sativa Linn.) mentioned in Unani system of medicine-a review. Int J Pharm Phytopharmacol Res. 4:47–49. [Google Scholar]
- Newman DJ, Cragg GM.. 2012. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 75(3):311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor NA, Fahmy HM, Mohammed FF, Elsayed AA, Radwan NM.. 2015. Nigella sativa ameliorates inflammation and demyelination in the experimental autoimmune encephalomyelitis-induced Wistar rats. Int J Clin Exp Pathol. 8:6269. [PMC free article] [PubMed] [Google Scholar]
- Phillipson JD. 2001. Phytochemistry and medicinal plants. Phytochemistry. 56(3):237–243. [DOI] [PubMed] [Google Scholar]
- Rahman MT. 2020. Potential benefits of combination of Nigella sativa and Zn supplements to treat COVID-19. J Herb Med. 23:100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana Keyhanmanesh LP, Omrani H, Mirzamohammadi Z, Shahbazfar AA.. 2014. The effect of single dose of thymoquinone, the main constituents of Nigella sativa, in guinea pig model of asthma. BioImpacts: BI. 4:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxas M, Jurenka J.. 2007. Colds and influenza: a review of diagnosis and conventional, botanical, and nutritional considerations. Alternat Med Rev. 12:25–49. [PubMed] [Google Scholar]
- Salem ML, Hossain MS.. 2000. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 22(9):729–740. [DOI] [PubMed] [Google Scholar]
- Salem ML. 2005. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 5(13–14):1749–1770. [DOI] [PubMed] [Google Scholar]
- Sekiou O, Bouziane I, Bouslama Z, Djemel A.. 2020. In-silico identification of potent inhibitors of COVID-19 main protease (Mpro) and angiotensin converting enzyme 2 (ACE2) from natural products: quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy-chloroquine against COVID-19 main protease active site and ACE2. ChemRxiv. 12181404:v1 [Google Scholar]
- Sethi G, Ahn KS, Aggarwal BB.. 2008. Targeting nuclear factor-κB activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 6:1059–1070. [DOI] [PubMed] [Google Scholar]
- Shanmugaraj B, Malla A, Phoolcharoen W.. 2020. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 9(2):110–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke JW, Borish L.. 2001. Th2 cytokines and asthma—interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2(2):66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D-L, Lu Z-M, Shen M-N, Li X, Sun L-Y.. 2012. Roles of pro-and anti-inflammatory cytokines in the pathogenesis of SLE. BioMed Res Int. 2012:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tembhurne S, Feroz S, More B, Sakarkar D.. 2014. A review on therapeutic potential of Nigella sativa (kalonji) seeds. J Med Plants Res. 8(3):167–177. [Google Scholar]
- Umar S, Shah MA, Munir MT, Yaqoob M, Fiaz M, Anjum S, Kaboudi K, Bouzouaia M, Younus M, Nisa Q, et al. . 2016. Synergistic effects of thymoquinone and curcumin on immune response and anti-viral activity against avian influenza virus (H9N2) in turkeys. Poult Sci. 95:1513–1520. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Miller SD.. 2002. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2:85–95. [DOI] [PubMed] [Google Scholar]
- Wu D, Meydani M, Leka LS, Nightingale Z, Handelman GJ, Blumberg JB, Meydani SN.. 1999. Effect of dietary supplementation with black currant seed oil on the immune response of healthy elderly subjects. Am J Clin Nutr. 70:536–543. [DOI] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. . 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan NT, Shumilina E, Qadri SM, Götz F, Lang F.. 2010. Effect of thymoquinone on mouse dendritic cells. Cell Physiol Biochem. 25:307–314. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ, Baric RS, Enjuanes L, Gallagher T, et al. . 2014. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci. 111:4970–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]