Abstract
In this study, we investigated the presence of tick-borne pathogens in ticks removed from tick-bitten humans in the southwestern provinces of the Republic of Korea (ROK). We identified 33 ticks from three tick species, namely Amblyomma testudinarium (60.6%), Haemaphysalis longicornis (27.3%), and Ixodes nipponensis (12.1%) in order of occurrence via morphology and 16S rDNA-targeting polymerase chain reaction (PCR). Tick-borne pathogens were detected in 16 ticks using pathogen-specific PCR. From the results, 12 ticks (36.4%) tested positive for spotted fever group (SFG) Rickettsia: Rickettsia monacensis (1/12), R. tamurae (8/12), and Candidatus Rickettsia jingxinensis (3/12). Three ticks (9.1%) were positive for Anaplasma phagocytophilum. In addition, three ticks (9.1%) tested positive for Babesia gibsoni (1/3) and B. microti (2/3). In conclusion, we identified three tick species; the most common species was A. testudinarium, followed by H. longicornis and I. nipponensis. SFG Rickettsia, A. phagocytophilum, and Babesia spp. were the most frequently detected pathogens in ticks removed from tick-bitten humans. To our knowledge, this is the first report of R. tamurae and Ca. R. jingxinensis detection in Korea. The present results will contribute to the understanding of tick-borne infections in animals and humans in the ROK.
Introduction
Ticks are major vectors of pathogens, such as bacteria, viruses, and protozoans. These arthropods and protozoans can transmit a variety of diseases in humans and animals [1]. Tick-borne diseases are caused by viral or bacterial pathogens transmitted through tick bites. Several tick-borne diseases, such as Lyme disease (caused by Borrelia spp.), spotted fever group rickettsioses (caused by Rickettsia spp.), anaplasmosis (caused by Anaplasma phagocytophilum), bartonellosis (caused by Bartonella spp.), Q fever (caused by Coxiella burnetii), and babesiosis (caused by Babesia spp.) have been reported in the Republic of Korea (ROK) [2].
The incidence of tick-borne diseases in the ROK is increasing due to global warming, increased conduct of outdoor activities, and increased international travel. The growing number of tick bites each year poses an escalating risk of tick-borne diseases [2]. A tick survey conducted by the Korea Centers for Disease Control and Prevention (KCDC) from 2013 to 2015 reported that, of the 29,992 ticks collected from 29 sites, Haemaphysalis longicornis and H. flava were distributed nationwide, while Amblyomma testudinarium was predominantly located in the southwestern area of the ROK [2]. The geographical distribution of chigger mites is mainly distributed in the area where scrub typhus is prevalent [3]. However, few studies have investigated the prevalence of tick-borne pathogens in ticks removed from tick-bitten humans in the ROK. Therefore, it is necessary to determine the extent of tick-borne pathogens in the ROK and to characterize them.
The present study aimed to investigate the presence of tick-borne pathogens in ticks removed from humans in the southwest provinces of the ROK. Our study detected the DNA of tick-borne pathogens in ticks using pathogen-specific nested PCR. The results of this study will contribute to the understanding of the interactions between ticks and pathogens that cause diseases in humans. Additionally, this study could assist in describing the potential for human tick attachment to the public health practitioners of ROK.
Materials and methods
Ethics statement
This study was approved by the Ethics in Human Research Committee of Chosun University Hospital under an institutional review board (IRB), which approved all the experiments that used ticks removed from tick-bitten humans (approval no. CHOSUN NON2019-001). The IRB approved the protocol with verbal consent for the use of ticks and not human subjects. We have read and explained the verbal version of the consent form to use samples for research purposes and obtained individual consent.
Tick samples
Ticks were removed by individuals or by the physicians in Gwangju Metropolitan City and the province of Jeollanam, which are in the southwest provinces of the ROK, between March 2014 and September 2017 (S1 Fig). Ticks were collected from patients visiting Chosun University Hospital located in this area. All ticks were morphologically identified according to species and life stage using a microscope and standard taxonomic keys [4]. The ticks were washed in 70% ethanol, rinsed twice with sterile phosphate-buffered saline (PBS), added to a hard tissue grinding MK28 tube (Bertin Technology, Rockville, MD, USA) containing 800 μL of PBS with 1× PC/SM (penicillin and streptomycin), ground using a FastPrep®-24 Classic instrument (MP Biomedicals, Solon, OH, USA), and stored at -80 °C until used for DNA extraction.
DNA extraction
We mixed 150 μL of the ground tick with 150 μL ATL (animal tissue lysis solution) buffer and 20 μL proteinase K, incubated at 56 °C overnight for lysis, and genomic DNA was extracted using a QIAamp Tissue & Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA was eluted into 50 μL TE buffer and stored at -20 °C until PCR amplification.
Polymerase Chain Reaction (PCR)
To detect the presence of Rickettsia DNA, the outer membrane protein A gene (ompA), citrate synthase gene (gltA), and the 17 kDa protein gene (17 kDa) of the spotted fever group Rickettsia species were targeted. The heat shock protein gene (groEL) and ankyrin-related protein gene (ankA) were used to detect A. phagocytophilum. To detect the presence of Borrelia DNA, the CTP synthase gene (pyrG) was targeted. The 16S-23S internal transcribed spacer region (ITS) was used to detect Bartonella species. The htpAB-associated repetitive element IS1111 was used to detect Coxiella species. To detect the presence of Babesia species, the sequence of its 18S rDNA was targeted. To identify tick species, conventional PCR targeting of the mitochondrial 16S rRNA gene (16S rDNA) was performed. All PCR primers used for detecting tick-borne pathogens, PCR conditions, and the corresponding product sizes, are listed in Table 1. Conventional PCR (C-PCR) was performed in 20 μL reaction volumes using AccuPowerR PCR PreMix (Bioneer Corp., Korea). Each PCR mixture consisted of 16 μL of distilled water, 1 μL of each primer (10 pmol/μL), and 2 μL of genomic DNA as the template. For 16S rDNA C-PCR and 18S rDNA nested PCR (N-PCR), we performed PCR using AmpliTaq Gold 360 Master Mix (Applied Biosystems, CA, USA) instead of AccuPowerR PCR PreMix.
Table 1. Oligonucleotide primers and PCR conditions used for the detection of tick-borne pathogens in ticks removed from tick-bitten humans.
| Identification | Target genea | Primer name | Nucleotide sequence (5’-3’) | Product size (bp) | PCR conditions (°C/sec) | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Denaturation | Annealing | Extension | Cycles | ||||||
| Rickettsia species | ompA | RR190.70F | ATGGCGAATATTTCTCCAAAAA | 634 | 94/30 | 50/30 | 72/60 | 40 | [5, 6] |
| RR190.701R | GTTCCGTTAATGGCAGCATCT | ||||||||
| RR190.70F | ATGGCGAATATTTCTCCAAAAA | 535 | 94/30 | 50/30 | 72/30 | 5 | [5] | ||
| RR190.602R | AGTGCAGCATTCGCTCCCCCT | 94/30 | 54/30 | 72/30 | 30 | ||||
| gltA | GLTA1F | GACGGTGATAAAGGAATCTTG | 1022 | 95/20 | 47/30 | 72/60 | 40 | [7] | |
| GLTA1R | CATTTCTTTCCATTGTGCCATC | ||||||||
| GLTA2F | CTACGAACTTACCGCTATTAG | 446 | 95/20 | 43/30 | 72/30 | 5 | |||
| GLTA2R | GACCAAAACCCATTAACCTAAAC | 95/20 | 48/30 | 72/30 | 30 | ||||
| 17 kDa | Rr17k.1p | TTTACAAAATTCTAAAAACCAT | 539 | 95/30 | 57/60 | 72/120 | 35 | [8] | |
| Rr17k.539n | TCAATTCACAACTTGCCATT | ||||||||
| Rr17k.90p | GCTCTTGCAACTTCTATGTT | 450 | 95/30 | 57/60 | 72/120 | 35 | |||
| Rr17k.539n | TCAATTCACAACTTGCCATT | ||||||||
| Anaplasma phagocytophilum | groEL | GRO607F | GAAGATGCWGTWGGWTGTACKGC | 688 | 95/30 | 54/30 | 72/60 | 30 | [9] |
| GRO1294R | AGMGCTTCWCCTTCWACRTCYTC | ||||||||
| GRO677F | ATTACTCAGAGTGCTTCTCARTG | 445 | 95/30 | 57/30 | 72/60 | 30 | |||
| GRO1121R | TGCATACCRTCAGTYTTTTCAAC | ||||||||
| ankA | ANK-F1 | GAAGAAATTACAACTCCTGAAG | 705 | 95/30 | 53/30 | 72/60 | 35 | [10] | |
| ANK-R1 | CAGCCAGATGCAGTAACGTG | ||||||||
| ANK-F2 | TTGACCGCTGAAGCACTAAC | 664 | 95/30 | 52/30 | 72/60 | 5 | |||
| ANK-R2 | ACCATTTGCTTCTTGAGGAG | 95/30 | 54/30 | 72/60 | 25 | ||||
| Borrelia species | pyrG | pyrG-1F | ATTGCAAGTTCTGAGAATA | 801 | 94/20 | 45/30 | 72/30 | 30 | [11] |
| pyrG-1R | CAAACATTACGAGCAAATTC | ||||||||
| pyrG-2F | GATATGGAAAATATTTTATTTATTG | 707 | 95/30 | 45/30 | 72/30 | 5 | |||
| 95/30 | 47/30 | 72/30 | 5 | ||||||
| pyrG-2R | AAACCAAGACAAATTCCAAG | 95/30 | 49/30 | 72/30 | 25 | ||||
| Bartonella species | ITS | ITS_OF | TTCAGATGATGATCCCAAGC | 639 | 95/30 | 48/30 | 72/60 | 5 | [12] |
| ITS_OR | AACATGTCTGAATATATCTTC | 95/30 | 50/30 | 72/60 | 30 | ||||
| ITS_IF | CCGGAGGGCTTGTAGCTCAG | 499 | 95/30 | 41/30 | 72/60 | 30 | |||
| ITS_IR | CACAATTTCAATAGAAC | ||||||||
| Coxiella burnetii | IS1111 | IS111F1 | TACTGGGTGTTGATATTGC | 485 | 95/15 | 52/5 | 72/30 | 35 | [13] |
| IS111R1 | CCGTTTCATCCGCGGTG | ||||||||
| IS111F2 | GTAAAGTGATCTACACGA | 260 | 95/15 | 56/15 | 72/15 | 30 | |||
| IS111R2 | TTAACAGCGCTTGAACGT | ||||||||
| Babesia species | 18S rDNA | Bab5 | AATTACCCAATCCTGACACAGG | 485 | 94/60 | 55/60 | 72/120 | 35 | [14] |
| Bab8 | TTTGGCAGTAGTTCGTCTTTAACA | ||||||||
| Bab6 | GACACAGGGGGTAGTGACAAGA | 407 | 94/60 | 55/60 | 72/120 | 30 | |||
| Bab7 | CCCAACTGCTCCTATTAACCATTAC | ||||||||
| Ticks | 16S rDNA | 16S+1-F | CTGCTCAATGAATATTTAAATTGC | 450 | 95/45 | 55/60 | 72/90 | 40 | [15] |
| 16S+1-R | CGGTCTAAACTCAGATCATGTAGG | ||||||||
a ompA, outer membrane protein A gene; gltA, citrate synthase gene; 17 kDa, 17 kDa protein gene; groEL, heat shock protein gene; ankA, ankyrin-related protein gene; pyrG, CTP synthase gene; ITS, 16S-23S internal transcribed spacer region; IS1111, htpAB-associated repetitive element; 18S rDNA, 18S ribosomal RNA gene; 16S rDNA, 16S ribosomal RNA gene.
The reaction mixture for N-PCR was identical to that used in C-PCR, except that the first PCR product was used as template DNA, and that N-PCR primers were included. For each PCR run, a positive and negative control (molecular grade water) were included. The detection limit of PCR used in this study was > 5 × 102 copies/μL.
All amplifications were performed in an AB thermal cycler (Applied Biosystems, Foster City, CA, USA). The amplified products were separated via electrophoresis on a 1.2% agarose gel and stained with ethidium bromide for visualization.
Sequencing and phylogenetic analysis
The amplified PCR products were purified using QIAquick PCR purification kits (QIAGEN, Hilden, Germany) and sequenced using PCR primers at Solgent Inc. (Daejeon, Korea). The sequences obtained in this study were compared to GenBank sequences using BLAST. Gene sequences, excluding the primer regions, were aligned using the multisequence alignment program in Lasergene version 8 (DNASTAR, USA). The nucleotide sequences obtained from the PCR amplifications performed in this study were registered and assigned the following GenBank accession numbers: Tick1 (MW481245) and Tick29 (MW481246) for the ankA gene; Tick29 (MW481247), Tick2 (MW481248), Tick17-1 (MW481249), Tick17-2 (MW481250), Tick15 (MW481251), Tick5 (MW481252), Tick6 (MW481253), Tick7 (MW481254), Tick18 (MW481255), Tick30 (MW481256), Tick19 (MW481257), Tick21 (MW481258), and Tick24 (MW481259) for the gltA gene; Tick25 (MW475155), Tick12 (MW475156), and Tick19 (MW475157) for the 18S rDNA.
Phylogenetic trees were constructed using ClustalW of the MegAlign Program (DNASTAR, USA) based on the alignments of positive gene sequences using the neighbor-joining method. Bootstrap analysis (1,000 replicates) was performed according to the Kimura 2-parameter method. Pairwise alignments were performed with an open-gap penalty of 10 and a gap extension penalty of 0.5.
Results
Tick identification
We obtained 33 ticks from 30 tick-bitten humans. Of these, 15 ticks (45.5%) were adults, namely 12 females and 3 males, and 18 ticks (54.5%) were nymphs. Based on morphological examination using a microscope, the ticks were identified as Amblyomma testudinarium (20, 60.6%; 7 adults and 13 nymphs), Haemaphysalis longicornis (9, 27.3%; five adults and four nymphs), and Ixodes nipponensis (4, 12.1%; three adults and one nymph), as described in Table 2. Tick identification using 16S rDNA C-PCR and DNA sequencing yielded the same results as the microscopic examination, except for four samples without tick DNA (Table 3). There were not enough tick lysates to extract DNA because we tried to isolate pathogens using the cell lines and mice.
Table 2. Developmental stages and species of ticks removed from tick-bitten humans determined from both morphological identification and 16S rDNA-targeting conventional PCR.
| Tick species | Amblyomma testudinarium | Heamaphysalis longicornis | Ixodes nipponensis | |
|---|---|---|---|---|
| Development stage | Adult female | 4 | 5 | 3 |
| Adult male | 3 | 0 | 0 | |
| Nymph | 13 | 4 | 1 | |
| Larva | 0 | 0 | 0 | |
| Total No. (%) | 20 (60.6 %) | 9 (27.3 %) | 4 (12.1 %) | |
| 33 (100 %) | ||||
Table 3. Characteristics of the 33 ticks using the DNA of tick-borne pathogens obtained from tick-bitten humans.
| Patient no. | Patient age/sex | Tick species identified by a microscopy | Development stage (sex) | Tick engorgement | Identification of ticks by 16S rDNA PCR | Detected tick-borne pathogens in ticks | ||
|---|---|---|---|---|---|---|---|---|
| SFG Rickettsia | A. phagocytophilum | Babesia spp. | ||||||
| 1 | 83/F | A. testudinarium | Nymph | +a | NA | - | A. phagocytophilum | - |
| 2 | 46/M | A. testudinarium | Nymph | + | NA | R. tamurae | - | - |
| 3 | 4/M | A. testudinarium | Nymph | + | A. testudinarium | - | - | - |
| 4 | NA | A. testudinarium | Nymph | NAb | A. testudinarium | - | - | - |
| 5 | 65/M | A. testudinarium | Nymph | + | A. testudinarium | R. tamurae | - | - |
| 6 | 74/M | A. testudinarium | Nymph | NA | A. testudinarium | R. tamurae | - | - |
| 7 | 58/F | A. testudinarium | Nymph | NA | A. testudinarium | R. tamurae | - | - |
| 8 | 52/F | A. testudinarium | Nymph | NA | A. testudinarium | - | - | - |
| 9 | 62/F | A. testudinarium | Nymph | + | A. testudinarium | - | - | - |
| 10 | 60/M | A. testudinarium | Nymph | + | A. testudinarium | - | - | - |
| 11 | 55/F | A. testudinarium | Nymph | + | A. testudinarium | - | - | - |
| 12 | 30/F | A. testudinarium | Nymph | + | A. testudinarium | - | B. microti | |
| 13 | 71/M | A. testudinarium | Nymph | + | A. testudinarium | - | - | - |
| 14 | 64/M | A. testudinarium | Adult (female) | NA | NA | - | - | - |
| 15 | NA | A. testudinarium | Adult (female) | NA | A. testudinarium | Ca. R. jingxinensis | - | - |
| 16 | 60/F | A. testudinarium | Adult (female) | + | A. testudinarium | - | - | - |
| 17 | 54/F | A. testudinarium | Adult (female) | NA | A. testudinarium | R. tamurae | - | - |
| A. testudinarium | Adult (male) | NA | A. testudinarium | R. tamurae | - | - | ||
| 18 | 53/F | A. testudinarium | Adult (male) | + | A. testudinarium | R. tamurae | - | - |
| 19 | 78/F | A. testudinarium | Adult (male) | + | A. testudinarium | R. tamurae | - | B. gibsoni |
| 20 | 60/F | H. longicornis | Nymph | NA | H. longicornis | - | - | - |
| H. longicornis | Nymph | NA | NA | - | - | - | ||
| 21 | 83/F | H. longicornis | Nymph | NA | H. longicornis | Ca. R. jingxinensis | - | - |
| 22 | 72/F | H. longicornis | Nymph | + | H. longicornis | - | - | - |
| 23 | 76/F | H. longicornis | Adult (female) | + | H. longicornis | - | - | - |
| H. longicornis | Adult (female) | + | H. longicornis | - | - | - | ||
| 24 | 77/F | H. longicornis | Adult (female) | + | H. longicornis | Ca. R. jingxinensis | - | - |
| 25 | 5/M | H. longicornis | Adult (female) | NA | H. longicornis | - | - | B. microti |
| 26 | 54/F | H. longicornis | Adult (female) | NA | H. longicornis | - | - | - |
| 27 | 77/F | I. nipponensis | Nymph | + | I. nipponensis | - | - | - |
| 28 | 72/M | I. nipponensis | Adult (female) | NA | I. nipponensis | - | - | - |
| 29 | 53/F | I. nipponensis | Adult (female) | + | I. nipponensis | - | A. phagocytophilum | - |
| 30 | 81/F | I. nipponensis | Adult (female) | + | I. nipponensis | R. monacensis | A. phagocytophilum | - |
a Presence.
b NA: not available.
Molecular detection of tick-borne pathogens in ticks removed from humans
We examined 33 ticks for the detection of tick-borne pathogens using pathogen-specific nested PCR. Tick-borne pathogens were detected in 16 ticks. From the results, 12 ticks (36.4%) were positive for spotted fever Rickettsia species, namely R. monacensis (1 of 33, 3.0%), R. tamurae (8 of 33, 24.2%), and Candidatus Rickettsia jingxinensis (3 of 33, 9.1%). Three ticks (9.1%) were positive for A. phagocytophilum, while the other three ticks (9.1%) were positive for either B. gibsoni (1 of 33, 3.0%) or B. microti (2 of 33, 6.0%) (Table 4). All ticks were negative for Borrelia spp., Bartonella spp., and C. burnetii.
Table 4. Detection of tick-borne pathogens in ticks via pathogen-specific nested PCR.
| Detected pathogens | Positive tick numbers | /Total numbers | PCR positivity (%) |
|---|---|---|---|
| Spotted fever group Rickettsia species | 12 | /33 | 36.4 |
| R. monacensis | 1 | /33 | 3.0 |
| R. tamurae | 8 | /33 | 24.2 |
| Candidatus Rickettsia jingxinensis | 3 | /33 | 9.1 |
| Anaplama phagocytophilum | 3 | /33 | 9.1 |
| Babesia species | 3 | /33 | 9.1 |
| B. gibsoni | 1 | /33 | 3.0 |
| B. microti | 2 | /33 | 6.0 |
| Borrelia species | 0 | /33 | 0 |
| Bartonella species | 0 | /33 | 0 |
| Coxiella burnetii | 0 | /33 | 0 |
Of the three A. phagocytophilum-positive ticks, one tick was identified as A. testudinarium, and two ticks were identified as I. nipponensis. Of the 12 SFG Rickettsia-positive ticks, nine ticks were identified as A. testudinarium, two ticks were identified as H. longicornis, and one tick was identified as I. nipponensis. R. tamurae was identified only in A. testudinarium. Of the three ticks detected with Babesia spp., two were A. testudinarium and one was H. longicornis. Among the 33 ticks, one I. nipponensis (adult female) was co-infected with A. phagocytophilum and R. monacensis. In addition, co-infections of R. tamurae and Babesia spp. were identified in A. testudinarium (adult males), as presented in Table 3.
Sequencing and phylogenetic analysis
The amplified PCR products were sequenced, and the sequencing results were aligned with the sequences obtained from the GenBank database to identify known sequences with a high degree of similarity using ClustalW. A neighbor-joining tree was constructed using the Kimura 2-parameter model (1,000 bootstrap replicates).
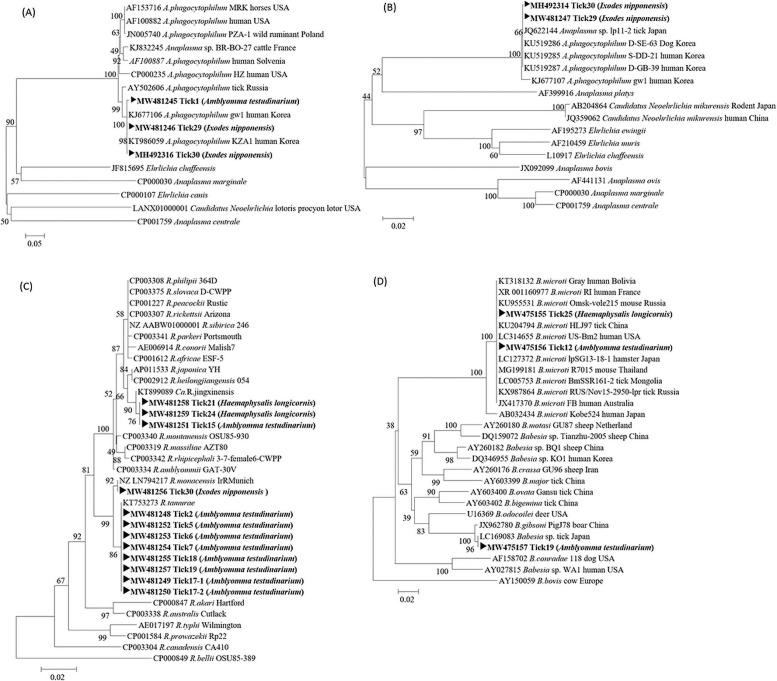
Partial ankA sequences obtained from A. phagocytophilum-positive ticks demonstrated 99% similarity with A. phagocytophilum (accession no. KJ677106 and KT986059, 98% bootstrap support; Fig 1A). The partial ankA sequences formed a cluster with A. phagocytophilum strains isolated from humans in the ROK. The partial groEL sequences obtained from A. phagocytophilum-positive ticks demonstrated 99% similarity with the A. phagocytophilum strain isolated from humans and dogs in the ROK (accession no. KU519286, 66% bootstrap support; Fig 1B).
Fig 1. Phylogenetic trees based on partial nucleotide sequences obtained from A. phagocytophilum-, spotted fever group Rickettsia-, and Babesia-positive ticks in this study and from GenBank.
(A) 560 bp portion of the ankA gene, (B) 330 bp portion of the groEL gene sequences for A. phagocytophilum, (C) 420 bp portion of the gltA gene sequences for SFG Rickettsia, and (D) 370 bp portion of the 18S rRNA gene sequences for Babesia species.
The partial 17 kDa, ompA, and gltA sequences obtained from SFG Rickettsia-positive ticks showed 99%–100% similarity with R. tamurae, R. monacensis, and Ca. R. jingxinensis. Phylogenetic analysis grouped partial gltA sequences with R. tamurae (accession no. KT753273, 86% bootstrap support; Fig 1C) and R. monacensis (accession no. NZ LN794217, 92% bootstrap support; Fig 1C), and Ca. R. jingxinensis (accession no. KT899089; 76% bootstrap support; Fig 1C).
The partial 18S rDNA sequences obtained from two Babesia species-positive ticks (Tick 12 and Tick 25) showed 99% similarity with a B. microti strain isolated from humans in the USA and a tick in China (accession no. KU204794 and LC314655, with 100% bootstrap support, Fig 1D). Another partial 18S rDNA sequence obtained from Tick19 had 99% similarity with B. gibsoni and 100% similarity with Babesia spp., which were clustered with a B. gibsoni strain isolated from a boar in China (accession no. JX962780, 100% bootstrap support, Fig 1D) and Babesia spp. from a tick in Japan (accession no. LC169083, 96% bootstrap support; Fig 1D).
Discussion
Previous studies that investigated the prevalence of infectious agents in ticks collected by dragging and flagging grass vegetation in the ROK showed that A. phagocytophilum was detected in 1.9% of H. longicornis ticks [16] and 0.1% of I. persulcatus ticks, while Rickettsia spp. was detected in 1.7% of H. longicornis ticks [17]. One study reported that a pool of H. longicornis, H. flava, and I. nipponensis ticks collected by dragging vegetation in the ROK were positive for Rickettsia spp. 17 kDa antigen (60/311, 19.3%) and ompA (53/311, 17.04%) [18]. In the present study, the infection prevalence of Rickettsia species (R. monacensis, R. tamurae, and Ca. R. jingxinensis) and A. phagocytophilum in ticks collected from humans were higher than those of ticks collected from vegetation. Thus, we suggest that further studies be conducted to compare the infection prevalence of tick-borne pathogens, including Rickettsia spp., A. phagocytophilum, and Babesia, between ticks isolated from humans and ticks collected from grass vegetation.
A. phagocytophilum infection was first reported using serological evidence from humans in 2002, and it is currently the most frequently reported tick-borne bacterial infection in the ROK [19]. The detection of Anaplasma spp. in ticks from grazing cattle collected from all ROK provinces has been reported [20]. Another study confirmed the presence of human granulocytic anaplasmosis (HGA) caused by A. phagocytophilum in a patient from the ROK who had a history of tick bites, clinical symptoms, and positive laboratory findings [21]. The present results showed that A. phagocytophilum was detected in A. testudinarium and I. nipponensis ticks. The amplicon sequences of the partial ankA gene in A. testudinarium (Tick 1) and I. nipponensis (Tick 29 and Tick 30) showed > 99% similarity. In the phylogenetic analysis, the sequences of the ankA gene from different types of ticks clustered together showed > 99% similarity with A. phagocytophilum strains isolated from humans in the ROK (Fig 1A). In this study, of the 30 patients who were bitten by ticks, 8 patients showed systemic symptoms (26.7%) including fever, diarrhea, headache, general weakness and chill. In particular, we detected the same pathogen with A. phagocytophilum in Tick30 and the blood of these tick-bitten patients. It has been identified via PCR and increased antibody levels (IgG/IgM) using an indirect immunofluorescence assay according to the sample collection date of these patients [22]. Further study will need to be conducted to analyze patient characteristics from clinical data of more ticks and these tick-bitten patients.
The first isolation of R. monacensis from ticks in the ROK was reported in 2013 [23]. A previous study from the ROK reported that I. nipponensis was infected with the human pathogen R. monacensis and that H. longicornis and H. flava were infected with unknown SFG Rickettsia pathogens [18]. Our results confirmed the presence of R. monacensis in I. nipponensis ticks removed from humans. In addition, our results indicated that I. nipponensis ticks are most likely the vectors responsible for transmitting R. monacensis infections in the ROK. Therefore, further studies are needed to determine the role of I. nipponensis in the transmission of R. monacensis pathogens to humans, and that the blood of patients bitten by I. nipponensis ticks and the ticks themselves should be investigated for the presence of R. monacensis.
R. tamurae was first isolated from A. testudinarium ticks collected in Japan in 1993. R. tamurae was formally identified as a novel species via genetic and phylogenetic analyses in 2006 [24]. In 2011, its first case of human infection was confirmed via molecular and serological analyses in Japan [25]. The presence of SFG Rickettsia, including R. tamurae, was found in Amblyomma and Dermacentor ticks in Thailand [26] and in Haemaphysalis ticks in Peninsular Malaysia [27]. In addition, R. tamurae was found in Amblyomma ticks from an area endemic for Brazilian spotted fever in Brazil [28]. Supporting these previous studies, our results showed the presence of R. tamurae in A. testudinarium ticks.
The presence of potentially novel species of Ca. R. jingxinensis was proposed in H. longicornis nymphs from Jingxin in Northeastern China in 2016 [29] and was detected in H. longicornis ticks in Xi’an, China in 2017 [30]. However, in the ROK, the pathogenicity of Ca. R. jingxinensis is still unclear. Therefore, further assessment of its potential pathogenicity in humans and animals is warranted.
There have been no previous reports on R. tamurae or Ca. R. jingxinensis from ticks in the ROK. Here, we report the first identification of R. tamurae and Ca. R. jingxinensis from ticks obtained from tick-bitten humans. The discovery of new pathogens in ticks removed from humans suggests that the risk of disease outbreaks may increase in the future.
Babesia was first discovered in animals by Babes in 1988, and more than 100 species have been identified to date. In the ROK, Babesia spp. have been isolated from cattle and other mammals (raccoons, deer, and badgers) since the 2000s [31–33]. Babesia spp. are mainly carried by Ixodes ticks. Previous studies using ticks collected from grass and vegetation in the ROK reported that H. longicornis was the most common tick species infected with Babesia [17, 20]. Our results showed that B. microti was found in both H. longicornis and A. testudinarium. In the USA, the primary vector for the transmission of B. microti to humans is the tick Ixodes scapularis in the nymphal stage [34]. The present results suggest that further studies are needed to determine the type of ticks that are vectors for the transmission of B. microti to humans in the ROK.
B. gibsoni was first identified in the nymphs of Rhipicephalus sanguineus ticks from infected dogs in Asia [35]. In this study, B. gibsoni was detected in A. testudinarium ticks. The first case of human babesiosis (KO1) was reported in 2007 in the ROK and was highly related to Chinese ovine Babesia spp. [14]. Based on the phylogenetic analysis of the 18S rDNA gene in our study, the pathogen clustered with a group of Babesia spp. isolated from a tick in Japan, which diverged from the KO1 strain (Fig 1D). The present results indicate that Babesia spp. may vary according to their geographical distribution.
This study was limited because only a few samples were obtained from the southwestern area of the ROK. Chosun University Hospital is located in Gwangju Metropolitan City, a province in the southwest of the ROK, and patients who visit this hospital are mainly residents of Gwangju Metropolitan City and Jeollanam-do province, which are located nearby (S1 Fig). Therefore, the sampling site of ticks removed from tick-bitten humans is limited to the southwestern region of the ROK. Further studies should be conducted to investigate pathogen prevalence in ticks removed from tick-bitten humans in more areas. Such studies could enable the identification of geographic regions with a high risk of tick-borne diseases. Additionally, further investigation is needed to determine the difference between pathogens found in ticks isolated from humans and ticks collected from grass vegetation. In addition, transmission studies should be conducted to determine whether the pathogens found in ticks are the same as those found in humans bitten by ticks. Serological testing of the blood of tick-bitten patients and ticks will be necessary to confirm the transmission of pathogens from ticks to humans. A study has reported the seroprevalence of A. phagocytophilum in human populations analyzed based on global data published from 1994 to 2018. Its seroprevalence was highest in the high-risk population (13.8%) and lowest in the healthy population (5.0%). The estimated seroprevalences of A. phagocytophilum in febrile patients, tick-bitten, and tick-borne disease populations were 6.4%, 8.0%, and 9.0%, respectively [36]. A serological study of R. japonica showed 19.88% seropositivity in 3,401 patients with acute febrile illness, and another reported 8% R. sibirica seropositivity and 14.34% R. coronii seropositivity in 3,362 patients with the same condition [37, 38]. Further experiments and correlation analyses using blood samples from tick-bitten humans and ticks isolated from them may help predict the transmission of tick-borne diseases.
In conclusion, we confirmed the presence of three tick species carrying tick-borne pathogens, the most common of which was A. testudinarium, followed by H. longicornis and I. nipponensis. These ticks were positive for SFG, Rickettsia, A. phagocytophilum, and Babesia. To our knowledge, this is the first report of the presence of R. tamurae and Ca. R. jingxinensis in ticks removed from tick-bitten humans in the southwestern area of the ROK.
Supporting information
Dark red color, Gwangju Metropolitan City and Chosun University Hospital located in this area; light red color, Jeollanam Provinces.
(PDF)
Acknowledgments
We sincerely appreciate the generous help regarding tick collection from the subjects and physicians at Chosun University Hospital.
Data Availability
We shared our data to figshare with DOI https://doi.org/10.6084/m9.figshare.14716458.v1. Furthermore, the sequence information in Fig 1 can be referred to as NCBI GenBank accession number presented in our manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.de la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Peña A, et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front Cell Infect Microbiol. 2017;7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Im JH, Baek JH, Durey A, Kwon HY, Chung MH, Lee JS. Current Status of Tick-Borne Diseases in South Korea. Vector-Borne Zoonotic Dis. 2019;19: 225–233. doi: 10.1089/vbz.2018.2298 [DOI] [PubMed] [Google Scholar]
- 3.Roh JY, Song BG, Park W Il, Shin EH, Park C, Park M-Y, et al. Coincidence between Geographical Distribution of Leptotrombidium scutellare and Scrub Typhus Incidence in South Korea. 2014. doi: 10.1371/journal.pone.0113193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker AR, Bouattour A, Horak I. Ticks of Domestic Animals in Africa: a Guide to Identification of Species. 2003. [Google Scholar]
- 5.Regnery RL, Spruill CL, Plikaytis2 BD, Branch RZ. Genotypic Identification of Rickettsiae and Estimation of Intraspecies Sequence Divergence for Portions of Two Rickettsial Genes. J Bacteriol. 1991. Available: http://jb.asm.org/ doi: 10.1128/jb.173.5.1576-1589.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roux V, Fournier P-E, Raoult D. Differentiation of Spotted Fever Group Rickettsiae by Sequencing and Analysis of Restriction Fragment Length Polymorphism of PCR-Amplified DNA of the Gene Encoding the Protein rOmpA Downloaded from. J Clin Microbiol. 1996. Available: http://jcm.asm.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, Sprong H. Molecular Detection of Tick-Borne Pathogens in Humans with Tick Bites and Erythema Migrans, in the Netherlands. PLoS Negl Trop Dis. 2016;10: 1–15. doi: 10.1371/journal.pntd.0005042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguti N, Tipton VJ, Keegan HL, Toshioka S. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ Sci Bull. 1971. [Google Scholar]
- 9.Pritt BS, Sloan LM, Hoang Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, et al. Emergence of a New Pathogenic Ehrlichia Species, Wisconsin and Minnesota, 2009. Public Health; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massung RF, Levin ML, Munderloh UG, Silverman DJ, Lynch MJ, Gaywee JK, et al. Isolation and Propagation of the Ap-Variant 1 Strain of Anaplasma phagocytophilum in a Tick Cell Line. J Clin Microbiol. 2007;45: 2138–2143. doi: 10.1128/JCM.00478-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapi E, Pabbati N, Datar A, Davies EM, Rattelle A, Kuo BA. Improved Culture Conditions for the Growth and Detection of Borrelia from Human Serum. Int J Med Sci. 2013;10: 362–376. doi: 10.7150/ijms.5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko S, Kang JG, Kim HC, Klein TA, Choi KS, Song JW, et al. Prevalence, Isolation and Molecular Characterization of Bartonella Species in Republic of Korea. Transbound Emerg Dis. 2016;63: 56–67. doi: 10.1111/tbed.12217 [DOI] [PubMed] [Google Scholar]
- 13.Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J Clin Microbiol. 2004;42: 4919–4924. doi: 10.1128/JCM.42.11.4919-4924.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J-Y, Cho Shin-Hyeong, Joo H-N, Tsuji M, Cho S-R, Park I-J, et al. First Case of Human Babesiosis in Korea: Detection and Characterization of a Novel Type of Babesia sp. (KO1) Similar to Ovine Babesia. J Clin Microbiol. 2007;45: 2084–2087. doi: 10.1128/JCM.01334-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Z, Liu G, Xie J, Yin H, Luo J, Zhang L, et al. Discrimination between Haemaphysalis longicornis and H. qinghaiensis based on the partial 16S rDNA and the second internal transcribed spacer (ITS-2). Exp Appl Acarol. 2011;54: 165–172. doi: 10.1007/s10493-010-9423-3 [DOI] [PubMed] [Google Scholar]
- 16.Oh JY, Moon BC, Bae BK, Shin EH, Ko YH, Kim YJ, et al. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju island, Korea. J Bacteriol Virol. 2009;39: 257–267. doi: 10.4167/jbv.2009.39.4.257 [DOI] [Google Scholar]
- 17.Kim CM, Yi YH, Yu DH, Lee MJ, Cho MR, Desai AR, et al. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol. 2006;72: 5766–5776. doi: 10.1128/AEM.00431-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noh Y, Lee YS, Kim HC, Chong ST, Klein TA, Jiang J, et al. Molecular detection of Rickettsia species in ticks collected from the southwestern provinces of the Republic of Korea. Parasites and Vectors. 2017;10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heo E-J, Park J-H, Koo J-R, Park M-S, Park M-Y, Stephen Dumler J, et al. Serologic and Molecular Detection of Ehrlichia chaffeensis and Anaplasma phagocytophila (Human Granulocytic Ehrlichiosis Agent) in Korean Patients Downloaded from. J Clin Microbiol. 2002;40: 3082–3085. doi: 10.1128/JCM.40.8.3082-3085.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang SW, Doan HTT, Choe SE, Noh JH, Yoo MS, Reddy KE, et al. Molecular investigation of tick-borne pathogens in ticks from grazing cattle in Korea. Parasitol Int. 2013;62: 276–282. doi: 10.1016/j.parint.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Yi J, Oh WS, Kim NH, Choi SJ, Choe PG, et al. Human granulocytic anaplasmosis, South Korea, 2013. Emerg Infect Dis. 2014;20: 1708–1711. doi: 10.3201/eid2010.131680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Shin NR, Kim CM, Park S, Yun NR, Kim DM, et al. First identification of Anaplasma phagocytophilum in both a biting tick Ixodes nipponensis and a patient in Korea: a case report. BMC Infect Dis. 2020;20: 826. doi: 10.1186/s12879-020-05522-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KM, Choi YJ, Shin SH, Choi MK, Song HJ, Kim HC, et al. Spotted fever group rickettsia closely related to Rickettsia monacensis isolated from ticks in South Jeolla province, Korea. Microbiol Immunol. 2013;57: 487–495. doi: 10.1111/1348-0421.12062 [DOI] [PubMed] [Google Scholar]
- 24.Fournier PE, Takada N, Fujita H, Raoult D. Rickettsia tamurae sp. nov., isolated from Amblyomma testudinarium ticks. Int J Syst Evol Microbiol. 2006;56: 1673–1675. doi: 10.1099/ijs.0.64134-0 [DOI] [PubMed] [Google Scholar]
- 25.Imaoka K, Kaneko S, Tabara K, Kusatake K, Morita E. The First Human Case of Rickettsia tamurae Infection in Japan. Case Rep Dermatol. 2011;3: 68–73. doi: 10.1159/000326941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nooroong P, Trinachartvanit W, Baimai V, Ahantarig A. Phylogenetic studies of bacteria (Rickettsia, Coxiella, and Anaplasma) in Amblyomma and Dermacentor ticks in Thailand and their co-infection. Ticks Tick Borne Dis. 2018;9: 963–971. doi: 10.1016/j.ttbdis.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 27.Kho KL, Koh FX, Hasan LIM, Wong LP, Kisomi MG, Bulgiba A, et al. Rickettsial seropositivity in the indigenous community and animal farm workers, and vector surveillance in Peninsular Malaysia. Emerg Microbes Infect. 2017;6: e18–9. doi: 10.1038/emi.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guedes E, Leite RC, Pacheco RC, Silveira I, Labruna MB. Rickettsia species infecting Amblyomma ticks from an area endemic for brazilian spotted fever in Brazil. Rev Bras Parasitol Vet. 2011;20: 308–311. doi: 10.1590/s1984-29612011000400009 [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Li Q, Zhang X, Li Z, Wang Z, Song M, et al. Characterization of rickettsiae in ticks in northeastern China. Parasites and Vectors. 2016;9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo WP, Huang B, Zhao Q, Xu G, Liu B, Wang YH, et al. Human-pathogenic Anaplasma spp., and Rickettsia spp. in animals in Xi’an, China. PLoS Negl Trop Dis. 2018;12: 1–12. doi: 10.1371/journal.pntd.0006916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SH, Kim TS, Lee HW, Tsuji M, Ishihara C, Kim JT, et al. Identification of newly isolated Babesia parasites from cattle in Korea by using the Bo-RBC-SCID mice. Korean J Parasitol. 2002;40: 33–40. doi: 10.3347/kjp.2002.40.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han JI, Lee SJ, Jang HJ, Na KJ. Asymptomatic Babesia microti-like parasite infection in wild raccoon dogs (Nyctereutes procyonoides) in South Korea. J Wildl Dis. 2010;46: 632–635. doi: 10.7589/0090-3558-46.2.632 [DOI] [PubMed] [Google Scholar]
- 33.Hong SH, Kim HJ, Jeong Y Il, Cho SH, Lee WJ, Kim JT, et al. Serological and molecular detection of toxoplasma gondii and babesia microti in the blood of rescued wild animals in Gangwon-do (Province), Korea. Korean J Parasitol. 2017;55: 207–212. doi: 10.3347/kjp.2017.55.2.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vannier E, Krause PJ. Human Babesiosis. n engl j med. 2012. doi: 10.1056/NEJMra1202018 [DOI] [PubMed] [Google Scholar]
- 35.Chao LL, Liao HT, Ho TY, Shih CM. First detection and molecular identification of Babesia gibsoni from Rhipicephalus sanguineus ticks. Acta Trop. 2017;166: 356–362. doi: 10.1016/j.actatropica.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Yan M, Liu A, Chen T, Luo L, Li L, et al. The seroprevalence of Anaplasma phagocytophilum in global human populations: A systematic review and meta-analysis. Transbound Emerg Dis. 2020;67: 2050–2064. doi: 10.1111/tbed.13548 [DOI] [PubMed] [Google Scholar]
- 37.Jang W-J, Kim J-H, Choi Y-J, Jung K-D, Kim Y-G, Lee S-H, et al. First Serologic Evidence of Human Spotted Fever Group Rickettsiosis in Korea. J Clin Microbiol. 2004;42: 2310–2313. doi: 10.1128/JCM.42.5.2310-2313.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang WJ, Choi YJ, Kim JH, Jung KD, Ryu JS, Lee SH, et al. Seroepidemiology of spotted fever group and typhus group rickettsioses in humans, South Korea. Microbiol Immunol. 2005;49: 17–24. doi: 10.1111/j.1348-0421.2005.tb03635.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dark red color, Gwangju Metropolitan City and Chosun University Hospital located in this area; light red color, Jeollanam Provinces.
(PDF)
Data Availability Statement
We shared our data to figshare with DOI https://doi.org/10.6084/m9.figshare.14716458.v1. Furthermore, the sequence information in Fig 1 can be referred to as NCBI GenBank accession number presented in our manuscript.