ABSTRACT
Aluminum (Al) toxicity in acidic soils severely reduces crop production worldwide. Sorghum (Sorghum bicolor L.) is an important agricultural crop widely grown in tropical and subtropical regions, where Al toxicity is prevalent. ATP-binding cassette (ABC) transporters play key roles in the development of plants and include the member sensitive to aluminum rhizotoxicity 1 (STAR1), which is reported to be associated with Al tolerance in a few plant species. However, a STAR1 homolog has not been characterized in sorghum with respect to Al tolerance. Here, we identified and characterized a SbSTAR1 gene in sweet sorghum encoding the nucleotide-binding domain of a bacterial-type ABC transporter. The transcriptional expression of SbSTAR1 is induced by Al in a time- and dosage-dependent manner in root, especially in root tip, which is the key site of Al toxicity in plants. The typical Al-associated transcription factor SbSTOP1 showed transcriptional regulation of SbSTAR1. SbSTAR1 was present at both the cytoplasm and nuclei. Overexpression of SbSTAR1 significantly enhanced the Al tolerance of transgenic plants, which possibly via regulating the hemicellulose content in root cell wall. This study provides the first ABC protein in sorghum implicated in Al tolerance, suggesting the existence of a SbSTAR1-mediated Al tolerance mechanism in sorghum.
KEYWORDS: Aluminum toxicity, ATP-binding cassette transporter, star1, transcriptional regulation, sorghum
Introduction
Crop aluminum (Al) toxicity is a primary limiting factor for crop yields in acidic soils, which cover nearly 50% of the world’s potential arable land.1 Sorghum (Sorghum bicolor L.) is an important agricultural crop and also a good source of fiber and fuel that is widely grown in many tropical and subtropical regions, where acidic soils are widespread, and Al toxicity is prevalent; thus, characterization of important Al tolerance genes in sorghum is expected to be helpful for breeders in increasing sorghum yields.2
Many Al tolerance genes have been identified in other plant species, and are mainly involved in two main types of Al resistance mechanisms, external exclusion and internal tolerance mechanisms.3,4 In contrast, few Al tolerance genes in sorghum have been studied. Among them, a multidrug and toxic compound extrusion (MATE) family member, SbMATE, encoding a citrate transporter that is involved in external mechanism, has been fully clarified.2,5,6 For the internal mechanism of Al tolerance in sorghum, identified genes are rare. Recent studies in other plant species may provide key potential genes, e.g., genes encoding ATP-binding cassette (ABC) transporters.
Plants possess a particularly large and diverse complement of the ABC protein superfamily in comparison with other organisms, whereas the functions of most members of this family await discovery. A unified nomenclature grouped eukaryotic ABC proteins into ABCA to ABCI subfamilies, with plants lacking ABCH cluster but, in particular, containing an ABCI subfamily incorporating bacterial-type ABCs that are absent from most animals.7 Most ABC proteins have both nucleotide-binding domains (NBDs) and transmembrane domains (TMDs), forming full-size proteins (containing two NBDs and two TMDs) or half-size proteins (containing one NBD and one TMD), while plant ABCI proteins contain only an NBD or TMD.7,8 In 2009, sensitive to aluminum rhizotoxicity 1 (STAR1) was reported to be required for Al tolerance in rice, which is subjected to the ABCI subfamily, encoding only NBD of a bacterial-type ABC transporter. OsSTAR1 interacts with STAR2 (encoding only a TMD), and the complex is thought to transport UDP-glucose into the cell wall, presumably altering the cell wall composition and thus limiting Al accumulation.9 AtSTAR1 in Arabidopsis was soon discovered in 2010.8 Until recently, the third STAR1 homolog, FeSTAR1, in buckwheat was characterized to be associated with Al resistance.10 In another study, FeSTAR2 was also reported to form a complex with FeSTAR1, 11 although the exact role of STAR1/STAR2 in these species needs further study. In addition, it is unclear whether STAR1 is a universal gene with respect to Al tolerance in monocots, since rice is the most Al-tolerant species among grain cereal crops, 12 whereas sorghum is relatively sensitive to Al compared with rice.
In this study, SbSTAR1 was identified in sweet sorghum, a variant of grain sorghum. SbSTAR1 shares high identity with OsSTAR1. The transcriptional expression of SbSTAR1 is induced by Al stress and regulated by the typical Al-associated transcription factor SbSTOP1. In addition, overexpression of SbSTAR1 significantly enhanced the Al tolerance of transgenic plants, which possibly via regulating the hemicellulose metabolism in root cell wall, suggesting the existence of a SbSTAR1-mediated Al tolerance mechanism in sorghum.
Materials and methods
Plant material and growth conditions
The sweet sorghum cultivar POTCHETSTRM was used and cultured as previously described.13 In brief, seeds were surface-sterilized, germinated for 2 d, transplanted into 0.5 mM CaCl2 solution (pH 4.5) for 3 d, and then were exposed to various treatments. The seedlings were grown in a growth chamber with a 14 h light (400 μmol m−2 s−1)/10 h dark photoperiod, 26 °C day/22 °C night temperatures and 60% relative humidity.
In the time-course assay, seedlings were exposed to 0.5 mM CaCl2 solution (pH 4.5) with 15 μM AlCl3 for the indicated time, and then 15 root tips (0–1 cm) were excised. A similar operation was used in the Al dosage-dependent assay, except that seedlings were treated with indicated concentrations of AlCl3 for 24 h. In the spatial expression assay, seedlings were treated for 24 h, and then roots (0–1 cm, 1–2 cm, or 2–3 cm) and shoots were excised. In the metal treatments, seedlings were exposed to 15 μM AlCl3, 0.5 μM CuCl2 or 10 μM LaCl3 for 24 h (pH 4.5), and then the root tips (0–1 cm) were cut.
Sequence analysis
Sequences of SbSTAR1 and its homologs were analyzed using BLAST in NCBI (https://www.ncbi.nlm.nih.gov/) and the sorghum genome database (http://pgsb.helmholtzmuenchen.de/plant/sorghum/index.jsp). Sequence alignment was performed in DNASTAR and displayed in GeneDoc. The phylogenetic tree was constructed using the neighbor-joining method in MEGA 7.
Expression assays
RNA isolation, reverse transcription of cDNA and quantitative real-time PCR (qRT-PCR) were performed as previously described.13,14 The expression of SbSTAR1 was detected using the following primers: forward primer, 5′-CTGCTGGATGAGCCGACG-3′, and reverse primer, 5′-GCTTCACGCTGTGGGAGAC-3′. The housekeeping gene β-actin (GenBank ID: X79378) was used as an internal control.14 qRT-PCR was performed using SYBR Premix ExTaq (Takara) in an Mx3005P qPCR system (Stratagene, USA). The relative expression level of the gene was calculated using the 2−ΔΔCT method.15
Transcriptional regulation analysis
The effector plasmid (CMV::SbSTOP1-Myc) and the reporter plasmid (pSbSTAR1::Firefly Luc-SV40::Renilla Luc) were constructed. Both cytomegalovirus (CMV) promoter and simian virus 40 (SV40) promoter are constitutive promoters in mammalian expression system. HEK293 (human embryonic kidney) cells were cultured and transfected as previously described.16 Briefly, the cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) contains FBS (10%) and penicillin/streptomycin (1%), and incubated in an incubator at 37 °C with 5% CO2 in air. Two constructed plasmids were cotransfected using the calcium phosphate transfection method. After 30 to 48 h, the transfected cells were ready for test. SbSTOP1-Myc protein was detected by an immunoblot assay using anti-Myc antiboday (MBL, M192-3). HSP90 protein was detected as loading control using anti-HSP90 antibody (Agrisera, AS11 1629).
The dual-luciferase reporter assay was conducted according to the technical manual (Promega, E1910) and a previous report.13 Briefly, after removing the culture medium, the transfected cells were lysed in 1× Passive Lysis Buffer. Firefly luciferase activity of the lysate was measured by adding Luciferase Assay Reagent II to generated luminescent signal. This reaction was quenched, and the Renilla luciferase activity was measured by adding Stop & Glo® Reagent. Both luminescent signals were measured with a luminometer (Berthold LB960). Relative luciferase activity was calculated as the ratio between Firefly luciferase activity and Renilla luciferase activity.
Subcellular localization assays
The transient plant expression plasmid (35s::GFP-SbSTAR1 or 35s::GFP) was transformed into Arabidopsis protoplasts, which were isolated from 4-week-old plants using the PEG-mediated method as reported.17 The protoplasts were then incubated in darkness at room temperature for approximately 16 h. Fluorescence images were captured under a fluorescence microscope (Axio Observer A1, Zeiss).
Aluminum tolerance phenotype analysis
The open reading frame of SbSTAR1 was cloned into the vector (35S::LUC-SbSTAR1). The vector was transformed into Arabidopsis wild type (WT, col-4) using the Agrobacterium tumefaciens-mediated floral dip method.18 The transgenic seedlings were first screened with the Basta herbicide. Protein expression was then confirmed by an immunoblot assay using anti-LUC antiboday (Sigma, L2164). Transgenic seeds (T3) were sterilized and germinated on MS medium (pH 5.8) vertically for 5 d, and then seedlings were transplanted to medium containing 1 mM CaCl2 and 1% (w/v) sucrose at pH 5.0, with or without 50 μM AlCl3 for 5 d. Root growth was measured, and the relative root elongation was calculated as the ratio between the root elongation with and without Al treatment.
Root cell wall extraction, fractionation and hemicellulose determination
Plants were cultured as previous description with minor modifications.19 One-week-old plants were removed from MS medium (pH 5.8) to 1/10 strength Hoagland solution (pH 5.8) for 3 weeks and treated with or without 50 μM AlCl3 for 24 h. Root crude cell wall materials were extracted as previous study.10 Briefly, roots were ground in liquid nitrogen and homogenized with ethanol (75%) for 20 min on ice, then the sample was centrifuged (17,000 g, 10 min) and the supernatant was removed. The pellet was washed with acetone, methanol: chloroform (1:1), methanol respectively, then dried and stored at 4 °C. Cell wall materials was fractionated as reported.20 Briefly, after removing pectin, the remaining pellet were extracted twice in a solution containing KOH (4%) and NaBH4 (0.1%) for 12 h. The supernatant was hemicellulose fraction (hemicellulose 1 as reported) and was determined using the phenol sulfuric acid method with glucose as a standard.10
Results
Sequence analysis of SbSTAR1
Using the amino acid sequence of OsSTAR1 as a query, a STAR1 homolog was identified in the sorghum genome database. The full-length SbSTAR1 (Sb10g028530.1) coding region was obtained in sweet sorghum, which is 807 bp in length, encoding a protein of 268 amino acids. There is no homolog of SbSTAR1 in the sorghum genome. According to genomic sequence analysis, SbSTAR1 has 4 exons and 3 introns, which is similar to OsSTAR1 but different from AtSTAR1 (Figure 1a).
Figure 1.
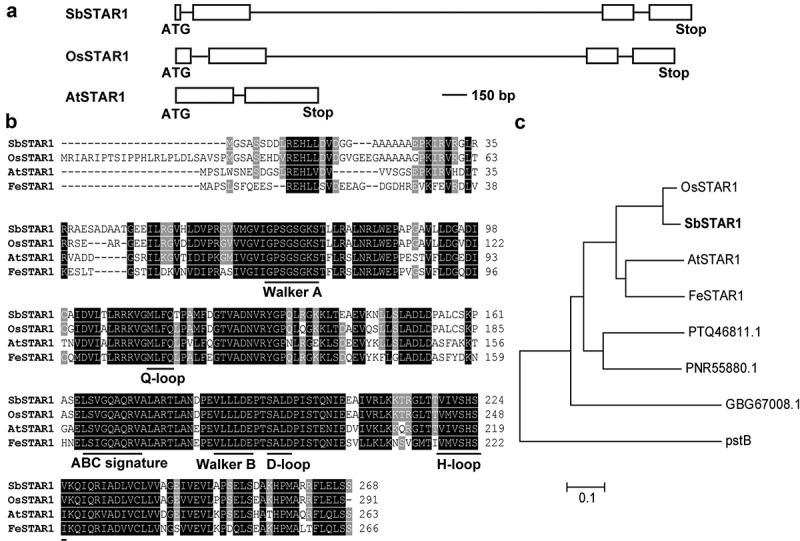
Gene structure and amino acid sequence analysis of SbSTAR1 in sweet sorghum. (a) Gene structure of SbSTAR1 and homologous genes. Box, exon; line, intron. (b) Sequence alignment of SbSTAR1 and homologous proteins from other species, including Oryza sativa (OsSTAR1, Os06g48060.1), Arabidopsis thaliana (AtSTAR1, At1g67940.1) and Fagopyrum esculentum (FeSTAR1, AYK27446.1). Horizontal lines indicate conserved motifs of nucleotide-binding domain (NBD) in ABC transporters. (c) Phylogenic analysis of SbSTAR1 (XP_002438933.1) and its homologs in Oryza sativa, Arabidopsis thaliana, Fagopyrum esculentum, Marchantia polymorpha (PTQ46811.1), Physcomitrium patens (PNR55880.1), Chara braunii (GBG67008.1) and Escherichia coli (pstB, EGM3813836.1). The phylogenetic tree was constructed using the neighbor-joining method in MEGA 7
At present, only three STAR1s’ functions have been well characterized; thus, we further compared the SbSTAR1 protein sequence with them. Amino acid sequence analysis revealed that SbSTAR1 exhibits 90.9%, 69.5%, and 65.0% identity with OsSTAR1, AtSTAR1 and FeSTAR1, respectively. SbSTAR1 contains only the nucleotide-binding domain (NBD) of an ABC transporter protein, as its homolog OsSTAR1 is categorized into the ABCI subfamily, which bears high similarity with bacterial multimeric-type ABC proteins.7,21 SbSTAR1 has all characteristic motifs of NBD in an ABC transporter, including walker A and B motif, ABC signature motif, Q-loop, H-loop, and D-loop (Figure 1b), suggesting similar mechanisms in ABC proteins for ATP binding and contact with the other monomer.22
Phylogenetic analysis showed that SbSTAR1 clusters closely with OsSTAR1 (Figure 1c). Putative STAR1 homologs could also be found in initial land plants, such as liverwort (Marchantia polymorpha) and moss (Physcomitrium patens). Even in charophytic algae (Chara braunii), which is the origin of land plants, 23 there is one homolog with 42.9% identity. In addition, SbSTAR1 also showed similarity to the bacterial phosphate transporter pstB, suggesting the early origin of the STAR1 protein (Figure 1c).9
Detection of the SbSTAR1 expression pattern
The expression pattern of SbSTAR1 was characterized comprehensively using quantitative real-time PCR. A time-course experiment revealed that the expression of SbSTAR1 was significantly induced by Al in the root tips (0–1 cm) during 24 h Al treatment (Figure 2a). Furthermore, the mRNA accumulation of SbSTAR1 increased in a dose-dependent manner when seedlings were exposed to increasing Al concentrations (Figure 2b). We next examined the spatial expression of SbSTAR1 under Al stress. SbSTAR1 was predominantly expressed in roots rather than shoots. In roots, the transcriptional abundance of SbSTAR1 was greater in the root (1–2 cm) and root (2–3 cm) sections than in the root tip (0–1 cm), in both the presence and absence of Al; however, significant Al-induced transcriptional change of SbSTAR1 was only detected in the root tip (0–1 cm) (Figure 2c), implying the potential role of SbSTAR1 in the root under Al stress, especially in the root tip, which is the site of Al toxicity.3 We also compared the expression of SbSTAR1 in response to Al stress with that of other metals. Its expression was induced by Al but not by Cu and La (Figure 2d).
Figure 2.
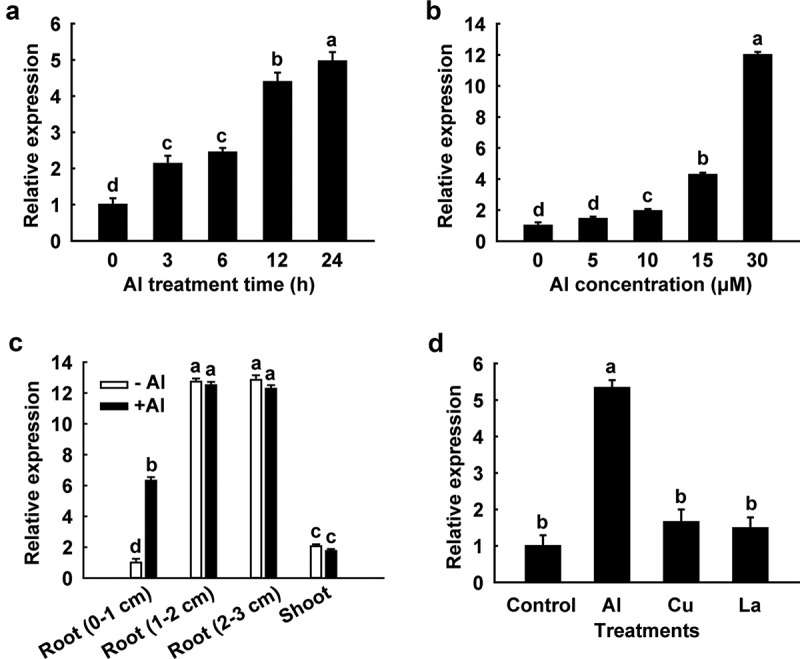
Quantitative real-time PCR analysis of the SbSTAR1 expression pattern. (a) Relative expression of SbSTAR1 in sweet sorghum root tips (0–1 cm) in response to 15 μM Al stress for different treatment times. (b) Relative expression of SbSTAR1 in root tips exposed to different Al concentrations for 24 h. (c) Relative expression of SbSTAR1 in root tip (0–1 cm), root (1–2 cm), root (2–3 cm) and shoot sections in the absence (-Al) or presence (+Al, 15 μM) of Al treatment for 24 h. (d) Relative expression of SbSTAR1 in root tips in response to AlCl3 (15 μM), CuCl2 (0.5 μM) and LaCl3 (10 μM) for 24 h. Data represent the means with SD (n = 3). Columns with different letters are significantly different at P < .05
The transcriptional regulation of SbSTAR1
Since the transcriptional levels of SbSTAR1 were induced by Al stress, we further identified the transcription factor regulating its expression. SbSTOP1 plays an important role in Al resistance in sweet sorghum, 13 and its homologs, such as OsART1 in rice and AtSTOP1 in Arabidopsis, positively regulate numerous Al resistance genes.24–27 Therefore, we examined the transcriptional regulation of SbSTOP1 to SbSTAR1 using a dual-luciferase reporter assay in a HEK293 expression system.13 Two vectors were constructed (Figure 3a). For an effector, full-length SbSTOP1 was constructed under the control of the cytomegalovirus (CMV) promoter. For a reporter, the SbSTAR1 promoter (−1476 bp from the translation start site) was introduced to drive the firefly luciferase reporter gene (Firefly Luc), with the Renilla luciferase reporter gene (Renilla Luc) driven by simian virus 40 (SV40) promoter as an internal control. The effector and reporter were cotransfected into HEK293 cells.16 The SbSTOP1-Myc fusion protein was first examined by immunoblotting (Figure 3b), and then the luciferase activity was examined. The SbSTAR1 promoter-driven reporter showed significantly higher luciferase activity in the presence of the SbSTOP1 effector compared to the empty vector, demonstrating that SbSTOP1 regulating the transcriptional expression of SbSTAR1 (Figure 3c).
Figure 3.
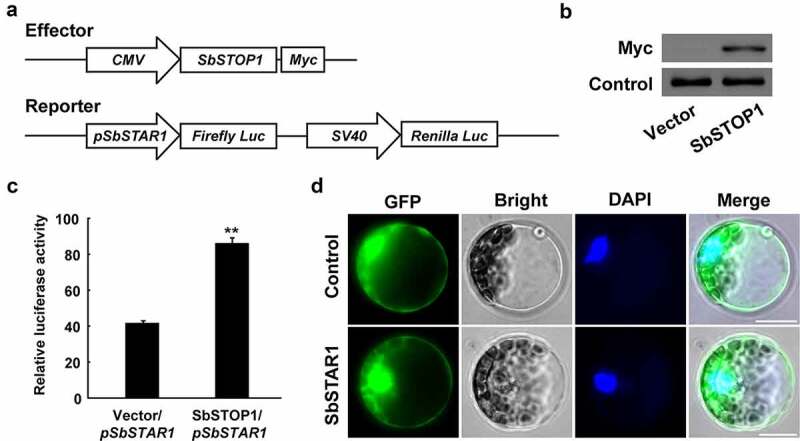
Transcriptional regulation of SbSTAR1 and protein subcellular localization. (a) Schematic diagram of the effector and reporter in the HEK293 expression system. In the effector, SbSTOP1 was driven by the CMV promoter; Myc, encoding Myc protein tag. In the reporter, the firefly luciferase reporter gene (Firefly Luc) was driven by the SbSTAR1 promoter (pSbSTAR1), and the Renilla luciferase reporter gene (Renilla Luc), as the internal control, was driven by the SV40 promoter. The CMV and SV40 promoter are constitutive promoters commonly used in mammalian expression vectors to drive gene expression. (b) Immunoblot analysis of the SbSTOP1-Myc fusion protein in HEK293 cells. (c) Transcriptional regulation of SbSTAR1 by SbSTOP1 in HEK293 cell. Relative luciferase activity, the luciferase activity of the reporter (Firefly Luc) was normalized to the internal control reporter (Renilla Luc). Data represent the means with SD (n = 3). ** represents significant differences from the vector-only control at P < .01. (d) Subcellular localization of SbSTAR1. Transient expression of GFP-SbSTAR1 fusion protein or GFP control in Arabidopsis protoplasts. GFP, GFP fluorescence; Bright, bright field; DAPI, nuclear signal. Scale bar indicates 20 μm
The subcellular localization of SbSTAR1
To investigate the subcellular localization of SbSTAR1, the GFP-SbSTAR1 fusion protein was expressed in Arabidopsis protoplasts. As shown in Figure 3d, the GFP-SbSTAR1 protein signal was observed in both the cytoplasm and nuclei, which was similar to the distribution of the GFP protein (control) only. Thus, the results indicate that SbSTAR1 is a soluble protein without specific subcellular localization.
SbSTAR1 overexpression in Arabidopsis confers aluminum resistance
To further analyze the function of SbSTAR1, SbSTAR1 was expressed in Arabidopsis under the control of the CaMV 35S promoter. The LUC-SbSTAR1 fusion protein was detected by immunoblotting (Figure 4a). Two independent transgenic lines (T3) were selected for phenotypic analysis. Rapid inhibition of plant root growth is the main symptom of Al toxicity.28,29 Thus, the root phenotype of the wild-type (WT) and two SbSTAR1 transgenic lines was observed under Al stress. As shown in Figure 4b and C, in the absence of Al, there were no differences in root growth between the WT and two SbSTAR1 transgenic lines. In the presence of Al, the root growth of both WT and transgenic lines was inhibited; however, SbSTAR1 overexpression lines had significantly longer roots than those of the WT. The relative root elongation for the WT was 50.8%, while that for the two transgenic lines was 72.6% and 76.8%, respectively. The present result indicated the positive role of SbSTAR1 in Al resistance.
Figure 4.
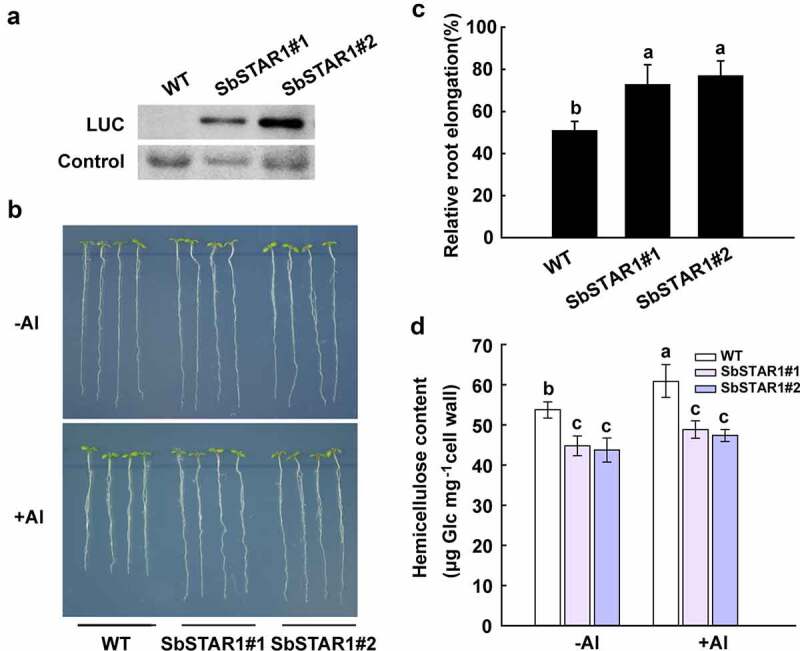
Transgenic Arabidopsis overexpressing SbSTAR1 shows improved tolerance to Al stress and lower hemicellulose content in root cell wall. (a) Immunoblot analysis of the LUC-SbSTAR1 fusion protein in two independent transgenic lines. (b) Root Al-sensitive phenotype of WT (Col-4) and SbSTAR1 transgenic lines. Five-day-old seedlings were cultured on MS medium and then transferred to medium containing 1 mM CaCl2 and 1% (w/v) sucrose at pH 5.0, with or without 50 μM AlCl3. (c) Relative root elongation (root elongation with Al treatment/root elongation without Al treatment) of WT and two SbSTAR1 transgenic lines. Data are shown as the means with SD (n ≥ 20). Columns with different letters indicate significant differences between plants at P < .05. Experiments were repeated three times. (d) Hemicellulose content in root cell wall of WT and two SbSTAR1 transgenic lines. The hemicellulose was extracted from root cell wall of four-week-old plants with or without 50 μM Al treatment for 24 h. Data are shown as the means with SD (n = 3). Columns with different letters indicate significant differences at P < .05
SbSTAR1 regulates plant Al resistance possibly via hemicellulose metabolism
The cell wall has been recognized as the main target of Al toxicity, and hemicellulose in cell wall matrix is the major component for Al accumulation (Yang, 2011). SbSTAR1 shares high similarity with OsSTAR1, which is involved in transporting UDP-glucose into the cell wall, presumably altering the cell wall composition.9 Therefore, we examined whether the Al resistant phenotype of SbSTAR1 transgenic lines is related to hemicellulose metabolism in cell wall. As shown in Figure 4d, hemicellulose content in SbSTAR1 transgenic lines was significantly lower than WT, especially under Al treatment. Thus, SbSTAR1 improved the Al resistance of plant possibly via regulating the hemicellulose content in root cell wall.
Discussion
Members of the ABC superfamily are established as key players in the physiology and development of plants.7 The ABCI subfamily is further created for plants, and is expected to undergo the most substantial changes since nomenclature, 7 while studies on the STAR1 protein may provide new viewpoints. OsSTAR1 is a member of the ABCI subfamily, which is required for detoxification of Al in rice and was first identified in 2009.9,21 However, over 10 y, few homologs have been characterized in both dicots and monocots, except AtSTAR1 in Arabidopsis, 9 and recently discovered FeSTAR1 in buckwheat.10,11 In the present study, we characterized a STAR1 homolog, SbSTAR1, with respect to Al tolerance in sweet sorghum, which is an Al-sensitive species compared with rice, indicating the widespread of STAR1 in both Al-tolerant and Al-sensitive monocots.
SbSTAR1 shares high identity with clarified homologs (Figure 1), especially OsSTAR1 (90.9%). SbSTAR1 contains all conserved motifs of NBD in ABC transporters, similar to its homologs, suggesting similar functions. Furthermore, overexpression of SbSTAR1 significantly improved the Al resistance of transgenic plants, which possibly due to SbSTAR1 is involved in regulating the hemicellulose metabolism of root cell wall (Figure 4). The results demonstrated that SbSTAR1 is a functional homolog of OsSTAR1 in terms of Al tolerance.
Despite functional similarity, SbSTAR1 has its distinguishing feature. First, compared with its homologous genes, 8–11 the expression pattern of SbSTAR1 has a lot in common with that of monocot species gene OsSTAR1 but is different from dicot species genes AtSTAR1 and FeSTAR1 (Figure 2). Second, the expression of SbSTAR1 is regulated by SbSTOP1 (Figure 3c), which is similar to that in rice but differs from that in Arabidopsis, suggesting the existence of the SbSTOP1-SbSTAR1-mediated Al signaling pathway in sorghum.26,27 Third, the subcellular localization of SbSTAR1 is consistent with FeSTAR1, 10 but differs from OsSTAR1.9 However, the localization of STAR1 may change when STAR1 interacts with its partner, STAR2. For instance, FeSTAR1 was changed to be localized to the membrane when coexpressed with FeSTAR2.11 Thus, in the future, a putative SbSTAR2 has to be identified and their complex localization needs to be detected.
Few Al tolerance genes in sorghum have been characterized to date, except SbMATE, SbNrat1, SbSTOP1 and SbGlu1.5,6,13,14,30 Among them, SbMATE-mediated citrate excretion is a key Al tolerance external mechanism in sorghum, 5,6 while our study suggested the existence of another path, the SbSTAR1-mediated Al tolerance mechanism in sorghum. In rice, the mechanism by which the OsSTAR1-OsSTAR2 complex resists Al stress was further clarified, whereas the exact role of them remains unclear, which is also an issue encountered for other homologs, such as AtSTAR1 and FeSTAR1. FeSTAR1 is involved in Al resistance via possibly cell wall matrix polysaccharides metabolism in plants, especially hemicellulose 1 content.8–11,31 While our results also suggested the overexpression of SbSTAR1 in plants reduced the hemicellulose content of root cell wall, although both in the presence and absence of Al due to the constitutive expression of SbSTAR1 under the control of the CaMV 35S promoter (Figure 4). Further work is still required to investigate the exact mechanism of STAR1.
In addition to the study of the function and mechanism of SbSTAR1 and its homologs, these results also lead to some interesting thinking, and our study may provide some clues. First, ABCI proteins share similarities with bacterial multimeric-type ABC proteins, which are ABC proteins in bacteria and are rare in animals but appear in some plants and algae.22 Does this special group of ABC proteins exist universally or only in certain plants? In the present study, STAR1 homologs are found from initial land plants to higher plants (Figure 1). Furthermore, land plants evolved from charophytic algae, and there is also a STAR1 homolog. In addition, the similarity of STAR1 to bacterial pstB is higher than that to any other plant ABC transporter (Figure 1).9 These results suggest the universal existence of STAR1 and imply the endosymbiotic origin of STAR1, 7,22 although the functions of other SbSTAR1 homologs with respect to Al tolerance are unclear. Second, in eukaryotes, it seems that ABCI genes were inherited from a common ancestor in plants but lost in animals, 7,22 therefore leading us to think about an evolutionary issue. Is this a group of genes that have not yet been lost in plants or an efficient strategy specially conserved by plants? It was implied that AtSTAR1 may interact with other proteins in different tissues to fulfill its function.8 Therefore, further study of STAR1 and its potential partners may be helpful to reveal the above question. In this regard, the sessile nature of plants and their increased requirement to cope with biotic and abiotic stresses (e.g., the soil environment) need to be taken into account.
In summary, we identified the SbSTAR1 gene in sweet sorghum that encodes NBD of a bacterial-type ABC transporter. The expression of SbSTAR1 in roots was upregulated by Al stress. Heterologous expression of SbSTAR1 enhanced the Al tolerance of plants. And SbSTAR1 improved the Al resistance of plant possibly via regulating the hemicellulose content in root cell wall. The results extend the understanding of STAR1 proteins regulation of Al tolerance in different plant species.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [31972508, 31572192]; Fundamental Research Funds for the Central Universities. We thank American Journal Experts (https://www.aje.com/) for its linguistic assistance during the preparation of this manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China[31972508, 31572192]; Fundamental Research Funds for the Central Universities.
Disclosure of interest
The authors report no conflict of interest.
References
- 1.HRv U, Mutert E.. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171(1):1–8. doi: 10.1007/BF00009558. [DOI] [Google Scholar]
- 2.Hufnagel B, Guimaraes CT, Craft EJ, Shaff JE, Schaffert RE, Kochian LV, Magalhaes JV.. Exploiting sorghum genetic diversity for enhanced aluminum tolerance: allele mining based on the AltSB locus. Sci Rep. 2018;8(1):10094. doi: 10.1038/s41598-018-27817-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochian LV, Piñeros MA, Liu J, Magalhaes JV. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol. 2015;66(1):571–598. doi: 10.1146/annurev-arplant-043014-114822. [DOI] [PubMed] [Google Scholar]
- 4.Ma JF. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000;41(4):383–390. doi: 10.1093/pcp/41.4.383. [DOI] [PubMed] [Google Scholar]
- 5.Magalhaes JV, Liu J, Guimarães CT, Lana UGP, Alves VMC, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet. 2007;39(9):1156–1161. doi: 10.1038/ng2074. [DOI] [PubMed] [Google Scholar]
- 6.Melo JO, Martins LGC, Barros BA, Pimenta MR, Lana UGP, Duarte CEM, Pastina MM, Guimaraes CT, Schaffert RE, Kochian LV, et al. Repeat variants for the SbMATE transporter protect sorghum roots from aluminum toxicity by transcriptional interplay in cis and trans. Proc Natl Acad Sci U S A. 2019;116(1):313–318. doi: 10.1073/pnas.1808400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, et al. Plant ABC proteins–a unified nomenclature and updated inventory. Trends Plant Sci. 2008;13(4):151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Huang CF, Yamaji N, Ma JF. Knockout of a Bacterial-Type ATP-Binding Cassette Transporter Gene, AtSTAR1, results in increased Aluminum sensitivity in Arabidopsis. Plant Physiol. 2010;153(4):1669–1677. doi: 10.1104/pp.110.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. The Plant Cell. 2009;21(2):655–667. doi: 10.1105/tpc.108.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu JM, Lou HQ, Jin JF, Chen WW, Wan JX, Fan W, Yang JL. A half-type ABC transporter FeSTAR1 regulates Al resistance possibly via UDP-glucose-based hemicellulose metabolism and Al binding. Plant Soil. 2018;432(1–2):303–314. doi: 10.1007/s11104-018-3805-4. [DOI] [Google Scholar]
- 11.Che J, Yamaji N, Yokosho K, Shen RF, Ma JF. Two genes encoding a bacterial-type ATP-binding cassette transporter are implicated in aluminum tolerance in buckwheat. Plant Cell Physiol. 2018;59(12):2502–2511. doi: 10.1093/pcp/pcy171. [DOI] [PubMed] [Google Scholar]
- 12.Ma JF, Shen R, Zhao Z, Wissuwa M, Takeuchi Y, Ebitani T, Yano M. Response of rice to Al stress and identification of quantitative trait Loci for Al tolerance. Plant Cell Physiol. 2002;43(6):652–659. doi: 10.1093/pcp/pcf081. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Gao J, You J, Liang Y, Guan K, Yan S, Zhan M, Yang Z. Identification of STOP1-like proteins associated with aluminum tolerance in sweet sorghum (Sorghum bicolor L.). Front Plant Sci. 2018;9:258. doi: 10.3389/fpls.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Shi WL, You JF, Bian MD, Qin XM, Yu H, Liu Q, Ryan PR, Yang ZM. Transgenic Arabidopsis thaliana plants expressing a beta-1,3-glucanase from sweet sorghum (Sorghum bicolor L.) show reduced callose deposition and increased tolerance to aluminium toxicity. Plant Cell Environ. 2015;38(6):1178–1188. doi: 10.1111/pce.12472. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Wang X, Zhang M, Bian M, Deng W, Zuo Z, Yang Z, Zhong D, Lin C. Trp triad-dependent rapid photoreduction is not required for the function of Arabidopsis CRY1. Proc Natl Acad Sci U S A. 2015;112(29):9135–9140. doi: 10.1073/pnas.1504404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Yan S, Yu H, Zhan M, Guan K, Wang Y, Yang Z. Sweet sorghum (Sorghum bicolor L.) SbSTOP1 activates the transcription of a β-1,3-glucanase gene to reduce callose deposition under Al toxicity: a novel pathway for Al tolerance in plants. Biosci Biotechnol Biochem. 2019;83(3):446–455. doi: 10.1080/09168451.2018.1540290. [DOI] [PubMed] [Google Scholar]
- 18.Clough SJ, Bent AF. Floral dip: a simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 19.Lei GJ, Yokosho K, Yamaji N, Fujii-Kashino M, Ma JF. Functional characterization of two half-size ABC transporter genes in aluminium-accumulating buckwheat. New Phytol. 2017;215(3):1080–1089. doi: 10.1111/nph.14648. [DOI] [PubMed] [Google Scholar]
- 20.Yang JL, Zhu XF, Peng YX, Zheng C, Li GX, Liu Y, Shi YZ, Zheng SJ. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 2011;155(4):1885–1892. doi: 10.1104/pp.111.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefèvre F, Boutry M. Towards identification of the substrates of ATP-binding cassette transporters. Plant Physiol. 2018;178(1):18–39. doi: 10.1104/pp.18.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiyama T, Sakayama H, De Vries J, Buschmann H, Saint-Marcoux D, Ullrich KK, Haas FB, Vanderstraeten L, Becker D, Lang D, et al. The Chara genome: secondary complexity and implications for plant terrestrialization. Cell. 2018;174(2):448–464.e424. doi: 10.1016/j.cell.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 24.Fan W, Lou HQ, Yang JL, Zheng SJ. The roles of STOP1-like transcription factors in aluminum and proton tolerance. Plant Signal Behav. 2016;11(2):e1131371. doi: 10.1080/15592324.2015.1131371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Magalhaes JV, Shaff J, Kochian LV. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57(3):389–399. doi: 10.1111/j.1365-313X.2008.03696.x. [DOI] [PubMed] [Google Scholar]
- 26.Sawaki Y, Iuchi S, Kobayashi Y, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009;150(1):281–294. doi: 10.1104/pp.108.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma FJ. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell. 2009;21(10):3339–3349. doi: 10.1105/tpc.109.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foy CD. Plant adaptation to acid, aluminum-toxic soils. Commun Soil Sci Plant Anal. 1988;19(7–12):959–987. doi: 10.1080/00103628809367988. [DOI] [Google Scholar]
- 29.Kochian LV, Hoekenga OA, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol. 2004;55(1):459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- 30.Lu M, Wang Z, Fu S, Yang G, Shi M, Lu Y, Wang X, Xia J. Functional characterization of the SbNrat1 gene in sorghum. Plant Sci. 2017;262:18–23. doi: 10.1016/j.plantsci.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Dong J, Piñeros MA, Li X, Yang H, Liu Y, Murphy AS, Kochian LV, Liu D. An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol Plant. 2017;10(2):244–259. doi: 10.1016/j.molp.2016.11.001. [DOI] [PubMed] [Google Scholar]


