Abstract
Introduction
Recruitment and retention are two of the most important factors in successfully running clinical trials. Many trials encounter problems with both, causing delays or preventing study progress. These issues are greater in older adults and patients with cancer.
Materials and methods
We assessed recruitment and retention in a large, multicentre, observational breast cancer study in older female patients (>70 years, N = 3440). Data collected by the Age Gap study were used to assess rates of, and reasons for, patients not being recruited or retained. Statistical analysis assessed the impact of age as a predictor of recruitment and retention.
Results
Between February 2013 and June 2018, 6876 patients were screened and 3456 were consented across 56 United Kingdom (UK) breast units. Reasons for non-recruitment included ineligibility, clinician issues, staffing resource issues, patients' lack of interest or time and trial burden. In comparison with the age demographics of patients with breast cancer in the UK, women aged 70–75 years were over-represented compared to older age groups. Logistic regression demonstrated that older age significantly reduced the odds of consent (OR = 0.96, CI: 0.938–0.982; p < 0.001). Multivariate analysis showed that age (p < 0.001), markers of poor functional ability (Eastern Cooperative Oncology Group Performance Status (p = 0.011)) and instrumental activities of daily living (p = 0.026) were significant predictors of withdrawal.
Discussion
This study has demonstrated that selection and attrition bias for age are apparent despite a range of ‘age friendly’ study design measures. Exploration of the underlying reasons for this and development of measures to address this should be the focus of further research.
Keywords: Breast cancer, Clinical trial, Recruitment, Retention, Age distribution, Age bias
1. Introduction
Recruitment is the greatest practical problem in clinical trials. Less than a third of publicly funded trials meet their recruitment targets and over half require an extension [1]. Recruiting older patients is particularly challenging [[2], [3], [4], [5], [6]] but due to increased interest in representative trial data [[7], [8], [9], [10]], effective ways of recruiting older patients are needed. This is increasingly important with aging global populations [11].
Evidence suggests that, if approached, older patients are as likely to participate in research as younger patients [12]. Older patients have similar attitudes towards enrolment, stating motivators such as altruism and hope for better treatments [13]. Despite this, older patients with cancer are less likely to be offered a trial than younger patients [[4], [5], [6],[13], [14], [15]]. Difficulties in recruitment and retention of older participants are often caused by strict eligibility criteria [3], such as age and co-morbidity restrictions. Trialists have concerns that older patients will have higher rates of treatment morbidity and other causes of mortality, which may undermine any gains from new treatments. Co-morbidity, concurrent medications and organ function impairment are often defined as exclusion criteria which introduces age bias, as older patients have higher rates of organ function impairment [16,17]. Other issues include lower levels of literacy and numeracy, increased prevalence of dementia and altered preferences for information provision (older patients are less keen on online materials, complex percentages and graphic displays [18]). Pragmatic trial design and minimally invasive recruitment and data collection methods may help overcome this [3,9,10]. Another factor is health care professionals' attitudes to recruitment, with clinicians wishing to avoid burdening older patients, concerns about their fitness for trial interventions or feeling they are unable to understand trial processes [12].
Attrition through patient withdrawal and loss to follow-up can also have a negative effect on trial outcomes [19]. It is important that clinical trials represent the populations treatments are designed to be used in [7] and if attrition is weighted towards certain characteristics, attrition bias may result [6,20]. In addition, findings from selected subpopulations may not extrapolate to the real world [21,22].
Similar problems arise where recruited participants do not complete the study which is a particular problem in patients with poor functional status and dementia [23]. Patient health deterioration or patient choice are often cited as reasons for discontinuation amongst older age groups [24]. Older patients may also have mobility issues and struggle to attend appointments. Lengthy follow-up schedules may also see patients become too frail to maintain engagement.
Accurate recording of reasons for non-recruitment or retention, which should be an integral component of good quality research [25], is often inadequately reported. This limits our understanding of remediable issues.
The Age Gap study was designed to recruit older patients with cancer [26]. It was a multicentre, observational study specifically recruiting women over the age of 70 with operable breast cancer. The trial assessed the oncological impacts of breast cancer treatments when adjusted for baseline variations in fitness, age and frailty using propensity score matching. The trial was designed to enhance recruitment and retention using a range of strategies suggested following the premature closure of previous older age specific randomised trials due to poor recruitment [27]. These included an observational design with propensity score matched adjustment of baseline variables instead of randomisation, use of enhanced, age appropriate, patient friendly trial literature, an inclusive recruitment strategy and varying levels of participation to suit a patient's wishes.
The aim of this work was to determine the impact of age and health status on recruitment and retention within the Age Gap study.
2. Materials and methods
2.1. Aims
To determine the impact of age and health status on recruitment and retention to a large prospective observational cohort study in a population of older women with operable breast cancer (the Age Gap study cohort). In addition, the pattern of recruitment was compared to the age demography of the wider UK population of women with breast cancer, (derived from the UK Cancer Registry website from 2015 to 2017, which was within the Age Gap study recruitment period from 2013 to 2018). Details of the parent study are given below followed by details of this planned subsidiary analysis.
2.2. Regulatory approval
Ethics approval and research governance approval was obtained (IRAS: 12 LO 1808).
2.3. Study design
A prospective, multicentre, observational cohort study. Participation at three levels: full participation, partial (no requirement to complete quality-of-life assessments) or by proxy (minimal data collection for women with dementia). Methodology for the main Age Gap study has been previously published [26] and is briefly summarised below.
3. Sites
56 UK breast units (Table ST1).
Inclusion criteria:
-
1.
Female, aged ≥70, with operable, invasive breast cancer with no upper limit for age or health status.
-
2.
Ability to provide informed consent or have a proxy give consent if cognitively impaired.
Exclusion criteria:
-
1.
Previous invasive breast cancer within five years
3.1. Screening data collection
At various stages of the recruitment process data were collected about potential participants and why they did not progress to the next stage of recruitment. Stages were defined as:
-
•
Screening: Remote case review in multidisciplinary team meetings (MDTs) or clinic
-
•
Approach: Patient or caregiver contacted to assess initial interest or not
-
•
Eligibility Assessment: Detailed review of eligibility criteria
-
•
Consent: Signed consent obtained.
At each stage details of patient age and ethnicity were recorded along with whether they were approached about the study (and reasons if not) and their interest in being involved in the study (and reasons if not) using a specially developed case report form with closed and open questions relating to participation or not, and reasons for withdrawal.
3.2. Baseline data collection
The following data were collected at baseline:
-
•
Charlson co-morbidity index, (CCI) [28]
-
•
Activities of Daily Living (ADL) [29]
-
•
Instrumental Activities of Daily Living (IADL) [30]
-
•
Mini Mental State Examination (MMSE) [31]
-
•
Eastern Cooperative Oncology Group Performance Status (ECOG-PS)
-
•
Quality-of-life using a range of validated questionnaires [32,33].
-
•
Tumour characteristics (grade, stage, receptor status)
-
•
Cancer treatment details.
3.3. Follow-up and outcomes
Participants were followed-up at 6 weeks and 6, 12, 18 and 24 months. Pragmatic flexibility was allowed around visit timing: ±2 weeks for the 6 week visit and ± 1 month for all other study visits. Data on patient screening, recruitment and discontinuation from the study were collected using a mixture of closed and open questions to determine reasons for withdrawal. Discontinuation of participation was due to death, voluntary withdrawal (patient/caregiver/Health Care Professional (HCP) decision and reason), involuntary withdrawal (inability to comply and reason) and loss to follow-up. Reporting of methods was compliant with STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [34].
3.4. Research engagement, recruitment and retention optimisation strategies
The Age Gap study was designed to be ‘older patient friendly’ with multiple features to enhance uptake and retention including:
-
•
Observational cohort design with propensity score matching to adjust for allocation bias rather than randomisation (previous randomised trials, including those conducted by our group [27], in this age demographic have failed to recruit)
-
•
No eligibility limits for comorbidity, frailty, or cognitive impairment. Women with cognitive impairment were permitted to be consented by proxy
-
•
Flexible data collection time points
-
•
Availability of telephone and postal follow-up to optimise for older patients [3]
-
•
Making elements of the trial optional such as quality-of-life form completion
-
•
Extensive user involvement in trial design to optimise for older patients
-
•
Direct follow-up limited to two years with longer-term follow-up via the UK cancer registry.
3.5. Statistical analysis
Data analyses were performed using Excel and SPSS (Version 25). P-values of <0.05 were considered significant. Medians and ranges were used to report age due to the skewed age range [35]. Mann-Whitney U tests were used to determine whether eligibility, consent and withdrawal were associated with patient characteristics. Age was categorised for the purposes of graphical displays but treated as continuous in analyses.
Multivariable logistic regression was used to determine the effect of age, participation level, ECOG-PS, cognitive capacity (MMSE, categorised as normal/mild/moderate/severe impairment) and functional capacity (ADL and IADL) on the likelihood of trial withdrawal.
For comparison of population sampling relative to the wider UK population of women with breast cancer, age specific incidence data from the Office for National Statistics (derived for 2015–2017) was compared with age strata from the Age Gap study (2013–2018).
4. Results
4.1. Screened to recruited ratios and reasons for non-recruitment
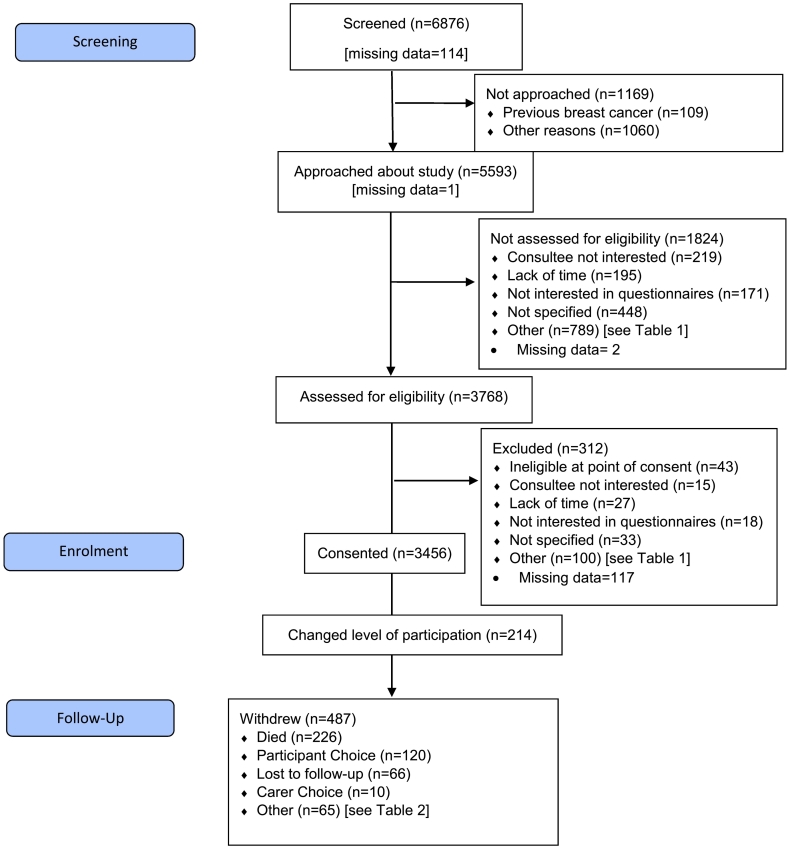
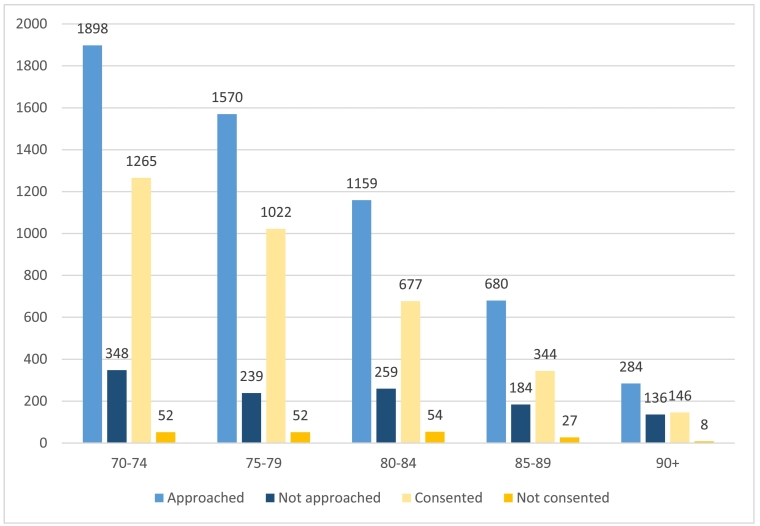
The study was recruited between February 2013 and June 2018. Patient flows through screening and recruitment are illustrated in Fig. 1, alongside reasons for not approaching (Table 1a), not formally assessing eligibility (Table 1b), not consenting (Table 1c) and for discontinuation (Table 2). The median age of women screened (77, range 70–102) was younger than those not screened (79, range 69–101; p < 0.001, Fig. 2).
Fig. 1.
Flow Diagram showing patient numbers at each stage of trial recruitment and follow-up.
Table 1.
Reasons patient were not screened for eligibility, not eligibility assessed or consented.
| Reason patient not screened | Count (%) [N = 1169] |
|---|---|
| Previous breast cancer and therefore ineligible | 109 (9.3) |
| Other | 1060 (90.7) |
| Already started treatment | 279 (23.9) |
| Clinical decision not to approach | 109 (9.3) |
| Co-morbidities | 35 (3.0) |
| Ineligible (other than started treatment) | 354 (30.3) |
| Research Staffing/Resource issues | 115 (9.8) |
| Treatment at another hospital/declined treatment | 47 (4.0) |
| Contact issues | 15 (1.3) |
| Could not be contacted | 9 (0.8) |
| Did not attend appointment | 6 (0.5) |
| Lack of consultee for patient with dementia | 34 (2.9) |
| Language barrier | 12 (1.0) |
| Not interested | 12 (1.0) |
| Trial enrolment would be ‘Too much’ for them | 33 (2.8) |
| Patient died | 2 (0.2) |
| No reason | 5 (0.4) |
| Unable to categorise | 8 (0.7) |
| Reason patient not assessed for eligibility | Count (%) [N = 1824] |
| Consultee not interested | 219 (12.0) |
| Lack of Time | 195 (10.7) |
| Not interested in questionnaires | 171 (9.4) |
| Not specified | 448 (24.6) |
| Other: | 789 (43.3) |
| Already started treatment | 176 (9.6) |
| Co-morbidities: | 35 (1.9) |
| Visual impairment | 4 (0.2) |
| Frailty or ‘Age’ | 5 (0.3) |
| Other co-morbidities | 26 (1.4) |
| Ineligible (other than started treatment) | 60 (3.3) |
| Staffing/Resource issue | 21 (1.2) |
| Treatment at another hospital/declined treatment | 92 (5.0) |
| Private care | 8 (0.4) |
| Treatment at another hospital | 74 (4.1) |
| GP care only | 1 (0.1) |
| No treatment/follow-up | 9 (0.5) |
| Declined | 126 (6.9) |
| Unable to contact patient | 43 (2.4) |
| Patient declined | 83 (4.6) |
| Trial Burden | 247 (13.5) |
| Too much | 232 (12.7) |
| Transport issues | 8 (0.4) |
| Acts as a carer | 7 (0.4) |
| Could not be consented due to lack of consultee | 10 (0.5) |
| Could not be consented due to language barrier | 4 (0.2) |
| Unable to categorise | 20 (1.1) |
| Reason patient not consented, (where given, more than one reason possible) | Count (%) [N = 193] |
| Consultee not interested | 15 (7.8) |
| Lack of Time | 27 (14.0) |
| Not interested in questionnaires | 18 (9.3) |
| Not specified | 33 (17.1) |
| Other: | 100 (51.8) |
| Trial burden | 58 (30.1) |
| Too much | 55 (28.5) |
| Transport | 3 (1.6) |
| Already started treatment | 14 (7.3) |
| Staffing/Resource issue | 8 (4.1) |
| Not Interested in trial | 10 (5.2) |
| Treatment at another hospital/declined treatment | 3 (1.6) |
| Private hospital | 2 (1.0) |
| Other hospital | 1 (0.5) |
| Co-morbidities | 2 (1.0) |
| More time needed to consider trial | 1 (0.5) |
| Language barrier | 1 (0.5) |
| Did not attend appointment | 1 (0.5) |
| Unable to categorise | 2 (1.0) |
Table 2.
Reasons patients did not complete the study.
| Reason for discontinuation | Count (%) [N = 487] |
|---|---|
| Patient died | 226 (46.4) |
| Breast cancer recorded as primary cause | 91 (19) |
| Breast cancer not recorded as primary cause | 123 (25) |
| Cause of death uncertain | 12 (2) |
| Participant choice | 113 (23) |
| No longer feels able to take part due to health reasons | 41 (8.4) |
| Questionnaires too difficult | 4 (0.8) |
| Questionnaires too time consuming | 11 (2.3) |
| Other | 25 (5.1) |
| No reason given | 32 (6.6) |
| Lost to follow-up | 66 (14) |
| Carer choice | 10 (2.1) |
| No longer feels able to take part due to health reasons | 7 (1.4) |
| Carer feels the study is too difficult/intrusive | 1 (0.2) |
| Other | 2 (0.4) |
| Other⁎ | 65 (13) |
| Ineligible | 20 (4.1) |
| Co-morbidities | 9 (1.8) |
| Staffing/Resource issue | 24 (4.9) |
| Patient moved | 12 (2.5) |
Initially 83 ‘other’ records were identified however 18 were categorised into the given options.
Fig. 2.
Graph to illustrate the number of patients screened for entry (total number is patients presenting at site) or consented (total number is patients who are eligible) by age category.
A total of 5593 women were approached about the study, of whom 3768 had a formal eligibility assessment (67%) and of these 3456 (92%) were consented. An additional sixteen patients were found to be ineligible after consent when baseline data was collected, leaving 3440/3768 (91%) fully eligible women. The median age of women consented was younger than those not consented (77, range 70–102 versus 79, range 70–95; p < 0.001) (Fig. 2).
Of the 3440 participants, 214 (6%) changed their level of participation. This included 209/214 (98%) participants who dropped from full to partial participation and 5/214 (2%) who changed from partial to data collection only. In total 487/3440 (14%) participants discontinued the study for a variety of reasons (Table 2).
In terms of age and comorbidity related exclusions, co-morbidities accounted for 35/1824 (1.9%) patients not screened, of these only 5/1824 (0.3%) were due to frailty/age (Table 1b). Trial burden was reported by 247/1824 (13.5%) patients as the reason they were not interested in the trial, 232/1824 (12.7%) stating the trial was too much for them, 8/1824 (0.4%) reporting transport tissues and 7/1824 (0.4%) due to their responsibilities as a caregiver. A lack of appropriate consultees was reported for 10/1824 (0.5%) patients with cognitive impairment. Decline of the trial was reported for 126/1824 (6.9%) patients with 83/1824 (4.6%) being direct decline by patient and 43/1824 (2.4%) where the patient could not be contacted. Of the patients that declined, one patient did not wish to have their information reviewed and several commented that the photo on the patient information sheet ‘looked too old’ and they therefore didn't associate themselves with the trial's age group (the photograph was subsequently changed). Five patients (0.3%) were not happy to be assessed for the trial due to frailty or age, 4 of these reporting that they felt they were too old, representing only 0.3% of those not assessed for eligibility.
Of the patients who were assessed for eligibility and formally invited to take part, 193/3768 (5%) did not consent, the most common reason was trial burden reported by 58/193 (30.1%) patients (Table 1c) such as completion of sometimes lengthy questionnaires. Lack of consultant interest was cited as a reason for not assessing eligibility in 219 of 1824 (12%).
4.2. Trial discontinuation
A total of 487/3456 participants (14%) did not complete the study, of these 226/487 (46.4%) died while on study, 120/487 (24.6%) withdrew themselves, 66/487 (13.6%) were lost to follow-up, 10/487 (2.1%) participants were withdrawn by their carer and 65/487 (13.3%) participants did not complete for other reasons. Only 9/487 (1.8%) participants withdrew due to comorbidities, although a larger number stated they were withdrawing for health reasons (41/487, 8.4%). Other reasons included finding the questionnaires too time consuming (11/487, 0.8%), or too difficult (4/487, 0.8%). Of the participants that were withdrawn by their caregiver 7/487 (1.4%) felt the participant could not continue due to health problems. Only 1/487 (0.2%) had a caregiver who felt the study was too difficult/intrusive. These are summarised in Table 2.
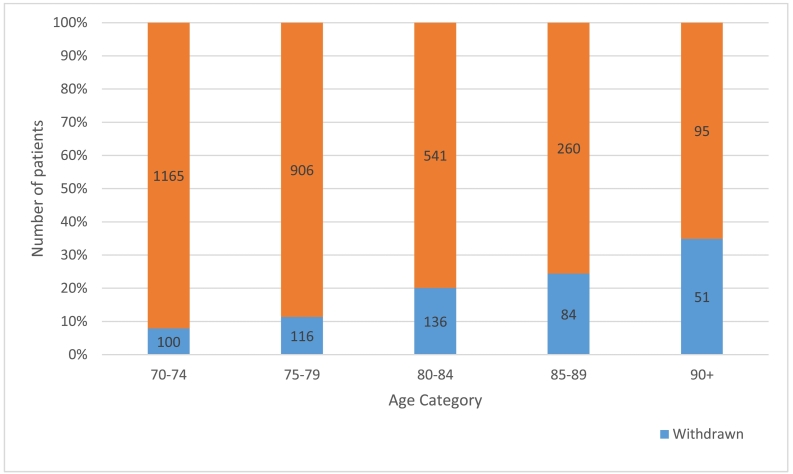
Patients who withdrew were generally older than women who did not withdraw (median age of 80 (range 70–101) versus 76 (70–102); p < 0.001, Fig. 3).
Fig. 3.
Graph to illustrate the percentage of patients who discontinued the study for each age category.
4.3. Population sampling for the Age Gap Study
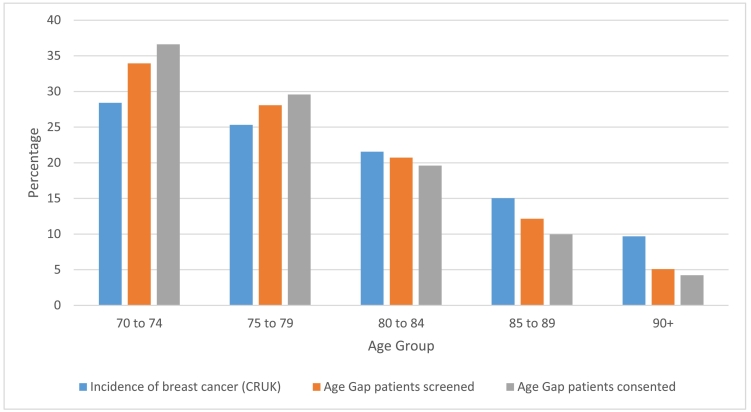
A comparison of UK age-specific population distribution for breast cancer, derived from the UK Office for National Statistics, was compared with the age-distribution of the Age Gap trial to determine whether sampling was representative. This demonstrated that the younger age groups were overrepresented in this trial, whereas the older age groups were under-represented (Fig. 4).
Fig. 4.
Graph of Incidence of breast cancer in the over 70 age group (CRUK percentage data derived from the UK Office for National Statistics) compared to Age Gap screened and consented percentages of corresponding age groups.
4.4. Logistic regression analysis of the impact of age on trial participation
Logistic regression showed age was a significant predictor of whether patients were screened, assessed for eligibility, consented or withdrawn (Table 3, Table 4).
Table 3.
Univariate logistic regression of age as a predictor for patients being screened, assessed for eligibility, consented and discontinued from the study.
| Independent Variable | Sample size | Dependent Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|---|
| Age | 6876 | Screened for eligibility | 0.960 | 0.951–0.969 | <0.001 |
| 3768 | Eligibility assessment | 0.966 | 0.958–0.975 | <0.001 | |
| 3456 | Consented to study | 0.960 | 0.938–0.982 | <0.001 | |
| 487 | Discontinued study | 1.095 | 1.095–1.112 | <0.001 |
Table 4.
Results of univariate logistic regression: An assessment of age group as a predictor for patients being screened, assessed for eligibility, consented or withdrawn.
| Independent Variable | Dependent Variable | Age Category | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|---|
| Age category | Screened (N = 6757) | 70–75 | 1.0 | N/A | <0.001 |
| 75–80 | 1.204 | 1.008–1.439 | 0.400 | ||
| 80–85 | 0.820 | 0.688–0.979 | 0.028 | ||
| 85–90 | 0.678 | 0.555–0.827 | <0.001 | ||
| 90+ | 0.383 | 0.303–0.484 | <0.001 | ||
| Eligibility (N = 5590) | 70–75 | 1.0 | N/A | <0.001 | |
| 75–80 | 0.943 | 0.814–1.092 | 0.431 | ||
| 80–85 | 0.773 | 0.661–0.904 | <0.001 | ||
| 85–90 | 0.537 | 0.448–0.644 | <0.001 | ||
| 90+ | 0.543 | 0.420–0.700 | <0.001 | ||
| Consented (N = 3647) | 70–75 | 1.0 | N/A | 0.007 | |
| 75–80 | 0.808 | 0.545–1.197 | 0.288 | ||
| 80–85 | 0.515 | 0.348–0.763 | <0.001 | ||
| 85–90 | 0.524 | 0.324–0.846 | 0.008 | ||
| 90+ | 0.750 | 0.350–1.610 | 0.461 | ||
| Withdrawn (N = 3454) | 70–75 | 1.0 | N/A | <0.001 | |
| 75–80 | 1.492 | 1.126–1.976 | 0.005 | ||
| 80–85 | 2.929 | 2.219–3.866 | <0.001 | ||
| 85–90 | 3.764 | 2.734–5.182 | <0.001 | ||
| 90+ | 6.254 | 4.206–9.301 | <0.001 |
This shows that a 90-year-old patient would have 0.44 odds ratio (56% reduced odds) of being screened compared to a 70-year-old patient. For consent, a 90-year-old patient would have 0.44 odds ratio (56% reduced odds) of being consented compared to a 70-year-old patient. For trial discontinuation, a 90-year-old patient having 6.14 odds ratio of discontinuation compared to a 70-year-old patient.
4.5. Multivariate analysis
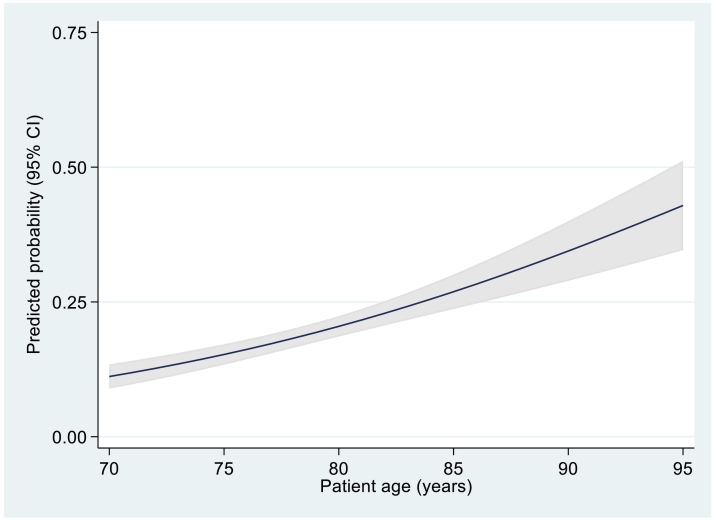
Multivariate analysis (Table 5) showed that, of the baseline characteristics assessed, only age (p < 0.001), ECOG-PS (p = 0.011) and IADL (p = 0.026) were significant predictors of trial discontinuation. Age increased the likelihood of withdrawal (Fig. 5), with an odds ratio of 1.079 of being withdrawn per year of increasing age. Similarly, performance status had a significant effect, with an odds ratio of 1.383 representing a 38.3% increase in odds of withdrawal with each reduction in level of functional ability. Increased IADL score was associated with a reduction in the odds of being withdrawn from the study, (odds ratio = 0.843) representing a 15.7% reduced odds of withdrawal with each increasing point score.
Table 5.
Results of multivariate analysis: An assessment of health status and age predictors of patients being withdrawn.
| Independent Variable | Dependent Variable | N | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|---|
| Withdrawn | Age | 3454 | 1.079 | 1.054–1.104 | <0.001 |
| Participation Level | 3456 | 0.768 | 0.547–1.078 | 0.128 | |
| MMSE | 2291 | 1.274 | 0.981–1.653 | 0.069 | |
| ECOG-PS | 3232 | 1.383 | 1.076–1.776 | 0.011 | |
| ADL | 3037 | 1.073 | 0.970–1.188 | 0.170 | |
| IADL | 2984 | 0.843 | 0.726–0.980 | 0.026 |
MMSE: Mini Mental State Examination, ECOG-PS: European Cooperative Oncology Group-Performance Status, ADL: Activities of Daily Living, IADL: Instrumental Activities of Daily Living.
Fig. 5.
Predicted risk of withdrawal by age plus 95% confidence intervals.
5. Discussion
The Age Gap trial has demonstrated that large-scale recruitment in the older population is feasible but has confirmed a number of factors affecting recruitment and discontinuation. Overall, 50.3% of patients screened were recruited to the study which is in line with breast cancer specific literature [36], as well as the general expectations for all trials. Failure to achieve recruitment targets is common across multi centre, publicly funded trials [1]. As with over half of publicly funded trials, the Age Gap trial required an extension due to slower than anticipated recruitment.
The Age Gap trial made efforts to counter the issues that reduce recruitment in the older patient (listed above). Our own experiences with the Endocrine or Surgical Therapy for Elderly womEn with Mammary cancer randomised trial (ESTEEM, surgery versus PET) and the Adjuvant Cytotoxic Chemotherapy in Older Women randomised trial (ACTION, chemotherapy in older women) provided a valuable insight into the barriers and facilitators of recruitment and informed the design of Age Gap [27]. Trial design avoided restrictive eligibility criteria, which reduced recruitment rates in the older patient [3], and consequently few patients were excluded due to this. Not meeting eligibility criteria accounted for only 30.3% of those screened being ineligible, suggesting that a range of other potentially unknown issues influenced recruitment and potentially introduced bias. Comorbidities are a common reason for older patients to be excluded from clinical trials [12]. Age Gap deliberately wished to recruit the widest range of fitness levels possible to permit propensity score matching for health status and as a result, comorbidity only accounted for 3% of those screened not being assessed for eligibility.
Retention in older patients is challenging, especially in longer trials, however participant choice only accounted for approximately one in two withdrawals (45.8%). The length of follow-up affects attrition, especially in older patients [37], however we have shown that it is feasible to follow patients up for two years with only a 13.1% attrition rate due to the low trial burden and pragmatic approach to follow-up. To keep burdensome direct follow-up to a minimum, longer-term follow-up was via the cancer registry, which increased follow-up duration with minimal cost and patient burden.
All analyses demonstrated that age was a significant predictor of whether patients were screened, assessed for eligibility, consented or withdrawn. Increasing age resulted in patients being less likely to be screened, assessed for eligibility or consented and an increasing likelihood of being withdrawn. As risk of death increases with age [11], this will have an effect on withdrawals.
Representation of different age groups in line with age specific cancer incidence in England also confirmed skewed age specific recruitment, with only half the expected patients over age 90 screened (Fig. 4) [38]. This may relate to staff, patient and relative reticence to subject frail and potentially vulnerable patients to the burden of trial participation. Staff reticence is shown in the age differential in screening and eligibility assessment where patients and carers have not even been approached at this stage of the recruitment process. Reduced trial representation at the extremes of age may have an impact on trial analysis in these age groups due to the smaller sample size. This could be mitigated by proactively identifying women in this older group to compensate for this [24], to have quota for each age range, or to adjust at the analysis stage by allocating greater weight to data from under-represented subgroups as a sensitivity analysis [39]. None of these approaches are likely to fully correct for volunteer bias (either in respect to age or to other characteristics), but nevertheless a partial adjustment is arguably better than none at all [39]. Lastly, steps to minimise the burden to patients and to embed research within routine care can make trials more appealing to patients who may otherwise be too “frail” to participate [10]. The design of the trial already included an ‘Age Gap Lite’ option, where participants could elect not to complete trial questionnaires and a proxy arm for patients lacking cognitive capacity where simple data collection only was an option to help address this issue.
Age bias from health care professionals (both doctors and nurses) is one factor which trial design struggles to mitigate for. Health care professionals act as gatekeepers and may create a barrier to recruitment [12]. Health care professional decisions can account for 50–80% of patients not being offered trial participation [37]. Whilst this may not be purely age bias, the close link between age, fitness, frailty and cognitive status makes this difficult to tease apart. Rates of health care professional exclusion in Age Gap were much lower, at only 9.3% of those not screened. Data suggests that clinicians assume that older patients are not interested in the trials [12]. However, only 0.2% of those not assessed for eligibility and 0.5% of those not consented stated their age as being the factor that put them off the trial, which is in line with the literature that the older population are generally happy to be involved in research [13]. Carer reticence, due to a wish to protect a vulnerable relative, may also be a factor. There are also social factors that may influence participation and other as yet unknown factors that justify further research into this issue.
Further to clinician and eligibility barriers, patient and funding barriers can also have an effect on recruitment and retention. The consequence of funding barriers were represented in this study by the number of potentially eligible patients that were not screened (9.8%), assessed for eligibility (1.2%) or consented (4.1%) due to staffing or resource issues (such as lack of availability of research nurses which was an issue in some centres). These issues unfairly disadvantage older patients who are less inclined to attend additional study specific visits due to co-morbidities and transport issues [12,37] so need to be seen during their clinic visit, which requires research staff flexibility.
It has been suggested that older patients are less informed about trials and this is a reason why recruitment rates are lower in the older population [40]. Although basic information such as years of schooling and overall cognition was collected through the MMSE questionnaire, specific information on health literacy especially in relation to trials, was not collected. Several campaigns have shown that it is possible to increase clinical trial awareness [41], and these methods could be used in the older population to potentially increase recruitment rates. Some inferences can be made about patient perceptions of the trial from overall recruitment numbers and the reasons patients provided for not being interested in the trial, however more detailed data collection forms and in-depth qualitative methods could provide more information in future trials.
It has been suggested that cognitive and performance status data are collected routinely to allow easier comparisons between studies [24]. This is due to poor ECOG-PS and high symptom burden being predictors of drop-out. Indeed, ECOG-PS was a significant predictor of withdrawal in this study. This could be useful for future studies to produce a more accurate projection of anticipated attrition, allow sample size calculations and recruitment targets to be adjusted for this. It is also important to ensure that inclusion and exclusion criteria closely reflect the clinical population demographics and do not restrict age and performance status unless absolutely necessary. One option to address this is to conduct trials specifically recruiting in this older age group, although as we have shown, skewed recruitment still occurs, so this solution is not perfect.
Acceptable trial design can be aided by patient and public involvement (PPI) in trial conduct and design which increases recruitment, especially when PPI members have personal experience of the disease [42]. The Age Gap PPI group fed into the design of the trial to ensure design and materials were acceptable to patients. Making patients aware that other patients in their age group have helped to design and run the trial may also be an incentive for participation.
5.1. Generalisability and implications for practice
The gradual under-representation of patients as they get older reduces the generalisability of the trial. Methods to make trial participants more representative need to be developed urgently.
6. Conclusion
The Age Gap trial has shown that it is feasible to recruit and retain older patients on a large scale where the study scope and design is appropriate. It has highlighted that there are still a large number of potential threats to representative recruitment and retention and further progress is needed. This project highlights some issues that may be adjusted for at trial design stage.
Table showing the Age Gap Trial recruiting centres and the name of the local principle investigator.
Trial sponsor
Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust, Clinical Research Office, First Floor 'C' Block, Doncaster Royal Infirmary, Armthorpe Road, Doncaster, DN2 5LT, UK
Disclaimer
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference number RP-PG-1209-10,071). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Author contributions
Study concepts: Lynda Wyld, M W Reed, Jenna Morgan, Annaliza Todd
Study design: Lynda Wyld, Annaliza Todd, M W Reed, Jenna Morgan, Stephen Walters, Tim Chater, Jacqui Gath, Tom Robinson
Data acquisition: Lynda Wyld, Annaliza Todd, Jenna Morgan, Charlene Martin, Maria Burton, Tim Chater, Kirsty Pemberton, KL Cheung, Riccardo Audisio, Alistair Ring
Quality control of data and algorithms: Annaliza Todd, Lynda Wyld, Charlene Martin, Tim Chater, Stephen Walters, Esther Herbert, Mike Bradburn, Jacqui Gath, Tracy Green
Data analysis and interpretation: Annaliza Todd, Lynda Wyld, Esther Herbert, Mike Bradburn, Stephen Walters
Statistical analysis: Annaliza Todd, Esther Herbert, Mike Bradburn, Stephen Walters, Lynda Wyld
Manuscript preparation: Lynda Wyld, Annaliza Todd, Jenna Morgan, Mike Bradburn, Stephen Walters.
Manuscript editing: ALL
Manuscript review: ALL
We confirm that all authors have made a significant contribution to this manuscript, have seen and approved the final manuscript, and agree to its submission to the Journal of Geriatric Oncology.
Declaration of Competing Interest
The authors declare no conflict of interest. Jenna Morgan, Stephen Walters and Thompson Robinson are all funded or part funded by the NIHR.
References
- 1.Francis D., Elbourne Diana R., Cook Jonathan A. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7(1):9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyld L., Reed M.W.R. The need for targeted research into breast cancer in the elderly. Br J Surg. 2003;90(4):388–399. doi: 10.1002/bjs.4124. [DOI] [PubMed] [Google Scholar]
- 3.Carroll C.B., Zajicek J.P. Designing clinical trials in older people. Maturitas. 2011;68(4):337–341. doi: 10.1016/j.maturitas.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Murthy V.H., Krumholz H.M., Gross C.P. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 5.Stewart J.H., Bertoni A.G., Staten J.L., Levine E.A., Gross C.P. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–3334. doi: 10.1245/s10434-007-9500-y. [DOI] [PubMed] [Google Scholar]
- 6.Freedman R.A., Foster J.C., Seisler D.K. Accrual of older patients with breast cancer to alliance systemic therapy trials over time: protocol A151527. J Clin Oncol. 2017;35(4):421–431. doi: 10.1200/JCO.2016.69.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.POST PooSaT-. POST PooSaT-Clinical Trials 2011. 2011. https://researchbriefings.parliament.uk/ResearchBriefing/Summary/POST-PN-390#fullreport
- 8.Rothwell P.M. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 9.Loudon K., Treweek S., Sullivan F., Donnan P., Thorpe K.E., Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. Bmj. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 10.Oude Rengerink K., Kalkman S., Collier S. Series: pragmatic trials and real world evidence: paper 3. Patient selection challenges and consequences. J Clin Epidemiol. 2017;89:173–180. doi: 10.1016/j.jclinepi.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 11.ONS How has life expectancy changed over time? 2015. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/articles/howhaslifeexpectancychangedovertime/2015-09-09 (accessed 16 July 2019)
- 12.MMP Kemeny, Bercedis L., Kornblith Alice B., Muss Hyman B., Judith Wheeler, Ellis Levine. Barriers to clinical trial participation by older women with breast cancer. Journal of Clinical Oncology. 2003;21(12) doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 13.Ayodele O., Akhtar M., Konenko A. Comparing attitudes of younger and older patients towards cancer clinical trials. J Geriatr Oncol. 2016;7(3):162–168. doi: 10.1016/j.jgo.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Al-Refaie W.B., Vickers S.M., Zhong W., Parsons H., Rothenberger D., Habermann E.B. Cancer trials versus the real world in the United States. Ann Surg. 2011;254(3):438–442. doi: 10.1097/SLA.0b013e31822a7047. [discussion 42-3] [DOI] [PubMed] [Google Scholar]
- 15.Paeck T., Ferreira M.L., Sun C., Lin C.W., Tiedemann A., Maher C.G. Are older adults missing from low back pain clinical trials? A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66(8):1220–1226. doi: 10.1002/acr.22261. [DOI] [PubMed] [Google Scholar]
- 16.Bayer A., Tadd W. Unjustified exclusion of elderly people from studies submitted to research ethics committee for approval: descriptive study. BMJ. 2000;321(7267):992–993. doi: 10.1136/bmj.321.7267.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugeja G., Kumar A., Banerjee A.K. Exclusion of elderly people from clinical research: a descriptive study of published reports. BMJ. 1997;315(7115):1059. doi: 10.1136/bmj.315.7115.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton M., Kilner K., Wyld L. 2017. Information needs and decision-making preferences of older women offered a choice between surgery and primary endocrine therapy for early breast cancer. [DOI] [PubMed] [Google Scholar]
- 19.Adamson J., Hewitt C.E., Torgerson D.J. Producing better evidence on how to improve randomised controlled trials. Br Med J. 2015;351:h4923. doi: 10.1136/bmj.h4923. [DOI] [PubMed] [Google Scholar]
- 20.Dumville J.C., Torgerson D.J., Hewitt C.E. Reporting attrition in randomised controlled trials. BMJ. 2006;332(7547):969. doi: 10.1136/bmj.332.7547.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santacatterina M., Bottai M. Inferences and conjectures in clinical trials: a systematic review of generalizability of study findings. J Intern Med. 2016;279(1):123–126. doi: 10.1111/joim.12389. [DOI] [PubMed] [Google Scholar]
- 22.Wang W., Ma Y., Huang Y., Chen H. Generalizability analysis for clinical trials: a simulation study. Stat Med. 2017;36(10):1523–1531. doi: 10.1002/sim.7238. [DOI] [PubMed] [Google Scholar]
- 23.Chatfield M.D., Brayne C.E., Matthews F.E. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005;58(1):13–19. doi: 10.1016/j.jclinepi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Hui D., Glitza I., Chisholm G., Yennu S., Bruera E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2013;119(5):1098–1105. doi: 10.1002/cncr.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott R.J., Counsell C.E., Gillespie W.J. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess (Winch Eng) 1999;3(20):1–143. [PubMed] [Google Scholar]
- 26.Collins K., Reed M., Lifford K. Bridging the age gap in breast cancer: evaluation of decision support interventions for older women with operable breast cancer: protocol for a cluster randomised controlled trial. BMJ Open. 2017;7(7) doi: 10.1136/bmjopen-2016-015133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed M.W., Wyld L., Ellis P., Bliss J., Leonard R. Breast cancer in older women: trials and tribulations. Clin Oncol (R Coll Radiol) 2009;21(2):99–102. doi: 10.1016/j.clon.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 30.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 31.Folstein M.F., Folstein S.E., McHugh P.R. “mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Aaronson N.K., Ahmedzai S., Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 33.Johnson C., Fitzsimmons D., Gilbert J. Development of the European Organisation for Research and Treatment of Cancer quality of life questionnaire module for older people with cancer: the EORTC QLQ-ELD15. Eur J Cancer. 2010;46(12):2242–2252. doi: 10.1016/j.ejca.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 34.von Elm E., Altman D.G., Egger M. Strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treweek S., Loudon K. Incomplete reporting of recruitment information in breast cancer trials published between 2003 and 2008. J Clin Epidemiol. 2011;64(11):1216–1222. doi: 10.1016/j.jclinepi.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Lara P.N., Higdon R., Lim N. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 38.CRUK Breast cancer statistics. 2019. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero (accessed 7 July 2019)
- 39.O’Muircheartaigh C.H.L. Generalizing from unrepresentative experiments: a stratified propensity score approach. Royal Statistical Soc. 2014;63(2):195–210. [Google Scholar]
- 40.McDonald A., Treweek S., Shakur H. Using a business model approach and marketing techniques for recruitment to clinical trials. Trials. 2011;12(1) doi: 10.1186/1745-6215-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackenzie I.S., Wei L., Rutherford D. Promoting public awareness of randomised clinical trials using the media: the ‘get randomised’ campaign. Br J Clin Pharmacol. 2010;69(2):128–135. doi: 10.1111/j.1365-2125.2009.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crocker J.C., Ricci-Cabello I., Parker A. 2018. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table showing the Age Gap Trial recruiting centres and the name of the local principle investigator.