ABSTRACT
The World Health Organization (WHO) introduced the new dengue classification in 2009. We aimed to assess the association of clinical signs and symptoms with WHO severe dengue classification in clinical practice. A systematic literature search was performed using the databases of PubMed, Embase, and Scopus between 2009 and 2018 according to PRISMA guideline. Meta-analysis was performed with the RevMan software. A random or fixed-effect model was applied to pool odds ratios and 95% confidence intervals of important signs and symptoms across studies. Thirty nine articles from 1790 records were included in this review. In our meta-analysis, signs and symptoms associated with higher risk of severe dengue were comorbidity, vomiting, persistent vomiting, abdominal pain or tenderness, pleural effusion, ascites, epistaxis, gum bleeding, GI bleeding, skin bleeding, lethargy or restlessness, hepatomegaly (>2 cm), increased HCT with decreased platelets, shock, dyspnea, impaired consciousness, thrombocytopenia, elevated AST and ALT, gall bladder wall thickening and secondary infection. This review shows new factors comorbidity, epistaxis, GI and skin bleeding, dyspnea, gall bladder wall thickening and secondary infection may be useful to refine the 2009 classification to triage severe dengue patients.
KEYWORDS: Severe dengue, signs, symptoms, predictive performance, meta-analysis
Introduction
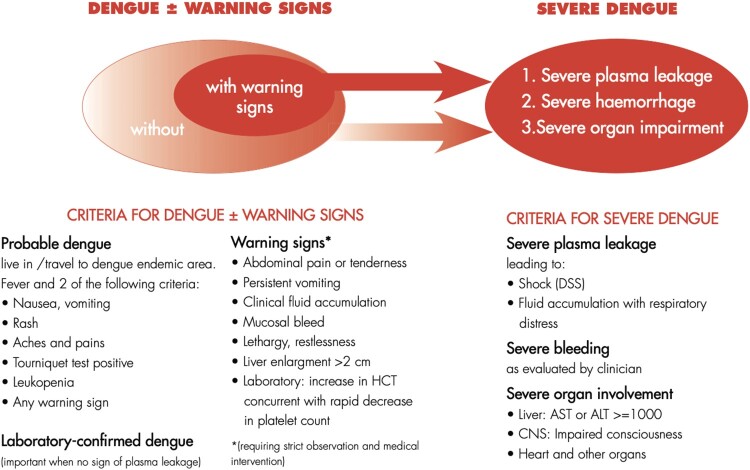
Dengue is the fastest spreading mosquito-borne viral disease globally, affecting 50 million individuals every year [1]. In the vast majority of individuals, dengue fever is a self-limiting disease that requires minimal supportive treatment. However, in less than 1% of patients, symptoms of severe dengue, including clinical fluid accumulation, shock, and multiple organ dysfunction could spell impending demise if left untreated. The new 2009 WHO classification for dengue was hence created to allow clinicians to triage patients easily according to their clinical presentations for more effective clinical management (Figure 1) [1]. This new classification is intended to bring greater clarity on the severity of clinical presentations compared to the 1997 classification of dengue into undifferentiated fever, dengue fever [1] and dengue hemorrhagic fever.
Figure 1.
The 2009 WHO revised dengue case classification.
The 1997 classification was proven to underestimate the severity of dengue infection [2]. Multiple studies had shown that plasma leakage causing clinical fluid accumulation, transaminitis and thrombocytopenia were more indicative of severe dengue instead of clinical manifestations of bleeding, as was prioritized in the old classification [3, 4]. In febrile travelers returning from endemic regions, one study showed that a significant number of cases of severe dengue would have been missed if the WHO diagnostic criteria for dengue haemorrhagic fever would have been applied [3]. While many studies have effectively highlighted the shortcomings of the 1997 classification, there is a paucity of studies done today to ascertain if the clinical utility of the current 2009 classification has improved clinical diagnosis and management of dengue infections.
Previous review has reported that the new 2009 classification has a higher sensitivity and specificity compared with the 1997 classification [5]. However, there was a question for applicability in clinical practice and usefulness for triage using the revised dengue classification [6]. Several studies have assessed the association of clinical factors with severe dengue [7–10]. However, risk factors reported among severe dengue patients remained inconsistent [7–10]. The objective of this review was to synthesize the best of available evidence by conducting meta-analysis to assess the factors associated with severe dengue patients. This systematic review and meta-analysis therefore investigate the likelihood of new factors associated with severe dengue, which may be useful to further revise the existing dengue 2009 classifications for more accurate triaging of patients.
Materials and methods
Search strategy
This review was conducted according to the standards outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11]. No documented review protocol exists for this meta-analysis. The year 2009 was selected as the start date of searching articles as the introduction of new WHO dengue case classification in 2009 [1]. The search was performed in three databases: PubMed, Embase, and Scopus; covering literature between the period of January 2009 and December 2018. Manual search for reference lists of included studies was performed to check additional studies relevant to the topic. The keywords used in search are “dengue” OR “severe dengue” OR “dengue severity” AND “diagnosis” OR “clinical diagnosis” OR “warning signs.” All the references were imported and removed duplicates by using bibliographical software package, EndNote version X7 (Thomas Reuters, New York, NY, USA). The studies were screened independently against the inclusion and exclusion criteria by two authors (TP and XZ), and a third author (PJ) resolved disagreement between the two reviewers regarding eligibility of a study.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) any type of studies (retrospective, prospective, or cohort, case–control, cross-sectional studies) reporting severe dengue (defined with 2009 WHO diagnosis criteria) compared with dengue fever; (2) studies that distinguished clinical signs and symptoms and/or laboratory features of severe dengue and dengue fever with or without warning signs; (3) studies that published on and after 2009; (4) studies that classified dengue severity according to new 2009 WHO classification; (5) studies that included either children or adults only or both children and adults. We excluded studies if they were narrative review, letters to editors, case reports and case series, incomplete information to extract data and not written in English.
Quality assessment
Two of the authors (TP and XZ) independently assessed the quality of each included study using the Newcastle-Ottawa Scale (NOS) [12]. NOS is the risk assessment tool developed to assess the quality of non-randomized studies used in systematic review and meta-analysis. It consists of three parameters of quality i.e. selection, comparability, and exposure with maximum of 4 points for selection of study groups, 2 points for comparability of groups and 3 points for exposures and outcomes. The NOS scores were divided into low quality (scores 1–3), intermediate quality (scores 4–6), and high-quality (scores 7–9) [13]. When any difference in opinion of quality assessment between the two authors happened, it was solved by a third author (PJ) via discussion and consensus.
Data extraction
The data were extracted from each study through structured data extraction forms. Items extracted for the characteristics of studies included the authors, year of publication, country, setting of study, study design, study population (children, adult or both), numbers of patients for dengue fever (with or without warning signs) and severe dengue, and diagnosis of dengue. Outcome data (clinical signs and symptoms and/or laboratory features) for severe dengue and dengue fever were extracted and compiled in the summary tables by one author (HTP), and cross-checked by another author (XZ) for accuracy and relevance.
Data analyses
Data were analyzed using RevMan software (Review Manager Version 5.3.5, The Nordic Cochrane Centre, Copenhagen). Dichotomous data was analysed using the Mantel–Haenszel (M-H) method; odds ratio (OR) with 95% confidence interval (CI) was calculated using either a fixed-effect or random-effect model with at least four or more studies though only 2 studies are needed for a meta-analysis theoretically. The test of overall effect was assessed using z-statistics at P < 0.05. Heterogeneity between studies was evaluated using the Cochrane Q (χ2 test) and I2 test. I2 value considered to 0% as no, 25% as low, 50% as moderate and 75% as high heterogeneity [14]. The statistical significance for heterogeneity was set with a P value < 0.10. The fixed-effects model with weighting of the studies was used when there was a lack of significant heterogeneity (P > 0.10), while the random-effects model with weighting of the studies was used when there was heterogeneity between studies (P < 0.10) [14]. Sensitivity testing to identify the effect of the subgroups was performed by subgroup analysis based on study population. Subgroup analysis was performed to (1) explore the potential sources of heterogeneity among the studies and (2) evaluate the effect in a specific subgroup. The predefined subgroups were study population (children, adult, or both) and dengue severity (severe dengue or dengue fever with or without warning signs).
Results
Study characteristics and quality
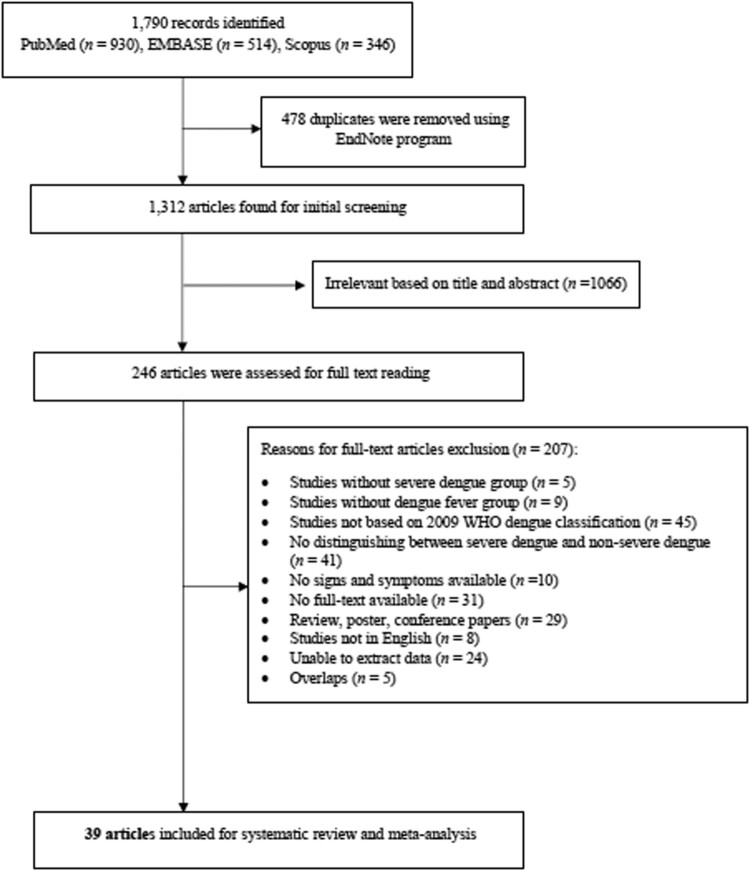
Figure 2 illustrates searching articles and the selection process. A total of 1790 records were identified, whereas a total of 478 duplicates were removed. The initial screening yielded 1312 articles, of which 246 articles were assessed for full text reading. A total of 207 full-text articles were excluded for the reasons mentioned in the study flow chart (Figure 2). Finally, 39 articles [2, 7–10, 15–48] were selected for inclusion in this meta-analysis according to the WHO classification for dengue, namely dengue without warning signs, dengue with warning signs and severe dengue, as well as unclassified signs or laboratory features. The date set for searching was 2009, all the studies were published after 2009.
Figure 2.
Selection of studies for inclusion in the systematic review and meta-analysis.
Table 1 provides a summary characteristic of prospective study (n = 16), retrospective study (n = 21) and case control study (n = 2). Sample sizes were varied among the studies, ranging from 8 to 2060 cases. This study included a population of children (n = 18), adult (n = 14) and both (n = 7) and they are from varying locations: Asia (n = 31), Brazil (n = 4), Germany (n = 1), Mexico (n = 2), and Spain (n = 1). Most studies were performed in hospital settings (n = 36) than healthcare network (n = 1), medical education and research institute (n = 1), tertiary care unit (n = 1). Comorbidities were reported in ten studies, the proportion of comorbidity varied from 0% to 100% in severe dengue and 13% to 55.7% in dengue fever with or without warning signs. Nineteen studies reported day of presentation of illness or fever, whereas median day of illness ranged from 3.5 to 5 days in severe dengue and 2–5 days in dengue fever with or without warning signs. Dengue infection was confirmed by clinically in two studies, whereas serology, ELISA, PCR, HIA, viral isolation, and nucleotide detection was used together with clinical diagnosis in 37 studies for confirmation of dengue infection. Assessing the quality of the studies by Newcastle-Ottawa Scale, 5 studies were high quality (scores 7–9), 33 studies were intermediate quality (scores 4–6) and only one study has low quality (scores 1–3).
Table 1.
Characteristics of included studies.
| Author, year | Country, setting | Study design | Population | Sample size (n) | Comorbidity | Day of presentation (days) | Diagnosis of dengue | Quality score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | DF | SD | DF | SD | DF | ||||||
| Adam et al. 2018 | Indonesia, hospital | Retrospective descriptive-analytic study | Children | 28 | 112 | NR | NR | 4–5 | 4–5 | Serology | 4 |
| Agarwal et al. 2018 | India, hospital | Retrospective study | Children | 52 | 136 | NR | NR | NR | NR | ELISA | 4 |
| Alvarado-Castro et al. 2016 | Mexico, hospital | Retrospective case series study | Children | 56 | 77 | NR | NR | 5 | 4.6 | Clinical diagnosis | 5 |
| Andries et al. 2016 | Cambodia, hospital | Case-control study | Children | 22 | 24 (without WS) 62 (with WS) |
NR | NR | 4 | 2 (without WS) 4 (with WS) |
Serology, PCR, HIA | 8 |
| Athira et al. 2018 | India, hospital | Retrospective cross-sectional study | Children | 11 | 7 (without WS) 16 (with WS) |
NR | NR | NR | NR | ELISA | 4 |
| Aung et al. 2013 | Thailand, hospital | Retrospective study | Adult | 90 | 193 | 25.6% | 20.2% | 4 | 4 | PCR, Serology | 5 |
| Bhaskar et al. 2015 | India, hospital | Retrospective study | Adult | 128 | 510 | 13% | 13% | NR | NR | ELISA | 6 |
| Carrasco et al., 2014 | Singapore, hospital | Retrospective cohort study | Adult | 96 | 500 | 21% | 19% | 3.9 | 4.3 | PCR, Serology | 6 |
| de Cavalcanti et al. 2013 | Brazil, hospital | Retrospective cross- sectional study | Both | 52 | 4 (without WS) 28 (with WS) |
NR | NR | NR | NR | ELISA, PCR, Viral isolation | 5 |
| Giraldo et al. 2011 | Brazil, hospital | Retrospective cohort study | Children | 30 | 151 | 23.3% | 29.1% | NR | NR | Clinical diagnosis, Serology | 7 |
| Hoffmeister et al. 2015 | Germany, hospital | Retrospective study | Adult | 6 | 30 (without WS) 11 (with WS) |
0% | 23% (without WS) 18.1% (with WS) |
NR | NR | ELISA, PCR, Serology | 4 |
| Jayaratne et al. 2012 | Sri Lanka, hospital | Prospective study | Adult | 40 | 144 | NR | NR | NR | NR | ELISA, PCR | 6 |
| Kumar et al. 2014 | Spain, hospital | Prospective study | Children | 20 | 95 | Exclude other febrile illness | 3.5 | 3 | ELISA | 5 | |
| Lee et al. 2016 | Taiwan, hospital | Retrospective study | Adult | 37 (≤4 days) 18 (> 4days) |
593 (≤4 days) 415 (> 4days) |
54.5% | 24.3% | 5 | 3 | PCR, Serology | 5 |
| Lin et al. 2016 | China, hospital | Prospective study | Adult | 8 | 130 | NR | NR | NR | NR | ELISA, PCR | 6 |
| Macedo et al. 2014 | Brazil, hospital | Retrospective study | Children | 107 | 18 (without WS) 142 (with WS) |
NR | NR | 4 | 5 (without WS) 5 (with WS) |
PCR, Serology | 6 |
| Michels et al. 2013 | Indonesia, hospital | Prospective observational study | Adult | 11 | 55 | Exclude concurrent chronic disease and pregnancy | NR | NR | PCR, Serology | 5 | |
| Nguyen et al. 2017 | Vietnam, hospital | Prospective study | Children | 117 | 1943 | NR | NR | NR | NR | ELISA, PCR, Serology | 7 |
| Pereira et al. 2018 | India, hospital | Retrospective study | Adult | 101 | 449 | Exclude concomitant febrile illness | NR | NR | ELISA, Serology | 5 | |
| Phakhounthong et al. 2018 | Cambodia, hospital | Retrospective study | Children | 38 | 160 | Exclude acquired healthcare associated infection | 4.1 | 4.3 | ELISA | 5 | |
| Pozo-Aguilar et al. 2014 | Mexico, hospital | Prospective cross-sectional study | Both | 115 109 |
374 380 |
NR | NR | NR | NR | ELISA, PCR, Serology | 7 |
| Prasad et al. 2013 | India, hospital | Prospective study | Children | 45 | 10 | NR | NR | NR | NR | ELISA, PCR | 6 |
| Ramabhatta et al. 20 17 | India, hospital | Prospective cross-sectional study | Children | 194 | 66 (without WS) 308 (with WS) |
NR | NR | NR | NR | Serology | 6 |
| Rathakrishnan et al. 2014 | Malaysia, hospital | Prospective descriptive study | Adult | 5 | 64 (without WS) 388 (with WS) |
NR | NR | 5 | 5 | ELISA, PCR, HIA | 6 |
| Roy et al. 2013 | India, hospital | Prospective study | Children | 73 | 15 (without WS) 32 (with WS) |
Exclude concomitant infections and liver disease | NR | NR | ELISA | 5 | |
| Sahana et al. 2014 | India, hospital | Prospective observational study | Children | 20 | 39 (without WS) 22 (with WS) |
NR | NR | 4.6 | 4.6 | Serology | 6 |
| Singh et al. 2015 | India, hospital | Prospective study | Children | 17 | 7 (without WS) 48 (with WS) |
Exclude other infections | NR | NR | ELISA, Serology | 3 | |
| Soundravally et al. 2015 | India, medical education and research institute | Nested case-control study | Both | 20 | 13 (without WS) 15 (without WS) |
Exclude known cases | NR | NR | ELISA, PCR | 5 | |
| Sreenivasan et al. 2018 | India, tertiary care center | Prospective analytical study | Children | 93 | 266 | Exclude co-infections and co-morbidities | 5 | 5 | ELISA | 6 | |
| Tai et al. 2017 | Australia, healthcare networks | Retrospective case series study | Both | 1 | 123 (without WS) 84 (with WS) |
100% | 7.3% (without WS) 13.1% (with WS) |
4 | 4.5 (without WS) 4 (with WS) |
PCR, Serology | 5 |
| Tamibmaniam et al. 2016 | Malaysia, hospital | Retrospective study | Both | 59 | 657 | 22% | 22% | 5 | 5 | Not reported | 4 |
| Temprasertrudee et al. 2018 | Thailand, hospital | Retrospective cohort study | Adult | 38 | 319 | 21.1% | 14.1% | 4 | 3 | Serology | 5 |
| Thanachartwet et al. 2015 | Thailand, hospital | Prospective study | Adult | 216 | 132 | Exclude mixed infection and underlying medical illness | NR | NR | ELISA, PCR | 6 | |
| Thanachartwet et al. 2016 | Thailand, hospital | Prospective observational study | Adult | 20 | 105 | Exclude mixed infection, underlying medical illness and pregnancy | 5 | 4 | ELISA, PCR | 6 | |
| Thein et al. 2013 | Singapore, hospital | Retrospective study | Both | 65 | 248 | NR | NR | 4 | 4 | PCR | 4 |
| Tsai et al. 2013 | Taiwan, hospital | Retrospective study | Both | 7 | 64 77 |
71.4% | 6.3% (without WS) 49.4% (with WS) |
4.4 | 3.8 (without WS) 3.6 (with WS) |
PCR, Serology, HIA | 4 |
| Van de Weg et al. 2012 | Indonesia, hospital | Prospective study | Children | 104 | 69 | NR | NR | 4 | 3 | PCR, Serology | 6 |
| Wakimoto et al. 2017 | Brazil, hospital | Retrospective case-control study | Children | 69 | 164 | NR | NR | NR | NR | ELISA | 7 |
| Zhang et al. 2017 | China, hospital | Retrospective study | Adult | 38 | 174 | Exclude chronic medical illness | NR | NR | Serology, Viral isolation, Nucleotide detection | 4 | |
Notes: DF = Dengue fever; SD = Severe dengue; ELISA = Enzyme-linked immunosorbent assay; PCR = Polymerase chain reaction; HIA = Hemagglutination inhibition assay; NR = Not reported; WS = Warning signs; Day of presentation = day of illness or fever prior to admission/first contact with health services
Potential predictive factors of severe dengue
A total of 39 factors were analyzed when there are four or more studies to perform a regression analysis (Table 2; Figure 3). The fixed effect model was used in 12 factors (nausea, headache, retro-orbital pain, arthralgia, myalgia, hematuria, cough, diarrhea, splenomegaly, shock, dyspnea, gall bladder wall thickening), while the random effect model was used in 27 factors (gender: male and female, comorbidity, fever, vomiting, rash, tourniquet test (+), leucopenia, abdominal pain or tenderness, persistent vomiting, pleural effusion, ascites, epistaxis, gum bleeding, gastrointestinal bleeding (hematemesis and/or melena), vaginal bleeding, lethargy or restlessness, hepatomegaly > 2 cm, increased HCT with decreased platelets, skin bleeding (petechiae, purpura, ecchymosis), impaired consciousness, thrombocytopenia (platelets < 150*109/L), elevated ALT (>40 u/l), elevated AST (>40 u/l), hypoalbuminemia, primary infection, secondary infection). Of these factors, a total of 21 factors were found to be significantly associated with severe dengue and dengue fever with or without warning signs.
Table 2.
Results of meta-analysis for the clinical characteristics between severe dengue and dengue fever with or without warning signs.
| Clinical characteristics | Number of studies | Total events | Odds ratio | Z | Test for OR | Test of heterogeneity | Model | |
|---|---|---|---|---|---|---|---|---|
| SD/DF | (95% CI) | P-value | I2 (%) | P-value | ||||
| Demographic characteristics | ||||||||
| Gender (Male) | 22 | 584/2483 | 0.95 (0.77–1.16) | 0.53 | 0.60 | 33 | 0.04 | Random |
| Gender (Female) | 17 | 485/1199 | 1.30 (0.95–1.77) | 1.66 | 0.10 | 62 | <0.001 | Random |
| Comorbidity | 8 | 100/545 | 2.03 (1.09–3.78) | 2.24 | 0.03 | 70 | <0.001 | Random |
| Probable dengue | ||||||||
| Fever | 14 | 951/3076 | 0.74 (0.34–1.60) | 0.77 | 0.44 | 52 | 0.01 | Random |
| Nausea | 8 | 140/461 | 0.92 (0.66–1.27) | 0.53 | 0.60 | 13 | 0.32 | Fixed |
| Vomiting | 19 | 849/2275 | 2.18 (1.50–3.16) | 4.12 | <0.001 | 77 | <0.001 | Random |
| Rash | 22 | 395/1569 | 1.07 (0.84–1.37) | 0.55 | 0.58 | 41 | 0.01 | Random |
| Headache | 18 | 505/2388 | 0.84 (0.70–1.00) | 2.00 | 0.05 | 28 | 0.11 | Fixed |
| Retro-orbital pain | 13 | 172/726 | 0.99 (0.75–1.30) | 0.10 | 0.92 | 0 | 0.73 | Fixed |
| Arthralgia | 16 | 281/1566 | 1.10 (0.89–1.36) | 0.86 | 0.39 | 0 | 0.55 | Fixed |
| Myalgia | 17 | 451/2498 | 1.01 (0.83–1.24) | 0.11 | 0.92 | 0 | 0.53 | Fixed |
| Tourniquet test (+) | 7 | 108/349 | 0.52 (0.19–1.44) | 1.27 | 0.21 | 68 | <0.01 | Random |
| Leucopenia | 14 | 275/1578 | 0.82 (0.59–1.15) | 1.15 | 0.25 | 35 | 0.06 | Random |
| Warning signs | ||||||||
| Abdominal pain or tenderness | 33 | 1338/2554 | 2.00 (1.49–2.68) | 4.62 | <0.001 | 75 | <0.001 | Random |
| Persistent vomiting | 12 | 296/465 | 2.57 (1.40–4.73) | 3.04 | 0.002 | 80 | <0.001 | Random |
| Clinical fluid accumulation | ||||||||
| Pleural effusion | 14 | 397/264 | 6.20 (3.66–10.51) | 6.77 | <0.001 | 65 | <0.001 | Random |
| Ascites | 15 | 420/266 | 5.20 (3.27–8.29) | 6.94 | <0.001 | 54 | 0.002 | Random |
| Gall bladder wall thickening | 4 | 141/80 | 5.61 (2.73–11.53) | 4.69 | <0.001 | 31 | 0.19 | Fixed |
| Mucosal bleeding | ||||||||
| Epistaxis | 9 | 73/110 | 2.23 (1.04–4.77) | 2.07 | 0.04 | 65 | 0.001 | Random |
| Gum bleeding | 10 | 48/208 | 3.34 (1.60–6.98) | 3.21 | <0.01 | 49 | 0.02 | Random |
| GI bleeding (hematemesis and/or melena) | 10 | 104/89 | 14.56 (5.38–39.39) | 5.27 | <0.001 | 74 | <0.001 | Random |
| Hematuria | 4 | 4/22 | 2.48 (0.75–8.25) | 1.48 | 0.14 | 0 | 0.53 | Fixed |
| Vaginal bleeding | 4 | 20/21 | 6.62 (0.38–114.64) | 1.30 | 0.19 | 75 | <0.01 | Random |
| Skin bleeding (petechiae, purpura, ecchymosis) | 19 | 386/723 | 2.12 (1.53–3.19) | 4.22 | <0.001 | 62 | <0.001 | Random |
| Lethargy or restlessness | 13 | 464/755 | 4.32 (1.86–10.04) | 3.40 | <0.001 | 89 | <0.001 | Random |
| Hepatomegaly > 2 cm | 25 | 796/730 | 3.34 (2.38–4.68) | 7.00 | <0.001 | 66 | <0.001 | Random |
| Increased HCT with decreased platelets | 7 | 170/224 | 3.19 (1.36–7.46) | 2.68 | 0.007 | 59 | 0.02 | Random |
| Severe dengue | ||||||||
| Severe plasma leakage | ||||||||
| Shock | 6 | 235/3 | 47.51 (14.80 -152.50) | 8.85 | <0.001 | 35 | 0.15 | Fixed |
| Dyspnea | 6 | 99/44 | 11.19 (6.91–18.11) | 9.82 | <0.001 | 0 | 0.56 | Fixed |
| Severe organ involvement | ||||||||
| Elevated ALT (>40 u/L) | 7 | 290/582 | 3.24 (1.87–5.61) | 4.19 | <0.001 | 51 | 0.04 | Random |
| Elevated AST (>40 u/L) | 8 | 338/790 | 3.75 (2.11–6.68) | 4.49 | <0.001 | 51 | 0.03 | Random |
| Impaired consciousness | 5 | 37/30 | 29.81 (4.08–217.94) | 3.34 | <0.001 | 74 | 0.002 | Random |
| Splenomegaly | 6 | 34/75 | 1.33 (0.81–2.18) | 1.14 | 0.25 | 0 | 0.76 | Fixed |
| Others | ||||||||
| Cough | 6 | 36/398 | 1.08 (0.73–1.59) | 0.39 | 0.70 | 0 | 0.60 | Fixed |
| Diarrhea | 12 | 71/704 | 1.02 (0.76–1.36) | 0.13 | 0.89 | 0 | 0.99 | Fixed |
| Thrombocytopenia (platelets <150*109/L) | 18 | 893/3282 | 2.70 (1.60–4.55) | 3.73 | <0.001 | 68 | <0.001 | Random |
| Hypoalbuminemia | 7 | 152/776 | 2.25 (0.85–5.92) | 1.64 | 0.10 | 78 | <0.001 | Random |
| Primary infection | 4 | 11/83 | 0.43 (0.09–2.04) | 1.07 | 0.29 | 64 | 0.03 | Random |
| Secondary infection | 5 | 96/310 | 1.93 (1.25–2.97) | 2.96 | 0.003 | 0 | 0.50 | Random |
Notes: SD = Severe dengue; DF = Dengue fever; HCT = Hematocrit; ALT = Alanine transaminase; AST = Aspartate transaminase.
Figure 3.
Forest plots comparison of signs and symptoms for severe dengue and dengue fever.
Socio-demographic characteristics
Socio-demographic characteristics including gender difference (male and female) showed no significant association with severe dengue (P > 0.05). Pooling of eight studies, comorbidity was positively associated with severe dengue (OR: 2.03, CI: 1.09–3.78, z = 2.24, P = 0.03).
Probable dengue without warning signs
The symptoms listed for probable dengue without warning signs include fever, nausea, vomiting, rash, headache, retro-orbital pain, arthralgia, myalgia, positive tourniquet test and leucopenia. Amongst all listed symptoms, vomiting was positively associated with severe dengue (OR: 2.18, CI: 1.50–3.16, z = 4.12, P < 0.001) in 19 studies.
Dengue with warning signs
The symptoms listed for dengue with warning signs include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation (pleural effusion, ascites, gallbladder wall thickening), mucosal bleeding (epistaxis, gum bleeding, gastrointestinal bleeding, hematuria, vaginal bleeding, skin bleeding), lethargy or restlessness, hepatomegaly >2 cm and increased hematocrit with decreased platelets. Of the listed symptoms, pleural effusion (OR: 6.20, CI: 3.66–10.51, z = 6.77, P < 0.001), ascites (OR: 5.20, CI: 3.27–8.29, z = 6.94, P < 0.001), gallbladder wall thickening (OR: 5.61, CI: 2.73–11.53, z = 4.69, P < 0.001), and gastrointestinal bleeding as a manifestation of mucosal bleeding (OR: 14.56, CI: 5.38–39.39, z = 5.27, P < 0.001) were highly associated with severe dengue for a patient being diagnosed with dengue with warning signs. In addition, of the warning signs, abdominal pain or tenderness (OR: 2.00, CI: 1.49–2.68, z = 4.62, P < 0.001), persistent vomiting (OR: 2.57, CI: 1.40–4.73, z = 3.04, P = 0.002), epistaxis (OR: 2.23, CI: 1.04–4.77, z = 2.07, P = 0.04), gum bleeding (OR: 3.34, CI: 1.60–6.98, z = 3.21, P < 0.01), skin bleeding (OR: 2.12, CI: 1.53–3.19, z = 4.22, P < 0.001), lethargy or restlessness (OR: 4.32, CI: 1.86–10.04, z = 3.40, P < 0.001), hepatomegaly >2 cm (OR: 3.34, CI: 2.38–4.68, z = 7.00, P < 0.001) and raising hematocrit (OR: 3.19, CI: 1.36–7.46, z = 2.68, P = 0.007) were moderately associated with severe dengue.
Severe dengue
The symptoms listed for severe dengue include shock, fluid accumulation leading to dyspnea, severe bleeding on clinical evaluation, impaired consciousness and transaminitis (aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥1000 units/L) and organ failure. Symptoms of shock (OR: 47.51, CI: 14.80–152.50, z = 8.85, P < 0.001), dyspnea (OR: 11.19, CI: 6.91–18.11, z = 9.82, P < 0.001) and impaired consciousness (OR: 29.81, CI: 4.08–217.94, z = 3.34, P < 0.001) had remarkably higher odds for severe dengue. Elevated ALT (OR: 3.24, CI: 1.87–5.61, z = 4.19, P < 0.001), elevated AST (OR: 3.75, CI: 2.11–6.68, z = 4.49, P < 0.001) were moderately associated with severe dengue.
Other signs and symptoms and laboratory features
Other symptoms of cough and diarrhoea in association with dengue infection were analysed but yielded non-significant results. Associated laboratory features of thrombocytopenia (OR: 2.70, CI: 1.60–4.55, z = 3.73, P < 0.001) was positively associated with severe dengue while hypoalbuminemia found no association with severe dengue (P > 0.05). The presence of a secondary dengue infection (a patient having a second or more dengue infection) was also statistically significant in the odds of being diagnosed with dengue infection (OR: 1.93, CI: 1.25–2.97, z = 2.96, P < 0.01).
Discussion
Our detailed meta-analysis comprises studies encompassing numerous countries globally suggests the current 2009 WHO clinical classification optimally identifies severe dengue infection.
Our study lies in its detailed meta-analysis of a wide range of studies encompassing numerous countries globally. We found that patients with comorbidity had 2-times higher risk of progression into severe dengue. This finding is in line with previous study indicating that pre-existing comorbidities were risk factors of severe organ involvement in dengue patients [49]. Digestive factors of vomiting, persistent vomiting, abdominal pain or tenderness were indicative of severe dengue in our study, which is consistent with previous study showing that vomiting and abdominal pain were most prevalent warning signs which occur prior to severe dengue [50]. Bleeding manifestations include mucosal bleeding (epistaxis, gum bleeding), GI bleeding (hematemesis and/or melena) and skin bleeding (petechiae, purpura, ecchymosis) were shown as valuable predictors of severe dengue in our study except for hematuria and vaginal bleeding. Consistent with previous meta-analyses, four kinds of bleeding: epistaxis, gum bleeding, hematemesis, and melena were related to the risk of development of patients with severe dengue [13]. Among bleeding factors, gastrointestinal bleeding proved highly indicative of severe dengue. A study also showed that patients with gastrointestinal bleeding had the highest risk of progressing into severe disease [23]. Notably, pleural effusion and ascites were significantly associated with severe dengue. Plasma leakage causing fluid accumulation, during which fever transitions into defervesence, was cited as a critical indicator of progression to severe dengue [33]. Concurrent increase in haemotocrit and rapid decrease in platelet count, vomiting and abdominal distention were significant in predicting the likelihood of severe plasma leakage as a warning sign of dengue [22]. In one Singaporean study, concurrent increase in haemotocrit and decrease in platelet count were found to be predictive of severe haemorrhage [22], which is consistent with our result.
Liver damage is a common complication of dengue, liver enzymes are valuable markers during dengue infection [51]. In our results, hepatomeagly (>2 cm), elevated AST and ALT were significantly different between severe dengue and dengue with or without warning signs. These findings are similar to the previous studies that liver enlargement and liver enzymes (AST and ALT) were significantly higher in severe dengue patient [48, 52]. Interestingly, four articles highlighted the presence of gallbladder wall thickening as a clinical sign of dengue infection and which was found to be associated with severe dengue. In multiple studies, this was characteristic only for severe dengue [25, 53]. One study showed that gallbladder thickening was present even before serological tests were positive [54] and as potential early predictors [53]. While thrombocytopenia was a significant predictor for severe dengue in many studies [52, 55], our result revealed that platelet count less than 150000/mm3 has value in ruling in dengue infection. However, one study surprisingly showed that it was unlikely to be a direct precipitant for clinical manifestations of bleeding [38]. Our analysis showed association of secondary dengue infection with severe dengue. As proven by other studies, patients presenting with a secondary dengue infection were associated with a higher risk of developing severe dengue [33, 56], which suggests that the clinical presentation of severe dengue was affected by both host factors (secondary immune response and viral load) [57].
Our review has several limitations. Firstly, there was variability among the included studies in terms of study designs, study population, diagnoses, comorbidities and day of presentation of illness or fever, which weakens the comparison among different studies. The definitions and cutoff values of warning signs and severity were widely varied within the studies [58], which brought heterogenous application to rule out the cases. Some identified studies were performed on individuals of one demographic, such as being either from the paediatric or adult age group, which can lead to unaccounted variation in presenting signs or symptoms. Secondly, research conducted in regions endemic for dengue infection, especially countries near the equator, constituted an overwhelming majority in our study. Therefore, studies of dengue infection in less endemic countries could have been elided over, conferring selection bias for our study. Thirdly, in our meta-analysis for dengue without warning signs, it was unfortunate that a majority of the listed symptoms did not prove significant. Many listed symptoms that we studied also did not stem from an acceptable level of heterogeneity.
Our finding identified significant association between 21 factors (comorbidity, vomiting, persistent vomiting, abdominal pain or tenderness, pleural effusion, ascites, epistaxis, gum bleeding, GI bleeding, skin bleeding, lethargy or restlessness, hepatomegaly (>2 cm), increased HCT with decreased platelets, shock, dyspnea, impaired consciousness, thrombocytopenia, elevated AST and ALT, gall bladder wall thickening and secondary infection) and severe dengue.
Therefore, these clinical signs and symptoms may be useful for triaging potential severe dengue in patients and may further guide further enhancement of the current WHO dengue severity classifications, though heterogenicity was considerably high. More large-scale multicenter studies may be carried on identifying the association of with severe dengue using standard definitions and classification.
Supplementary Material
Appendices.
Appendix 1. Indexed and keyword terms for searching in three databases
| Databases | Indexed and keyword terms |
|---|---|
| Pubmed | ((((“Dengue”[Mesh]) OR dengue)) AND (((“Severe Dengue”[Mesh]) OR severe dengue) OR dengue severity)) AND (((“Diagnosis”[Mesh]) OR clinical diagnosis) OR warning signs) Filters: Publication date from 2009/01/01 to 2018/12/31 |
| Embase | ((“dengue”/exp OR “dengue”) AND “severe dengue”/exp OR “severe dengue” OR “dengue severity”) AND (“diagnosis”/exp OR “diagnosis” OR “clinical diagnosis” OR “warning signs”) AND (2009–2018) |
| Scopus | (TITLE-ABS-KEY (dengue AND severe AND dengue)) AND (diagnosis OR warning AND signs) (2009–2018) |
Appendix 2. Quality assessment of studies using Newcastle-Ottawa scale.
| Author, Year | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort or case | Selection of the non-exposed cohort or control | Ascertainment of exposure or adequate case definition | Outcome of interest was not present at the start of studyor definit ion of control | Assessment of outcome or exposure | Follow up long enough for outcomes to occur or ascertainment for case and control | Adequacy of follow up or non-response rate | |||
| Adam et al. 2018 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Agarwal et al. 2018 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Alvarado- Castro et al. 2016 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Andries et al. 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Athira et al. 2018 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Aung et al. 2013 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Bhaskar et al. 2015 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 6 |
| Carrasco et al., 2014 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 6 |
| de Cavalcanti et al. 2013 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Giraldo et al. 2011 | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 7 |
| Hoffmeister et al. 2015 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Jayaratne et al. 2012 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Kumar et al. 2014 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 |
| Lee et al. 2016 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Lin et al. 2016 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Macedo et al. 2014 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 6 |
| Michels et al. 2013 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 |
| Nguyen et al. 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Pereira et al. 2018 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Phakhounth ong et al. 2018 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Pozo- Aguilar et al. 2014 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Prasad et al. 2013 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Ramabhatta et al. 20 17 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Rathakrishn an et al. 2014 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Roy et al. 2013 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 |
| Sahana et al. 2014 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| Singh et al. 2015 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| Soundravall y et al. 2015 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 5 |
| Sreenivasan et al. 2018 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| Tai et al. 2017 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Tamibmani am et al. 2016 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 4 |
| Temprasertr udee et al. 2018 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Thanachart wet et al. 2015 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| Thanachart wet et al. 2016 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| Thein et al. 2013 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Tsai et al. 2013 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Van de Weg et al. 2012 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Wakimoto et al. 2017 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Zhang et al. 2017 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
Funding Statement
This work was supported by Centre of Infectious Disease Epidemiology and Research under Saw Swe Hock School of Public Health, research program funded Ministry of Defence, Singapore [grant number N-608-000-065-001].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Geolocation information
Asia, Europe, America.
References
- 1.World Health Organization . Dengue guidelines for diagnosis, treatment, prevention and control. Geneva: WHO Press; 2009; Available from: https://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. [PubMed] [Google Scholar]
- 2.Macedo GA, Gonin ML, Pone SM, et al. Sensitivity and specificity of the World Health Organization dengue classification schemes for severe dengue assessment in children in Rio de Janeiro. PLoS One. 2014;9(4):e96314. DOI: 10.1371/journal.pone.0096314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srikiatkhachorn A, Gibbons RV, Green S, et al. Dengue hemorrhagic fever: the sensitivity and specificity of the WHO definition in identifying severe dengue cases in Thailand, 1994–2005. Clin Infect Dis. 2010;50(8):1135. DOI: 10.1086/651268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichmann O, Gascon J, Schunk M, et al. Severe dengue virus infection in travelers: risk factors and laboratory indicators. J Infect Dis. 2007;195(8):1089–1096. DOI: 10.1086/512680. [DOI] [PubMed] [Google Scholar]
- 5.Horstick O, Jaenisch T, Martinez E, et al. Comparing the usefulness of the 1997 and 2009 WHO dengue case classification: a systematic literature review. Am J Trop Med Hyg. 2014;91(3):621–634. DOI: 10.4269/ajtmh.13-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barniol J, Gaczkowski R, Barbato EV, et al. Usefulness and applicability of the revised dengue case classification by disease: multi-centre study in 18 countries. BMC Infect Dis. 2011;11(1):1–12. DOI: 10.1186/1471-2334-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakimoto MD, Camacho LAB, Gonin ML, et al. Clinical and laboratory factors associated with severe dengue: a case-control study of hospitalized children. J Trop Pediatr. 2018;64(5):373–381. DOI: 10.1093/tropej/fmx078. [DOI] [PubMed] [Google Scholar]
- 8.Thanachartwet V, Oer-Areemitr N, Chamnanchanunt S, et al. Identification of clinical factors associated with severe dengue among Thai adults: a prospective study. BMC Infect Dis. 2015;15(1):1–11. DOI: 10.1186/s12879-015-1150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira MS, Kudru CU, Nair S, et al. Factors associated with severity of illness in patients with dengue fever in a tertiary care hospital in southern India. Asian J Pharm Clin Res. 2018;11(3):272–276. DOI: 10.22159/ajpcr.2018.v11i3.23496. [DOI] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. DOI: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottwwa Hospital Research Institute, 2012. Available from: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf.
- 12.Zhang H, Zhou YP, Peng HJ, et al. Predictive symptoms and signs of severe dengue disease for patients with dengue fever: a meta-analysis. Biomed Res Int. 2014;359308; DOI: 10.1155/2014/359308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions. September 2020, 2nd Edition [cited. www.training.cochrane.org/handbook].
- 14.Adam AS, Pasaribu S, Wijaya H.. Clinical profile and warning sign finding in children with severe dengue and non-severe dengue. IOP Conf Series Earth Environ Sci. 2018;125(1):012038. DOI: 10.1088/1755-1315/125/1/012038. [DOI] [Google Scholar]
- 15.Agarwal N, Roy MP, Singh MK.. Clinical and biochemical findings in confirmed pediatric dengue cases in Delhi. J Pediatr Infect Dis. 2018;13(1):15–19. DOI: 10.1055/s-0037-1602844. [DOI] [Google Scholar]
- 16.Alvarado-Castro VM, Ramirez-Hernandez E, Paredes-Solis S, et al. Clinical profile of dengue and predictive severity variables among children at a secondary care hospital of Chilpancingo, Guerrero, Mexico: case series. Bol Med Hosp Infant Mex. 2016;73(4):237–242. DOI: 10.1016/j.bmhimx.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Andries AC, Duong V, Cappelle J, et al. Proteinuria during dengue fever in children. Int J Infect Dis. 2017;55:38–44. DOI: 10.1016/j.ijid.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Athira PP, Jagan OA, Umadevi P, et al. A retrospective study of paediatric dengue cases in a tertiary care hospital in southern India. J Clin Diagn Res. 2018;12(7):SC01–SC06. DOI: 10.7860/JCDR/2018/34710.11756. [DOI] [Google Scholar]
- 19.Aung KL, Thanachartwet V, Desakorn V, et al. Factors associated with severe clinical manifestation of dengue among adults in Thailand. SE Asian J Trop Med Public Health. 2013;44(4):602–612. [PubMed] [Google Scholar]
- 20.Bhaskar E, Sowmya G, Moorthy S, et al. Prevalence, patterns, and factors associated with bleeding tendencies in dengue. J Infect Dev Ctries. 2015;9(1):105–110. doi: 10.3855/jidc.5031. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco LR, Leo YS, Cook AR, et al. Predictive tools for severe dengue conforming to World Health Organization 2009 criteria. PLoS Negl Trop Dis. 2014;8:7. DOI: 10.1371/journal.pntd.0002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Cavalcanti LPG, Martins Mota LA, Lustosa GP, et al. Evaluation of the WHO classification of dengue disease severity during an epidemic in 2011 in the state of Ceará, Brazil. Mem Inst Oswaldo Cruz. 2014;109(1):93–98. DOI: 10.1590/0074-0276140384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giraldo D, Sant'Anna C, Perisse AR, et al. Characteristics of children hospitalized with dengue fever in an outbreak in Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg. 2011;105(10):601–603. DOI: 10.1016/j.trstmh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmeister B, Suttorp N, Zoller T.. The revised dengue fever classification in German travelers: clinical manifestations and indicators for severe disease. Infection. 2014;43(1):21–28. DOI: 10.1007/s15010-014-0688-z. [DOI] [PubMed] [Google Scholar]
- 25.Jayaratne SD, Atukorale V, Gomes L, et al. Evaluation of the WHO revised criteria for classification of clinical disease severity in acute adult dengue infection. BMC Res Notes. 2012;5(1):1–8. DOI: 10.1186/1756-0500-5-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Gittens-St Hilair M, Jason V, et al. The clinical characteristics and outcome of children hospitalized with dengue in Barbados, an English Caribbean country. J Infect Dev Ctries. 2015;9(4):394–401. DOI: 10.3855/jidc.5566. [DOI] [PubMed] [Google Scholar]
- 27.Lee IK, Liu JW, Chen YH, et al. Development of a simple clinical risk score for early prediction of severe dengue in adult patients. PLoS One. 2016;11(5):e0154772. DOI: 10.1371/journal.pone.0154772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YP, Luo Y, Chen Y, et al. Clinical and epidemiological features of the 2014 large-scale dengue outbreak in Guangzhou city, China. BMC Infect Dis. 2016;16(1):1–8. DOI: 10.1186/s12879-016-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michels M, Sumardi U, de Mast Q, et al. The predictive diagnostic value of serial daily bedside ultrasonography for severe dengue in Indonesian adults. PLoS Negl Trop Dis. 2013;7(6):e2277. DOI: 10.1371/journal.pntd.0002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen MT, Ho TN, Nguyen VV, et al. An evidence-based algorithm for early prognosis of severe dengue in the outpatient setting. Clin Infect Dis. 2017;64(5):656–663. DOI: 10.1093/cid/ciw863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phakhounthong K, Chaovalit P, Jittamala P, et al. Predicting the severity of dengue fever in children on admission based on clinical features and laboratory indicators: application of classification tree analysis. BMC Pediatr. 2018;18(1):1–9. DOI: 10.1186/s12887-018-1078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pozo-Aguilar JO, Monroy-Martínez V, Díaz D, et al. Evaluation of host and viral factors associated with severe dengue based on the 2009 WHO classification. Parasit Vectors. 2014;7(1):1–11. DOI: 10.1186/s13071-014-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad D, Kumar C, Jain A, et al. Accuracy and applicability of the revised WHO classification (2009) of dengue in children seen at a tertiary healthcare facility in northern India. Infection. 2013;41(4):775–782. DOI: 10.1007/s15010-013-0405-3. [DOI] [PubMed] [Google Scholar]
- 34.Ramabhatta S, Palaniappan S, Hanumantharayappa N, et al. The clinical and serological profile of pediatric dengue. Indian J Pediatr. 2017;84(12):897–901. DOI: 10.1007/s12098-017-2423-0. [DOI] [PubMed] [Google Scholar]
- 35.Rathakrishnan A, Klekamp B, Wang SM, et al. Clinical and immunological markers of dengue progression in a study cohort from a hyperendemic area in Malaysia. PLoS One. 2014;9(3):e92021), DOI: 10.1371/journal.pone.0092021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A, Sarkar D, Chakraborty S, et al. Profile of hepatic involvement by dengue virus in dengue infected children. N Am J Med Sci. 2013;5(8):480–485. DOI: 10.4103/1947-2714.117313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahana KS, Sujatha R.. Clinical profile of dengue among children according to revised WHO classification: analysis of a 2012 outbreak from Southern India. Indian J Pediatr. 2015;82(2):109–113. DOI: 10.1007/s12098-014-1523-3. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Meena JK, Verma CR, et al. A hospital-based study of hepatic dysfunction in children with dengue fever. Asian Pac J Trop Dis. 2015;5(12):964–967. DOI: 10.1016/S2222-1808(15)60965-3. [DOI] [Google Scholar]
- 39.Soundravally R, Sherin J, Agieshkumar BP, et al. Serum levels of copper and iron in dengue fever. Rev Inst Med Trop Sao Paulo. 2015;57(4):315–320. DOI: 10.1590/s0036-46652015000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreenivasan P, Geetha S, Sasikala K.. Development of a prognostic prediction model to determine severe dengue in children. Indian J Pediatr. 2018;85(6):433–439. DOI: 10.1007/s12098-017-2591-y. [DOI] [PubMed] [Google Scholar]
- 41.Tai AY, McGuinness SL, Robosa R, et al. Management of dengue in Australian travellers: a retrospective multicentre analysis. Med J Aust. 2017;206(7):295–300. DOI: 10.5694/mja16.01056. [DOI] [PubMed] [Google Scholar]
- 42.Tamibmaniam J, Hussin N, Cheah WK, et al. Proposal of a clinical decision tree algorithm using factors associated with severe dengue infection. PLoS One. 2016;11(8):e0161696), DOI: 10.1371/journal.pone.0161696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Temprasertrudee S, Thanachartwet V, Desakorn V, et al. A multicenter study of clinical presentations and predictive factors for severe manifestation of dengue in adults. Jpn J Infect Dis. 2018;71(3):239–243. DOI: 10.7883/yoken.JJID.2017.457. [DOI] [PubMed] [Google Scholar]
- 44.Thanachartwet V, Wattanathum A, Oer-areemitr N, et al. Diagnostic accuracy of peripheral venous lactate and the 2009 WHO warning signs for identifying severe dengue in Thai adults: a prospective observational study. BMC Infect Dis. 2015;16(1):1–10. DOI: 10.1186/s12879-016-1386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thein TL, Gan VC, Lye DC, et al. Utilities and limitations of the World Health Organization 2009 warning signs for adult dengue severity. PLoS Negl Trop Dis. 2013;7(1):e2023. DOI: 10.1371/journal.pntd.0002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai CY, Lee IK, Lee CH, et al. Comparisons of dengue illness classified based on the 1997 and 2009 World Health Organization dengue classification schemes. J Microbiol Immunol Infect. 2013;46(4):271–281. DOI: 10.1016/j.jmii.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 47.van de Weg CA, van Gorp EC, Supriatna M.. Evaluation of the 2009 WHO dengue case classification in an Indonesian pediatric cohort. Am J Trop Med Hyg. 2012;86(1):166–170. DOI: 10.4269/ajtmh.2012.11-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Xie WZ, Xie SX, et al. A novel predictor of patients with severe dengue: The aspartate aminotransferase/platelet count ratio index (APRI). Hepatol Int. 2018;90(5):803–809. DOI: 10.1007/s12072-016-9783-9. [DOI] [PubMed] [Google Scholar]
- 49.Pang J, Hsu JP, Yeo TW, et al. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: a matched case-control study. Sci Rep. 2017;7(1):1–10. DOI: 10.1038/srep39872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad MH, Ibrahim MI, Mohamed Z, et al. The sensitivity, specificity and accuracy of warning signs in predicting severe dengue, the severe dengue prevalence and its associated factors. Int J Environ Res Public Health. 2018;15:9. DOI: 10.3390/ijerph15092018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Souza LJD, Nogueira RMR, Soares LC.. The impact of dengue on liver function as evaluated by aminotransferase levels. Braz J Infect Dis. 2007;11(4):407–410. DOI: 10.1590/S1413-86702007000400007. [DOI] [PubMed] [Google Scholar]
- 52.Agrawal VK, Prusty BSK, Reddy CS, et al. Clinical profile and predictors of severe dengue disease: a study from South India. Caspian J Intern Med. 2018;9(4):334–340. DOI: 10.22088/cjim.9.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moras EC, Raj N, Achappa B, et al. Hyperferritinemia and gallbladder wall oedema as early markers of a severe dengue infection in a developing nation. Res Squ. 2020. DOI: 10.21203/rs.3.rs-36751/v1 [DOI] [Google Scholar]
- 54.Chatterjee R, Mysore A, Ahya K, et al. Utility of sonography in clinically suspected dengue. Pediatr Infect Dis J. 2012;4(3):107–111. DOI: 10.1016/j.pid.2012.07.006. [DOI] [Google Scholar]
- 55.Lora AJ M, Fernandez J, Morales A, et al. Disease severity and mortality caused by dengue in a Dominican pediatric population. Am J Trop Med Hyg. 2014;90(1):169–172. DOI: 10.4269/ajtmh.13-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hegazi MA, Bakarman MA, Alahmadi TS, et al. Risk factors and predictors of severe dengue in Saudi population in Jeddah, Western Saudi Arabia: a retrospective study. J Trop Med Hyg. 2020;102(3):613–621. DOI: 10.4269/ajtmh.19-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran L, Radwan I, Low SK, et al. Role of cytokines produced by T helper immune-modulators in dengue pathogenesis: a systematic review and meta-analysis. Acta Trop. 2021;105823. DOI: 10.1016/j.actatropica.2021.105823. [DOI] [PubMed] [Google Scholar]
- 58.Morra ME, Altibi AMA, Iqtadar S, et al. Definitions for warning signs and signs of severe dengue according to the WHO 2009 classification: systematic review of literature. Rev in Med Virol. 2018;28(4):e1979. DOI: 10.1002/rmv.1979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.