Abstract
In the recent past, the sirtuins have been under intense investigation for their roles in biology and disease, including cancer. The sirtuin SIRT6 is comparatively a lesser studied member of this family of seven proteins. Like certain other sirtuins, SIRT6 is emerging to have an oncogenic function as well as tumor suppressor roles in cancer. Limited studies have been conducted assessing the role and functional significance of SIRT6 in melanoma and non-melanoma skin cancers. In this review, we have attempted to critically dissect the potential role and significance of SIRT6 in skin carcinogenesis. With limited available information to date, SIRT6 appears to have a pro-proliferative function in non-melanoma skin cancers (NMSCs), including squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). In addition, SIRT6 is also emerging to have an oncogenic function in melanoma. Moreover, we have provided information regarding the available SIRT6 inhibitors. Conclusively, it appears that additional comprehensive studies are needed to establish the role of SIRT6 in skin biology and skin diseases, including cancer. Further, concerted efforts are needed to characterize the stage-specific role of SIRT6 in skin cancers.
Keywords: SIRT6, Skin, Melanoma, Skin Cancer
Introduction
Over the past decade, the role of Sirtuin-6 (SIRT6) in cellular processes has been rapidly evolving. Researchers have become interested in defining the role of this sirtuin in important cellular functions and disease conditions, including metabolism, aging, and cancer. SIRT6 is one of seven members of the histone deacetylase (HDAC) class III, sirtuin clan (SIRT1-SIRT7), an ancient family of proteins that have been conserved through evolution from prokaryotic to eukaryotic cell types. Sirtuins were first discovered in yeast as the Silent Information Regulator 2 (SIR2), which functioned as an essential gene silencing complex1. The catalytic mechanism of sirtuins differs from the rest of the HDACs due to nicotinamide adenine dinucleotide (NAD+) requirement as an enzymatic co-factor in order to catabolize the deacetylation of a given substrate2. The key feature of NAD+ requirement facilitates the function of sirtuins in cellular metabolism and the modulation of histones and transcription factors. Each sirtuin is characterized by an amino acid catalytic core domain of approximately 275 amino acids and also by unique N- and/or C-terminal sequences of variable length.
Interestingly, each sirtuin is biologically unique based on their cellular location, and thus, their functions (Figure 1). Moving from the nucleus to the cytoplasm, sirtuins participate in critical cellular functions, thereby regulating a variety of mechanisms for cellular homeostasis. In mammals, sirtuins have shown to undergo nuclear-cytoplasmic shuttling, where their functions within the cell are suggested to change depending on each tissue and cell type3. For instance, SIRT1, 6, and 7 are actively located in the nucleus, accompanied by the roles they each possess in DNA repair, transcription, and epigenetics3. SIRT1, as well as SIRT2, can function in both the nucleus and cytoplasm3. SIRT2 is mostly found in the cytoplasm, where it performs key functions associated to microtubule stabilization, gluconeogenesis, and inflammatory response4. SIRT3, 4, and 5 are described as mitochondrial sirtuins, based on their localization and observed roles in cellular metabolism2. Altogether, despite the structural similarities among the sirtuins, each sirtuin has its own role, regulating critical mechanisms within the cell that help maintain homeostasis.
Figure 1: Sirtuin localization and function.
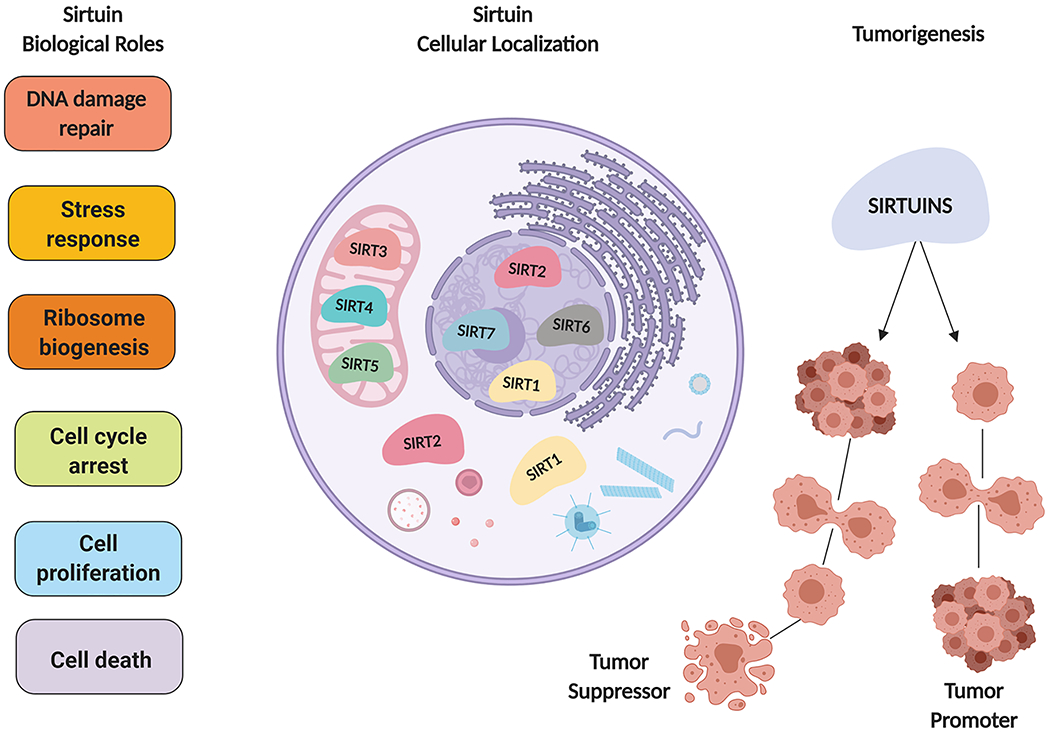
Each sirtuin is biologically unique based on their cellular location and function. With controversial roles in cancer sirtuins possess important biological functions that are necessary for cellular homeostasis.
Extensive studies have been undertaken in order to understand the function of sirtuins in different biological systems. Nonetheless, a deeper understanding of the mechanisms underlying the functions of each sirtuin, in distinct biological processes, remained unexplored. Formerly, SIRT6 was only studied as a chromatin regulator, now as a nascent topic of study, more researchers have become interested in defining the role of SIRT6 in cancers, including skin neoplasms. It is critical to explore the mechanistic workings of SIRT6 in skin, since it may be a key regulator to engendering strategies that may have significant impact in the field of cutaneous biology and towards development of new therapeutic approaches for skin diseases and skin cancers.
Essential Biochemical Functions of SIRT6
Elucidating the specific molecular and biochemical roles of SIRT6 is challenging, mostly due to its three distinctive enzymatic activities in histone-deacetylation5, adenosine diphosphate (ADP)-ribosylation6 and most recently found lysine defatty-acylation (Figure 2)7. Typically, SIRT6 has been shown to deacetylate targets sites on histone 3 (H3), specifically lysines H3K9ac, H3K18ac, and H3k56ac5. Observations of SIRT6 auto-mono-ADP-ribosylation were first observed when an incubation with NAD was conducted in vitro6. This was followed by reports of SIRT6 dependent mono-ADP ribosylation of poly adenosine diphosphate-ribose polymerase 1(PARP1) polymerase and Kruppel-associated box associated protein-1 (KAP1)8,9. These reports suggested that the diverse enzymatic activities conducted by SIRT6 could be specific to certain substrates as well as physiological conditions. Intriguingly, new studies in SIRT6 biology highlighted that SIRT6 can also efficiently catalyze the removal of certain long-chain fatty-acyl groups from lysine in vitro, suggesting additional important physiological functions conducted by SIRT6. Initial studies detected defatty-acylation activity of SIRT6 in in vitro assays on tumor necrosis factor alpha (TNF-α), lysine K19 or K20, and H3 peptides with 6- to 16-carbon chain fatty-acyl groups on lysine K910,11. Recently, SIRT6 was shown to remove long chain octenoyl-groups on H3K9, H3K18 and H3K2712. Nonetheless, a deeper understanding of SIRT6 substrate specific activities is still needed in order to link these processes to important cellular functions.
Figure 2: SIRT6 biochemical functions associated with aging and cancer.
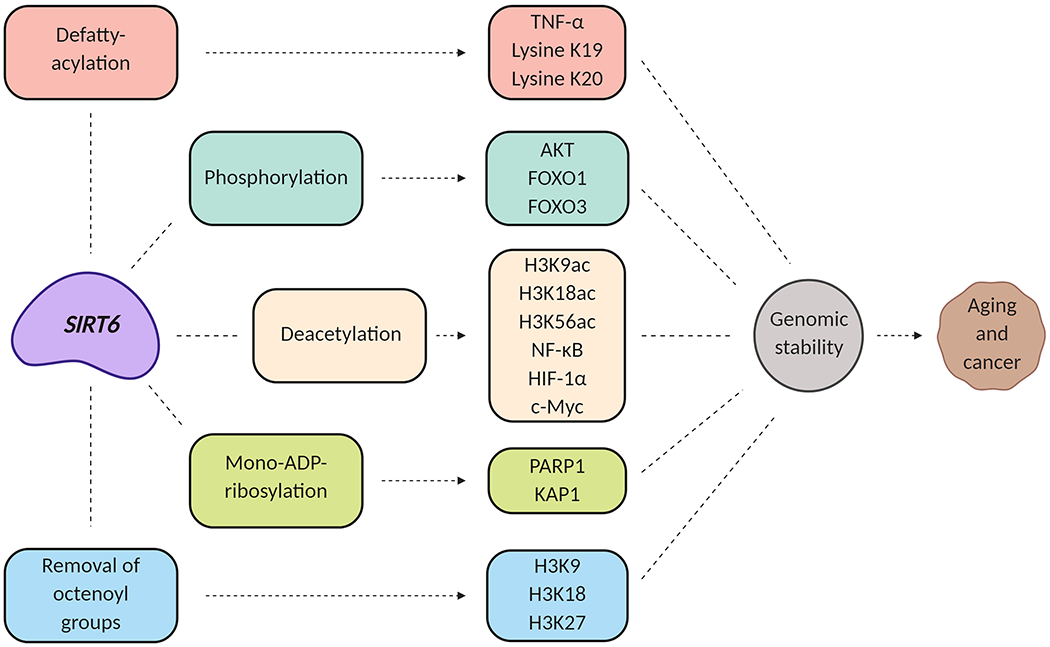
SIRT6 interacts with multiple molecular targets that are involved in aging and potentially carcinogenesis.
SIRT6 Connection between Aging and Cancer
Because of the important biochemical functions of SIRT6 in biological systems, SIRT6 plays a critical role as a modulator of transcription factors that are associated with important cellular functions such as telomere maintenance, inflammation, metabolism, disease development and aging (e.g., nuclear factor-kappa B (NF-κB), hypoxia inducible factor 1 subunit alpha (HIF-1α), and cellular-Myelocytomatosis (c-Myc))(Figure 2)5. Unfortunately, aging has been linked to the development of various diseases, including cancer13. The likelihood of developing neoplasms increases with aging. Nevertheless, aging as a process remains complex, as it encompasses a variety of factors13. These factors include elements that affect lifespan, cellular changes, and longevity14. Because of the role of SIRT6 in longevity, it may be viewed as a potential connection between aging and cancer. Throughout the years, sirtuins have been associated with epigenetic changes that maybe linked with aging and cancer. Indeed, loss of genomic stability is an important aspect of cancer. In an important study, Tennen and colleagues demonstrated a role for SIRT6 in regulating an ageing-associated epigenetic silencing process and provided evidence suggesting that SIRT6 is required for maintenance of telomere position effect in human cells15. In another study, Michishita and colleagues proposed that SIRT6 contributes to the propagation of a specialized chromatin state at telomeres, required for proper telomere metabolism and function. This study linked chromatin regulation by SIRT6 to telomere maintenance and organismal aging16. In another recent study, Tasselli et al demonstrated that SIRT6 deacetylates H3K18ac at pericentric chromatin to prevent mitotic errors and cellular senescence17. Their study unraveled a new function for SIRT6 that provides a connection between SIRT6 and aging- and cancer- related dysfunction in mammalian cells. Moreover, Endish et al showed that reduced levels of SIRT6 lead to Hutchinson-Gilford Progeria Syndrome (HGPS), a human premature aging disorder18. Both HGPS cells and SIRT6 deficient cells showed abnormalities in pericentric heterochromatin, aberrant transcription and dysfunction of telomeres18. Additionally, the group demonstrated that restoring SIRT6 expression in HGPS cells may partially impede senescence and the formation of dysmorphic nuclei. In fact, the authors implied that lamin A/C (LMNA) point mutation, the established main molecular pathology underlying HGPS, occurs downstream of SIRT6 events. In all, understanding the different mechanisms involved in SIRT6 heterochromatin dysfunction could lead to novel discoveries in aging associated diseases.
At organism levels, SIRT6 was shown to be necessary to maintain health and to prevent the development of several progeroid pathologies in mice19. SIRT6 deficiency in mice was shown to result in premature aging phenotypes leading to death at about four weeks of age19. A key factor in the regulation of lifespan is the insulin-like growth factor 1 (IGF-1) signaling pathway. Mostoslavsky and colleagues showed that serum IGF-1 levels were severely reduced in SIRT6 knockout (KO) mice, IGF-1 levels were low in 12 day old mice before any other phenotypes were detected proposing SIRT6 function is essential for glucose homeostasis and normal IGF-1 levels19. In contrast, overexpression of SIRT6 in male mice increased lifespan20. Interestingly, SIRT6 overexpression extended lifespan only in males, potentially by reducing IGF-1 signaling specifically in white adipose tissue (WAT)20. To understand the changes observed in IGF1 signaling, the authors analyzed different key targets of this pathway. The analysis encompassed the phosphorylation levels of protein-serine/threonine kinase (AKT) activation sites (Thr 308 and Ser 473), FOXO1 (Thr 24) and FOXO3 (Thr 32). The most significantly decreased phosphorylation levels were observed in the perigonadal WAT of SIRT6 transgenic males in comparison to wildtype (WT) males. The levels of AKT on both activation sites, forkhead box protein O1 (FOXO1) and forkhead box protein O3 (FOXO3) in WAT were lower in the transgenic mice. The phosphorylation levels of the IGF-1 receptor (Tyr 1135) and S6 (Ser 235/236) were lower in SIRT6 transgenic males than in the WT male littermates. Interestingly, no significant change was found in the phosphorylation levels observed in female mice20. Overall, decrease in the phosphorylation levels of AKT and FOXO proteins in male SIRT6 mice demonstrated that lifespan was regulated by changes in IGF-1 signaling20. Thus, it would be interesting to further study the relationship between IGF-1 signaling and SIRT6 expression in additional animal models of both genders to determine whether the effect SIRT6 has on IGF-1 is strictly gender dependent or time dependent. In another important study, SIRT6 was shown to link H3K9 deacetylation to NF-κB-dependent gene expression and organismal life span21. SIRT6 regulates the signaling of an important target in charge of aging-associated changes in gene expression, namely NF-κB21. SIRT6 was shown to interact with v-rel avian reticulo-endotheliosis viral oncogene homolog A (RELA) subunit of NF-κB and deacetylate H3K9 at NF-κB target gene promoter site21.
In the context of skin, a recent study conducted by Kim et al found a reduction in the expression of SIRT6, as well as Sirtuin-1 (SIRT1) in human dermal fibroblasts (HDF)22. SIRT6 was classified as a late aging biomarker since statistical correlation studies revealed that procollagen Types I and VII, fibrillin-1, along with SIRT6 had a strong correlation with the cellular stiffness of HDFs as the passage number of the cells increased22. This was directly associated with key aging biomarkers found in fibroblasts22. The group proposed reduced levels of SIRT6 expression in older fibroblasts, which had a direct impact in age development of the cell22. In another study, it was found that SIRT6 knockdown in HDFs influence the synthesis and degradation of collagen by hyperactive NF-κB signaling, leading to a decrease in dermal collagen fibrils, suggesting a role of SIRT6 in skin anti-aging23. To further amplify these arguments, Sharma et al determined that human dermal fibroblasts, retrieved from an older human participant, possessed a higher resistance towards reprogramming by classic Yamanaka factors24. Nonetheless, older cells with increased levels of SIRT6 expression during reprogramming, permitted the cells to undergo the process more effectively. Further, increase expression of SIRT6 was found in the younger cells24. Suggesting, SIRT6 is essential for cellular development.
Overall, these findings support a potential role for SIRT6 regulating longevity and indicate that manipulation of SIRT6 levels could possibly lead to a new avenue of treatments for age-related conditions. Very limited information is available regarding the role of SIRT6 in skin aging and skin health. Additional detailed studies are needed to dissect the skin aging pathways modulated by SIRT6.
Tumor Suppressor and Oncogenic properties of SIRT6
The exact role of SIRT6 in cancer remains complex with available evidence for both oncogenic and tumor suppressor properties25–29 (Figure 3).SIRT6 has been shown to be dysregulated in several cancers. Based on published studies, SIRT6 has been shown to imparts its tumor suppressor properties by repressing transcriptional activity of key pathways such as NF-κB, c-Myc, and HIF-1α in osteosarcoma, ovarian, liver, lung, colorectal, bladder, endometrial, and pancreatic cancers30. Wu et al demonstrated that inhibition of SIRT6 lead to cell invasion, proliferation and migration of adrenocortical carcinoma cells that was associated with upregulated toll-like receptor 4(TLR4) and phosphorylation of NF-κB subunit p6531. In another study, SIRT6 was shown to inhibit colon cancer progression by modulating phosphatase and tensin/AKT (PTEN/AKT) signaling32. Further, repression of N-cadherin by SIRT6 has been shown to prevent epithelial-mesenchymal transition linked to metastasis33. Liu et al found that SIRT6 inhibited colorectal cancer stem cell proliferation by targeting cell division cycle 25A (CDC25A) by reducing H3K9 levels34.
Figure 3: SIRT6 tumor suppressor and oncogenic functions.
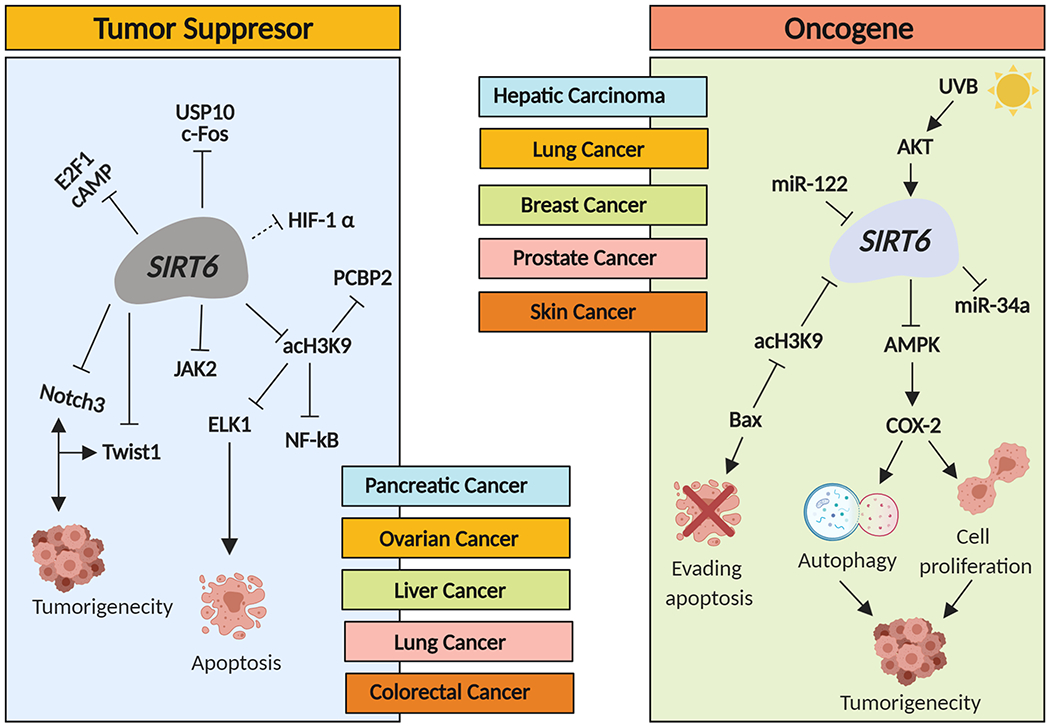
SIRT6 can act as both a tumor promoter and oncogene in cancer by targeting different cellular pathways.
In a very detailed study, Kugel and colleagues found that SIRT6 suppressed pancreatic ductal adenocarcinoma (PDAC) by modulating Lin28b, a negative regulator of the let-7 microRNA35. SIRT6 loss was shown to result in histone hyperacetylation at the Lin28b promoter, Myc recruitment, and induction of Lin28b and let-7 target genes, high mobility group at-hook 2 (HMGA2), insulin-like growth factor binding protein 1 (IGF2BP1), and insulin-like growth factor binding protein 3 (IGF2BP3). Overall, SIRT6 was suggested to be an important PDAC tumor suppressor35. Another study by Lin et al. found that the ubiquitin-specific peptidase 10 (USP10) antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation36. In this study, the downregulation of USP10 was found to activate SIRT6 instability and negatively control activity of the c-Myc oncogene that inhibits cell growth, cell-cycle progression, and tumor formation36. Additional studies suggested a tumor suppressor role of SIRT6 in ovarian cancer37, non-small cell lung cancer38, and glioma39. Wu and colleagues demonstrated that the transcription factor E2F1 enhances glycolysis by suppressing SIRT6 transcription in cancer cells, suggesting a tumor suppressor function of this HDAC40.
On the other hand, SIRT6 has also been shown to have oncogenic function via multiple mechanisms, in several cancers such as hepatic-, lung-, breast-, esophageal-, prostate-and skin-cancers. Huang et al demonstrated that SIRT6 plays an oncogenic role by downregulating genes involved in the NF-κB pathway as well as the phosphatidylinositol-3 Kinase-AKT (PI3K-AKT) pathway, which can also contribute to autophagy induction41. SIRT6 overexpression was shown to be required for the activation of transforming growth factor (TGF)-β1 and H2O2/HOCl reactive oxygen species (ROS) which help to facilitate tumorigenesis of hepatocellular carcinoma (HCC) cells42.Cagnetta and colleagues recently demonstrated that depletion of SIRT6 enhanced the vulnerability of acute myeloid leukemia (AML) cells to DNA-damaging agents43.
Indeed, the role of SIRT6 is extremely complex and needs to be carefully investigated. A very interesting study by Elhanati and colleagues suggested that a reciprocal regulation between SIRT6 and microRNA-3122 (miR-122) controls liver metabolism and predicts hepatocarcinoma prognosis44. In this study, SIRT6 was shown to downregulate miR-122 by deacetylating H3K56 in the promoter region. In addition, miR-122 was shown to bind to three sites on the SIRT6 3’ untranslated region(UTR) and reduces its levels. SIRT6 and miR-122 were shown to oppositely regulate a similar set of metabolic genes and fatty acid β-oxidation. In addition, loss of a negative correlation between SIRT6 and miR-122 expression in hepatocellular carcinoma patients was shown to be significantly associated with better prognosis44.
Thus, overall SIRT6 plays critical roles in the regulation of tumorigenesis through different biological pathways and can either act as an oncogene or a tumor suppressor. Below, we have provided a discussion regarding the potential role of SIRT6 in skin cancers.
SIRT6 in Melanoma and Non-Melanoma Skin Cancers
Molecular and genetic alterations and environmental stress play an important role in the pathogenesis of skin cancers45. The most commonly studied forms of skin cancer are divided into two main categories: melanoma, and non-melanoma skin cancers (NMSC). Incidence of both melanoma and NMSC continue to increase46. One of the causes of skin cancer is the presence of alterations in oncogenes and/or tumor suppressor genes. Particularly, B-rapidly accelerated fibrosarcoma kinase (BRAF), PTEN, and TP53 are some of the important genes mutated in skin cancer47. These genetic modifications can cause cells to proliferate uncontrollably. NMSCs are derived from both cutaneous squamous cells and basal cells, and are further classified into basal cell carcinoma (BCC) or squamous cell carcinoma (SCC)48. The NMSCs are most common cancers and have been associated with significant morbidity. However, they rarely metastasize and are not a liked to high mortality among skin cancers.
The melanomas originate from melanocytes and are a dangerous type of skin cancers. It is estimated that by the end of this year 96,480 individuals will be diagnosed with melanoma46. In contrast to NMSC, melanoma can metastasize to other organs in the body, making this a fatal skin cancer47. If not diagnosed at an early stage, the anticipated 5-year survival of melanoma patients is only 23%46. Moreover, approximately 7,230 Americans with melanoma will die in 201946. Ultraviolet (UV) radiation has been recognized as the most probable environmental causative factor of melanoma as well as NMSCs49. Melanin can act as a barrier against UV radiation, blocking their penetration into skin layers50. Thus, melanin production and content are factors in determining individual’s susceptibility to melanoma. Indeed, metastatic melanomas are very difficult to treat, often acquiring resistance to chemotherapy. Therefore, new treatments for patients with melanoma are continuously evolving, including immunotherapies and targeted therapies51. Notwithstanding, these treatments are often associated with relapse of the malignancy. In the case of BRAF inhibitors, half of the patients have been shown to develop recurrence within less than 6 months51. Consequently, there is an urgent need to find new targets to effectively treat melanoma.
In the recent past, SIRT6 is being actively investigated for its potential role in skin cancers as well as its potential targetability for anti-skin cancer drug development (Figure 4). Unfortunately, limited research has been done regarding defining the role and functional significance of SIRT6 in skin cancer. SIRT6 has been shown to be upregulated in human SCC. Ming et al found that in keratinocytes, SIRT6 functions as an oncogene acting as a downstream target of AKT activation after UVB exposure29. AKT has been suggested to serve as a mediator of UVB-induced SIRT6 expression through c-fos52, because AKT activation induces c-fos expression53, and c-fos induces SIRT6 transcription26. UVB-induced cycloocygenase-endoperoxide synthase 2 (COX-2) upregulation interferes with AKT signaling29, in a manner that SIRT6 and AKT/COX-2 signaling form a network cascade leading to skin carcinogenesis.
Figure 4: SIRT6 functions as an oncogene in skin cancers.
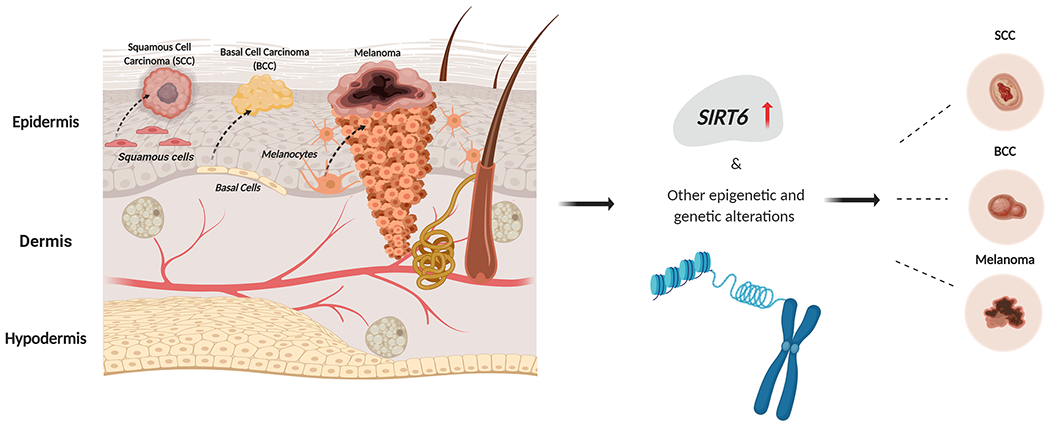
The skin is divided into epidermis, dermis and hypodermis. Within the skin squamous cells, basal cells and melanocytes can give rise to different types of skin cancers. SIRT6 aids proliferation of non-melanoma and melanoma skin cancers.
Lefort et al demonstrated that microRNA-34a (miR-34a) suppresses SIRT6 in keratinocyte differentiation27. In this study, the expression of SIRT6 was found to be inversely correlated to mir-34a in keratinocyte-derived tumors and keratinocyte differentiation. The authors demonstrated that miR-34a is induced with keratinocyte differentiation, and inhibition in cutaneous SCCs can be justified through loss of p53 function54. Interestingly, miR-34a regulates neurogenic locus notch homolog protein 1 (NOTCH1) expression through p53. NOTCH1, is a transmembrane receptor known to regulate keratinocyte differentiation, suggesting pro-differentiation function associated with miR-34a is due to a crosstalk between NOTCH1 and p5354. These studies suggest that SIRT6 is important role in keratinocytes and their differentiation, potentially acting as a pro-proliferative sirtuin in keratinocytic cancers.
Very limited information is currently available regarding the role of SIRT6 in BCC. In one study, Temel et al investigated the expression profile of all mammalian sirtuins in non-tumoral and tumor tissues with BCC, in a total of twenty-seven patients. Expression levels of SIRT6 mRNA were not altered in tumor tissues compared with non-tumor tissue samples55. Nonetheless, probably because of a lack of enough samples, this study did not focus of a stage-specific differences in the expression of SIRT655. However, the authors speculated that SIRT6 expression could increase with UVB-induced DNA damage repair in BCC. While it is possible that SIRT6 has tumor modulatory functions in BCC, additional studies are needed55.
A few studies have been focused on defining the role of SIRT6 in melanoma. Recently, we demonstrated that SIRT6 is upregulated, both at mRNA and protein levels, in melanoma cell lines, as well as clinical tissue samples of human melanoma56. Further, lentiviral short hairpin RNA (shRNA)-mediated transient knockdown of SIRT6 in human melanoma cells was found to induce cellular senescence, accompanied by a marked accumulation of cells in G1 phase arrest56. In addition, our data showed that SIRT6 inhibition significantly alters genes and pathways related to autophagy in melanoma cells and reduced the conversion of microtubule-associated protein 1a/1b-light chain 3 (LC3) protein from its free form LC3-I to phosphatidylethanolamine-conjugated form LC3-II, which is known to initiate autophagosome formation56. This study from our laboratory was supported by another study where Wang et al demonstrated that aberrant expression of SIRT6 contributes to melanoma growth57. The authors suggested that the effects of SIRT6 on autophagy in melanoma are mediated by IGF-AKT signaling. In melanoma, autophagy has been shown to suppress tumorigenesis by degrading oncogenic and toxic proteins at initial stages58 or promote tumor development in established tumors by reducing cancer cell vulnerability to stress and maintaining tumor cells homeostasis58. Thus, based on these studies, there seems to be a positive association between SIRT6 expression and the levels of autophagy in melanoma56,57. Wang et al, provides strong evidence supporting IGF-AKT as one of the intermediary players between SIRT6 and autophagy in melanoma, where the deacetylase activity of SIRT6 appears to be indispensable57. AKT has been shown to translocate to the plasma membrane upon PI3K activation, stimulating a wide range of downstream signaling targets in melanoma that regulate cell cycle, apoptosis, DNA repair, glucose metabolism, cell growth, invasion and angiogenesis53. The main target of AKT is mTOR, which has a central role in PI3K-AKT pathway in cancer53. Thus, it would be interesting to explore the association between SIRT6 and PI3K-AKT-mTOR signaling pathway as it may serve as a new avenue for melanoma treatments.
Based on the limited number of studies done so far, SIRT6 seems to affect a number of important processes, which are relevant to cutaneous neoplasms, including BCC, SCC, and melanomas (Figure 5). Indeed, at the mechanistic level, a number of targets and processes have been linked with SIRT6 in the skin (Figure 6 and Table 1). Nevertheless, these published studies point towards a pro-proliferative role of SIRT6 in skin cancers, promoting cellular proliferation and survival53,56,58.
Figure 5: SIRT6 modulates cellular mechanisms and processes relevant to skin carcinogenesis.
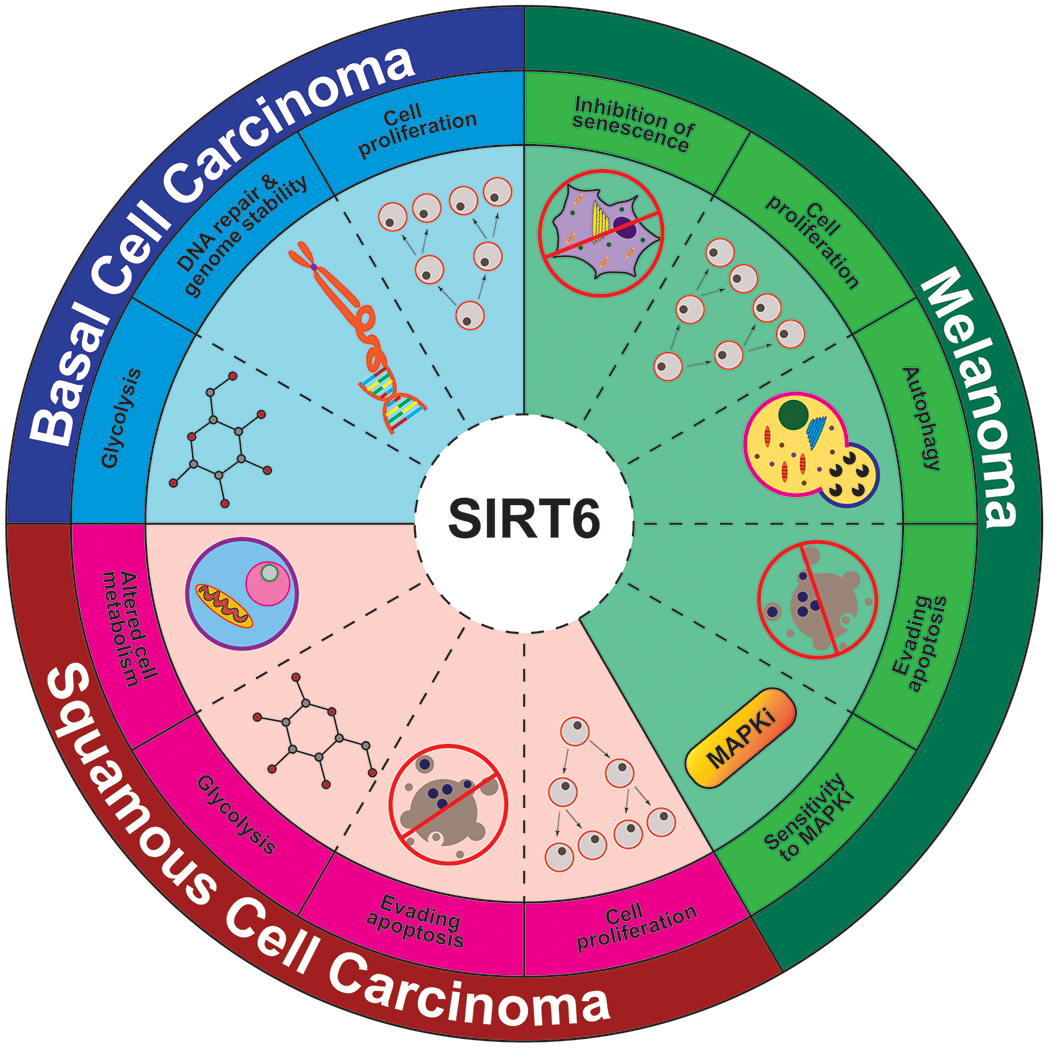
The important cellular processes which are known to be linked to SIRT6 and are relevant to development and/or progression of melanoma and non-melanoma skin cancers are depicted.
Figure 6: SIRT6cellular functions and their impact on skin cancer.
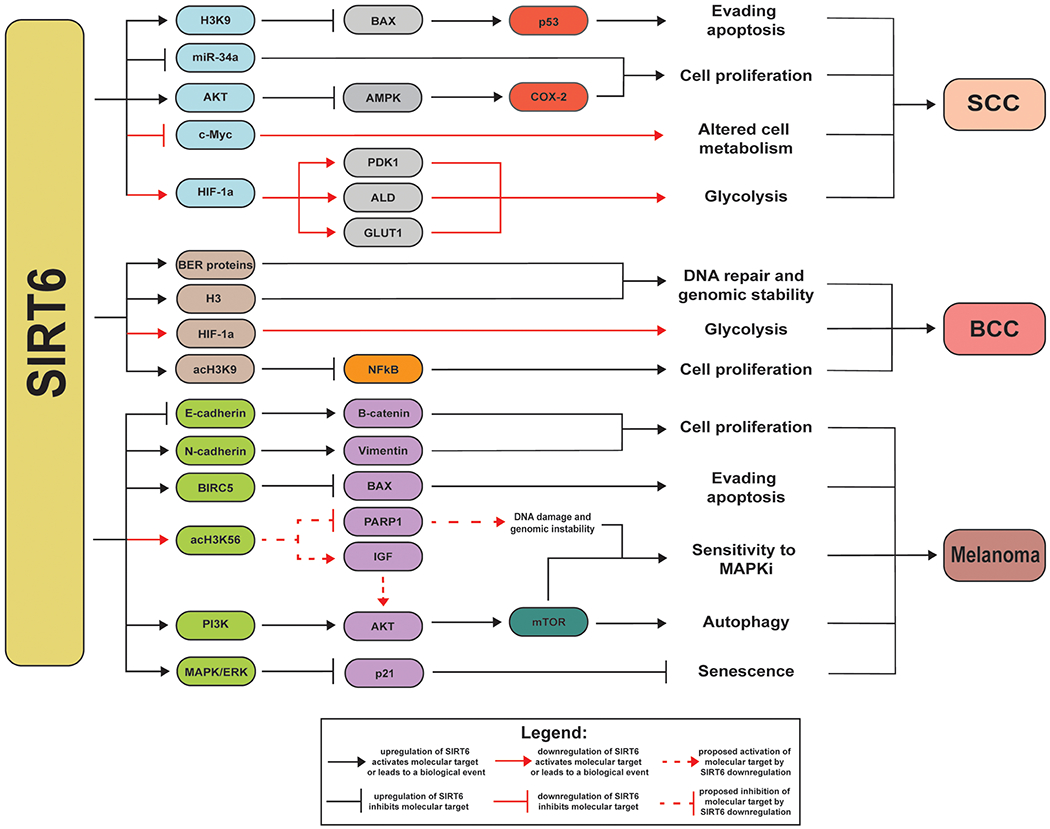
The known links between SIRT6 and other molecular targets, affecting important cellular processes, relevant to development and/or progression of melanoma, and non-melanoma skin cancers are depicted.
Table 1:
The known mechanistic information of SIRT6 in skin cancer.
| Skin Cancer Type | Substrates and Targets | Function(s) |
|---|---|---|
| SCC | H3K9ac, BAX, p53, miR-34a, AKT, AMPK, COX-2, HIF-1α, PDK1, ALD, GLUT-1 | • SIRT6 interacts with H3K9ac suppressing BAX to alter p53, avoiding apoptosis67. • Tp53 promotes accumulation of SIRT6, leading to SCC54. • SIRT6 contributes to SCC tumorigenesis by altering the differentiation of skin cells54. • SIRT6 upregulation decreases miR-34a levels in SCC. miR-34a controls the differentiation of squamous cells, downregulation leads to uncontrollable cell proliferation27. • UV radiation activates AKT pathway and AKT and SIRT6 form a positive feedback loop to inhibit Adenosine Monophosphate-Activated Protein Kinase (AMPK), which activates COX-2, leading to SCC29. • SIRT6 deficiency activates HIF-1α. HIF-1α can further activate glycolytic genes including PDK1, ALD, and GLUT-168. |
| BCC | Histones (e.g. H3, H3K9ac), base excision repair (BER) proteins, HIF-1α, NF-κB | • SIRT6 interacts with histones and BER proteins, preventing genetic alterations and genomic instability along with DNA repair55,69. • After UV exposure, upregulation of SIRT6 is initiated by DNA damage55. • Low levels of SIRT6 induce a metabolic switch, in which, the Warburg Effect takes place due to HIF-1α activation70. • SIRT6 deacetylates H3K9ac promoting NF-κBexpression71. • NF-κB activation leads to cell proliferation in the skin72. |
| Melanoma | E-cadherin, N-cadherin, vimentin, β-catenin, BIRC5, BAX, H3K56ac, PARP1, IGF, PI3K, AKT, mTOR, p21 | • SIRT6 interacts with epithelial-mesenchymal transition (EMT) markers, to initiate metastasis73. SIRT6 blocks E-cadherin (epithelial marker) upregulating β-catenin activating N-cadherin and vimentin, (mesenchymal markers) causing melanoma cells to proliferate uncontrollably74. • In advanced stages of melanoma,SIRT6 promotes BIRC5 activity by suppressing apoptosis, which helps cancerous cells to proliferate57. • SIRT6 activates the MAPK/ERK pathway which then blocks p21, inhibiting senescence71. • Evasion from apoptosis has been demonstrated by a reduction in proapoptotic proteins (e.g.Bcl-2-associated X protein(BAX))57. • SIRT6 depletion increases H3K56ac inducing DNA damage59. • Alterations in SIRT6 can prevent PARP1 from aiding in DNA repair8. • Downregulation of SIRT6 upregulates IGF, activating AKT, leading to DNA damage and increase sensitivity to MAPKi59. • SIRT6 upregulation activates PI3K/AKT/mTOR pathway57, initiating autophagy which promotes melanoma survival and migration75. |
The potential targetability of SIRT6 for the management of skin cancers is being actively investigated in our laboratory as well as by others. In another very interesting study, Strub et al demonstrated that haploinsufficiency of SIRT6 promotes insulin-like growth factor binding protein 2 (IGFBP2) expression via increased chromatin accessibility, H3K56 acetylation at the IGFBP2 locus, and consequent activation of the insulin-like growth factor 1 receptor (IGF1R) and downstream AKT signaling allowing melanoma cells to persist in the presence of mitogen activated protein kinase inhibitors (MAPKi)59. Using matched melanoma samples derived from patients receiving dabrafenib + trametinib, the authors found that IGFBP2 is a potential biomarker for MAPKi resistance59. This study identified not only an epigenetic mechanism of drug resistance, but also provided a rationale for a new combination therapy that could overcome resistance to standard-of-care therapy for BRAFV600-mutant melanoma patients59. In contrast to SIRT6, Sirtuin-5 (SIRT5) does not improve sensitivity to BRAF inhibitors in BRAFV600-mutant melanoma animals59,60. Given the targetable properties of SIRT6 in melanoma and non-melanoma skin cancers, the development of SIRT6 specific inhibitors accompanied by detailed molecular insights into the mechanistic and therapeutic tangibility of SIRT6 in skin is essential for the development of novel strategies to modulate skin cancer59.
SIRT6 Specific Inhibitors: An Avenue of Opportunities For Drug Development
Because of the potential oncogenic function of SIRT6 in certain cancers as well as its importance in other conditions, it has become an attractive target for the development of small molecule inhibitors. A broad range of therapeutic applications are foreseen for SIRT6 inhibitors, with potential applications in diabetes (as blood glucose downregulation agents), immune-mediate disorders, and cancers (including as chemosensitizers)61. Moreover, the development of these inhibitors could be used to complement the available genetic tools to embark on studies defining the biological importance of SIRT6 as well as its mechanisms, interactions, and downstream targets. Unfortunately, only a small number of SIRT6 inhibitors are currently available, and some of these inhibitors are not very well characterized, especially regarding their structure activity relationships. Pseudopeptides, cyclic pentapeptides, peptides, and thiomyristoyl peptides have been reported to inhibit SIRT6 activity; however, these lack a strong selectivity for SIRT6 versus other sirtuins62,63. Some small molecule inhibitors, with salicylate-based structures that are not related to peptides, have been better characterized. These seem to hold promise for their future use and towards the development of next-generation SIRT6 inhibitors63,64. Some of the existing SIRT6 inhibitors are briefly discussed below.
Parenti et al synthesized and tested a total of twenty different compounds for their potential for SIRT6 inhibition, of which only three (viz. SYN17739303, BAS13555470, BAS00417531) achieved reasonable inhibitory effects at a low micromolar range (Figure 7)63. SIRT6, unlike the rest of the sirtuin family, lacks a co-factor binding loop that allows different conformations dependent on the ligands bound to the active site65. This distinguishing feature of SIRT6 requires the presence of modifications to the active site that interferes with the design of selective inhibitors63. Fortunately, the three compounds identified by Parenti et al, are quite promising, because of being structurally diverse and nonpeptide selective SIRT6 inhibitors. Additionally, the SIRT6/SIRT1 selectivity ratio for two of the compounds identified show an IC50 towards SIRT6 that is 17- and 19- fold lower than those toward SIRT163. This is very promising given the fact that deacetylase activity of purified SIRT6 protein in vitro is 1000-fold lower for SIRT6 when compared to SIRT1. Biologically, these compounds were shown to reduce TNF-α secretion, as should be expected based on studies regarding the role of SIRT6 in TNF-α production63. Moreover, a strong increase in glucose transporter 1 (GLUT1) expression was followed by an increase in glucose uptake in culture cells treated with these compounds, in accordance with the roles SIRT6 has shown to have in glucose homeostasis63. Interestingly, Sociali et al determined the pharmacological effects of one of these compounds, commercially known as SIRT6-inhibitor-compound-1 (SIRT6-IN-1) also called, 2,4,-dioxo-N-(4-(pyridine-3-yloxy)phenyl)-1,2,3,4-tetrahydroquinazoline-6-sulfonamide, on a mouse model for Type 2 diabetes mellitus in a in a mouse model (high-fat-diet–fed animals)66. The data from this study demonstrated that SIRT6-IN-1 resulted in a significant improvement in glucose regulation, notable increase in glucose transporters and triglycerides, as well as reduced cholesterol and levels of insulin66. Although SIRT6 has many significant biological roles, the biggest drawback, when developing inhibitors, is the identification of structural features that can be associated with SIRT6 specific inhibition. Evidently, successful development of SIRT6-specific inhibitors may lead to the development of new pharmacological applications.
Figure 7: SIRT6 Specific Inhibitors Structures and SIRT6 Inhibition.
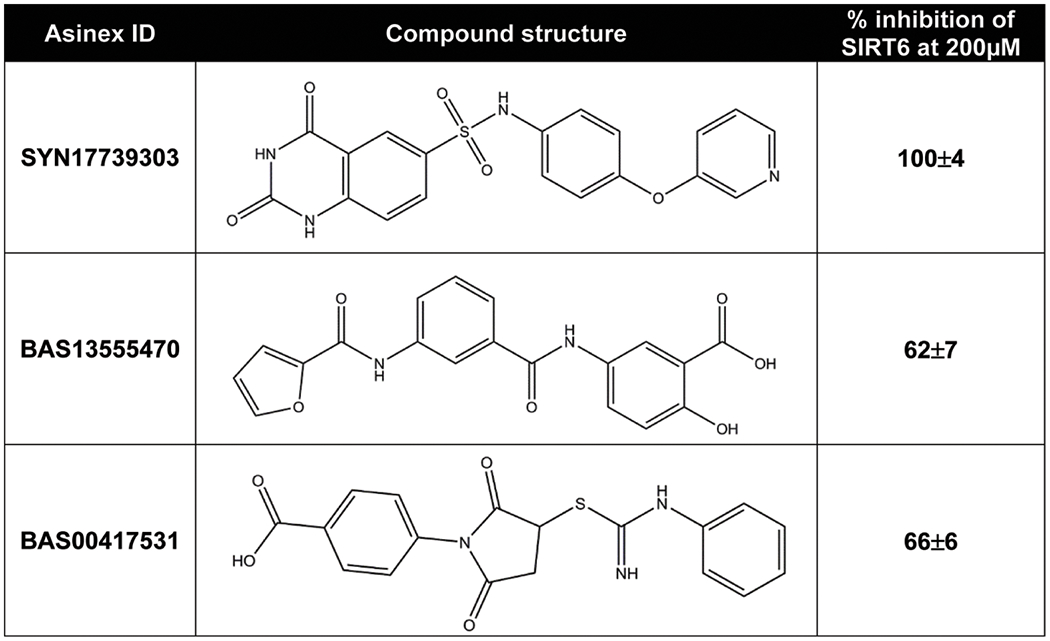
Current SIRT6 inhibitors with inhibitory effects at a low micromolar range are depicted. It is important to note that this figure was modified from Parenti et al.
Conclusions
Based on studies, in the recent past, sirtuins have emerged to be important in skin biology. The role of SIRT6 in skin biology and disease, including skin cancers is beginning to be explored. With limited available information to date, it appears that in skin neoplasms, including SCC, BCC, and melanomas, SIRT6 plays a pro-proliferative function. However, detailed investigations are needed to determine the stage specific behavior of SIRT6, during the process of skin carcinogenesis. This is mainly based on the evidence showing an early lower expression of SIRT6 in pre-cancerous keratinocytic neoplasms. It appears that there may be a connection between SIRT6 with aging and cancer, which needs to be explored in detail. Further, improved SIRT6 inhibitors are needed, which could be tested in robust pre-clinical and clinical studies. Addressing the knowledge gaps regarding the connections and association between SIRT6 and other driver pathways of neoplastic transformation, could be useful towards the management of both melanoma as well as non-melanoma skin cancers.
Acknowledgements:
This work was partially supported by funding from the NIH (R01AR059130 and R01CA176748 to NA), and the Department of Veterans Affairs (VA Merit Review Awards I01CX001441 and 1I01BX004221-01; and a Research Career Scientist Award IK6BX003780 to NA). We also acknowledge support from the Skin Diseases Research Center (SDRC) Core Grant P30AR066524 from NIH/NIAMS, as well as the University of Wisconsin Carbone Cancer Center Support Grant P30CA014520.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to declare
References
- 1.Shore D, Squire M, Nasmyth KA. Characterization of two genes required for the position-effect control of yeast mating-type genes. EMBO J. 1984;3(12):2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Ven RAH, Santos D, Haigis MC. Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol Med. 2017;23(4):320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–2921. [DOI] [PubMed] [Google Scholar]
- 4.Park SH, Zhu Y, Ozden O, et al. SIRT2 is a tumor suppressor that connects aging, acetylome, cell cycle signaling, and carcinogenesis. Transl Cancer Res. 2012;1(1):15–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci. 2014;39(2):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280(22):21313–21320. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Khan S, Jiang H, et al. Identifying the functional contribution of the defatty-acylase activity of SIRT6. Nat Chem Biol. 2016;12(8):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332(6036):1443–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Meter M, Kashyap M, Rezazadeh S, et al. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat Commun. 2014;5:5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288(43):31350–31356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Khan S, Wang Y, et al. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496(7443):110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang WW, Zeng Y, Wu B, Deiters A, Liu WR. A Chemical Biology Approach to Reveal Sirt6-targeted Histone H3 Sites in Nucleosomes. ACS Chem Biol. 2016;11(7):1973–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passarino G, De Rango F, Montesanto A. Human longevity: Genetics or Lifestyle? It takes two to tango. Immun Ageing. 2016;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tennen RI, Bua DJ, Wright WE, Chua KF. SIRT6 is required for maintenance of telomere position effect in human cells. Nat Commun. 2011;2:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452(7186):492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasselli L, Xi Y, Zheng W, et al. SIRT6 deacetylates H3K18ac at pericentric chromatin to prevent mitotic errors and cellular senescence. Nat Struct Mol Biol. 2016;23(5):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endisha H, Merrill-Schools J, Zhao M, et al. Restoring SIRT6 Expression in Hutchinson-Gilford Progeria Syndrome Cells Impedes Premature Senescence and Formation of Dysmorphic Nuclei. Pathobiology. 2015;82(1):9–20. [DOI] [PubMed] [Google Scholar]
- 19.Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. [DOI] [PubMed] [Google Scholar]
- 20.Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. [DOI] [PubMed] [Google Scholar]
- 21.Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KS, Park HK, Lee JW, Kim YI, Shin MK. Investigate correlation between mechanical property and aging biomarker in passaged human dermal fibroblasts. Microsc Res Tech. 2015;78(4):277–282. [DOI] [PubMed] [Google Scholar]
- 23.Baohua Y, Li L. Effects of SIRT6 silencing on collagen metabolism in human dermal fibroblasts. Cell Biol Int. 2012;36(1):105–108. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Diecke S, Zhang WY, et al. The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem. 2013;288(25):18439–18447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastian C, Zwaans BM, Silberman DM, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151(6):1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min L, Ji Y, Bakiri L, et al. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14(11):1203–1211. [DOI] [PubMed] [Google Scholar]
- 27.Lefort K, Brooks Y, Ostano P, et al. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013;32(16):2248–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Xie QR, Wang B, et al. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein Cell. 2013;4(9):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ming M, Han W, Zhao B, et al. SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer. Cancer Res. 2014;74(20):5925–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demir IE, Ceyhan GO, Friess H. Epigenomic therapies: the potential of targeting SIRT6 for the treatment of pancreatic cancer. Expert Opin Ther Targets. 2017;21(1):1–3. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Tian H, Xue L, Wang L. SIRT6 abrogation promotes adrenocortical carcinoma through activation of NF-kappaB signaling. Mol Cell Biochem. 2019;458(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- 32.Tian J, Yuan L. Sirtuin 6 inhibits colon cancer progression by modulating PTEN/AKT signaling. Biomed Pharmacother. 2018;106:109–116. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Qu Y, Zhou Q, Ma Y. SIRT6 inhibits proliferation and invasion in osteosarcoma cells by targeting N-cadherin. Oncol Lett. 2019;17(1):1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Wu M, Du H, Shi X, Zhang T, Li J. SIRT6 inhibits colorectal cancer stem cell proliferation by targeting CDC25A. Oncol Lett. 2018;15(4):5368–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kugel S, Sebastian C, Fitamant J, et al. SIRT6 Suppresses Pancreatic Cancer through Control of Lin28b. Cell. 2016;165(6):1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Z, Yang H, Tan C, et al. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5(6):1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Yin XJ, Xu CJ, et al. The histone deacetylase SIRT6 inhibits ovarian cancer cell proliferation via down-regulation of Notch 3 expression. Eur Rev Med Pharmacol Sci. 2015;19(5):818–824. [PubMed] [Google Scholar]
- 38.Han Z, Liu L, Liu Y, Li S. Sirtuin SIRT6 suppresses cell proliferation through inhibition of Twist1 expression in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7(8):4774–4781. [PMC free article] [PubMed] [Google Scholar]
- 39.Feng J, Yan PF, Zhao HY, Zhang FC, Zhao WH, Feng M. SIRT6 suppresses glioma cell growth via induction of apoptosis, inhibition of oxidative stress and suppression of JAK2/STAT3 signaling pathway activation. Oncol Rep. 2016;35(3):1395–1402. [DOI] [PubMed] [Google Scholar]
- 40.Wu M, Seto E, Zhang J. E2F1 enhances glycolysis through suppressing Sirt6 transcription in cancer cells. Oncotarget. 2015;6(13):11252–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang N, Liu Z, Zhu J, et al. Sirtuin 6 plays an oncogenic role and induces cell autophagy in esophageal cancer cells. Tumour Biol. 2017;39(6):1010428317708532. [DOI] [PubMed] [Google Scholar]
- 42.Feng XX, Luo J, Liu M, et al. Sirtuin 6 promotes transforming growth factor-beta1/H2O2/HOCl-mediated enhancement of hepatocellular carcinoma cell tumorigenicity by suppressing cellular senescence. Cancer Sci. 2015;106(5):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cagnetta A, Soncini D, Orecchioni S, et al. Depletion of SIRT6 enzymatic activity increases acute myeloid leukemia cells’ vulnerability to DNA-damaging agents. Haematologica. 2018;103(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elhanati S, Ben-Hamo R, Kanfi Y, et al. Reciprocal Regulation between SIRT6 and miR-122 Controls Liver Metabolism and Predicts Hepatocarcinoma Prognosis. Cell Rep. 2016;14(2):234–242. [DOI] [PubMed] [Google Scholar]
- 45.Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984. [DOI] [PubMed] [Google Scholar]
- 46.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 47.Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linares MA, Zakaria A, Nizran P. Skin Cancer. Prim Care. 2015;42(4):645–659. [DOI] [PubMed] [Google Scholar]
- 49.Liu-Smith F, Jia J, Zheng Y. UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer. Adv Exp Med Biol. 2017;996:27–40. [DOI] [PubMed] [Google Scholar]
- 50.Nasti TH, Timares L. MC1R, eumelanin and pheomelanin: their role in determining the susceptibility to skin cancer. Photochem Photobiol. 2015;91(1):188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malissen N, Grob JJ. Metastatic Melanoma: Recent Therapeutic Progress and Future Perspectives. Drugs. 2018;78(12):1197–1209. [DOI] [PubMed] [Google Scholar]
- 52.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. [DOI] [PubMed] [Google Scholar]
- 53.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–383. [DOI] [PubMed] [Google Scholar]
- 54.Dotto GP, Karine L. miR-34a/SIRT6 in squamous differentiation and cancer. Cell Cycle. 2014;13(7):1055–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Temel M, Koc MN, Ulutas S, Gogebakan B. The expression levels of the sirtuins in patients with BCC. Tumour Biol. 2016;37(5):6429–6435. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Peterson LM, Ndiaye MA, Singh CK, Chhabra G, Huang W, Ahmad N. SIRT6 histone deacetylase functions as a potential oncogene in human melanoma. Genes Cancer. 2017;8(9-10):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Guo W, Ma J, et al. Aberrant SIRT6 expression contributes to melanoma growth: Role of the autophagy paradox and IGF-AKT signaling. Autophagy. 2018;14(3):518–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gewirtz DA. Autophagy and senescence in cancer therapy. J Cell Physiol. 2014;229(1):6–9. [DOI] [PubMed] [Google Scholar]
- 59.Strub T, Ghiraldini FG, Carcamo S, et al. SIRT6 haploinsufficiency induces BRAF. Nat Commun. 2018;9(1):3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moon H, Zhu J, White AC. Sirt5 is dispensable for Braf(V600E) -mediated cutaneous melanoma development and growth in vivo. Exp Dermatol. 2019;28(1):83–85. [DOI] [PubMed] [Google Scholar]
- 61.Etchegaray JP, Zhong L, Mostoslavsky R. The histone deacetylase SIRT6: at the crossroads between epigenetics, metabolism and disease. Curr Top Med Chem. 2013;13(23):2991–3000. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Zheng W. Cyclic peptide-based potent human SIRT6 inhibitors. Org Biomol Chem. 2016;14(25):5928–5935. [DOI] [PubMed] [Google Scholar]
- 63.Parenti MD, Grozio A, Bauer I, et al. Discovery of novel and selective SIRT6 inhibitors. J Med Chem. 2014;57(11):4796–4804. [DOI] [PubMed] [Google Scholar]
- 64.Damonte P, Sociali G, Parenti MD, et al. SIRT6 inhibitors with salicylate-like structure show immunosuppressive and chemosensitizing effects. Bioorg Med Chem. 2017;25(20):5849–5858. [DOI] [PubMed] [Google Scholar]
- 65.Pan PW, Feldman JL, Devries MK, Dong A, Edwards AM, Denu JM. Structure and biochemical functions of SIRT6. J Biol Chem. 2011;286(16):14575–14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sociali G, Magnone M, Ravera S, et al. Pharmacological Sirt6 inhibition improves glucose tolerance in a type 2 diabetes mouse model. FASEB J. 2017;31(7):3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ran LK, Chen Y, Zhang ZZ, et al. SIRT6 Overexpression Potentiates Apoptosis Evasion in Hepatocellular Carcinoma via BCL2-Associated X Protein-Dependent Apoptotic Pathway. Clin Cancer Res. 2016;22(13):3372–3382. [DOI] [PubMed] [Google Scholar]
- 68.Kleszcz R, Paluszczak J, Krajka-Kuzniak V, Baer-Dubowska W. The inhibition of c-MYC transcription factor modulates the expression of glycolytic and glutaminolytic enzymes in FaDu hypopharyngeal carcinoma cells. Adv Clin Exp Med. 2018;27(6):735–742. [DOI] [PubMed] [Google Scholar]
- 69.Xu Z, Zhang L, Zhang W, et al. SIRT6 rescues the age related decline in base excision repair in a PARP1-dependent manner. Cell Cycle. 2015;14(2):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong L, D’Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140(2):280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vitiello M, Zullo A, Servillo L, et al. Multiple pathways of SIRT6 at the crossroads in the control of longevity, cancer, and cardiovascular diseases. Ageing Res Rev. 2017;35:301–311. [DOI] [PubMed] [Google Scholar]
- 72.Ali F, Khan BA, Sultana S. Wedelolactone mitigates UVB induced oxidative stress, inflammation and early tumor promotion events in murine skin: plausible role of NFkB pathway. Eur J Pharmacol. 2016;786:253–264. [DOI] [PubMed] [Google Scholar]
- 73.Chandrakesan P Cancer cell of origin controls epithelial-to-mesenchymal transition in skin squamous cell carcinoma. Stem Cell Investig. 2017;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia LM, Chhabra G, Ndiaye MA, Ahmad N. Abstract 546: The nuclear sirtuin SIRT6 promotes epithelial-mesenchymal transition in human melanoma cells. Cancer Research. 2018;78:546–546. [Google Scholar]
- 75.Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Potential therapeutic targets of epithelial-mesenchymal transition in melanoma. Cancer Lett. 2017;391:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]


