Abstract
Penile cancer is a rare but highly lethal cancer, and therapeutic options for patients presenting with lymph nodal disease are very limited. Adoptive cell therapy using tumor infiltrating lymphocytes (TIL) was shown to provide durable objective response in patients with metastatic melanoma and TIL have been expanded from solid tumors at rates between 70 and 90% depending on the specific diagnosis. We evaluated whether TIL could be expanded from surgical specimens of patients with penile cancer. Tumor samples from metastatic lymph nodes obtained at the time of inguinal lymph node dissection were collected, minced into fragments, placed in individual wells of a 24-well plate, and propagated in high dose IL-2 for four weeks. The phenotype of expanded TILs was assessed by flow cytometry and their anti-tumor reactivity was assessedby IFN-γ ELISA. TIL were expanded from 11 out of 12 (91.6%) samples of metastatic lymph nodes. Expanded TIL were predominantly CD3+ (mean 67.5%, SD 19.4%) with a mean of 46.8% CD8+ T cells (SD 21.1%). Five out of 11 samples (45.4%) from expanded TIL secreted IFN-γ in response to autologous tumor. TIL expansion and phenotype of expanded T cell lymphocytes were independent of previous HPV infection and treatment with neoadjuvant chemotherapy. This is the first report demonstrating successful expansion of tumor-reactive TIL from penile cancer patients, which support development of adoptive cell therapy strategies using TIL for the treatment of advanced and recurrent penile cancer.
Keywords: Adoptive cellular immunotherapy, Immunophenotyping, Penile cancer, T cells, Tumor-infiltrating lymphocytes
INTRODUCTION
Penile cancer is a rare but highly lethal cancer in men. In the US alone, the number of estimated deaths were 410 out of 2,080 new cases in 2019 (1). The inguinal lymph nodes (LN) are the first location of metastatic spread in penile cancer, and LN metastasis is the main prognostic factor for survival (2). In spite of surgical resection, the 5-year survival of patients with clinically evident inguinal nodal disease is between 30% and 50% (3). Patients with pelvic LN metastasis have a poor survival (67% at 1-year, and 21% at 5-year) and patients with distant metastasis are expected to live less than 18 months (3, 4). Neoadjuvant and first-line chemotherapies have limited efficacy with objective responses observed in about only half of the patients with locally advanced and metastatic penile cancer (5). Although immune checkpoint blockade has proven effective for treatment of several other solid tumors (6–8), there are currently no large studies or data regarding immunotherapy of penile cancer. Moreover, the very low incidence of penile cancer is a challenge for both translational research and clinical trial accrual, creating an unmet need for cancer research and novel therapies in penile cancer (9).
Adoptive cell therapy with tumor-infiltrating lymphocytes (TIL) begins with expansion of TILs from surgically resected human tumor samples, followed by rapid ex vivo expansion, and subsequent intravenous reinfusion of the expanded TIL product into patients following lymphodepletion (10). It has been proven effective in cervical cancer, ovarian cancer, and particularly in metastatic melanoma (11–13). In penile cancer, presence of CD8+ TIL in tumor microenvironment was associated with decreased LN metastasis (14). In contrast, presence of regulatory T cells and CD163+ macrophages were associated with poor cancer-specific survival (14, 15). There is a complex interplay within the penile tumor microenvironment, and given the data regarding CD8+ infiltration as a positive prognostic factor, ACT using TIL is a strategy worth exploring for penile cancer patients.
Inguinal lymph node dissection (ILND) is the cornerstone of treatment for penile cancer patients with palpable or radiologically visible inguinal LNs (16) and it involves removal of locoregional metastases to both superficial and deep inguinal LN (17). Despite the increase in utilization of chemotherapy over the last decade in the US, ILND was found to be the only endpoint to impact oncological outcomes (HR for overall survival, 0.64; 95% CI, 0.52–0.78) in patients with LN+ penile cancer, whereas chemotherapy and radiotherapy failed to demonstrate any overall survival benefit (18). ILND offers a unique opportunity to harvest and expand TIL to develop more effective systemic therapy options and potentiate anti-tumor immune response in penile cancer patients. In the present study, we evaluated the feasibility of TIL expansion from surgically resected metastatic LNs at the time of ILND in penile cancer patients. Our secondary aim was to assess the effect of infection with Human Papilloma Virus (HPV) and neoadjuvant chemotherapy (NAC) on expansion of TIL.
MATERIAL and METHODS
Patient selection and data collection
Patients with solid penile tumors and clinically palpable/visible inguinal LN treated with ILND between 2017 and 2020 were included. ILND was performed in a standardized manner with established surgical templates of dissection for both superficial and deep inguinal LNs and oncological principles of care as previously described (19). Nodal metastases were evaluated preoperatively by biopsy or imaging (CT or MRI of the pelvis with IV contrast) and confirmed by histopathological assessment following ILND. Patients with suspected skin or underlying infection were excluded. HPV detection was determined based on molecular detection of HPV DNA on formalin-fixed paraffin-embedded tissue using in situ hybridization (HPVHL, Mayo Clinic Laboratories, Rochester, MN) or PCR and fragment analysis (HPV DNA Tissue Testing, Neogenomics, Fort Myers, FL) ± p16 immunohistochemistry (clone E6H4 mouse, Ventana, Tucson, AZ). The study was approved by the Institutional Review Board (MCC50180 and MCC50280). Informed consent was obtained from all patients prior to tissue collection.
TIL culture
Inguinal LN metastases were minced into ~1–3 mm3 fragments and placed in 24 well plates with 2 mL of RPMI 1640 containing 2.05 mM L–glutamine (HyClone, Thermo Fisher Scientific, Waltham, MA), 10 % heat-inactivated human AB serum (Omega Scientific, Tarzana, CA), 55 μM 2-mercaptoethanol (Invitrogen), 50 μg/mL gentamicin (Invitogen), 100 I.U./mL penicillin, 100 μg/mL streptomycin, and 10 mM HEPES Buffer (Mediatech, Manassas, VA). Recombinant human interleukin-2 (IL-2, 6000IU/mL, Prometheus) was added to the media and supplemented with each media change. Some cultures were supplemented with 1 ug/mL anti-CD137 agonistic antibody after initial setup in 10 ug/mL anti-CD137 (Urelemab, BMS-663513, a kind gift from BMS). If available, excess tissue was physically and enzymatically digested as described below. TIL cultures were expanded for four weeks and confluent wells were split into additional wells. TIL from each independent fragment was counted manually by trypan blue exclusion.
Rapid expansion protocol (REP)
The initial TIL cultures were then stimulated with 30 ng/mL human anti-CD3 (OKT3, Ortho Pharmaceutical, CA-S 140608–64–6) in the presence of irradiated (5000 rad) allogenic PBMC feeder cells at a ratio of 1 TIL to 200 feeder cells as previously described (20). TIL were cultured in media containing RPMI 1640, 2.05 mM L–glutamine (HyClone, Thermo Fisher Scientific, MT10040CM), 10% heat-inactivated human AB serum (Omega Scientific, HS-25), 55 μM 2-mercaptoethanol (Invitrogen, 21985023), and 10 mM HEPES Buffer (Mediatech, MT25060CI). On day 5, 70% of the media was replaced with REP Media II comprised of a 1:1 (v:v) mixture of REP Media I and AIM V (Invitrogen, 12055083). Media was supplemented with 3000 I.U./mL rhIL-2 on days 2 and 5. Cells were collected on day 14 of REP.
Flow cytometry
The phenotype of expanded TIL was evaluated using flow cytometry. The antibody panel used included CD3 PerCpCy5.5 (Biolegend 300430), CD4 FITC (BD Biosciences 555346), CD8 BV510 (Biolegend 344732), and CD56 PE (BD Biosciences 555516). All cells were stained with a Live/Dead Near-IR viability stain (Invitrogen, L10119) and fixed in 2% paraformaldehyde. Data were acquired on an LSR II or Celesta flow cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar, Inc.). Cells expressing CD56 without CD3 (CD3−CD56+) were labelled NK cells whereas cells expressing T cell markers of CD3, CD4 and CD8 were considered CD3+ T cells, CD4+ T cells and CD8+ T cells respectively.
Co-culture assay
The tumor material remaining after initial TIL culture fragments were plated was mechanically and enzymatically digested using media containing 2% Collagenase Type IV and a GentleMACS Dissociator (Miltenyi, 130–093–235), and then stored in liquid nitrogen until the time required for downstream assays. Upon cell recovery, tumor cells were counted by trypan blue exclusion. TIL and autologous tumor digest were cultured at a 1:1 ratio (1×105 cells each) for 24 hours in round bottom 96-well plates in the absence or presence of a purified anti-human HLA-A,B,C (MHC Class I) antibody (clone: W6/32, 5 microgram/1 ml) (Biolegend, San Diego, CA). Supernatants were collected after 24 hours. IFN-γ concentration was measured using a Human IFN-γ Quantikine ELISA Kit (R&D Systems, SIF50). Optical density of each well was measured at 450 nm and IFN-γ concentration was calculated from the standard curve. IFN-γ concentration ≥ 100 pg/ml was the cut-off for reactivity.
Statistical analysis
Data represented as scatter plots show individual patient data points as well as error bars representing median values with interquartile range (Q1-Q3) or mean values with standard deviation (SD). The statistical analyses were performed using GraphPad Prism software. P values were two-sided and determined using GraphPad Prism software by the Mann Whitney test, or chi-square test where indicated. P < 0.05 was considered statistically significant.
RESULTS
Clinicopathological characteristics of penile cancer patients
Metastatic inguinal LN samples were harvested from 12 patients who underwent inguinal lymph node dissection (ILND) between 3/9/2017 and 6/3/2020. Briefly, 11 patients underwent bilateral ILND while one patient underwent unilateral salvage ILND. Two of the patients also underwent concomitant bilateral pelvic lymph node dissection. Six samples (50%) were collected from patients who had received neoadjuvant chemotherapy (NAC) prior to surgery. Two patients were also concurrently treated with neoadjuvant external beam radiotherapy. All penile tumors were squamous cell carcinomas. HPV infection status was determined via immunohistochemistry and/or in situ hybridization and 4 patients (33.3%) had HPV-positive tumors. Clinicopathological characteristics of the patients are shown in table 1.
Table 1.
Patient demographics and clinicopathological characteristics.
| ID | Age | Smoking status | Clinical stage | Neoadjuvant treatment | From chemo to surgery | Pathological stage | HPV status |
|---|---|---|---|---|---|---|---|
| 001 | 51 | current smoker | T1N2M0 | None | T1aN3 | Negative | |
| 002 | 81 | former smoker | T1aN2M0 | None | T1aN2 | Negative | |
| 003 | 43 | non-smoker | T1N1M0 | None | T2N3 | Negative | |
| 004 | 33 | occasional smoker | T3N2M0 | TIP x4 | 38 days | T3N3 | Negative |
| 005 | 62 | occasional smoker | T1bN2M0 | TIP x4 | 210 days | TxN2 | Negative |
| 006 | 52 | current smoker | T1aN3M0 | Cisplatin + EBRT | 205 days | 4N3 | Negative |
| 007 | 83 | non-smoker | T2N1M0 | None | T1aN3 | Negative | |
| 008 | 71 | former smoker | TaN3M0 | TIP x4 | 56 days | TaN3 | Positive |
| 009 | 47 | non-smoker | T3cN2M0 | TIP x4 | 46 days | T3bN3 | Positive |
| 010 | 79 | former smoker | T1bN1M0 | None | T1bN3 | Positive | |
| 011 | 58 | former smoker | T1bN2M0 | None | T1bN1 | Positive | |
| 012 | 76 | former smoker | T2N2M0 | TIP x4 + EBRT | 33 days | T2N2 | Negative |
Abbreviations: EBRT, external beam radiation therapy; TIP, Paclitaxel, ifosfamide and cisplatin.
Expansion and phenotype of TIL from metastatic inguinal lymph nodes
TIL growth was achieved in 11 out of 12 (91.6%) patients using metastatic LNs (Table 2 ). The mean (SD) number of plated and expanded fragments were 15.5 (8.9) and 9.2 (8.5), respectively. The mean number of expanded TIL was 1.34E+08 among 10 patients whose total number of expanded TIL could be measured. Expanded TIL were predominantly CD3+ (mean: 67.5%, SD: 19.4%, range: 26.4–98.0%) with a mean of 46.8% CD8+ T cells (SD: 21.1%, range: 11.2–98.8%) and a mean of 29.1% CD4+ T cells (SD: 16.6%, range: 1.0–57.4%). Natural killer cells (CD3−CD56+) were present as well (mean: 32.3%, SD: 18.5%, range: 15.1–73.8%) (Figure 1A and 1B).
Table 2.
Expansion of TIL from penile tumor fragments.
| Sample | Number of fragments plated | Number of fragments expanded | Total TIL number |
|---|---|---|---|
| 1 | 12 | 9 | 9.88E+06 |
| 2 | 36 | 34 | 6.16E+08 |
| 3 | 24 | 14 | >1.0E+06a |
| 4 | 12 | 10 | 4.29E+08 |
| 5 | 12 | 9 | 1.06E+08 |
| 6 | 6 | 0 | 0 |
| 7 | 6 | 6 | 3.60E+07 |
| 8 | 12 | 6 | 2.06E+07 |
| 9 | 6 | 5 | 5.64E+07 |
| 10 | 24 | 4 | 3.93E+07 |
| 11 | 24 | 4 | 9.74E+06 |
| 12 | 12 | 1 | 1.78E+07 |
| Mean | 15.5 | 9.2 | 1.34E+08 |
| SD | 8.9 | 8.5 |
TIL growth was observed; the exact number is unknown.
Figure 1. Phenotype of TIL expanded from lymph node metastasis of penile cancer.

A) At four weeks after the initiation of TIL cultures, TIL were collected from each fragment and the percentage of CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD3−CD56+ NK cells was measured by flow cytometry and analyzed according to the representative gating strategy shown. B) Each column represents the mean percentage of cells expanded from 11 evaluable patients.
There was no difference in the total numbers of TIL expanded from tumor samples treated with or without NAC (median; 5.64E+07 vs. 3.60E+07, respectively, p=0. 574) and tumors samples with or without HPV positivity (median; 2.99E+07 vs. 7.10E+07, respectively, p=0. 476, Figure 2).
Figure 2. Expansion of TIL stratified by (A) neoadjuvant chemotherapy and (B) HPV status.

The total number of expanded TIL from lymph node metastasis of penile cancer was measured at 4 weeks after initiation of culture. Total number of expanded TIL among evaluable 10 patients stratified by previous neoadjuvant chemotherapy (A) (5 chemo-naïve and 5 chemo-treated cases) and (B) HPV status (4 HPV-positive and 6 HPV-negative cases). Each point represents the total TIL generated from each fragment within an individual patient. Median and interquartile range. Mann-Whitney test.
We also measured the phenotype of expanded TIL, including T cells and NK cells by flow cytometry. Between patients treated with or without NAC, no difference in the expansion of CD3+ T cells (median, 69.5% vs. 70.0%, respectively, p=0.662), CD4+ T cells (median, 22.0% vs. 35.5%, respectively, p=0.329), CD8+ T cells (median, 53.9% vs. 41.2%, respectively, p=0.246), or NK cells (median, 37.4% vs. 28.3%, respectively, p=0.730) was noted (Figure 3A). Similarly, between tumors with or without HPV positivity, no difference in the expansion of CD3+ T cells (median, 72.5% vs. 69.5%, respectively, p=0.648), CD4+ T cells (median, 36.2% vs. 22.0%, respectively, p=0.163), CD8+ T cells (median, 42.6% vs. 47.9%, respectively, p=0.527) or NK cells (median, %30.7 vs. 24.1%, respectively, p= 0.904) was detected (Figure 3B). HPV status and pre-treatment with NAC appeared to have no impact on anti-tumor reactivity of expanded TIL. Thus, TIL expansion was feasible regardless of previous NAC or HPV infection. Likewise, the number of expanded TIL from LN were independent of age, smoking status, body mass index, pathological LN stage or tumor grade (data not shown).
Figure 3. Phenotype of TIL stratified by (A) neoadjuvant chemotherapy and (B) HPV status.
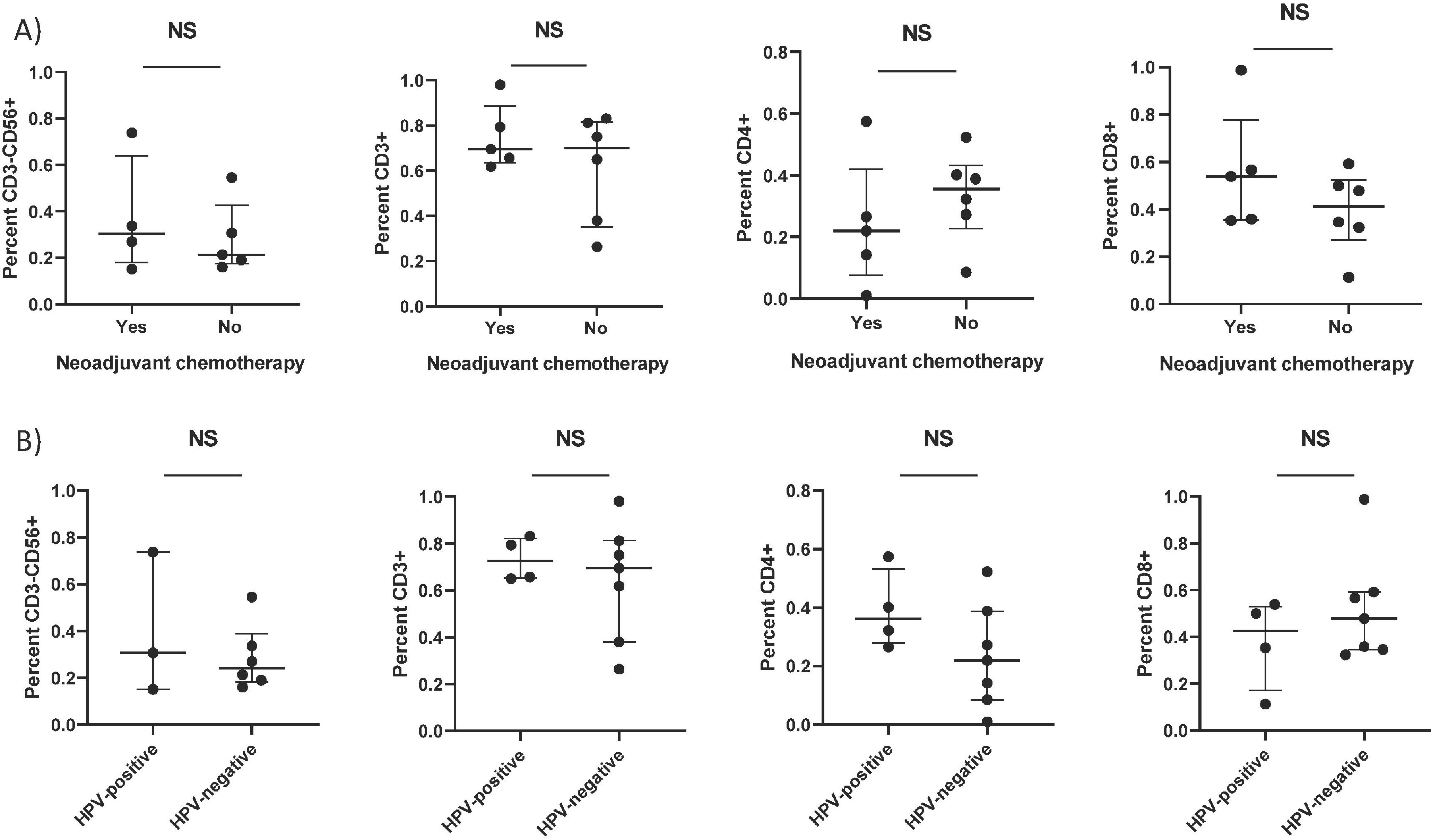
At four weeks after the initiation of TIL cultures, TIL were collected from each fragment and the percentage of CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD3−CD56+ NK cells was measured by flow cytometry. Mean percentage of cells expanded from 11 evaluable patients (A) stratified by neoadjuvant chemotherapy (6 chemo-naïve and 5 chemo-treated cases) and (B) HPV status (4 HPV-positive and 7 HPV-negative cases). Each point represents the mean percentage of cells generated from each fragment within an individual patient. Mann-Whitney test.
Anti-tumor reactivity of TIL
TIL expanded from each fragment was co-cultured with autologous tumor cells for 24 hours to measure the anti-tumor reactivity of expanded TIL. IFN-γ concentration in the supernatants was measured by ELISA. Among 11 expanded TIL, five TIL samples demonstrated anti-tumor reactivity in response to autologous tumor. Reactivity of two of these TIL samples is shown in Figure 4. Previously we demonstrated increased ability of TIL to secrete IFN-γ after REP in melanoma (21). For one sample, we performed a REP using TIL of one individual fragment. There was a 1446-fold expansion of TIL during the REP (not shown). We compared the percentages of T cells in pre- and post-REP products. As shown in Figure 5A, CD3+ and CD4+ T-cells were notably increased after REP. We also measured the anti-tumor reactivity in pre-REP and post-REP TIL. Reactivity against autologous tumor was increased in the final REP product (Figure 5B). This reactivity was specific as blocking MHC class I reduced the IFN-γ production.
Figure 4. Reactivity of expanded TIL from lymph node metastasis of penile cancer.

Expanded TIL was collected from individual tumor fragments at 4 weeks after initiation of culture. TILs were co-cultured at a 1:1 ratio with digested autologous tumor cells, or with digested autologous tumor cells at a 1:1 ratio and anti-HLA Class I antibody [W6/32] for 24 hours. The supernatants were collected and IFN-γ in supernatants was measured by ELISA. Two example graphs for co-culture assays from two different patients, in which assay results of all tumor fragments that harvested from the patient and subsequently grew TIL are shown.
Figure 5. Reactivity and phenotype of penile TIL before and after rapid expansion protocol (REP).

A) Phenotypes of TIL expanded from fragment 4 before and after REP. The percentage of immune cell phenotypes were measured by flow cytometry as described. B) IFN-γ levels before and after REP. Expanded TIL collected from individual fragment 4 (patient #9) underwent rapid expansion protocol (REP) as described. Pre-REP and Post-REP TIL was co-cultured in complete media alone (CM), with digested autologous tumor cells at a 1:1 ratio or with digested autologous tumor cells at a 1:1 ratio and anti-HLA Class 1 blocking antibody [W6/32] for 24 hours and supernatants were collected. IFN-γ in supernatants was measured by ELISA.
DISCUSSION
Penile cancer is a highly aggressive cancer and involvement of inguinal LNs confers a poor prognosis (22). ILND is performed with curative intent and it enables to resect all LNs potentially harboring metastasis of penile cancer located in the anatomical space bordered by adductor longus muscle medially, sartorius muscle laterally, and the inguinal ligament superiorly (17). Following therapeutic ILND, about 16% of patients with pathologic node-positive (pN+) disease experience nodal recurrence within a median of 5.7 months. These patients have a very dismal prognosis with a median survival of 4.5 months in spite of salvage treatment such as chemoradiotherapy (22). Moreover, none of the patients with pathologically confirmed metastasis in pelvic lymph nodes (pN3) survive more than 5 years after ILND (23).
To address this unmet need in the present study, we aimed to explore the feasibility of expanding TIL from resected metastatic LN of penile cancer patients. Although penile cancer, specifically clinically node-positive (cN+) disease is extremely rare (about 75 ILND procedures per year in the US)(18), we were able to collect 12 ILND samples from the patients treated in our institution, a quaternary referral center for penile cancer. We expanded anti-tumor reactive TIL from metastatic LN of penile cancer patients with a success rate over 90%. We believe that the resected inguinal LN available after ILND can be a valuable source for expansion of TIL and adoptive cell immunotherapy using TIL can be a potential therapeutic as an adjuvant or salvage treatment.
In the present study, NAC did not affect expansion of TIL in penile cancer patients. NAC provides an objective response in half of the patients with fixed and mobile bulky (≥ 4 cm) inguinal LNs (5) and it is recommended per National Comprehensive Cancer Guidelines (16). Half of the patients in our study were treated with NAC and received chemotherapy with a median of 51 (range, 33 –210) days before ILND. Nonetheless, TIL expansion was feasible from both chemo-naïve and NAC treated specimens, regardless of chemotherapy regimen or the duration between last chemotherapy cycle and TIL harvesting surgery. It is worth to mention that 3 other patients previously planned to enroll in our study had showed complete response to NAC and pathological evaluation of ILND specimen had revealed LNs without any metastatic disease. TIL expansion was not attempted from benign LNs per our study protocol. In such patients, primary penile tumors, concurrently resected with local excision or penectomy, can be an alternative source of TIL expansion in penile cancer, which warrants further investigation.
HPV infection is an important etiologic factor in penile cancer and about 50% of all penile cancer cases were attributed to HPV infection in population studies (24, 25). Patients with HPV-positive penile tumors have better disease outcomes and these tumors demonstrate distinct genomic features such as expression of viral E6 and E7 oncogenes, lack of TP53 tumor suppressor gene mutation, and low frequency of EGFR mutation compared to HPV-negative penile tumors (25, 26). In the present study, TIL expansion was feasible in patients regardless of HPV status. Total number of TIL expanded from HPV-negative tumors appeared to be higher than HPV-positive samples albeit statistically not significant. In a multi-center global phase II trial of refractory metastatic cervical carcinoma, a common HPV-associated malignancy, TIL therapy provided an ORR of 44% (27). Since HPV-positive penile squamous cell carcinoma is genetically and histologically similar to HPV-related cervical squamous cell carcinoma (25, 28), it is reasonable to expect similar high oncological efficacy from TIL therapy for HPV-positive penile cancer as well.
In the present study, penile TIL demonstrated antitumor reactivity against autologous tumor in 5 samples. The HPV-specific reactivity of expanded TILs remains to be explored in penile cancer. In another report of 9 patients with metastatic HPV+ cervical cancer, treatment with a single infusion of tumor-infiltrating T cells selected for HPV E6 and E7 reactivity provided two complete and one partial responses (11). Surprisingly, anti-tumor T-cell responses observed in those two complete responders were shown to be directed against mutated neoantigens and the cancer-germline antigens rather than HPV antigens (29). Further research is warranted to develop future assays for anti-tumor reactivity of TIL.
Interestingly, anti-tumor reactivity was increased after REP for one sample. The flow cytometric analysis of the post-REP TIL sample showed a decreased percentage of CD8+ T cells but increased percentage of CD4+ T-cells compared to pre-REP TIL sample. The historical conventional wisdom is that CD8+ T cells are instrumental for an anti-tumor response and CD4+ T cells are not needed for objective response in TIL therapy (30). Nonetheless, CD4+ lymphocytes were shown to account for 20% of objective response in patients with metastatic melanoma treated with CD8+- depleted TILs (31). As active CD4+ T cells can exclusively secrete IL-2 and IL-17 rather than IFN-γ and experimental co-culture assays are limited in terms of HLA class II expression, CD4+ T cells might potentially play a more important role in anti-tumor response than thought (31). In our lab, we are currently exploring intrinsic determinants of CD4+ TIL that leads to clinical efficacy, antigen recognition by CD4+ T cells and potential use of CD4+ TIL to rescue resistance to immunotherapy. Furthermore, particular T cell subtypes such as central memory and effector-memory T cells may play an important role during expansion phase of TIL and in mediating anti-tumor response of expanded penile TIL, which warrants further investigation.
CONCLUSIONS
In the present study, we demonstrated feasibility of expanding TIL from surgical samples of metastatic LN in penile cancer patients. Expanded TIL also showed anti-tumor reactivity. Expansion of penile TIL was feasible from tumor samples of penile cancer patients who were infected with HPV or treated with NAC. As the therapeutic armamentarium against penile cancer remains highly limited, we believe that adoptive T cell therapy using TIL expanded from metastatic LNs can be a therapeutic strategy for penile cancer, particularly for advanced and recurrent penile cancer which have dismal prognosis.
HIGHLIGHTS.
Expansion of tumor-infiltrating lymphocytes (TILs) from penile cancer is feasible.
Metastatic inguinal lymph nodes appears to be a great source for harvesting of TILs.
Expanded TILs are predominantly CD3+ and about half of them are CD8+ T cells.
Expansion of TIL is independent of HPV status and previous neoadjuvant chemotherapy.
Adoptive T cell therapy using TIL can be an invaluable therapeutic option for penile cancer.
Acknowledgments
This work was supported in part by the Tissue Core and the Flow Cytometry Core at the Moffitt Cancer Center, and in part by the Cancer Center Support Grant P30 CA076292 from the National Cancer Institute. We thank Shwetha Lakshmipathi, Shayna Smeltzer, Tanja Svrdlin, and Luz Nagle for their help in experimental work and data analysis, and Suzie McFarland for her assistance in the coordination of cases planned for TIL harvesting along with capture of their clinically relevant data parameters.
Funding
This work was funded by Swim Across America, the R.S. Evans Foundation and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. MH was supported by NCI-1F31CA250320–01. AAS was supported by NCI-5K23CA178083. SPT was supported by an American Cancer Society - Leo and Anne Albert Charitable Foundation Research Scholar Grant, (RSG-16-117-01-LIB).
Abbreviations:
- TIL
tumor-infiltrating lymphocyte
- BC
bladder cancer
- ILND
inguinallymph node dissection
- LN
lymph node
- SD
standard deviation
- HPV
Human Papilloma Virus
- REP
rapid expansion protocol
- NAC
neoadjuvant chemotherapy
- NK
natural killer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
PES is the vice-chair of the NCCN bladder and penile cancer panel as well as the president of the Global Society of Rare Genitourinary Tumors. Moffitt Cancer Center has licensed Intellectual Property (IP) related to the proliferation and expansion of tumor infiltrating lymphocytes (TILs) to Iovance Biotherapeutics. SPT, MH, and AAS are inventors on such Intellectual Property. SPT and AAS are listed as co-inventors on a patent application with Provectus Biopharmaceuticals. Moffitt has also licensed IP to Tuhura Biopharma. SPT is an inventor on such Intellectual Property. SPT participates in sponsored research agreements with Iovance Biotherapeutics, Provectus Biopharmaceuticals, Intellia Therapeutics, and Myst Therapeutics that are not related to this submitted work. Dr. Pilon-Thomas has received research support that is not related to this submitted work from the following entities: NIH-NCI (U01 CA244100–01 and R01 CA239219–01A1) and V Foundation. Additionally, Dr. Pilon-Thomas is a co-Investigator on NIH-NCI (U54 CA193489–01A1 and R01 CA241559) grants, which are not related to this submitted work. Dr. Sarnaik has received Ad hoc consulting fees from Iovance Biotherapeutics, Guidepoint, Defined health, and Gerson Lehrman Group. Dr. Sarnaik has received speaker fees from Physicians’ Educational Resource (PER) LLC.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Ficarra V, Akduman B, Bouchot O, Palou J, Tobias-Machado M. Prognostic factors in penile cancer. Urology. 2010;76(2 Suppl 1):S66–73. [DOI] [PubMed] [Google Scholar]
- 3.Graafland NM, van Boven HH, van Werkhoven E, Moonen LM, Horenblas S. Prognostic significance of extranodal extension in patients with pathological node positive penile carcinoma. J Urol. 2010;184(4):1347–53. [DOI] [PubMed] [Google Scholar]
- 4.Hegarty PK, Kayes O, Freeman A, Christopher N, Ralph DJ, Minhas S. A prospective study of 100 cases of penile cancer managed according to European Association of Urology guidelines. BJU Int. 2006;98(3):526–31. [DOI] [PubMed] [Google Scholar]
- 5.Azizi M, Aydin AM, Hajiran A, Lai A, Kumar A, Peyton CC, et al. Systematic Review and Meta-Analysis-Is there a Benefit in Using Neoadjuvant Systemic Chemotherapy for Locally Advanced Penile Squamous Cell Carcinoma? J Urol. 2020;203(6):1147–55. [DOI] [PubMed] [Google Scholar]
- 6.Borel C, Jung AC, Burgy M. Immunotherapy Breakthroughs in the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Cancers (Basel). 2020;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boegemann M, Aydin AM, Bagrodia A, Krabbe LM. Prospects and progress of immunotherapy for bladder cancer. Expert Opin Biol Ther. 2017;17(11):1417–31. [DOI] [PubMed] [Google Scholar]
- 8.Eso Y, Seno H. Current status of treatment with immune checkpoint inhibitors for gastrointestinal, hepatobiliary, and pancreatic cancers. Therap Adv Gastroenterol. 2020;13:1756284820948773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canter DJ, Nicholson S, Watkin N, Hall E, Pettaway C, In PEC. The International Penile Advanced Cancer Trial (InPACT): Rationale and Current Status. Eur Urol Focus. 2019;5(5):706–9. [DOI] [PubMed] [Google Scholar]
- 10.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, et al. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother. 2012;35(8):615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevanovic S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res. 1995;1(5):501–7. [PubMed] [Google Scholar]
- 14.Ottenhof SR, Djajadiningrat RS, Thygesen HH, Jakobs PJ, Jozwiak K, Heeren AM, et al. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front Immunol. 2018;9:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassallo J, Rodrigues AF, Campos AH, Rocha RM, da Cunha IW, Zequi SC, et al. Pathologic and imunohistochemical characterization of tumoral inflammatory cell infiltrate in invasive penile squamous cell carcinomas: Fox-P3 expression is an independent predictor of recurrence. Tumour Biol. 2015;36(4):2509–16. [DOI] [PubMed] [Google Scholar]
- 16.Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, et al. Penile cancer: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2013;11(5):594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta MK, Patel AP, Master VA. Technical considerations to minimize complications of inguinal lymph node dissection. Transl Androl Urol. 2017;6(5):820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi SS, Handorf E, Strauss D, Correa AF, Kutikov A, Chen DYT, et al. Treatment Trends and Outcomes for Patients With Lymph Node-Positive Cancer of the Penis. JAMA Oncol. 2018;4(5):643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ercole CE, Pow-Sang JM, Spiess PE. Update in the surgical principles and therapeutic outcomes of inguinal lymph node dissection for penile cancer. Urol Oncol. 2013;31(5):505–16. [DOI] [PubMed] [Google Scholar]
- 20.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chacon JA, Wu RC, Sukhumalchandra P, Molldrem JJ, Sarnaik A, Pilon-Thomas S, et al. Co-stimulation through 4–1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS One. 2013;8(4):e60031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graafland NM, Moonen LM, van Boven HH, van Werkhoven E, Kerst JM, Horenblas S. Inguinal recurrence following therapeutic lymphadenectomy for node positive penile carcinoma: outcome and implications for management. J Urol. 2011;185(3):888–93. [DOI] [PubMed] [Google Scholar]
- 23.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93(2):133–8. [DOI] [PubMed] [Google Scholar]
- 24.Olesen TB, Sand FL, Rasmussen CL, Albieri V, Toft BG, Norrild B, et al. Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 2019;20(1):145–58. [DOI] [PubMed] [Google Scholar]
- 25.Aydin AM, Chahoud J, Adashek JJ, Azizi M, Magliocco A, Ross JS, et al. Understanding genomics and the immune environment of penile cancer to improve therapy. Nat Rev Urol. 2020;17(10):555–70. [DOI] [PubMed] [Google Scholar]
- 26.Stankiewicz E, Prowse DM, Ng M, Cuzick J, Mesher D, Hiscock F, et al. Alternative HER/PTEN/Akt pathway activation in HPV positive and negative penile carcinomas. PLoS One. 2011;6(3):e17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jazaeri AA, Zsiros E, Amaria RN, Artz AS, Edwards RP, Wenham RM, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. Journal of Clinical Oncology. 2019;37(15_suppl):2538-. [Google Scholar]
- 28.Jacob JM, Ferry EK, Gay LM, Elvin JA, Vergilio JA, Ramkissoon S, et al. Comparative Genomic Profiling of Refractory and Metastatic Penile and Nonpenile Cutaneous Squamous Cell Carcinoma: Implications for Selection of Systemic Therapy. J Urol. 2019;201(3):541–8. [DOI] [PubMed] [Google Scholar]
- 29.Stevanovic S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356(6334):200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, et al. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16(24):6122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman KM, Prieto PA, Devillier LE, Gross CA, Yang JC, Wunderlich JR, et al. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J Immunother. 2012;35(5):400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


