ABSTRACT
The emerging evidence supports the use of prebiotics like herb-derived polysaccharides for treating nonalcoholic fatty liver disease (NAFLD) by modulating gut microbiome. The present study was initiated on the microbiota-dependent anti-NAFLD effect of Astragalus polysaccharides (APS) extracted from Astragalus mongholicus Bunge in high-fat diet (HFD)-fed mice. However, the exact mechanisms underlying the beneficial effects of APS on NAFLD formation remain poorly understood.
Co-housing experiment was used to assess the microbiota dependent anti-NAFLD effect of APS. Then, targeted metabolomics and metagenomics were adopted for determining short-chain fatty acids (SCFAs) and bacteria that were specifically enriched by APS. Further in vitro experiment was carried out to test the capacity of SCFAs-producing of identified bacterium. Finally, the anti-NAFLD efficacy of identified bacterium was tested in HFD-fed mice.
Our results first demonstrated the anti-NAFLD effect of APS in HFD-fed mice and the contribution of gut microbiota. Moreover, our results indicated that SCFAs, predominantly acetic acid were elevated in APS-supplemented mice and ex vivo experiment. Metagenomics revealed that D. vulgaris from Desulfovibrio genus was not only enriched by APS, but also a potent generator of acetic acid, which showed significant anti-NAFLD effects in HFD-fed mice. In addition, D. vulgaris modulated the hepatic gene expression pattern of lipids metabolism, particularly suppressed hepatic fatty acid synthase (FASN) and CD36 protein expression.
Our results demonstrate that APS enriched D. vulgaris is effective on attenuating hepatic steatosis possibly through producing acetic acid, and modulation on hepatic lipids metabolism in mice. Further studies are warranted to explore the long-term impacts of D. vulgaris on host metabolism and the underlying mechanism.
KEYWORDS: Gut microbiota, nonalcoholic fatty liver disease, obesity, D. vulgaris, acetic acid
Introduction
The human body is a “superorganism” with trillions of commensal bacteria in its gastrointestinal tract.1 Gut microbiota plays critical roles in modulating host physiology and metabolism,2–4 and therefore, gut dysbiosis contributes to the development of many kinds of human diseases, in particular obesity-related metabolic diseases such as nonalcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus and metabolic syndrome.5–7 The compositional alteration of intestinal bacteria in NAFLD patients was first reported in 1921 by Hoefert.8
The causal role of gut dysbiosis in NAFLD development has been extensively studied and validated by targeting gut microbiota manipulations such as probiotics, prebiotics9 or fecal microbiota transplants in either human patients or animal models.10–12 Although extremely challenging, increasing evidence has demonstrated that some specific bacterium could either promote or inhibit NAFLD development. For example, Wu et al. revealed that the anti-obesity effect of polysaccharides from Hirsutella sinensis was due to enrichment of the commensal bacterium Parabacteroides goldsteinii and further, oral gavage of live P. goldsteinii prevented body weight gain, improved intestinal integrity and reduced inflammation and insulin resistance.13 In contrast, Yuan et al. have demonstrated that patients with NASH accompanied with bacterially derived auto-brewery syndrome showed high abundance of Klebsiella pneumoniae in gut. Moreover, the increased Klebsiella pneumoniae was accounted for the alcohol production in these patients resulting to the occurrence of NAFLD.14 Although Klebsiella pneumoniae is a typical pneumonia-causing pathogen,15 our previous study revealed it metabolized melamine to cyanuric acid causing kidney damage and crystal formation in rats16 . Hence, the function of specific bacterium in gut is usually complex and largely unknown.
Our report here was initiated by the observation that Astragalus polysaccharides (APS) from Astragalus mongholicus Bunge, an herbal medicine, exhibited anti-NAFLD effects with efficacy in lowering plasma lipids, improving insulin sensitivity,17,18 and ameliorating metabolic risk in metabolically stressed transgenic mice.19 Our recent study demonstrated that APS was effective in attenuating high fat diet-induced metabolic disorders including reduced the extent of hepatic steatosis, inhibiting body weight gain, and improved insulin resistance.20 However, the mechanism underlying the preventive effect of APS on NAFLD was not elucidated. In our current study, we first observed that the anti-NALFD effect of APS was associated with gut microbiota modulation and increasing of acetic acid in serum and feces. Subsequent 16S rRNA gene sequencing and metagenomics analysis revealed that bacteria in the Desulfovibrio genus were depleted by high-fat diet (HFD) feeding and enriched in APS supplemented mice, in particular Desulfovibrio vulgaris (D. vulgaris) with the highest abundance within Desulfovibrio genus. Furthermore, we demonstrated that D. vulgaris was not only a potent generator of acetic acid, but also attenuated HFD-induced body weight gain, hepatic steatosis, highlighting the novel anti-NAFLD effect of D. vulgaris in mice.
Results
The anti-NAFLD effects of APS are associated with gut microbiota modulation
In line with our recent report,20 our current results first confirmed that APS supplementation substantially improved metabolic disorders in HFD mice including decreases in body weight (p < .01), fat index (p < .05), hepatic steatosis (p < .01), liver triglycerides (TG) level (p < .05), and pro-inflammatory cytokines expression (Il1β and Il6) in liver and white adipose tissue (p < .05) in the context of similar energy intake (p = .10) with HFD-fed mice (Figure 1a-g). Nevertheless, the hepatic IL-6 level was of no difference among the three groups (data not shown). Twelve weeks of APS supplementation reduced serum insulin level (p < .05), but not fasting blood glucose in HFD-fed mice (p = .23, Figure 1h-i). Expression of both fatty acid synthase (FASN) and carnitine palmitoyltransferase-1α (CPT1-α) proteins, the rate limiting enzymes for de novo synthesis and -oxidation of fatty acids, was also reduced in the livers of APS supplemented mice compared to HFD mice, respectively (p < .05, Figure 1j).
Figure 1.
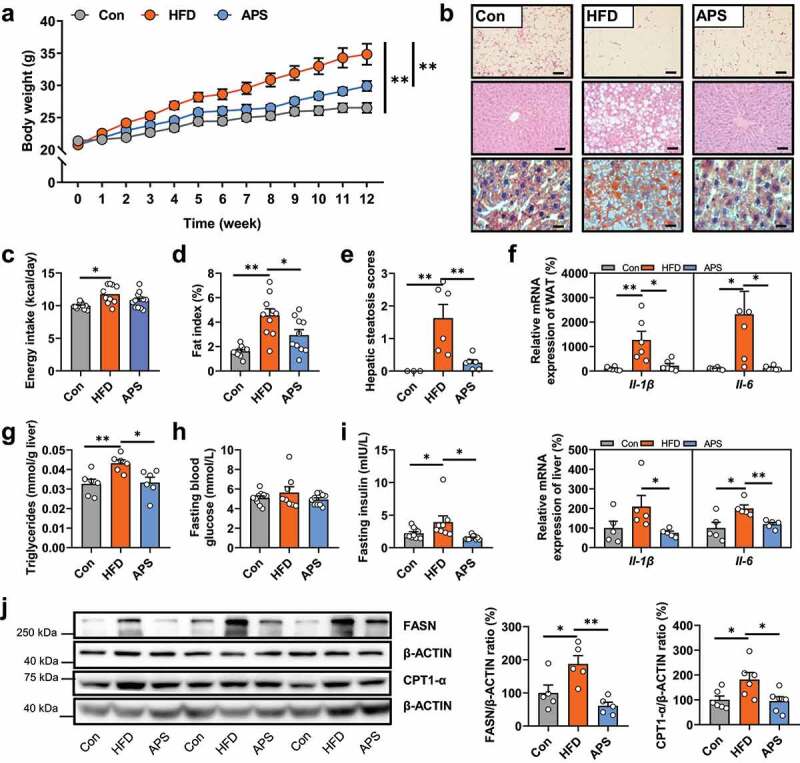
The anti-NAFLD effects of APS are associated with gut microbiota modulation. Male C57BL/6 J mice (4-week-old) were treated with normal chow diet (Con) or high-fat diet (HFD) with or without APS supplementation (4% APS in HFD) for 12 weeks (n = 10). (a) Body weight (g). (b) Representative photomicrographs of adipose tissues with H&E staining (magnification, ×200, 50 μm), and liver tissue with H&E (magnification, ×200, 50 μm) and oil red O staining (magnification, ×400, 25 μm). (c) Average energy intake per mouse (kcal/day). (d) Fat index (%, epididymal fat mass (g)/body weight (g) *100%). (e) Hepatic steatosis scores. (f) The relative mRNA expression of pro-inflammatory cytokines (Il1β and Il6) in white adipose tissues (WAT, %) and liver tissues (%). (g) Hepatic triglycerides levels (TG, mmol/g liver). (h) Fasting blood glucose (mmol/L). (i) Fasting serum insulin (mIU/L). (j) The protein expression of FASN and CPT1-α in liver tissues (%). Data are presented as the mean ± s.e.m.; *p < .05, **p < .01
Emerging evidence has demonstrated the well-established anti-obesity effect of polysaccharides from Ganoderma lucidum,9 Hirsutella sinensis,13 and Polygonatum odoratum21 through modulating gut microbiota. Our recent study indicated that APS supplementation also altered the composition of gut microbiota in HFD-fed mice.20 In addition, our in vitro data indicated that APS could not inhibit the TG accumulation in hepatocytes (data not shown). We therefore wondered whether the modulation on gut microbiota contributed to the anti-NAFLD effect of APS. The gut microbiota composition was compared among groups based on 16S rRNA gene sequencing. An average of 38621 ± 1058 (mean±s.e.m) valid reads were obtained that covered the majority of bacterial diversity (Supplementary Table 1). First of all, the results showed that the gut microbiome of HFD mice was characterized with reduced α diversity indexes including Shannon, Sobs, Chao and Ace compared to the Control group (Con, p < .001). APS supplementation significantly increased these indexes compared to the HFD group (p < .05, Supplementary Figure 1a), suggesting that APS supplementation increased the bacterial richness (increased Sobs, Chao, Ace and Shannon indexes) and evenness (increased Shannon index) in HFD-fed mice. Then, weighted UniFrac principal coordinate analysis (PCoA) showed that samples from both HFD and APS groups were separated from the Con group on PCoA 1 (p = .001), while HFD and APS groups were also separated with each other (p = .004, Supplementary Figure 1b). In addition, we observed significant changes in the relative abundance of the main phyla including increased Firmicutes and Proteobacteria (p < .01) and reduced Bacteroidetes (p < .001) in the HFD mice. APS supplementation mainly reduced the relative abundance of Firmicutes (p < .01) and increased Bacteroidetes (p < .001) leading to the reduction of Firmicutes/Bacteroidetes (F/B) ratio compared to the HFD group, while some unidentified bacteria in HFD and APS groups were not detected in Con group (Supplementary Figure 1c).
Next, to test whether the altered gut microbiota was secondary or causative for APS effect, we observed the impacts on gut microbiota by a short-term (2-day or 2-week) APS intervention in HFD-fed mice. The results showed that even short-term APS supplementation (either 2-day or 2-week) significantly altered the main composition of gut microbiota, specifically reduced Firmicutes and increased Bacteroidetes abundance leading to the decrease of F/B ratio (p < .05, Supplementary Figure 2). Therefore, the change of gut microbiota by short-term APS intervention suggested that the altered gut microbiota was not a consequence of APS effect in HFD-fed mice. Altogether, these results implied that the anti-NAFLD effect of APS was associated with the modulation of gut microbiota in HFD-fed mice.
The remodel of gut microbiota by APS is robust and sustained
Given the coprophagic character of mice, the bacteria transfer through fecal-oral route is highly probable.22 To further test the role of gut microbiota in the anti-NAFLD effect of APS, a co-housing experiment was performed, where two subgroups of mice from either HFD or APS supplemented groups were co-housed (HFD_CoH and APS_CoH) and kept on HFD for continued 8 weeks without APS supplementation in both APS and APS_CoH groups (Seeing experimental design in Figure 2a). Although energy intake was similar among groups, co-housing narrowed the difference in final body weight between HFD_CoH and APS_CoH, as well as serum alanine aminotransferase (ALT) levels (Figure 2b-d). The extent of hepatic steatosis in both HFD_CoH and APS_CoH groups was affected by each other characterized with reduced volume of fat cavitation compared to HFD group (Figure 2e). To test whether the phenotypic changes of co-housed mice were due to the bacteria transfer, the compositional structure of gut microbiota was compared based on 16S rRNA gene sequencing. First of all, the results revealed that the bacterial richness and evenness of APS supplemented mice was higher than HFD group, as well as the differences in bacterial composition (figure 2f-g, Supplementary Figure 3a). Co-housing mainly resulted in the assimilation of gut microbiota to that of APS supplemented mice in both α and β diversity, rather than HFD mice including the changes of indexes of Shannon, Chao, Ace and Sobs, and the diversified composition (figure 2f, Supplementary Figure 3a, Supplementary Table 2). The weighted UniFrac PCoA showed the HFD group was distinctly separated from the rest three groups on PCoA 1 (p = .004), as well as the separated trend with APS (p = .051) or HFD_CoH group (p = .034). However, samples from APS and APS_CoH groups showed no separation (p = .529) (Figure 2g). In addition, the data also showed the ratio of Firmicutes/Bacteroidetes (F/B) was consistently reduced in APS, HFD_CoH and APS_CoH groups compared to HFD group (Supplementary Figure 3b). These results demonstrated that the remodeled gut microbiota during co-housing contributed to the metabolic benefits of APS, and the impacts of APS on gut microbiota were also more robust and sustained than HFD.
Figure 2.
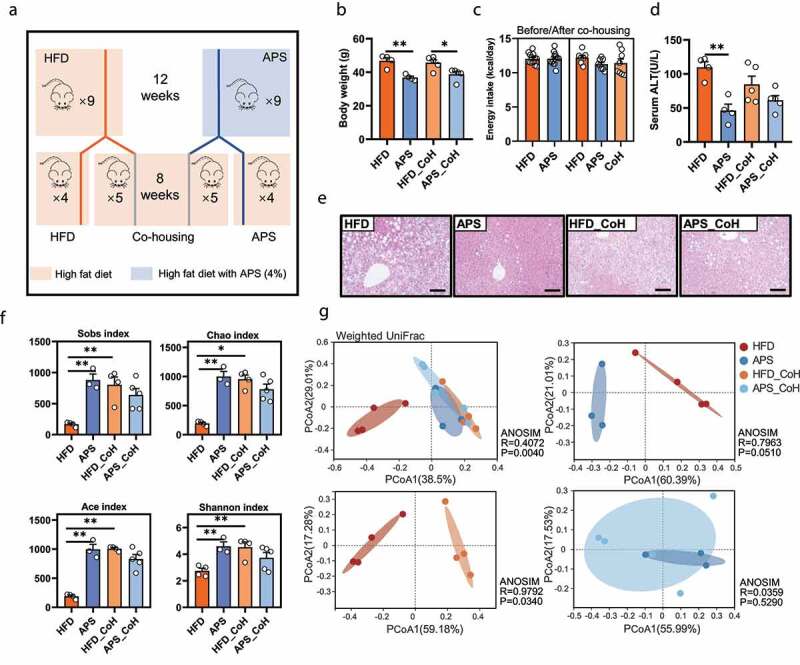
The co-housing experiment was conducted to examine the gut microbiota-dependent effect of APS. HFD_CoH or APS_CoH represents mice that are from either HFD or APS groups were co-housed within same cages for another 8 weeks to let their gut microbiota transferred naturally (a). (b) Body weight (g). (c) Average energy intake per mouse before and after co-housing (kcal/day). (d) Serum alanine aminotransferase (ALT) levels (U/L). (e) Representative photomicrographs of liver tissues with H&E (magnification, ×200, 50 μm). (f) Sobs, Chao, Ace, and Shannon index. (g) Weighted UniFrac based PCoA analysis among groups (ANOSIM, R = 0.4072, P = .004), between HFD and APS group (ANOSIM, R = 0.7963, P = .051), or HFD and HFD_CoH group (ANOSIM, R = 0.9792, P = .034), or APS and APS_CoH group (ANOSIM, R = 0.0359, P = .529). Data are presented as the mean ± s.e.m.; *p < .05, **p < .01
The anti-NAFLD effects of APS are associated with increased bacteria-derived acetic acid
Given the anti-NAFLD effect of APS is related with altered gut microbiota and some herb-derived polysaccharides are usually fermented into short-chain fatty acids (SCFAs, mainly acetic acid, propionic acid, and butyric acid) by gut microbiota,23 which play important roles in attenuating metabolic diseases,22,24,25 we hypothesized that the anti-NAFLD effect of APS was probably due to increased production of SCFAs. We then quantified the absolute contents of SCFAs in both fecal and serum samples of mice with authentic standards and calculated sample concentrations based on the derived standard curves (Supplementary Figure 4a-b). The results showed that acetic acid, propionic acid, and butyric acid were significantly reduced in both feces and serum of HFD mice, whereas APS supplementation increased the levels of acetic acid in both feces (2.072 ± 0.154 nmol/mg to 4.272 ± 0.356 nmol/mg) and serum (0.103 ± 0.034 mM to 0.219 ± 0.039 mM), and butyric acid in feces (0.055 ± 0.008 nmol/mg to 0.360 ± 0.056 nmol/mg) (Figure 3a). In addition, we found that the serum level of acetic acid, but not propionic acid and butyric acid, was increased in HFD_CoH group compared to HFD group (p < .001, Figure 3b).
Figure 3.
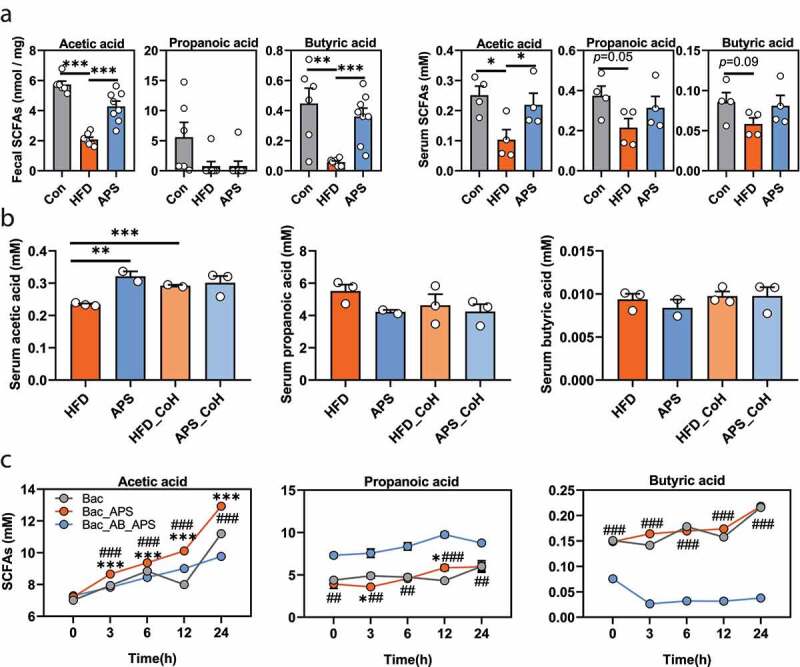
The anti-NAFLD effects of APS are associated with increased production of acetic acid. (a) Feces (n = 6–8) and serum (n = 4) samples of normal chow diet (Con) and HFD mice supplemented with or without APS were analyzed using targeted metabolomics for SCFAs (acetic acid, propionic acid, and butyric acid) levels by GC-TOF-MS. (b) Serum levels of SCFAs in mice in co-housing experiment (mM, n = 4–5). CoH represents co-housing. (c) Contents of SCFAs were measured at the 0, 3rd, 6th, 12th, and 24th h of anaerobic culturing in the presence of intestinal bacteria from HFD-fed mice with or without vancomycin treatment (500 mg/L in drinking water for 5 days). (Bac: Culture medium containing the bacteria from HFD mice without APS; Bac_APS: APS (200 mg/L) added ex vivo to medium containing the equal amount of bacteria from HFD mice; Bac_AB_APS: APS added ex vivo to culture medium containing the bacteria from HFD mice with antibiotic treatment, n = 2–3). Data are presented as the mean ± s.e.m.; *p < .05, **p < .01 ***p < .001. *p < .05, ***p < .001 vs Bac; ##p < .01, ###p < .001 vs BAC_AB_APS at Panel c
To further determine whether the increased acetic acid levels were derived from APS or gut microbiota itself, we detected SCFAs production by fecal bacteria from mice with or without oral antibiotic pre-treatment (500 mg/L vancomycin in drinking water for 5 days) under anaerobic culture condition. As expected, the SCFAs were accumulated in the medium containing fecal bacteria over time, in which acetic acid and propionic acid were found in higher concentrations than butyric acid (Figure 3c). The addition of APS to the bacterial medium further increased the contents of acetic acid time-dependently (p < .001) but not that of propionic acid or butyric acid. The capability for acetic acid and butyric acid production was abolished in bacteria that were pre-treated by antibiotics even in the presence of APS (p < .001, Figure 3c, Supplementary Figure 5). On the contrary, the levels of propionic acid were increased in bacteria from antibiotic-treated mice (p < .01, Figure 3c). These results indicated that APS was mainly fermented into acetic acid by gut microbiota, instead of propionic acid and butyric acid.
Since the physiological functions of acetic acid in metabolic diseases are sometimes discrepant,26–28 the action of acetate is supposed to be context dependent, such as mode and location of administration, metabolic and anatomic differences in species and so on.29 We then tested whether the acetic acid supplementation could produce similar effect with APS in HFD-fed mice. The results showed that acetic acid treatment significantly inhibited the HFD-induced body weight gain (p < .05), reduced the size of white adipocytes and fat index (p < .05), attenuated hepatic steatosis (p < .01) and reduced liver TG levels (p < .01) and serum ALT (p < .01), as well as fasting blood glucose (p < .01), with no difference in energy intake (p = .17, Figure 4a-h). Moreover, acetic acid also suppressed the expression of hepatic FASN protein (p < .05), but with minor impact on CPT1-α expression (p = .66, Figure 4i). Altogether, the results indicated that the anti-NAFLD effects of APS were mainly associated with the increased acetic acid derived by gut microbiota.
Figure 4.
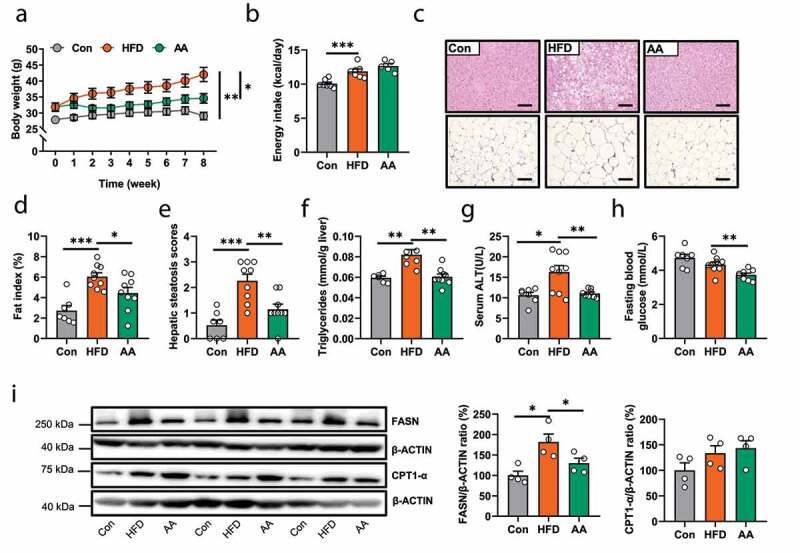
Male C57BL/6 J mice (4-week-old) were treated with either Con or HFD for 8 weeks and then mice in HFD group were subdivided into two groups with or without sodium acetate (AA, 3.7% in HFD) for another 8 weeks (n = 7–9). (a) Body weight (g). (b) Average energy intake per mouse (kcal/day). (c) Representative photomicrographs of liver and adipose tissues with H&E (magnification, ×200, 50 μm).(d) Fat index (%, epididymal fat mass (g)/body weight (g) *100%). (e) Hepatic steatosis scores. (f) Hepatic triglycerides levels (mmol/g liver). (g) Serum ALT level (U/L). (h) Fasting blood glucose (mmol/L). (i) The protein expression of FASN and CPT1-α in liver tissues (%). Data are presented as the mean ± s.e.m.; *p < .05, **p < .01, ***p < .001
D.vulgaris is a potent generator of acetic acid enriched by APS supplementation
Next, we expected to find the specific bacterium that was responsible for acetic acid generation by mining the 16S rRNA gene sequencing data. For such a purpose, only bacteria that were significantly enriched by APS supplementation were selected for further analysis as shown in Supplementary Figure 6 and Supplementary Table 3. There were nine enriched OTUs in the APS supplemented mice, in which only four of them were annotated at the genus level, including Desulfovibrio, Parabacteroides, Acetatifactor, and Alistipes. We then performed a Spearman’s correlation analysis between these 4 genera and metabolic disorder-related parameters, as well as acetic acid levels in both feces and serum. Among these 4 genera, Desulfovibrio was not only the highest in abundance, but also negatively correlated with most of the metabolic disorder-related parameters such as levels of liver TG level (p < .05), fasting serum insulin (p < .001), pro-inflammatory cytokines in liver or white adipose tissue (WAT, p < .05), and positively correlated with serum acetic acid level (p < .05, Figure 5a, Supplementary Table 4), suggesting that bacteria in the Desulfovibrio genus were associated with the activity of APS, especially acetic acid production. In agreement with the results based on OTUs analysis (Supplementary Figure 6), the relative abundance of the Desulfovibrio genus was significantly enriched in APS supplemented mice compared to the HFD group (Figure 5b). Then, we further explored the relative abundance changes of bacteria within the Desulfovibrio genus at species level with metagenomics data. A total of 27 Desulfovibrio species were identified, and most of them were reduced in the HFD group, and enriched by APS supplementation, while only some of them differed significantly across the three groups (p < .05) including D. vulgaris, D. desulfuricans, D. alaskensis, D. acrylicus and D. frigidus. In addition, D. vulgaris was the one with the highest relative abundance within Desulfovibrio genus (Figure 5c, Supplementary Table 5).
Figure 5.
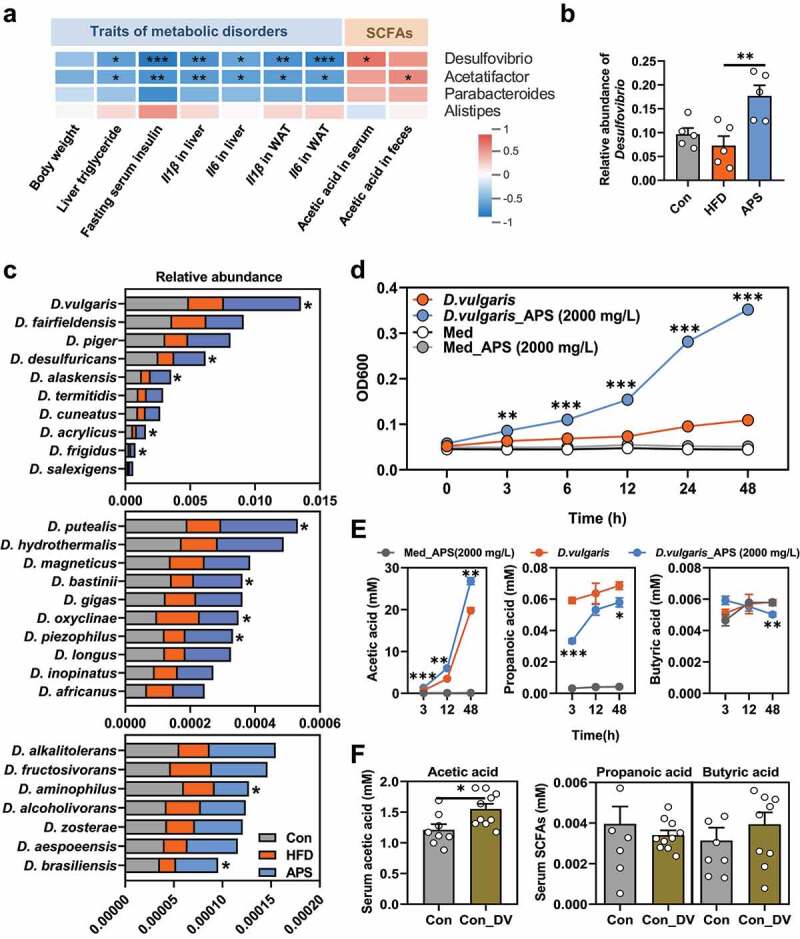
D. vulgaris is a potent generator of acetic acid that is enriched by APS supplementation. (a) Spearman’s correlation analysis between the 4 bacteria genera enriched by APS supplementation and hepatic steatosis traits (including body weight, liver triglyceride level, fasting serum insulin level, Il1β and Il6 gene expression in liver tissues, Il1β and Il6 gene expression in white adipose tissues (WAT), and acetic acid levels in serum and feces tissues. (b) Relative abundance of Desulfovibrio genus from 16S rRNA gene sequencing data in APS supplement expression. (c) Relative abundance of species from Desulfovibrio genus in cecum samples of mice analyzed by using metagenomics sequencing (*p < .05, One-way ANOVA, n = 3). (d) OD600 were measured at the 0, 3rd, 6th, 12th, 24th, and 48th h of anaerobic culturing of D. vulgaris with or without APS (2000 mg/L). (e) Contents of SCFAs (acetic acid, propionic acid, and butyric acid) were measured at the 3rd, 12th, and 48th h of anaerobic culturing of D. vulgaris with or without APS (2000 mg/L, n = 3). (f) Chow diet fed mice were supplemented phosphate buffer solution (PBS) or D. vulgaris for 1 week, and the targeted metabolomics for SCFAs level of serum samples were analyzed by UPLC/TOF-MS (mM). Data are presented as the mean ± s.e.m.; *p < .05, **p < .01, ***p < .001. *p < .05, **p < .01, ***p < .001 vs D. vulgaris at panel d, e
Since D. vulgaris was of the highest abundance among the enriched Desulfovibrio genus and its negative correlation with metabolic disorders, a specific strain of D. vulgaris (D. vulgaris Hildenborough, ATCC 29579) from ATCC was used and tested its capacity for SCFAs generation. Our results showed that D. vulgaris growth was significantly stimulated by APS from 3 to 48 h dose-dependently (p < .001, Figure 5d, Supplementary Figure 7a-b). In addition, we also tested whether the growth of D. vulgaris could be stimulated by other polysaccharides like inulin, a dietary herbal polysaccharide mainly consists of fructose with well-established benefits in metabolic disorders.30,31 Our results showed that inulin addition did not stimulate the growth of D. vulgaris at all even at very high concentration (Supplementary Figure 7c-d). Moreover, we observed that the concentration of acetic acid, but not propionic acid or butyric acid in D. vulgaris medium elevated time-dependently, and was further increased with addition of APS (p < .001, Figure 4e). In agreement with the short-term in vitro data, the in vivo study indicated that D. vulgaris gavage significantly increased the levels of serum acetic acid (p < .05), but not propionic acid or butyric acid (figure 5f, Supplementary Figure 8a-b).
D. vulgaris attenuates hepatic steatosis and its related metabolic disorders
D. vulgaris is a sulfate-reducing bacteria capable of producing hydrogen sulfide (H2S) in the mammalian intestinal tract,32 however, the physiological functions of sulfate-reducing bacteria in the mammalian gut are not well understood. Given its potent capacity for acetic acid production and its enrichment in APS-supplemented mice, we hypothesized that D. vulgaris might have anti-NAFLD effects in HFD mice. To test this hypothesis, male C57BL/6 J mice were maintained on either normal chow diet or HFD with or without D. vulgaris supplementation (DV) by oral gavage for 12 weeks. The results showed that D. vulgaris supplementation significantly reduced body weight gain (p < .05), increased the brown adipose tissue (BAT) index (p < .05), attenuated hepatic steatosis (p < .01) and TG accumulation in hepatocytes (p < .05), reduced serum ALT levels (p < .05), and reduced trend of liver index (p = .08) in HFD-fed mice, whereas no significant differences were found in the context of normal chow diet (p > .05, Figure 6a-g, Supplementary Figure 9). These results indicated that D. vulgaris supplementation attenuated HFD-induced hepatic steatosis and its related metabolic disorders in mice.
Figure 6.
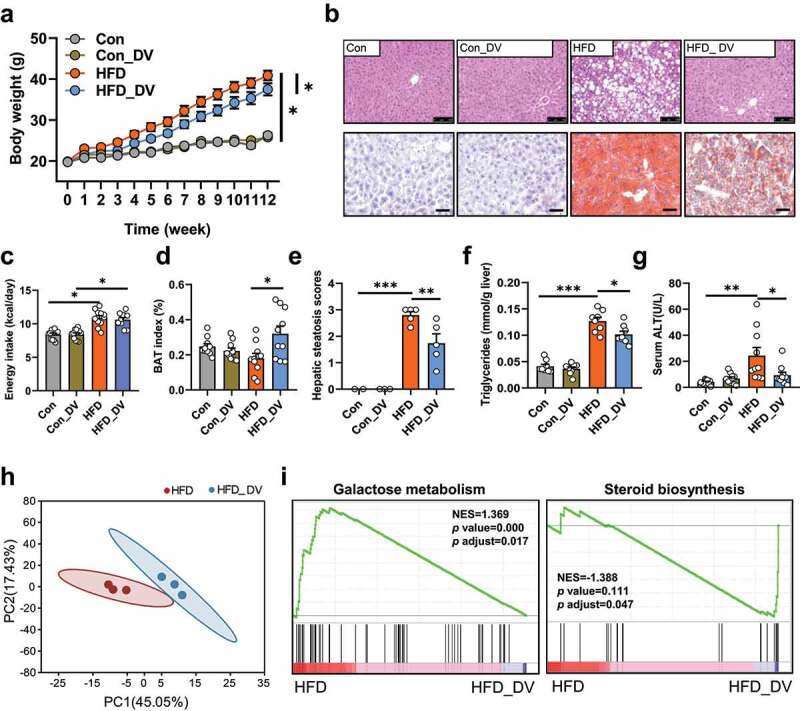
D. vulgaris attenuates hepatic steatosis and reduces body weight gain in HFD mice. Normal chow diet (Con) fed mice and HFD-fed mice were treated with phosphate buffer solution (PBS) or D. vulgaris bacteria liquid (1 × 109 colony-forming units, DV) by oral gavage for 12 weeks (n = 10). (a) Body weight (g). (b) Representative photomicrographs of liver tissues with H&E (magnification, ×200, 50 μm) and oil red O staining (magnification, ×400, 25 μm). (c) Average energy intake per mouse (kcal/day). (d) Brown adipose tissue (BAT) index (%, brown adipose tissue weight (g)/body weight (g) * 100%). (e) Hepatic triglycerides levels (mmol/g liver). (f) Hepatic steatosis scores. (g) Serum ALT level (U/L). RNA-seq was performed on liver tissues in Con or HFD with or without D. vulgaris supplement mice (n = 3). (h) Principal component analysis (PCA) between HFD and HFD_DV groups. (i) GSEA analyses of energy metabolism, carbohydrate metabolism, lipid metabolism, and metabolites biosynthesis between HFD group and HFD_DV group. Positive and negative NES indicate higher and lower expression in HFD group, respectively. Data are presented as the mean ± s.e.m.; *p < .05, **p < .01, ***p < .001
D. vulgaris regulates hepatic gene expression profiles in HFD-fed mice
To further understand the potential mechanism underlying the anti-NAFLD effect of D. vulgaris, the liver gene expression profiles were investigated in mice with or without D. vulgaris treatment in the context of either normal chow diet or HFD. First of all, the principal component analysis (PCA) showed a distinct separation among the 4 groups on PC1 with respect to the types of diets, and subsequent analysis showed that D. vulgaris treatment resulted in a clear separation in gene expression in the context of HFD, but not normal chow diet (Figure 6h, Supplementary Figure 10a). In agreement with our observation on the anti-NAFLD effect of D. vulgaris (Figure 6a-g), our transcriptomic analysis showed a substantial number of altered genes in the HFD_DV group compared to that of HFD group, but not between Con_DV and Con groups (Supplementary Figure 10b-c). Then, we performed gene set enrichment analysis (GSEA) based on all annotated genes to determine which pathways were regulated by D. vulgaris in HFD mice. Totally, there were 29 pathways in energy metabolism, carbohydrate metabolism, lipid metabolism, and metabolites biosynthesis (Supplementary table 6). Among them, galactose metabolism pathway had the highest positive normalized enrichment score (NES), suggesting this pathway was significantly enriched in HFD group (p adjust = 0.017). In addition, steroid biosynthesis pathway had the lowest negative NES, indicating this pathway was significantly enriched in HFD_DV group (p adjust = 0.047, Figure 6i, Supplementary Figure 10d). Moreover, the expression of genes or proteins that are directly involved in metabolic disorders including fatty acid metabolism, inflammation, and intestinal integrity was validated in liver and intestinal tissues. D. vulgaris treatment did not change the expression of genes for intestinal integrity (Ocln, Tjp1), SCFAs receptor (Ffar2), or inflammation (Il1β, Il6, Tnfα, Cd14) in either liver or intestine, as well as histological damage in intestine tissues (Figure 7a-d). Notably, D. vulgaris treatment significantly inhibited the expression of hepatic glucokinase (GCK), CD36, and FASN proteins (p < .05), as well as stimulation on Cpt1α and peroxisome proliferator-activated receptor-α (Pparα) mRNA expression (p < .01, Figure 7a, e), suggesting the anti-NAFLD effect of D. vulgaris was mainly associated with inhibition of fatty acid de novo synthesis and β-oxidation in liver of HFD-fed mice.
Figure 7.
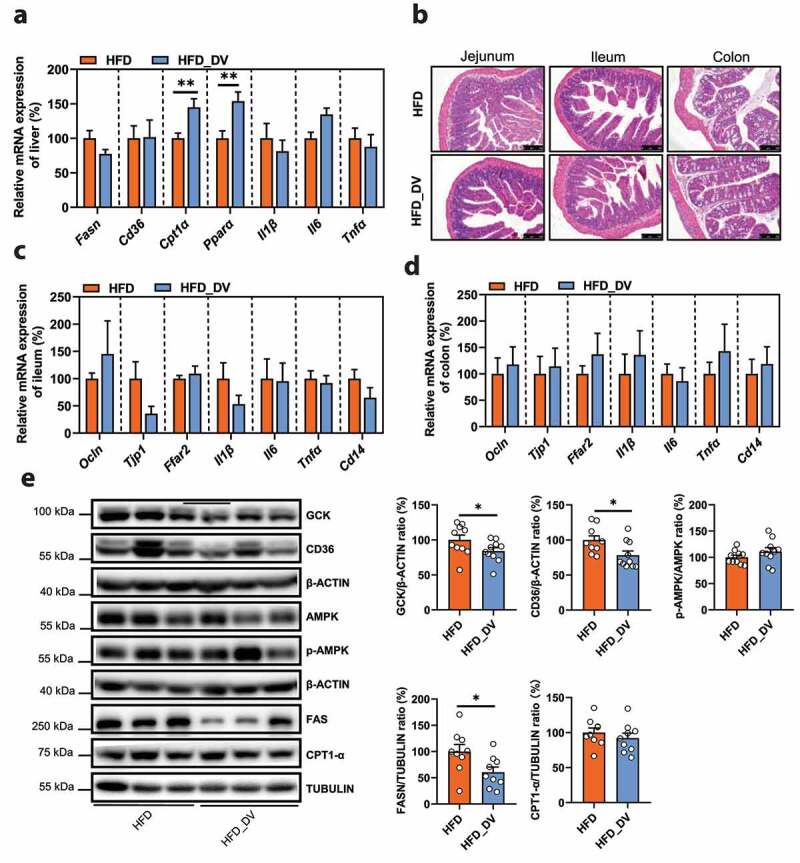
(a) The relative mRNA expression of genes for lipid metabolism (Fasn, Cd36, Cpt1α, Ppar-α) and pro-inflammatory cytokines (Il1β, Il6, Tnfα) in liver tissues (%, n = 10). (b) Representative photomicrographs of jejunum, ileum, and colon tissues with H&E staining (magnification × 200, 50 μm). (c) The relative mRNA expression of genes for intestinal integrity (Ocln, Tjp1), SCFAs receptor (Ffar2), and inflammation (Il1β, Il6, Tnfα, Cd14) in ileum tissues (%, n = 6). (d) The relative mRNA expression of genes for intestinal integrity (Ocln, Tjp1), SCFAs receptor (Ffar2), and inflammation (Il1β, Il6, Tnfα, Cd14) in colon tissues (%, n = 6). (e) The protein expression of GCK, CD36, p-AMPK/AMPK, FASN, and CPT1-α in liver tissues (%, n = 9–10). Data are presented as the mean ± s.e.m.; *p < .05, **p < .01
In summary, our current study revealed a bacterium, D. vulgaris, with potent capacity for acetic acid generation in APS treated mice. A novel role of D. vulgaris was preventing HFD-induced NAFLD formation in mice through inhibiting fatty acid de novo synthesis and stimulating β-oxidation of fatty acid was demonstrated.
Discussion
Although the underlying mechanism is largely unknown, gut dysbiosis has emerged as a central initiator of obesity-related diseases including NAFLD, type 2 diabetes and metabolic syndrome.7,10,33 Increasing evidence indicates that modulation of gut microbiota is a practical and effective way for the prevention or therapy of gut microbiota-related diseases by using probiotics or prebiotics.12,34,35 Some herb-derived polysaccharides also exhibit anti-obesity and -NAFLD effects by modulating gut microbiota such as polysaccharides from Ganoderma lucidum, Hirsutella sinensis or Astragalus membranaceus.9,13,20 Based on the results of our previous study as to the anti-NAFLD effects of APS,20 our current study aimed to identify the key bacterium responsible for attenuating NAFLD by investigating the bacteria that were specifically enriched in APS treated mice. In agreement with our previous report,20 our results further demonstrated that the anti-NAFLD effects of APS was associated with its modulation on gut microbiota resulting in dramatic increase of serum acetic acid in HFD mice. Moreover, by screening the bacteria that were specifically enriched by APS, we identified the relative abundance of D. vulgaris was enriched, which was demonstrated to be a potent generator of acetic acid and was effective for preventing NAFLD formation in HFD-fed mice.
Emerging evidence has demonstrated that the metabolic benefits of some herb-derived polysaccharides are associated with the production of SCFAs (acetic acid, propionic acid and butyric acid) by gut microbiota.36–38 We hypothesized that the anti-NAFLD effects of APS were probably associated with production of SCFAs by gut microbiota. The targeted metabolomics revealed increased SCFAs levels in both feces and serum of APS treated mice with only acetic acid being consistently and highly elevated in both (Figure 3a). In contrast, the latest research has revealed that dietary fructose supplement resulted in enhanced hepatic lipogenesis through microbial fermentation into acetic acid that served as the substrate acetyl-CoA for lipid production.39 These findings provide a new perspective on the relationship between diet, flora and host metabolism in fatty liver and other metabolic diseases. However, studies undertaken so far provide conflicting evidence concerning the role of acetic acid on host metabolism involving lipid synthesis, insulin secretion, hyperphagia and obesity,26,27,40,41 which is supposed to be context-dependent such as mode and location of administration, metabolic and anatomic differences in species, as well as metabolic phenotypes of host.29 Therefore, though our results indicated acetic acid production was stimulated by APS, it was uncertain whether increased acetic acid was accounted for the anti-NAFLD effects of APS in HFD mice. According to previous report,42 acetic acid treatment inhibited the HFD-induced body weight gain and hepatic lipids accumulation through up-regulating expression of hepatic Pparα, Cpt-1, acetyl-CoA oxidase (Aco) and uncoupling protein 2 (Ucp2) genes in mice. In line with the observations in mice42,43 and obese subjects,44 our results demonstrated that dietary supplement of acetic acid significantly prevented the formation of NAFLD and inhibited body weight gain in HFD mice, and inhibited hepatic FASN protein expression, the rate-limiting enzyme for de novo fatty acid synthesis in liver (Figure 4). These results gave supporting evidence for the anti-NAFLD effect of acetic acid and also suggested that increased acetic acid might account for the anti-NAFLD effects of APS. Moreover, we demonstrated that the quantity of microbiota-derived acetic acid was enhanced in the presence of APS and abolished by vancomycin pre-treatment ex vivo, suggesting that bacteria responsible for the fermentation of APS to acetic acid might be vancomycin sensitive.
The relative abundance of bacteria with well-established SCFAs-producing power such as Bacteroides, Roseburia, Akkemansia muciniphia, Streptococcus, and Bifidobacterium were first examined. Nevertheless, the alteration in relative abundance of these SCFAs-producing bacteria was either not consistent between short-term and long-term APS treatment, or not statistically different among groups (data not shown). We then paid attention to the bacteria that were significantly enriched in APS group compared to the HFD group. Desulfovibrio genus drew our attention because of its negative correlation with the traits of metabolic disorders and positive correlation with serum acetic acid levels (Figure 5a). Desulfovibrio bacteria are capable of producing H2S in the mammalian intestinal tract and are usually found in a variety of habitats including soil, intestine and feces of animals, and both salt and fresh water.45–47 In addition, either increased or decreased abundance of Desulfovibrio bacteria was observed in NAFLD animals,48–50 therefore, the exact role of Desulfovibrio bacteria in NAFLD is not clear. Our results revealed Desulfovibrio bacteria were positively correlated with acetic acid levels and negatively correlated with metabolic disorders suggesting Desulfovibrio bacteria were beneficial rather than detrimental bacteria for metabolic disease. Further analysis revealed D. vulgaris species was of the highest abundance within Desulfovibrio genus that was dramatically enriched by APS. Therefore, a specific strain of D. vulgaris species (D. vulgaris Hildenborough, ATCC 29579) was adopted for further functional trial. Interestingly, the growth of D. vulgaris was stimulated by APS dose- and time-dependently, but not by inulin, another type of polysaccharides consisting of fructose with well-established metabolic benefits such as improving insulin sensitivity, attenuating hepatic steatosis and modulating gut dysbiosis.51–53 The difference in affecting D. vulgaris growth between APS and inulin was possibly due to the fact that the compositional monosaccharides and chemical bonds are different between APS and inulin. The former is mainly composed of glucose through alpha-(1-4) linkage,54 while the latter is beta-(1-2)-D-fructosidic linkage.55 The alpha-type bonds in polysaccharides are usually broken down by alpha-amylases. It seems that alpha-amylases is encoded in some species of Desulfovibrio,56 but not inulinases. Therefore, it is possible that D. vulgaris could ferment APS due to the presence of alpha-amylases, but not inulin in our current observation (Supplementary Figure 7c-d). Since the monosaccharides composition of APS consists glucose (70.55%), arabinose (23.39%), galactose (3.61%), rhamnose (1.6%), and xylose (0.84%).20 It was completely different with the monosaccharide composition of inulin, or polysaccharides from Ganoderma lucidum and Hirsutella sinensis which also did not enrich Desulfovibrio in HFD mice,9,13 suggesting the interaction between D. vulgaris and polysaccharides was monosaccharides composition- and chemical bonds-dependent. Moreover, we observed increased contents of acetic acid and propionic acid in the medium of D. vulgaris, while only the concentration of acetic acid was further elevated with the addition of APS, but not propionic acid or butyric acid (Figure 5e). SCFAs (mainly including acetic acid, propionic acid and butyric acid) have tremendous functions on host, including energy source for colonocytes,57,58 as well as modulator of immune system and metabolism.25,26,41,42 The theory of “the acetate switch” describes the ability of bacterial cells to either deplete the acetic acid-producing carbon (acetogenic) sources like glucose and serine, or scavenge environmental acetic acid for survival when carbon sources are scarce.59 A recent study revealed the metabolic process for the degradation of a plant sugar, sulfoquinovose (6-deoxy-6-sulfoglucose) by an anaerobic co-culture of Escherichia coli K-12 and Desulfovibrio sp. Strain DF1 which resulted in the production of acetic acid and H2S.60 Since glucose is the main monosaccharide component of APS we used, and our in vitro experiment demonstrated that glucose also promoted the D. vulgaris growth similar to the effect of APS (Supplementary Figure 11), glucose may serve as the carbon source for D. vulgaris growth and acetic acid production.
Given the fact that either elevated or decreased abundance of Desulfovibrio bacteria in metabolic diseases was reported,61,62 the exact role of bacteria in Desulfovibrio genus like D. vulgaris in metabolic diseases was not clear. In line with the in vitro observation, we demonstrated that oral gavage of D. vulgaris in mice led to significant increase of serum acetic acid, but not propionic acid or butyric acid (figure 5f). Furthermore, our data indicated that D. vulgaris treatment protected C57BL/6 J mice against 12 weeks of HFD-induced substantial metabolic disorders including body weight gain, hepatic steatosis and TG accumulation, whereas no effect was observed in the context of normal chow diet (Figure 6). As a result, our current study indicated that D. vulgaris prevented mice from HFD-induced metabolic disorders. To the best of our knowledge, this is the first report on the anti-NALFD effect of D. vulgaris in mice.
In addition to acetic acid, D. vulgaris, as a sulfate reducing bacteria, is also a producer of H2S. Based on the existing evidence, H2S is regarded as a toxic gas for a long time,63 but also plays critical roles in host metabolism such as glucose and lipid homeostasis, oxidative stress reduction.64–66 Supplement of exogenous H2S in the form of NaHS, a H2S donor, or stimulation of endogenous H2S production from hepatocytes were beneficial for liver injury or chronic liver diseases including NAFLD etc.67–69 In contrast, Bilophila wadsworthia is also a producer of H2S,70 which aggravates metabolic dysfunction in HFD mice through promoting intestinal barrier dysfunction, bile acid dysmetabolism and decreasing butyrate production.71 Unlike the endogenously produced H2S in liver where H2S performs its multiple functions as a gas signaling molecule directly in host metabolism,72,73 the exact role of gut microbiota-derived H2S in host metabolism is poorly understood, except for the local impact on mucosal inflammation and tissue damage in inflammatory bowel disease.74,75 We therefore wondered whether the H2S levels were elevated by D. vulgaris with or without APS addition in vivo or in vitro. Our results first confirmed the capacity of H2S production of D. vulgaris, while APS addition substantially increased H2S content in medium of D. vulgaris in parallel to the stimulation on its growth (Supplementary Figure 12a, Figure 5d). Meanwhile, D. vulgaris treatment resulted in mild increase of H2S in either serum and liver tissue compared to HFD group, but of no statistical difference (Supplementary Figure 12b-c). However, we found that hepatic H2S was increased by HFD feeding compared to Con group, whereas no additional impact was observed in the presence of APS supplement on hepatic H2S level (Supplementary Figure 12d). Therefore, we believe that the anti-NAFLD effect of D. vulgaris in our current study had a weak connection with the capacity of H2S production by D. vulgaris. Nevertheless, further study is needed to determine whether the bacteria-derived acetic acid is critical for the effect of D. vulgaris in HFD-fed mice by knocking down the expression of gene ackA (gene ID: 2796447), encoding rate-limiting enzyme for acetic acid generation in D. vulgaris.
The subsequent transcriptomic analysis revealed that D. vulgaris treatment extensively altered the hepatic gene expression profiles in HFD mice. Energy and glycolipid metabolism-related pathways were significantly modulated by D. vulgaris treatment including galactose metabolism, steroid biosynthesis, nitrogen metabolism, citrate cycle (TCA cycle), starch and sucrose metabolism, sphingolipid metabolism, fructose and mannose metabolism, amino sugar and nucleotide sugar metabolism, butanoate metabolism, and glycerolipid metabolism (NES top 10, Supplementary Table 6). Previously, the interactions between gut microbiome and host lipids metabolism have been extensively investigated where the microbiota-derived acetic acid serves as precursor for fatty acid synthesis in liver and release of glycerophospholipid.76 However, given the diversified functions of acetic acid including as building block for fatty acid synthesis, or signaling molecule of host metabolism through its receptors,77 the altered fatty acid metabolism-related pathways in liver of D. vulgaris-treated mice suggested that bacteria-derived acetic acid might mediate the crosstalk between D. vulgaris and host metabolism. Further analysis indicated that D. vulgaris treatment significantly suppressed the expression of hepatic FASN, CD36 and GCK proteins, as well as upregulating expression of Cpt1α and Pparα mRNA (Figure 7a, e). Liver-specific Gck knockout mice showed decreased hepatic Fasn gene expression, in addition to altered glucose metabolism.78 CD36 is a transmembrane glycoprotein that takes up fatty acid and increased expression of CD36 involves in the development and progression of NAFLD.79 As a result, these data collectively indicated that D. vulgaris treatment prevented mice from HFD-induced NAFLD, which was associated with inhibition of fatty acid de novo synthesis and uptake, and stimulation on fatty acid β-oxidation in liver.
Limitation of the study
First, although the preventive effect of APS against metabolic disorders in HFD-fed mice was demonstrated, we were not sure whether APS could produce satisfactory therapeutic effect on hepatic steatosis, which is of vital significance for the potential application of APS as a prebiotic to treat NAFLD in future. Second, since D. vulgaris is a potent generator of both acetic acid and H2S, it was inconclusive on the exact role of these two important metabolites of D. vulgaris, and further studies are needed to determine the safety of a long-term usage of D. vulgaris, as well as the underlying mechanism. Third, although our current study demonstrated D. vulgaris was beneficial for preventing metabolic disorders, we should be cautious in interpreting these results in respect to its potential application due to the fact that D. vulgaris was not commonly present in human gut, as well as the huge gap of gut microbiome between human and rodents which hinders the clinical translation of bacteria species identified from rodent model. Finally, even within human beings, the taxonomic composition of gut microbiome is highly influenced by ethnicity, or geographic location,80 therefore, more efforts should be paid to the functional role of gut microbiota rather than identification of specific bacterium species in respect to gut microbiota-targeted disease management.
Conclusions
In conclusion, our current study identified a potent acetic acid-producing bacterium, D. vulgaris that was enriched in APS supplemented mice. We demonstrated that D. vulgaris treatment effectively attenuated HFD-induced NAFLD development in mice. This study highlights the novel role of D. vulgaris, a traditional sulfate-reducing bacterium, for improving metabolic disorders. Further studies are warranted to systemically evaluate the long-term impacts of D. vulgaris on host and gut microbiota by taking its capacity of producing both H2S and acetic acid into account.
Materials and methods
Mice
Male C57BL/6 J mice were provided by Shanghai Laboratory Animal Center (Shanghai, China) and housed in a 12-hour light (7 AM to 7 PM) and 12-hour dark (7 PM to 7 AM) cycle. 4-week-old mice were used in the study with free access to water and standard chow diet or HFD (60% fat, D12492, Research Diet). The caloric information of standard chow diet and HFD with or without APS supplement was provided in supplementary table 7. The experiments were conducted under the Guidelines for Animal Experiment of Shanghai University of Traditional Chinese Medicine and the protocol was approved by the institutional Animal Ethics Committee (Approval number: PZSHUTCM19010401, PZSHUTCM19010402, PZSHUTCM19010404, and PZSHUTCM190628018).
The monosaccharides composition of APS and animal intervention
The APS used in our current study was composed of 5 monosaccharides including rhamnose (1.6%), arabinose (23.39%), xylose (0.84%), glucose (70.55%) and galactose (3.61%). The preparation method and monosaccharides composition of APS were analyzed and described in our previous article.20
High-fat feed pellets were first broken to the powder form, next, APS was added in the high-fat feed powder to the finial concentration of 4%, and then mixed well and reshaped them from powder to pellets in the sterile condition. After an accommodation period of 1 week, mice were fed for 12 weeks with chow-diet, or HFD with or without APS supplementation (4% in HFD, n = 10). At the end of the experiment, mice were euthanized with 1% pentobarbital sodium solution by intraperitoneal injection. Serum, cecum contents, and tissue samples were collected, weighed, and immediately frozen in liquid nitrogen and stored at −80°C for further analysis. Additionally, part of the liver tissue and epididymal fat tissue were fixed with 10% neutral formalin.
Co-housing experiment
Mice after 12 weeks from either HFD or APS supplementation (n = 9) groups were subdivided into 4 groups. Mice in co-housing cages were defined as HFD_CoH (n = 5) and APS_CoH (n = 5), respectively, while the 2 sub-groups of mice from HFD (n = 4) or APS (n = 4) groups were maintained in separate cages as usual. Mice from either the HFD or APS group were co-housed and maintained within the same cages for another 8 weeks on HFD with no APS supplementation.81
Sodium acetate treatment in HFD-fed mice
High-fat feed pellets were first broken to the powder form, next, sodium acetate was added in the high-fat feed powder to the finial concentration of 3.7%, and then mixed well and reshaped them from powder to pellets in the sterile condition. After 1-week accommodation, 25 mice were first fed with either chow-diet (n = 7) or HFD (n = 18) for 8 weeks, then, HFD-fed mice were sub-divided into two groups (n = 9) with or without 3.7% sodium acetate supplementation for another 8 weeks.25 After that, all mice were euthanized with 1% pentobarbital sodium solution by intraperitoneal injection. Liver tissues were collected and either fixed in 10% neutral formalin for HE staining or frozen immediately in liquid nitrogen and stored at −80°C for subsequent analysis.
D. vulgaris cultivation and treatment
D. vulgaris (D. vulgaris Hildenborough, ATCC 29579) was grown at 30 °C in modified Baar’s medium for sulfate reducers with fresh anaerobic gas (100% N2). D. vulgaris was centrifuged, enriched, and resuspended in phosphate buffer solution (PBS). After an accommodation period of 1 week, 40 mice were fed with chow-diet or HFD with or without D. vulgaris supplementation (1 × 109 colony-forming units) by oral gavage for 12 weeks (daily for first 4 weeks, 3–4 times per week for next 4 weeks, twice a week for last 4 weeks, n = 10).82 Liver tissues and different intestinal segments were collected and either fixed in 10% neutral formalin separately for HE staining or frozen immediately in liquid nitrogen and stored at −80°C for subsequent analysis.
Gut microbiota analysis
The genomic DNA of gut microbiota was extracted using the QIAamp DNA Mini Kit (51304, QIAGEN) from cecum contents or feces for subsequent 16S rRNA gene sequencing. Raw fastq files of this study were deposited in Sequence Read Archive database (accession number SRP144843, SRP144852, PRJNA527182, and PRJNA615253). Detailed procedures and methods for data analysis of 16S rRNA gene sequencing are provided in the Supplemental Material.
Transcriptomics
The transcriptomics of liver tissue was performed by Shanghai Majorbio Bio-pharm Technology Co.,Ltd (China). The detailed procedure is provided in supplementary methods. The original sequencing data were deposited in Sequence Read Archive database (accession number PRJNA647305).
Statistical analysis
Data are shown as means ± s.e.m unless otherwise noted. Statistical significance of body weight was determined with the unpaired two-tailed Student’s t-test. The statistical significance of hepatic steatosis scores was evaluated with non-parametric the Kruskal-Wallis test followed by the Mann-Whitney U test. The 16S rRNA gene sequencing data were analyzed with Tukey’s honest significant difference post hoc test. Multiple comparisons were performed by using one-way ANOVA followed by Tukey’s honest significant difference post hoc test with SPSS software (20). p < .05 was considered statistically significant. For additional analysis details in the 16S rRNA gene sequencing analysis and liver transcriptomics analysis see supplementary materials.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China [81873059]; National Natural Science Foundation of China [82004016].
Declarations of interests
The authors disclose no conflicts.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Scott AJ, Alexander JL, Merrifield CA, Cunningham D, Jobin C, Brown R, Alverdy J, O’Keefe SJ, Gaskins HR, Teare J, et al. International cancer microbiome consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut. 2019;68(9):1624–20. doi: 10.1136/gutjnl-2019-318556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG.. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:7353642. doi: 10.1155/2016/7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 4.Bennett BJ, De Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes D. Gut microbiota: antidiabetic drug treatment confounds gut dysbiosis associated with type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12(2):61. doi: 10.1038/nrendo.2015.222. [DOI] [PubMed] [Google Scholar]
- 7.Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, Kautzky-Willer A, Paulweber B, Hackl E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8(4):545–556. doi: 10.3920/BM2016.0184. [DOI] [PubMed] [Google Scholar]
- 8.Hoefert B. Über die bakterienbefunde im duodenalsaft von gesunden und kranken. Zschr Klin Med. 1921;92:221–235. [Google Scholar]
- 9.Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6(1):7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwenger KJP, Bolzon CM, Li C, Allard JP. Non-alcoholic fatty liver disease and obesity: the role of the gut bacteria. Eur J Nutr. 2019;58(5):1771–1784. doi: 10.1007/s00394-018-1844-5. [DOI] [PubMed] [Google Scholar]
- 11.Porras D, Nistal E, Martinez-Florez S, Gonzalez-Gallego J, Garcia-Mediavilla MV. Intestinal microbiota S-CS. Modulation in obesity-related non-alcoholic fatty liver disease. Front Physiol. 2018;9:1813. doi: 10.3389/fphys.2018.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clement K. Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology. 2020;158(7):1881–1898. doi: 10.1053/j.gastro.2020.01.049. [DOI] [PubMed] [Google Scholar]
- 13.Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu CC, Young JD, Lai HC. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68(2):248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 14.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 2019;30(4):675–88 e7. doi: 10.1016/j.cmet.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Cortes G, Borrell N, De Astorza B, Gomez C, Sauleda J, Alberti S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun. 2002;70(5):2583–2590. doi: 10.1128/Iai.70.5.2583-2590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng XJ, Zhao AH, Xie GX, Chi Y, Zhao LJ, Li HK, Wang CR, Bao YQ, Jia WP, Luther M, et al. Melamine-induced renal toxicity is mediated by the gut microbiota. Sci Transl Med. 2013;5(172):ARTN 172ra22. doi: 10.1126/scitranslmed.3005114. [DOI] [PubMed] [Google Scholar]
- 17.Zou F, Mao XQ, Wang N, Liu J, Ou-Yang JP. Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta Pharmacol Sin. 2009;30(12):1607–1615. doi: 10.1038/aps.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke B, Ke X, Wan X, Yang Y, Huang Y, Qin J, Hu C, Shi L. Astragalus polysaccharides attenuates TNF-alpha-induced insulin resistance via suppression of miR-721 and activation of PPAR-gamma and PI3K/AKT in 3T3-L1 adipocytes. Am J Transl Res. 2017;9:2195–2206. [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YC, Tsay HJ, Lu MK, Lin CH, Yeh CW, Liu HK, Shiao YJ. Astragalus membranaceus-Polysaccharides Ameliorates obesity, Hepatic steatosis, Neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. Int J Mol Sci. 2017;18(12):18. doi: 10.3390/ijms18122746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong Y, Li B, Zheng N, Wu G, Ma J, Tao X, Chen L, Zhong J, Sheng L, Li H. Integrated metagenomic and metabolomic analyses of the effect of astragalus polysaccharides on alleviating high-fat diet–induced metabolic disorders. Front Pharmacol. 2020:11. doi: 10.3389/fphar.2020.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Fei Y, Liu L, Xiao Y, Pang Y, Kang J, Wang Z. Polygonatum odoratum polysaccharides modulate gut microbiota and mitigate experimentally induced obesity in rats. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19113587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin HV, Frassetto A, Kowalik EJ Jr., Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PloS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, He M, Xiao H, Liu X, Wang K, Zhang Y. Acetic acid influences BRL-3A cell lipid metabolism via the AMPK signalling pathway. Cell Physiol Biochem. 2018;45(5):2021–2030. doi: 10.1159/000487980. [DOI] [PubMed] [Google Scholar]
- 27.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Zhang P, Luo J, Shen L, Zhang S, Gu H, He J, Wang L, Zhao X, Gan M, et al. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes. 2021;13(1):1–19. doi: 10.1080/19490976.2020.1862612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canfora EE, Blaak EE. Acetate: a diet-derived key metabolite in energy metabolism: good or bad in context of obesity and glucose homeostasis? Curr Opin Clin Nutr Metab Care. 2017;20(6):477–483. doi: 10.1097/MCO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 30.Chambers ES, Byrne CS, Morrison DJ, Murphy KG, Preston T, Tedford C, Garcia-Perez I, Fountana S, Serrano-Contreras JI, Holmes E, et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019;68(8):1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar SA, Ward LC, Brown L. Inulin oligofructose attenuates metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Br J Nutr. 2016;116(9):1502–1511. doi: 10.1017/S0007114516003627. [DOI] [PubMed] [Google Scholar]
- 32.Kovac J, Vitezova M, Kushkevych I. Metabolic activity of sulfate-reducing bacteria from rodents with colitis. Open Med (Wars). 2018;13(1):344–349. doi: 10.1515/med-2018-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Oca P, Robles-Vera I, Sanchez-Roncero A, Escriva F, Perez-Vizcaino F, Duarte J, Alvarez C, Fernandez-Millan E. Gut DYSBIOSIS and altered barrier function precedes the appearance of metabolic syndrome in a rat model of nutrient-induced catch-up growth. J Nutr Biochem. 2020;81:108383. doi: 10.1016/j.jnutbio.2020.108383. [DOI] [PubMed] [Google Scholar]
- 34.Suk KT, Kim DJ. Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13(3):193–204. doi: 10.1080/17474124.2019.1569513. [DOI] [PubMed] [Google Scholar]
- 35.Xue L, He J, Gao N, Lu X, Li M, Wu X, Liu Z, Jin Y, Liu J, Xu J, et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. 2017;7(1):45176. doi: 10.1038/srep45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Du P, Cheng Y, Guo Y, Hu B, Yao W, Zhu X, Qian H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr Polym. 2021;256:117571. doi: 10.1016/j.carbpol.2020.117571. [DOI] [PubMed] [Google Scholar]
- 37.Sang T, Guo C, Guo D, Wu J, Wang Y, Wang Y, Chen J, Chen C, Wu K, Na K, et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr Polym. 2021;256:117594. doi: 10.1016/j.carbpol.2020.117594. [DOI] [PubMed] [Google Scholar]
- 38.Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, Nie S, Xie M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr Polym. 2020;235:115957. doi: 10.1016/j.carbpol.2020.115957. [DOI] [PubMed] [Google Scholar]
- 39.Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, Zeng X, Trefely S, Fernandez S, Carrer A, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579(7800):586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med. 2015;21(2):173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 41.Den Besten G, Lange K, Havinga R, Van Dijk TH, Gerding A, Van Eunen K, Muller M, Groen AK, Hooiveld GJ, Bakker BM, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G900–10. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- 42.Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem. 2009;57(13):5982–5986. doi: 10.1021/jf900470c. [DOI] [PubMed] [Google Scholar]
- 43.Den Besten G, Bleeker A, Gerding A, Van Eunen K, Havinga R, Van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPAR-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 44.Lim J, Henry CJ, Haldar S. Vinegar as a functional ingredient to improve postprandial glycemic control-human intervention findings and molecular mechanisms. Mol Nutr Food Res. 2016;60(8):1837–1849. doi: 10.1002/mnfr.201600121. [DOI] [PubMed] [Google Scholar]
- 45.Hagiya H, Kimura K, Nishi I, Yamamoto N, Yoshida H, Akeda Y, Tomono K. Desulfovibrio desulfuricans bacteremia: a case report and literature review. Anaerobe. 2018; 49:112–115. doi: 10.1016/j.anaerobe.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Dzierzewicz Z, Cwalina B, Gawlik B, Wilczok T, Gonciarz Z. Isolation and evaluation of susceptibility to sulphasalazine of Desulfovibrio desulfuricans strains from the human digestive tract. Acta Microbiol Pol. 1997;46:175–187. [PubMed] [Google Scholar]
- 47.Dzierzewicz Z, Cwalina B, Kurkiewicz S, Chodurek E, Wilczok T. Intraspecies variability of cellular fatty acids among soil and intestinal strains of Desulfovibrio desulfuricans. Appl Environ Microbiol. 1996;62(9):3360–3365. doi: 10.1128/AEM.62.9.3360-3365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Z, Chen L, Zhao Y, Wang C, Duan C, Yang G, Niu C, Li S. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol. 2020;104:5273–5282. doi: 10.1007/s00253-020-10633-9. [DOI] [PubMed] [Google Scholar]
- 49.Petrov PD, Garcia-Mediavilla MV, Guzman C, Porras D, Nistal E, Martinez-Florez S, Castell JV, Gonzalez-Gallego J, Sanchez-Campos S, Jover R, et al. Gut microbiota, circulating bile acids, and hepatic metabolism genes that protects against non-alcoholic fatty liver disease. Mol Nutr Food Res. 2019;63(20):e1900487. doi: 10.1002/mnfr.201900487. [DOI] [PubMed] [Google Scholar]
- 50.Zhao W, Xiao M, Yang J, Zhang L, Ba Y, Xu R, Liu Z, Zou H, Yu P, Wu X, et al. The combination of Ilexhainanoside D and ilexsaponin A1 reduces liver inflammation and improves intestinal barrier function in mice with high-fat diet-induced non-alcoholic fatty liver disease. Phytomedicine. 2019;63:153039. doi: 10.1016/j.phymed.2019.153039. [DOI] [PubMed] [Google Scholar]
- 51.Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, Theis S, Verbeke K, Raes J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968–1974. doi: 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao M, Gao C, Xu L, Jiang L, Zhu J, Chen G, Law BYK, Xu Y. Effect of Inulin-Type carbohydrates on insulin resistance in patients with Type 2 diabetes and obesity: a systematic review and meta-analysis. J Diabetes Res. 2019;2019:5101423. doi: 10.1155/2019/5101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weitkunat K, Stuhlmann C, Postel A, Rumberger S, Fankhanel M, Woting A, Petzke KJ, Gohlke S, Schulz TJ, Blaut M, et al. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci Rep. 2017;7(1):6109. doi: 10.1038/s41598-017-06447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Y, Ren W, Zhang L, Zhang Y, Liu D, Liu YA. Review of the pharmacological action of astragalus polysaccharide. Front Pharmacol. 2020;11:349. doi: 10.3389/fphar.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosak T, Schubotz F, De Santiago-torio A, Kuehl JV, Carlson HK, Watson N, Daye M, Summons RE, Arkin AP, Deutschbauer AM. System-wide adaptations of Desulfovibrio alaskensis G20 to phosphate-limited conditions. PLoS One. 2016;11(12):e0168719. doi: 10.1371/journal.pone.0168719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl. 1996;216(sup216):132–148. doi: 10.3109/00365529609094568. [DOI] [PubMed] [Google Scholar]
- 58.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 59.Wolfe AJ. The acetate switch. Microbiol Mol Biol Rev: MMBR. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burrichter A, Denger K, Franchini P, Huhn T, Muller N, Spiteller D, Schleheck D. Anaerobic degradation of the plant sugar Sulfoquinovose concomitant with H2S production: escherichia coli K-12 and Desulfovibrio sp. strain DF1 as co-culture model. Front Microbiol. 2018;9:2792. doi: 10.3389/fmicb.2018.02792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, et al. T cell-mediated regulation of the microbiota protects against obesity. Science. 2019;365(6451):365. doi: 10.1126/science.aat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiao X, Wang Y, Lin Y, Lang Y, Li E, Zhang X, Zhang Q, Feng Y, Meng X, Li B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem. 2019;64:88–100. doi: 10.1016/j.jnutbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Mishanina AV, Libiad M, Banerjee R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat Chem Biol. 2015;11(7):457–464. doi: 10.1038/nchembio.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo ZL, Tang LJ, Wang T, Dai RW, Ren JD, Cheng L, Xiang K, Tian FZ. Effects of treatment with hydrogen sulfide on methionine-choline deficient diet-induced non-alcoholic steatohepatitis in rats. J Gastroenterol Hepatol. 2014;29(1):215–222. doi: 10.1111/jgh.12389. [DOI] [PubMed] [Google Scholar]
- 65.Wu D, Zheng N, Qi K, Cheng H, Sun Z, Gao B, Zhang Y, Pang W, Huangfu C, Ji S, et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med Gas Res. 2015;5(1):1. doi: 10.1186/s13618-014-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carter RN, Morton NM. Cysteine and hydrogen sulphide in the regulation of metabolism: insights from genetics and pharmacology. J Pathol. 2016;238(2):321–332. doi: 10.1002/path.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sutti S, Locatelli I, Bruzzi S, Jindal A, Vacchiano M, Bozzola C, Albano E. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin Sci (Lond). 2015;129(9):797–808. doi: 10.1042/CS20150053. [DOI] [PubMed] [Google Scholar]
- 68.Sun L, Zhang S, Yu CY, Pan ZW, Liu Y, Zhao J, Wang XY, Yun FX, Zhao HW, Yan S, et al. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am J Physiol-Endocrinol Metabol. 2015;309(11):E925–E35. doi: 10.1152/ajpendo.00294.2015. [DOI] [PubMed] [Google Scholar]
- 69.Shirozu K, Tokuda K, Marutani E, Lefer D, Wang R, Ichinose F. Cystathionine gamma-lyase deficiency protects mice from galactosamine/lipopolysaccharide-induced acute liver failure. Antioxid Redox Signal. 2014;20(2):204–216. doi: 10.1089/ars.2013.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peck SC, Denger K, Burrichter A, Irwin SM, Balskus EP, Schleheck D. A glycyl radical enzyme enables hydrogen sulfide production by the human intestinal bacterium Bilophila wadsworthia. Proc Natl Acad Sci U S A. 2019;116(8):3171–3176. doi: 10.1073/pnas.1815661116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Natividad JM, Lamas B, Pham HP, Michel ML, Rainteau D, Bridonneau C, Da Costa G, Van Hylckama Vlieg J, Sovran B, Chamignon C, et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun. 2018;9(1):2802. doi: 10.1038/s41467-018-05249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mani S, Cao W, Wu LY, Wang R. Hydrogen sulfide and the liver. Nitric Oxide-Biology and Chemistry. 2014;41:62–71. doi: 10.1016/j.niox.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Norris EJ, Culberson CR, Narasimhan S, Clemens MG. The liver as a central regulator of hydrogen sulfide. Shock. 2011;36(3):242–250. doi: 10.1097/SHK.0b013e3182252ee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Figliuolo VR, Dos Santos LM, Abalo A, Nanini H, Santos A, Brittes NM, Bernardazzi C, De Souza HSP, Vieira LQ, Coutinho-Silva R, et al. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis. Life Sci. 2017;189:29–38. doi: 10.1016/j.lfs.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Dordevic D, Jancikova S, Vitezova M, Kushkevych I. Hydrogen sulfide toxicity in the gut environment: meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J Adv Res. 2021;27:55–69. doi: 10.1016/j.jare.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kindt A, Liebisch G, Clavel T, Haller D, Hormannsperger G, Yoon H, Kolmeder D, Sigruener A, Krautbauer S, Seeliger C, et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat Commun. 2018;9(1):3760. doi: 10.1038/s41467-018-05767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 78.Hayashi H, Sato Y, Li Z, Yamamura K, Yoshizawa T, Yamagata K. Roles of hepatic glucokinase in intertissue metabolic communication: examination of novel liver-specific glucokinase knockout mice. Biochem Biophys Res Commun. 2015;460(3):727–732. doi: 10.1016/j.bbrc.2015.03.097. [DOI] [PubMed] [Google Scholar]
- 79.He J, Lee JH, Febbraio M, Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med (Maywood). 2011;236(10):1116–1121. doi: 10.1258/ebm.2011.011128. [DOI] [PubMed] [Google Scholar]
- 80.gGaulke CA, Sharpton TJ. The influence of ethnicity and geography on human gut microbiome composition. Nat Med. 2018;24(10):1495–1496. doi: 10.1038/s41591-018-0210-8. [DOI] [PubMed] [Google Scholar]
- 81.Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10(1):104–116. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- 82.Huang K, Liu Y, Tang H, Qiu M, Li C, Duan C, Wang C, Yang J, Zhou X. Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front Pharmacol. 2019;10:107. doi: 10.3389/fphar.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


