Abstract
Dengue is transmitted mainly by the adult female Aedes aegypti mosquito. However, little is known about the impact of adult Aedes abundance on the risk of dengue transmission. Here we analysed nationally representative dengue case and vector surveillance data collected from Singapore, to determine the effect of adult Aedes abundance on the risk of dengue transmission. A case was an area with active dengue transmission as indicated by the presence of dengue cluster. A control was an area where no dengue cluster was reported. Using multivariate logistic regression, we analysed 88 cases and 602 controls and estimated the odds of dengue cluster formation at various adult Aedes abundance levels, estimated by the mean number of adult female Aedes per Gravitrap per week and categorised into Low, Moderate, High and Very High abundance level. We found that the risk of dengue cluster formation was positively associated with adult Ae. aegypti abundance. We observed a three to four-fold increase in the odds of dengue clusters forming in areas with High (AOR: 3.40, 95% CI: 2.09, 5.52) and Very High (AOR: 3.99, 95% CI: 2.46, 6.46) adult Aedes aegypti abundance level compared to those with low Ae. aegypti abundance level. Our study strengthens the evidence for the use of adult Aedes indices for dengue risk assessment and early warning for dengue outbreaks. Entomological indicators of adult Ae. aegypti could be used to anticipate and prioritize areas for dengue control.
Author summary
The House Index and Breteau index are the most widely used indices for vector surveillance. However, the appropriateness of these larval indices has been questioned as they have not been satisfactorily linked to disease transmission. Indices based on actual counts of adult Aedes are likely to be more useful in assessing transmission risk, but the adult Aedes population is rarely sampled as such sampling is perceived as time-consuming and difficult. In this study, which used national data from Singapore, we investigated the effect of adult Aedes abundance on the risk of dengue transmission. We found that areas with high Ae. aegypti abundance had a higher odds of dengue cluster formation. Our study findings suggest that the adult Ae. aegypti abundance can allow early identification of geographical localities at high risk of dengue transmission and thus, a useful indicator to anticipate and prioritize areas for dengue control.
Introduction
Dengue is currently regarded as one of the most important mosquito-borne viral diseases. The global incidence of dengue has been increasing drastically around the world in recent decades [1,2]. One study estimates that 390 million dengue infections occur worldwide each year, of which 96 million manifest clinically [3]. Dengue is endemic throughout the tropics, and almost half of the world’s population are at risk of infection, the majority of whom (70%) live in the Asia-Pacific region [1]. The geographical range of dengue is expected to further expand due to climate change and urbanisation [4].
Dengue is transmitted primarily by the vector Aedes aegypti, and a secondary vector Aedes albopictus [5]. Vector surveillance, recommended by the World Health Organization, is a routine practice in many dengue endemic countries to provide quantifiable measure of fluctuations in magnitude and geographical distribution of dengue vector populations [6]. The House Index and Breteau Index are the most widely used indices for vector surveillance [1,6]. However, the appropriateness of these indices had been questioned as they have not been satisfactorily linked to disease transmission [7,8]. These larval indices do not seem to reliably assess transmission risk, which policy makers typically rely on to define thresholds for dengue epidemic alerts and to set targets for vector control programs [9,10]. Indices based on actual counts of adult Ae. aegypti are likely to be more useful in assessing transmission risk, but the adult Ae. aegypti population is rarely sampled as such sampling is perceived as time-consuming and difficult [6,11]. Another difficulty for adult sampling is the variability linked to the adult sampling tools since each sampling tool has its own attractiveness and can provide very different numbers when used in the same location [12]. Thus, only a few studies have attempted to examine the relationship between the adult Ae. aegypti and dengue transmission [8]. This study therefore aimed to assess the effect of adult Aedes abundance on risk of dengue transmission using nationally representative surveillance data in Singapore.
Methods
Ethics statement
The study was granted approval by the Environmental Health Institute of the National Environment Agency, Singapore. The study did not involve human participants.
Study setting
Located within Southeast-Asia, Singapore is a city-state with a land area of 724.2km2 and an estimated population of 5.8 million [13]. Singapore experiences a tropical climate, characterized by abundant rainfall, high and uniform temperatures and high humidity throughout the year [14]. Dengue is hyper-endemic in this country with year-round circulation of all four dengue virus (DENV) serotypes and a cyclic epidemic pattern that oscillates between DENV-1 and DENV-2 as the dominant serotype [15]. In 2019 and 2020, Singapore experienced explosive dengue outbreaks, reporting 15,998 and 35,315 laboratory confirmed dengue cases respectively [16,17]. The low herd immunity levels of Singapore’s resident population and the increased human population density are factors that may have contributed to the population’s susceptibility to outbreaks [18,19].
There are three types of residences in Singapore: public high-rise apartment blocks, private high-rise apartment blocks and landed houses. Built by the government, the public high-rise apartment blocks are the most common housing type, accounting for close to 80% of Singapore residence [20]. These apartment blocks range from three to 50 storeys, with the majority between 12 and 30 storeys. The remaining residents live in private high-rise apartment blocks and landed houses built by private developers.
Entomological data
Gravitraps (Fig 1), which lure and trap gravid female Aedes mosquitoes, were used as a surveillance tool by the National Environment Agency (NEA), the lead government authority for vector control in Singapore. NEA has deployed Gravitraps across all public residential high-rise apartment blocks, to monitor the spatio-temporal variability of the adult Aedes population. Gravitraps were placed along the common corridors of the apartment blocks in the ratio of 1 Gravitrap for every 20 households (i.e. two Gravitraps on each of three floors per apartment block: lower floor(2nd), mid floor (5th or 6th) and high floor (10th or 11th). Mosquitoes were collected from the Gravitraps on a weekly basis and are entomologically identified in the laboratory using mosquito identification keys [21,22]. More than 50,000 Gravitraps were deployed as of 2019 [23].
Fig 1. Photo of a Gravitrap with mosquitoes trapped on the sticky lining.

Dengue case data
In Singapore, the reporting of all laboratory confirmed dengue infections is mandatory. These reports are consolidated through the national surveillance system by the Ministry of Health (Singapore) and transmitted to the NEA for the purpose of implementing disease control activities. Reported dengue infections are linked in space and time in order to determine areas of elevated dengue transmission requiring control activities such as house-to-house inspections to search and destroy mosquito breeding habitats and community engagement to create awareness for dengue and eliciting dengue prevention action [24]. Such areas are defined as “dengue clusters” and each area is formed when two or more reported infections fall within a 150-meter radius and with illness onset dates within a 14-day period of each other [25].
Statistical analysis
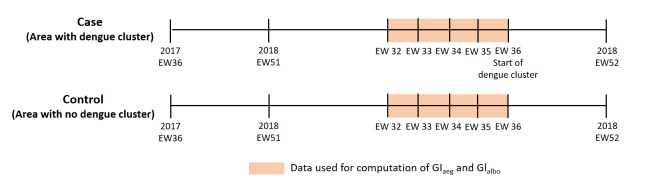
We obtained records of all dengue clusters and adult Aedes vector data between September 2017 and December 2018. We restricted our analysis to areas deployed with Gravitraps. We used an epidemiological “case-control” study design to examine the effect of adult Aedes abundance on the risk of dengue transmission. We defined a case as an area with a dengue cluster and a control as an area (i.e. a group of 10–15 public high-rise apartment blocks that are typically 150m apart and bounded by the roads) where no dengue cluster was reported throughout the study duration (Fig 2). The mean Ae. aegypti trap rate (GIaeg) and mean Ae. albopictus trap rate (GIalbo), defined as the mean number of adult female Ae. aegypti and Ae. albopictus trapped per Gravitrap per week respectively, were used to estimate the adult Aedes abundance in an area. We calculated the mean GIaeg and GIalbo of each dengue cluster, four weeks preceding its formation. Similarly, we calculated the mean GIaeg and GIalbo from the same four-week period for the controls (Fig 3). Wilcoxon rank-sum test was used to test for differences in the GIaeg and GIalbo between the two groups. To assess the association between adult Aedes abundance and risk of dengue transmission, we stratified the GIaeg and GIalbo into four levels (i.e. Low, Moderate, High and Very High) based on quartiles. We estimated the odds of dengue cluster formation at different Aedes abundance levels by fitting a multivariable logistic regression with the outcome measure being the presence of dengue clusters (cases) coded as “1” and the absence of dengue clusters (controls) as “0”, the GIaeg and GIalbo as the independent variable, and adjusting for seasonal effect and potential confounder such as the geographical districts. Due to data unavailability, we were not able to account for the effect of other confounders such as the localized initiatives for dengue prevention and control.
Fig 2. Map showing an example of a case (red) and controls (green). The figure was created with base layer obtained from https://landsatlook.usgs.gov/.
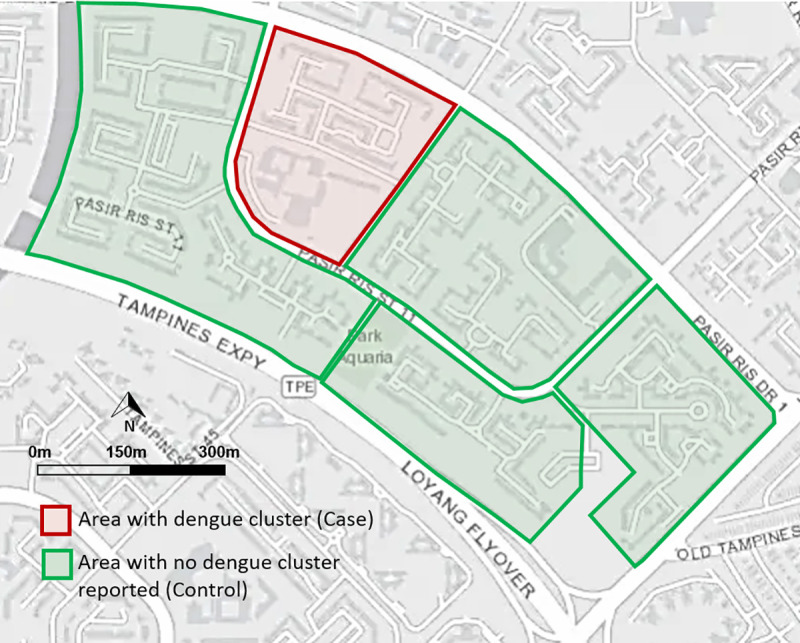
Fig 3. Illustration on the computation of mean GIaeg and GIalbo in cases (area with dengue cluster) and controls (area with no dengue cluster).
Results
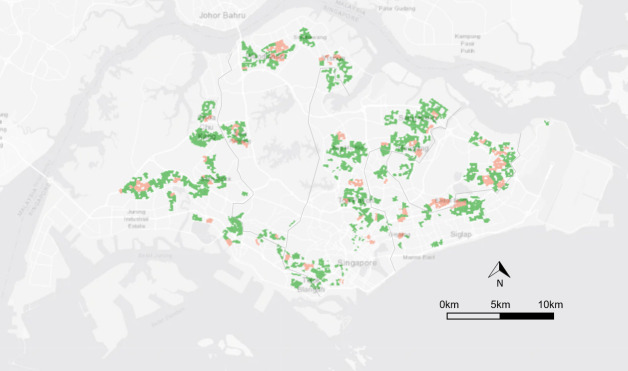
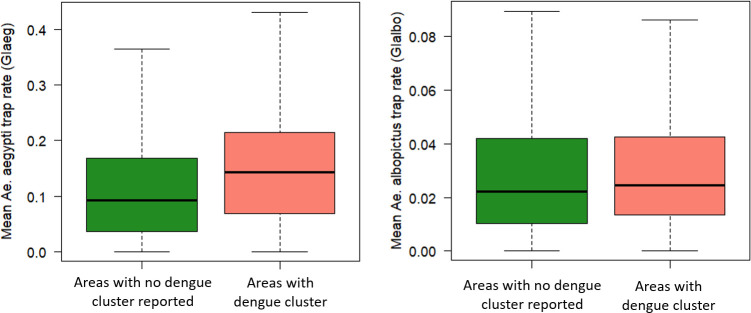
Over the study duration of 69 weeks, the GIaeg ranged between 0.00 and 0.98 and averaging at 0.12 while the GIalbo ranged between 0.00 to 0.79 and averaging at 0.03. There were small variations in the GIaeg and GIalbo throughout the year (S1 Fig). The GIaeg and GIalbo distributions were generally the same across the five geographical districts except the South-East district, which had a lower GIalbo (Tables 1 and 2). We analysed 88 cases and 602 controls. Fig 4 shows the spatial distribution of the cases and controls. The cases and controls are well distributed across the country and thus nationally representative. Areas with dengue cluster had a higher GIaeg than those with no dengue cluster. There was no significant difference in the Glalbo between the two groups (Fig 5).
Table 1. Summary statistics of the mean Ae. aegypti trap rates, GIaeg. Interquartile range refers to the 1st and 3rd quartile.
| Variable | Mean (SD) | Median | Interquartile Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Overall | 0.12 (0.09) | 0.10 | 0.05–0.17 | 0.00 | 0.98 |
| Geographical districts | |||||
| Central | 0.11 (0.09) | 0.10 | 0.05–0.16 | 0.00 | 0.91 |
| North-East | 0.13 (0.12) | 0.11 | 0.04–0.19 | 0.00 | 0.81 |
| North-West | 0.11 (0.10) | 0.09 | 0.04–0.16 | 0.00 | 0.74 |
| South-East | 0.11 (0.08) | 0.10 | 0.05–0.16 | 0.00 | 0.53 |
| South-West | 0.12 (0.11) | 0.10 | 0.04–0.17 | 0.00 | 0.98 |
Table 2. Summary statistics of the mean Ae. albopictus trap rates, GIalbo. Interquartile range refers to the 1st and 3rd quartile.
| Variable | Mean (SD) | Median | Interquartile Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Overall | 0.03 (0.03) | 0.02 | 0.01–0.04 | 0.00 | 0.79 |
| Geographical districts | |||||
| Central | 0.04 (0.03) | 0.03 | 0.01–0.05 | 0.00 | 0.29 |
| North-East | 0.03 (0.02) | 0.02 | 0.01–0.04 | 0.00 | 0.22 |
| North-West | 0.03 (0.03) | 0.02 | 0.01–0.04 | 0.00 | 0.24 |
| South-East | 0.02 (0.04) | 0.01 | 0.005–0.03 | 0.00 | 0.79 |
| South-West | 0.04 (0.03) | 0.03 | 0.01–0.05 | 0.00 | 0.41 |
Fig 4. Map showing the spatial distribution of the cases (red) and controls (green).
The grey boundary lines indicate the five geographical districts in Singapore. The figure was created using R software with base layer obtained from https://landsatlook.usgs.gov/.
Fig 5. Comparison of the GIaeg and GIalbo between cases (areas with dengue cluster) and controls (areas with no dengue cluster reported).
For the univariate analysis, the odds of dengue cluster forming in areas with Moderate, High and Very High GIaeg level was 2.22 (95% CI: 1.37, 3.60), 3.30 (95% CI: 2.07, 5.27) and 4.14 (95% CI: 2.63, 6.49) compared to that of areas with Low GIaeg (S1 Table). After adjusting for the effects of potential confounders, the risk of dengue transmission in areas with Moderate, High and Very High GIaeg level remained high. We observed a two to four-fold increase in the odds of dengue clusters in areas with Moderate GIaeg (AOR: 2.38, 95% CI: 1.45, 3.89), High GIaeg (AOR: 3.40, 95% CI: 2.09, 5.52) and Very High GIaeg (AOR: 3.99, 95% CI: 2.46, 6,46) compared to those with Low GIaeg (Table 3). There was no significant difference in the odds of dengue cluster among the different GIalbo levels (S2 Table). Among the geographical districts, North-East district (AOR: 2.95, 95% CI: 2.02, 4.31) had the highest odds of dengue cluster formation compared to the Central district. Among the epidemiological month, March, April, July and August had lower odds of dengue cluster formation relative to January (S2 Table).
Table 3. Adjusted odds ratios for factors associated with dengue cluster formation.
| Variable | Odds Ratio | 95% CI | |
|---|---|---|---|
| Mean Ae. aegypti trap rate (GIaeg) | |||
| Low: | GIaeg < 0.05 | Referent | |
| Moderate: | 0.05 ≤ GIaeg < 0.10 | 2.38 | 1.45–3.89 |
| High: | 0.10 ≤ GIaeg < 0.17 | 3.40 | 2.09–5.52 |
| Very High: | GIaeg ≥ 0.17 | 3.99 | 2.46–6.46 |
| Geographical district | |||
| Central | Referent | ||
| North-East | 2.95 | 2.02–4.31 | |
| North-West | 1.81 | 1.20–2.72 | |
| South-East | 1.10 | 0.59–2.03 | |
| South-West | 1.64 | 1.06–2.53 | |
Discussion
Various researchers have investigated the relationship between dengue transmission and the immature stages of Aedes—larval and pupal [26–28]. However, studies examining the relationship between dengue transmission and the adult Aedes remain scarce. In this present study, we examined the effect of adult Aedes abundance on risk of dengue transmission. After adjusting for the effect of potential confounders, we found that the risk of dengue transmission was positively associated with adult Ae. aegypti abundance. This finding was consistent with the few studies in existing literature. A study in Venezuela found a positive correlation between the number of dengue cases and abundance of Ae. aegypti adults [29]. Another study in Puerto Rico also showed that the number of adult Ae. aegypti was a significant risk factor for dengue infection [30]. Our study findings suggest that the entomological measures of adult Ae. aegypti can be a useful indicator to anticipate and prioritize areas for dengue control.
In our study, the adult Ae. albopictus abundance was not a significant predictor of dengue transmission. This is not surprising since Ae. albopictus is native and ubiquitous throughout Singapore [31]. Moreover, Ae. albopictus is generally known to be a less efficient vector of dengue than Ae. aegypti as it is not as well adapted to urban environments and is less anthropophilic than Ae. aegypti [32]. There were spatial differences in the transmission risk among the geographical districts of residence, with higher levels observed in the North-East district compared to the others. This appears to correspond with historical trends for dengue reports in Singapore which showed that North-East district had comparatively higher levels of reported dengue [31].
To the best of our knowledge, this is the first large scale, nationally representative study that examined the effect of adult Aedes abundance on risk of dengue transmission. Unlike previous studies, we used a case-control study design whereby entomological data were collected from areas of confirmed dengue cluster as well as control areas (i.e. no dengue cluster). We only used laboratory confirmed dengue infections, and thus the potential for case misclassification was low. Although unreported and asymptotic infections form the majority of dengue infections in Singapore [33], this is unlikely to lead to inaccurate quantifications of the effect of adult Aedes abundance on the risk of dengue transmission as the infection to case notification ratio is unlikely to vary with Aedes abundance. The Gravitrap, like all adult traps, catches only a fraction of the mosquito population. Despite that, it allows us to assess the relative abundance of Aedes across time and space. Furthermore, our study has shown that the Gravitrap is sensitive enough to indicate transmission risk, demonstrating the relevance of Gravitraps catches. We were unable to account for the effects of vector control activities on dengue transmission. Home inspections and community efforts aimed at removing larval habitats and the use of chemical control to reduce adult mosquito population could to some extent mask the true association between Aedes abundance and risk of dengue transmission.
With the disease burden of dengue increasing across the world, identifying specific locations where dengue outbreak risks are the highest is of critical importance [8]. Our study strengthens the evidence for the use of adult Ae. aegypti indices for dengue risk assessment and early warning for dengue outbreaks. We showed that the adult Ae. aegypti abundance can allow early identification of geographic localities at high risk of dengue transmission for advance vector control activities. Therefore, the use of adult Aedes entomological surveillance in conjunction with a disease surveillance system can benefit dengue control strategies in other cities where dengue is endemic.
Supporting information
(TIF)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank the following people who have contributed to the study; the staff of the Environmental Public Health Operations Department, the Control of Ops Branch 1 team for providing the dengue cluster and Gravitrap surveillance data, and the staff of the Environmental Health Institute, the Applied Entomology team for adult mosquito identification.
Data Availability
The data underlying the results presented in the study are owned by a third party. They are available upon reasonable request from the Environmental Public Health Operations Group of the National Environment Agency (email: Contact_NEA@nea.gov.sg).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization. Global strategy for dengue prevention and control 2012–2020.
- 2.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33 4:330–42. doi: 10.1016/s0188-4409(02)00378-8 [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496 7446:504 doi: 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messina JP, Brady OJ, Golding N, Kraemer MU, Wint GW, Ray SE, et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019;4 9:1508–15. doi: 10.1038/s41564-019-0476-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4 5:e646. doi: 10.1371/journal.pntd.0000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Focks DA. A review of entomological sampling methods and indicators for dengue vectors. World Health Organization; 2004. doi: 10.1603/0022-2585-41.6.1123 [DOI] [Google Scholar]
- 7.Romero-Vivas CM, Falconar AK. Investigation of relationships between Aedes aegypti egg, larvae, pupae, and adult density indices where their main breeding sites were located indoors. J. Am. Mosq. Control Assoc. 2005;21 1:15–21. doi: 10.2987/8756-971X(2005)21[15:IORBAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 8.Bowman LR, Runge-Ranzinger S, McCall PJ. Assessing the relationship between vector indices and dengue transmission: a systematic review of the evidence. PLoS Negl Trop Dis. 2014;8 5:e2848. doi: 10.1371/journal.pntd.0002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am. J. Trop. Med. Hyg. 2000;62 1:11–8. [PubMed] [Google Scholar]
- 10.Sanchez L, Vanlerberghe V, Alfonso L, del Carmen Marquetti M, Guzman MG, Bisset J, et al. Aedes aegypti larval indices and risk for dengue epidemics. Emerg Infect Dis. 2006;12 5:800. doi: 10.3201/eid1205.050866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anders KL, Hay SI. Lessons from malaria control to help meet the rising challenge of dengue. The Lancet Infect Dis. 2012;12 12:977–84. doi: 10.1016/S1473-3099(12)70246-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cansado-Utrilla C, Jeffries CL, Kristan M, Brugman VA, Heard P, Camara G, et al. An assessment of adult mosquito collection techniques for studying species abundance and diversity in Maferinyah, Guinea. Parasit Vectors. 2020; 13:1–6. doi: 10.1186/s13071-019-3862-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singapore Department of Statistics [Internet]. Population and Population Structure [cited 16 Nov 2020]. Available from: https://www.singstat.gov.sg/find-data/search-by-theme/population/population-and-population-structure/latest-data.
- 14.Meteorological Service Singapore. Climate of Singapore [cited 16 Nov 2020]. Available from: http://www.weather.gov.sg/climate-climate-of-singapore/.
- 15.Rajarethinam J, Ang L-W, Ong J, Ycasas J, Hapuarachchi HC, Yap G, et al. Dengue in Singapore from 2004 to 2016: Cyclical epidemic patterns dominated by serotypes 1 and 2. Am J Trop Med Hyg. 2018;99 1:204–10. doi: 10.4269/ajtmh.17-0819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Environment Agency, Singapore. NEA Urges Continued Vigilance At Start of 2021 As Aedes Aegypti Mosquito Population Remains High and Many Residents Continue To Work From Home. NEA News. 25 Jan 2021. [Cited 01 Mar 2021]. Available from: https://www.nea.gov.sg/media/news/news/index/nea-urges-continued-vigilance-at-start-of-2021-as-aedes-aegypti-mosquito-population-remains-high-and-many-residents-continue-to-work-from-home [Google Scholar]
- 17.National Environment Agency, Singapore. NEA Urges Continued Vigilance As Aedes Aegypti Mosquito Population And Number Of Dengue Cases Remain High At The Start Of 2020. NEA News. 15 Jan 2020. [Cited 01 Mar 2021]. Available from: https://www.nea.gov.sg/media/news/news/index/nea-urges-continued-vigilance-as-aedes-aegypti-mosquito-population-and-number-of-dengue-cases-remain-high-at-the-start-of-2020 [Google Scholar]
- 18.Singapore Department of Statistics [Internet]. Population trends 2019. [cited 01 Mar 2021]. Available from: https://www.singstat.gov.sg/find-data/search-by-theme/population/population-and-population-structure/publications-and-methodology [Google Scholar]
- 19.Low S-L, Lam S, Wong W-Y, Teo D, Ng L-C, Tan L-K. Dengue seroprevalence of healthy adults in Singapore: serosurvey among blood donors, 2009. Am J Trop Med Hyg. 2015;93 1:40–5 doi: 10.4269/ajtmh.14-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singapore Department of Statistics [Internet]. Households [cited 16 Nov 2020]. Available from: https://www.singstat.gov.sg/find-data/search-by-theme/households/households/latest-data.
- 21.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE, Richardson JH. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J Trop Med Public Health. 2010; 41:1–225. [PubMed] [Google Scholar]
- 22.Ong J, Chong C-S, Yap G, Lee C, Abdul Razak MA, Chiang S, et al. Gravitrap deployment for adult Aedes aegypti surveillance and its impact on dengue cases. PLoS Negl Trop Dis. 2020;14 8:e0008528. doi: 10.1371/journal.pntd.0008528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epidemiological News Bulletin [Internet]. Review of the Dengue Situation in Singapore [cited 16 Nov 2020]. Available from: https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/epidemiological-news-bulletin/enb-quarterly_apr-2018-vol-44-no-2-final.pdf.
- 24.Sim S, Ng LC, Lindsay SW, Wilson AL. A greener vision for vector control: The example of the Singapore dengue control programme. PLoS Negl Trop Dis. 2020;14 8:e0008428. doi: 10.1371/journal.pntd.0008428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ler TS, Ang LW, Yap GS, Ng LC, Tai JC, James L, et al. Epidemiological characteristics of the 2005 and 2007 dengue epidemics in Singapore–similarities and distinctions. Western Pac Surveill Response J. 2011;2 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham HV, Doan HT, Phan TT, Minh NN. Ecological factors associated with dengue fever in a Central Highlands province, Vietnam. BMC Infect. Dis. 2011;11 1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulaiman SA, Pawanchee ZA, Arifin Z, Wahab A. Relationship between Breteau and House indices and cases of dengue/dengue hemorrhagic fever in Kuala Lumpur, Malaysia. J Am Mosq Control Assoc. 1996;12:494–6. [PubMed] [Google Scholar]
- 28.Chadee DD. Dengue cases and Aedes aegypti indices in Trinidad, West Indies. Acta Trop. 2009;112 2:174–80. doi: 10.1016/j.actatropica.2009.07.017 [DOI] [PubMed] [Google Scholar]
- 29.Rubio-Palis Y, Pérez-Ybarra LM, Infante-Ruíz M, Comach G, Urdaneta-Márquez L. Influence of climatic variables on dengue cases and abundance of Aedes aegypti (Diptera: Culicidae) in Maracay, Venezuela. Boletin De Malariologia Y Salud Ambiental. 2011;51 2:145–57. [Google Scholar]
- 30.Rodriguez-Figueroa L, Rigau-Perez JG, Suarez EL, Reiter P. Risk factors for dengue infection during an outbreak in Yanes, Puerto Rico in 1991. Am. J. Trop. Med. Hyg. 1995;52 6:496–502. doi: 10.4269/ajtmh.1995.52.496 [DOI] [PubMed] [Google Scholar]
- 31.Ong J, Liu X, Rajarethinam J, Yap G, Ho D, Ng LC. A novel entomological index, Aedes aegypti Breeding Percentage, reveals the geographical spread of the dengue vector in Singapore and serves as a spatial risk indicator for dengue. Parasit Vectors. 2019;12 1:17. doi: 10.1186/s13071-018-3281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezza G. Aedes albopictus and the reemergence of Dengue. BMC public health. 2012;12 1:72. doi: 10.1186/1471-2458-12-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health Singapore [Internet]. Dengue [cited 03 May 2021]. Available from: https://www.moh.gov.sg/diseases-updates/dengue.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are owned by a third party. They are available upon reasonable request from the Environmental Public Health Operations Group of the National Environment Agency (email: Contact_NEA@nea.gov.sg).