Abstract
Preclinical cancer research increasingly demands sophisticated models for the development and translation of efficient and safe cancer treatments to clinical practice. In this regard, tumor-grafted chorioallantoic membrane (CAM) models are biological platforms that account for the dynamic roles of the tumor microenvironment and cancer physiopathology, allowing straightforward investigations in agreement to the 3Rs concept (the concept of reduction, refinement, and replacement of animal models). CAM models are the next advanced model for tumor biological explorations as well as for reliable assessment regarding initial efficacy, toxicity, and systemic biokinetics of conventional and emerging neoplasm treatment modalities. Here we report a standardized and optimized protocol for the production and biocharacterization of human papillomavirus (HPV)-negative head and neck chick chorioallantoic membrane models from a commercial cell line (SCC-25). Oral malignancies continue to have severe morbidity with less than 50% long-term survival despite the advancement in the available therapies. Thus, there is a persisting demand for new management approaches to establish more efficient strategies toward their treatment. Remarkably, the inclusion of CAM models in the preclinical research workflow is crucial to ethically foster both the basic and translational oncological research on oral malignancies as well as for the advancement of efficient cancer treatment approaches.
Keywords: chick chorioallantoic membrane, head/neck, cancer, alternative models, 3Rs principle, ethics
Head and neck squamous cell carcinomas (HNSCCs) represent a wide class of epithelial neoplasms localized in the oral and nasal cavities, paranasal sinuses, salivary glands, pharynx, and larynx and whose molecular mechanisms involved in the progression of the disease are still to be completely clarified.1,2 One of the leading causes of the development of HNSCCs is the long-term consumption of tobacco and alcohol.3 Another associated risk factor for the onset of HNSCCs is human papillomavirus (HPV) infection.4 The presence of HPV usually affects the prognosis of the disease, with more favorable outcomes for the patients.4,5 The general workup for staging and diagnosis of squamous cell carcinoma of the oral cavity, larynx, oropharynx, and hypopharynx includes physical examinations, medical history, blood test, MRI/CT imaging, and biopsy under local anesthesia.4 These features together with the evaluations on the genomic alterations or gene expression profile are important for the pathological staging and prognosis and to determine the type of treatment. In general, the usual management strategies for oral cavity, laryngeal, oropharyngeal, and hypopharyngeal cancers are different between locally advanced tumors and early stage tumors.6 Indeed, according with the most recent Clinical Practice Guidelines in Oncology (e.g., NCCN), the standard treatments in early stage disease, consisting of conservative surgery or radiotherapy, give similar locoregional control. Standard options for locally advanced HNSCC are either surgery plus adjuvant chemo/radiotherapy or primary chemoradiotherapy alone, even if the best treatment regime is chosen on a case-by-case analysis.4,7 In the field of prognostic estimation, it appears that cases of HNSCC that survive beyond 5 years after the initial diagnosis show decreased overall survival when compared to noncancer subjects of the same age.8 In general, stage I cases had improved survival compared to stage II–IV, where no particular difference was proved in long-term survival for cases alive 5 years after diagnosis. However, site, stage, smoking, and cardiovascular disease are significant factors determinant of mortality.8 Nevertheless, oral malignancies, including tongue cancer, continue to have severe morbidity with less than 50% long-term survival despite the advancement in the available and emerging therapies.4,5,9,10 In this regard, understanding the biological processes at the basis of oral malignancies and the development of new therapeutic strategies to improve the survival rate of patients while preserving the structure and function of the involved organs are crucial topics for their management.11 In this context, in vivo models are pivotal to foster the advancements in oncology by bridging the gap between preclinical investigations and human clinical trial on conventional and emerging therapeutic approaches as well as to understand tumor cell behaviors in a physiological environment.16 The most widely employed in vivo models are murine, and they have been crucial to establish most of the current models in pediatric oncology and to develop some drugs, among which topotecan and irinotecan.7 Moreover, murine models are particularly relevant for absorption–distribution–metabolism–excretion–toxicity (ADMET) investigations.113−115 However, genetically immunocompromised murine models have very high costs of maintenance, and the tumor engraftment may require up to 4 months. Engraftment failure can be high and cannot be usually determined before some months post-implantation, and their employment is increasingly discouraged by following the 3Rs concept (the concept of reduction, refinement, and replacement of animal models) and the European Parliament Directive 2010/63/EU.13,14 Among other biological models, the chick chorioallantoic membrane (CAM) is one of the most attractive and ethical in vivo models that jointly combines reliability, medium-/high-throughput screenings, and easy handling during imaging/treatment evaluations.15−16 Notably, the absence of a mature immune system of the embryo during the early developmental stage reduces the risk of tumor rejection after implantation.16 Thus, CAM models can develop visible solid tumors within 4–5 days after engraftment, in comparison to the approximately 3–6 weeks of murine models.7 Besides the usually high rate of success for tumor grafting, other major advantages of CAM models are the easy daily inspection of the tumor site and their flexibility of employment. Moreover, CAM models are ethical models in agreement with the 3Rs concept because the chick embryo does not develop pain perception before the 17th day of incubation.16 Indeed, investigations in CAM models usually do not require permissions or approval from ethics committees.16 Despite some difficulties in the application of CAM models to long-term investigations, their highly vascularized membrane together with the immature immune response allowed the low rejection rate grafting of several tissues and the study of various neoplasms, including osteosarcoma, glioblastoma, pancreatic carcinoma, and colon carcinoma.17−20 Several variable tumor engraftment methods for CAM model production have been reported, yet these studies focus on the tumor biology or the evaluation of a treatment avoiding reporting a standard protocol for the production of the models.21,22 In general, in the literature there is a serious lack of work regarding the standard production of CAM models. Thus, a detailed step-by-step procedure for the reliable CAM tumor model fabrication together with the standard biocharacterization is required to serve as guide for researchers interested to advance the field.
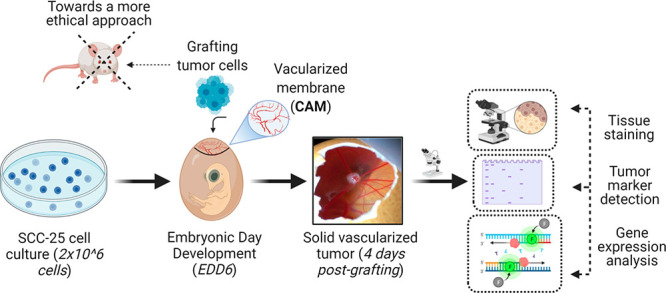
In order to address this demand, we report a standardized protocol for the composition as well as the cascade assays for the characterization of a commercial HPV-negative head and neck cell line (SCC-25) grafted on chick chorioallantoic membrane of fertilized Leghorn chicken eggs.
Materials
Reagents and Consumables
Fertilized red or white Leghorn chicken eggs
SCC-25 squamous cell carcinoma cell line (ATCC, catalog number CRL-1628)
DMEM/Ham’s F12 1:1 medium (DMEM/F12 Gibco, 21041025)
Fetal bovine serum, qualified, heat-inactivated (FBS, Thermo Fisher Scientific, 10500064)
l-Glutamine (Thermo Fisher Scientific, A2916801)
Hydrocortisone (Sigma-Aldrich, H0888)
Penicillin–streptomycin (Pen/Strep 100X, 5,000 U/mL) (Thermo Fisher Scientific, 15070063)
Phosphate-buffered saline without calcium and magnesium (PBS, Sigma-Aldrich, D8537)
Trypsin-EDTA (0.5%), phenol red (Thermo Fisher Scientific, 25300054)
Serological pipette, pipette tips, microcentrifuge tubes, conical tubes, and flasks
TC Dish150, Standard (83.3903, SARSTEDT)
Matrigel Matrix (Corning, ref 354234)
Sterile water
Fixative solution, i.e., 4% paraformaldehyde (PFA) in PBS
RNA extraction kit, Nucleospin RNA plus (740984.50 MACHEREY-NAGEL)
cDNA synthesis kit, iScript cDNA Synthesis (1708891 BIORAD)
iTaqUniversal SYBRGreen Supermix (1725121 BIORAD)
RIPA buffer (Pierce 89901)
Protease Inhibitors Cockatil Tablets (04693116001 Roche)
Bradford Reagent (B6916 Sigma-Aldrich)
Albumin Standard (23209 Thermo scientific)
Nitrocellulose membrane, TransBlot Turbo Midi-size nitrocellulose (1620167 BIORAD)
anti-TFRC primary antibody (SAB4200398 Sigma-Aldrich)
Goat anti-rabbit IgG (H+L)-HRP-conjugated secondary antibody, (170–6515 BIORAD)
Clarity Western ECL substrate (1705061 BIORAD)
Paraffin wax (melting point 56 °C)
Ethanol (70, 80, 95, and 100% alcohol)
Xylene
Mayer’s hematoxylin solution
Eosin Y aqueous solution 1%
Permount mounting medium
Equipment and Tools
Cell counter (Invitrogen Countess cell counter)
Optical microscope
Egg incubator, 37.5 °C/99.5 °F, 60% humidity, FIEM MG 140/200
Tilting egg racks
Sterile dissection scissors and tweezers
Soft tissue paper or cotton swab
Refrigerator or cold room at 4 °C
Analytical balance
Ruler
Adhesive tapes, preferably Scotch magic tape
Portable digital microscope, Dino-Lite AM7915MZT (or any camera-associated microscope)
DinoCapture 2.0 Software
Rotary microtome
Forced ventilation histology oven
Paraffin-embedding station
Light microscope equipped with RGB video camera
Methods
Experimental Outline
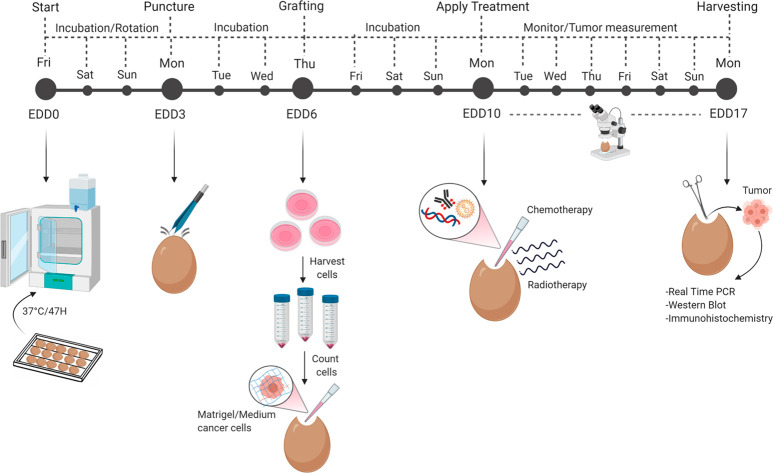
The establishment of the experimental design is the first important step to define a complete outline of the assay procedure. For the optimization of the CAM assay employing SCC-25 cells, the schedules of egg incubation and subsequent experimentations were based on the proposed scheme by Kleibeuker et al. (Figure 1).23 In general, the start of the incubation corresponds to the embryonic day of development 0 (EDD0). Starting from this moment, under appropriate environmental conditions, the fertilized eggs begin their embryonic growth. It is important to take into account that the biological window available to perform this assay must not exceed the 17 days of incubation to prevent the hatching of the eggs and avoid ethical restrictions.16
Figure 1.
Overview of the CAM schedule from EDD0 to EDD17. In general, chick embryo incubation is marked as embryonic day of development 0 (EDD0). The puncturing day (EDD3) allows the translocation of the natural air sac to the top of the egg. Grafting of 2 × 106 SCC-25 cells (EDD6) enables the generation of a solid visible tumor at 4 days post-grafting (EDD10). The topical treatment is applied on EDD10, and the tumor mass is monitored until EDD17, the last day of incubation (harvesting).
Cleaning the Working Area and Eggs (Start of Incubation)
It is highly recommended to work under sterile conditions and to adequately clean the working area with 70% ethanol and/or bleach to avoid any type of microorganism contamination. Carefully clean the shell of each egg with soft tissue paper soaked in distilled water before fitting them into the tray.230 Place the eggs horizontally next to each other and insert a steel spring in the ends of the columns to secure the eggs while the racks tilt (Figure 2A).231 Make sure that the temperature and humidity of the incubator reach the appropriate parameters before starting the incubation (37.5 °C, ∼47% humidity).232
Figure 2.
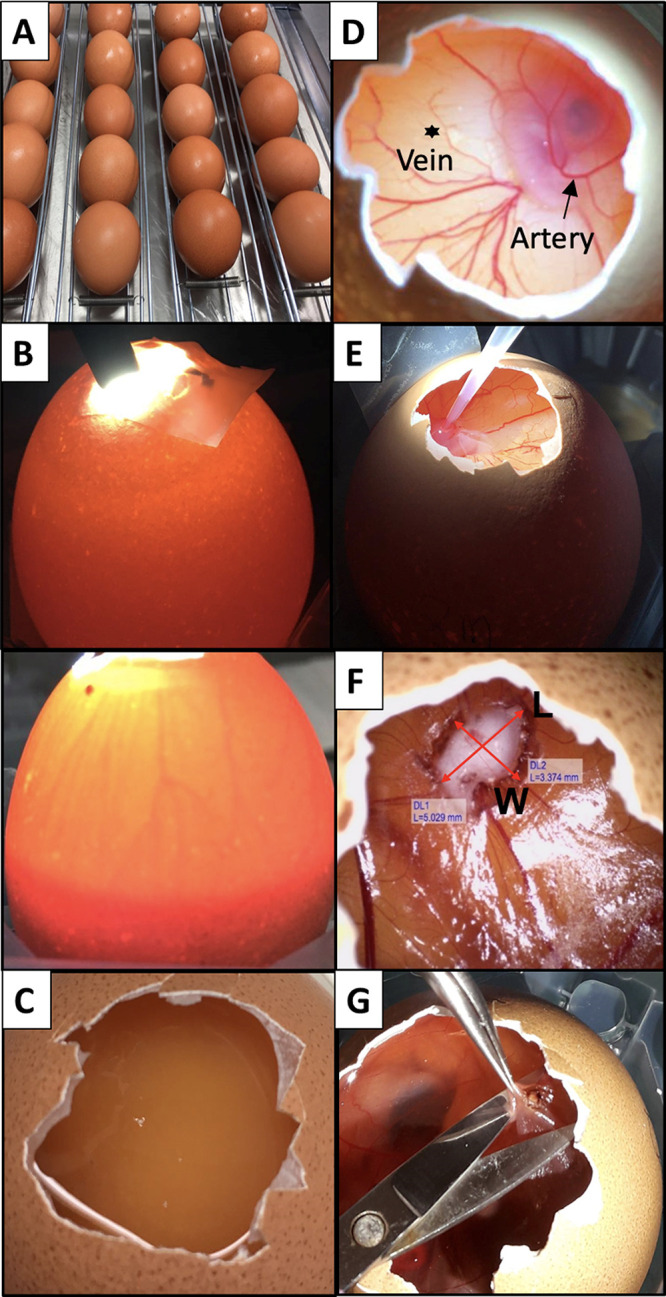
Images of the main steps of the CAM protocol. (A) Eggs are horizontally placed in the tilting trays before the incubation starts. (B) A light source placed on top/behind the egg allows identification of the location of the air chamber and the existence of a vascular network (Top, infertile egg; bottom, fertile egg). (C) An unfertilized egg is associated with the absence of the embryo and of the vascular network. (D) Distinction between artery (arrow) and vein (asterisk). Arteries are thicker and darker compared to veins.25 (E) Image showing the grafting procedure of SCC-25 cancer cells onto the bleeding blood vessel. (F) Tumor volume measurement determined by means of width and length. The length is associated with the longest diameter of the tumor mass. (G) Harvesting procedure of the tumor at EDD17. The CAM membrane is gently lifted with tweezers and the tumor is cut with scissors.
Puncturing
Take the eggs out after 3 days of incubation (EDD3) and put them in upright position so that the air sac will translocate to the top of the eggs.233 Gently scratch with tweezers the tip of each egg until the shell becomes fragile and breakable, finally making a small hole. Seal the hole with an adhesive tape to prevent dehydration and infection, and put the eggs back in the incubator in the stationary mode (no tilting).
Tumor Grafting
Selection of Eggs
At EDD6, the fertilized eggs must be accurately selected before proceeding with the grafting of the tumor cell suspension on the CAM. Remove the eggshell around the small hole to create a small window of about 1 cm2.234 Check the fertilization and discard the unfertilized eggs (Figure 2C). Label the eggs with the appropriate information (e.g., egg number, cell density, name of the cell line, and treatment conditions) to avoid confusion. Reseal the window, and put the eggs back in the incubator.
Preparation of Tumor Cell Suspension
Harvest the SCC-25 cells, and collect them in one 50 mL tube.235 Spin down the cells, and discard the supernatant. Resuspend the pellet in fresh cell culture medium. Count the cells, and adjust the concentration in order to have at least 2 × 106 cells per egg.236 Spin down the cells again, and discard the supernatant. Resuspend the pellet in a mixture of Matrigel/medium without FBS (in ratio 1:1) such that each egg will be dispensed with 25 μL of the cell suspension.237
Grafting
Open the sealed window. Roll a soft tissue paper and gently poke a small vein within the CAM region until it starts to bleed (Figure 2D).238 Pipette 25 μL of the cell suspension on top of the bleeding blood vessel. Reseal the window, and put the eggs in the incubator (Figure 2E). Always make sure that the humidifier and the tank are filled with water and that the incubator is still maintaining the right conditions of temperature and humidity.
Apply Treatment (EDD10)
A reference concentration of your drug/chemotherapeutic compound to be tested is recommended.239 The amount of the treatment solution to be prepared will depend on the number of tumor-grafted eggs to be treated, while the frequency of the treatment will depend on your own schedule and experimental objectives. To proceed, take the eggs out from the incubator, and mark each egg for the corresponding treatment to avoid confusion. For our experiments, each egg is topically administered with chemotherapeutic drugs/test compounds suspended in 30 μL of solution (serum-free medium).240
Tumor Growth Monitoring (EDD10–EDD17)
Place a ruler under the DinoLite camera and calibrate the software before starting to acquire images of the tumors. Although constantly noting the calibration by using the ruler can help in obtaining consistent and accurate measurements, it is also advised to take photographs at the same magnification to better visualize the changes in tumor dimensions. The complementary DinoLite software (DinoCapture 2.0) comes with length measurement tools. Measure the tumor sizes, in which the longer and shorter measurements are denoted as the length (L) and width (W), respectively (Figure 2F). From these measurements, the tumor volume is derived using a modified ellipsoid formula: 0.5(L × (W2)).241,19
Tumor Harvesting (EDD17)
On EDD17, take the eggs out of the incubator and place them in the cold room for at least 2 h to restrict the movements of the chick embryo. Thoroughly clean the dissection area, tweezers, and scissors with 70% ethanol and bleach. Prepare wash containers with ethanol and PBS for rinsing the tools to avoid cross-contamination among different tumors. Open the sealed window, and remove some eggshell around to make the tumor more accessible for cutting. Carefully lift the membrane with tweezers. Cut the tumor, and place it in a Petri dish with PBS. Take a photograph of the tumor, and place it in an empty microcentrifuge tube, and weigh it (Figure 2G).242 Store the harvested tumor samples directly in −80 °C or in formalin fixing solution for further analysis.
Downstream Assays
Quantitative Real Time-PCR
To extract the total RNA from the SCC-25 cell line, we used Nucleospin RNA plus Kit (740984.50 MACHEREY-NAGEL) following the manufacturer’s instruction. Briefly, the harvested tissue is minced in small pieces using a plastic pestle. The extracted RNA can be used immediately or stored at −80 °C. The quality control of RNA through agarose gel electrophoresis will avoid the likelihood of final problem solving, such as in cDNA reverse transcription and amplification of the gene target. For cDNA synthesis, 500 ng of RNA was reverse-transcribed with iScript cDNA Synthesis Kit (1708891 BIORAD). Dilute 500 ng of the total cDNA 1:10 in nuclease-free water in order to get 50 ng as final amount before using it for the PCR reaction. Quantitative real-time PCR is carried out using iTaqUniversal SYBRGreen Supermix (1725121 BIORAD). To prepare the PCR reaction mix in a final volume of 20 μL, use 1–2 μL of the diluted cDNA with around 5–10 ng of cDNA template. Use specific primers for your gene of interest and primers for a housekeeping gene. One of the commonly utilized housekeeping genes, which we also use, is GAPDH. All the samples are prepared in triplicate. The amplification curves are visualized by SYBR Green Analysis on Applied Biosystem Instrument (7300). The recommended thermal cycling for the amplification is as follows: 95 °C for 10 min, 40 cycles at 95 °C 15 s, 64 °C for 30 s, and 72 °C for 30 s. The 2–ΔΔCT method is used to calculate the relative expression level.24
Western Blotting
Cells pellets are resuspended in RIPA buffer (Pierce 89901) supplemented with protease inhibitors and mechanically minced using a 200 μL pipet tip. The lysates are then incubated for 30 min in ice, and the supernatants are collected after centrifugation for 30 min at 14 000 rpm. The protein concentration in the lysates is determined through the Bradford assay, using a standard calibration curve method prepared with bovine serum albumin (BSA) of known concentrations (2000 μg/mL). The absorbance values at 595 nm of the samples and the standards are noted. The formula y = mx + q is used to derive the protein concentration. Then, 30–50 μg of the total protein was separated via SDS-PAGE. Upon transferring the samples from the gel to a nitrocellulose membrane, the proteins are treated with a blocking solution (TBS 1× 5% powdered milk) for 1 h at room temperature. For the overnight primary antibody incubation at 4 °C, we used anti-TFRC primary antibody (SAB4200398 Sigma-Aldrich). The membrane was washed thrice with TBS 1×–0.1% Tween20, and the horseradish peroxidase (HRP)-conjugated secondary antibody was then added and incubated for 1 h at room temperature. Finally, after further washing in TBS 1×–0.1% Tween20, the bands are visualized through chemiluminescence using an enhanced chemiluminescence (ECL) kit (1705061 Biorad) and Image Quant LAS 4000 System.
Hematoxylin and Eosin (H&E) Staining
Tumor samples, fixed in 4% paraformaldehyde for 24–48 h, were rinsed in running tap water for 10–15 min. Then, dehydrate samples through increasing alcohol series, followed by three changes of 100% alcohol applied for 5 min each. The tissues were cleared in xylene for 12 min and then immersed in paraffin wax for 10 min, followed by other two paraffin changes, 5 min each, and finally embedded in paraffin blocks. Serial sections of 5–6 μm were cut with a microtome, placed on slides, and heated overnight at 40 °C in forced ventilation histology oven. The sections were cleared from paraffin by two changes in xylene, 6 min each. The tissue was hydrated through decreasing ethanol series and rinsed in distilled water for at least 5 min.
Slices were stained with Mayer’s hematoxylin solution for 5 min, followed by a 10 min rinse in running tap water, and finally stained in eosin Y aqueous solution for 2 min. Slides were rinsed in distilled water, heated at 40 °C for 40 min, dipped twice in xylene, and a coverslip was added placing a drop of Permount mounting medium.
Histological images (40×, 100×, 200×, and 400×) are acquired by light microscope (Olympus BX43, Japan) and digitized using a RGB video camera (Olympus DP 20, Japan).
Anticipated Results
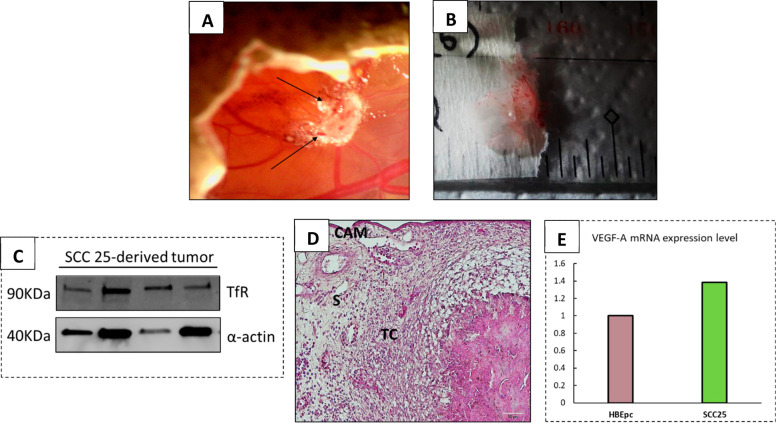
The reliable generation of a solid tumor on CAM is fundamental for both the comprehension of in vivo cancer cell behaviors and the efficacy/toxicity evaluation of emerging and conventional therapeutic strategies. Imaging of tumors provides detailed information about the quality and the presence/absence of blood vessel across the tumor itself; an important parameter to monitor during the time frame of the experiment and/or after the therapeutic treatments.26 Imaging also provides a practical method to identify the volume of the tumor mass without interfering with its spatial organization and allows for distinguishing the tumor from artifacts caused by the aggregation of the Matrigel solution. The primary goals in the production of CAM tumor models include the achievement of a high embryo survival rate and visible tumor grafting that allow the topical application of therapeutics. Our optimized protocol allows the development of solid vascularized SCC-25 tumor with high efficiency (∼80%) and with a volume of 5–20 mm3 at EDD10 (Figure 3A). It is important to notice that the assessment of tumor size following the harvesting can be affected by the wrinkling of the membrane after 2 h at 4 °C. However, the size of the excised tumors usually significantly correlates with their in vivo volume. Measuring the weight of the excised tumors is a facile and useful end-point evaluation that provides additional information on the aggressiveness and ability of cancer cells to grow and form solid tumors (Figure 3B). It is worth remembering that the identification and introduction of new molecular pathways involved in the neoplastic transition has rapidly expanded, considerably advancing the diagnostic techniques.27 In fact, the detection of specific biomolecular tumor markers represents a significant diagnostic screening approach that allows differentiation between cancer cells and the surrounding cells. Among the number of molecular mechanisms already identified in neoplasms, the expression of transferrin receptor (TfR) appears to be compromised in HNSCCs, leading to an overexpression of the receptor and therefore constituting a promising tumor marker.28,29 In this regard, to effectively prove the human origin of the harvested tissue, Western blot analysis has been carried out using an antihuman TfR antibody. The results demonstrate the presence of the TfR protein maker in the harvested tumor samples, confirming the significance of this approach to determinate the human origin of the grafted tumor (Figure 3C).
Figure 3.
Characterization of harvested SCC25 tumors. (A) Representative image of SCC-25 solid tumor grown onto the CAM. The arrows indicate the blood vessels across the tumor mass. (B) Example of solid and vascularized tumor harvested at EDD17. (C) Western blotting analysis depicting the expression of the TfR marker in SCC-25-tumor-derived cells. (D) H&E staining of SCC-25 tumor-derived cells showing the tissue structure and cells distribution (TC = tumor cells; S = stroma; scale bar = 20 μm). (E) Real-time PCR measurement of VEGF-A mRNA expression levels in SCC-25 cancer cells compared to HBEpc bronchial cell line.
H&E staining is another pivotal end-point assay that mainly provides qualitative information and a general overview on the structure of the tissues.30 Remarkably, this approach enables visualization of cellular morphology and the distribution pattern of distinct cells, as well as their density and consistency. The H&E staining of the collected SCC-25 tumor specimen showed a well-organized, preserved, and homogeneous structure, clearly identifiable from the surrounding stroma and the membrane border (Figure 3D).
Since genetic alterations are often events upstream of cell metabolism dysfunction, advances in the assessment of gene expression profile may be useful for the prognosis of various type of cancers, hence offering a wide overview about the gene interaction network in the development of the disease. In fact, mutations that lead to the alteration of gene expression level of cytokines, enzymes, and growth factors are the main responsible of tumorigenesis.31 The deregulation of the vascular endothelial growth factor-A (VEGF-A) was observed to be among the tumor promoting factors in SCC-25 cells. Indeed, VEGF-A is relatively overexpressed in SCC-25 cells compared to the level in normal cells (Figure 3E) and constitute a promising molecular therapeutic target for head and neck tumors. Overall, these findings aim to emphasize the use of the chick embryo as an in vivo model for the screening of anticancer compounds.
Summary
HNSCCs represent an aggressive class of neoplasms with a worldwide high incidence due to two main risk factors: tobacco/alcohol consumption and HPV infection.32 The translation of efficient therapeutic strategies in oncology requires a deep understanding beyond the physiopathology of neoplasms as well as ethical and reliable in vivo models that provide an analogous environment for an accurate exploration of the behavior of cancer tissues and their response to the therapeutic approaches. Due to its highly vascularized environment and immature immune responses, the CAM model provides an optimal environment for the grafting of cancer cell lines and patient-derived cancer cells.33
Here, we have presented a standardized and optimized protocol for the medium-/high-throughput production of solid tumors using the commercial HPV-negative head and neck cell line SCC-25. Each step of the protocol requires basic biological practice and experience. In general, the high survival rate of the embryos and tumor take rate are among the essential criteria considered for the efficiency of the protocol. Additionally, a detailed outline and explanation of the steps and further remarks have been included to provide a suitable guide for the generation of tumor grafts and subsequent imaging and biomolecular assays. The reported step-by-step method allows the establishment of a feasible in vivo model that can provide insights on the biological processes at the basis of oral malignancies and the development of new therapeutic strategies.100 The CAM model may push oncological research toward a more rapid evaluation and efficient screening/selection of conventional and emerging antitumor treatments.
Acknowledgments
This work is supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) under MFAG 2017, ID 19852 project (P.I.: V.V.). Figure 1 and the graphical abstract have been created with BioRender.com.
The authors declare no competing financial interest.
References
- Kim N.; Ryu H.; Kim S.; et al. (2019) CXCR7 promotes migration and invasion in head and neck squamous cell carcinoma by upregulating TGF-β1/Smad2/3 signaling. Sci. Rep. 9 (1), 1–11. 10.1038/s41598-019-54705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Bian X.-y.; Chen Q.; Yao X.-f.; Wang X.-d.; Zhang W.-c.; Tao Y.-j.; Jin R.; Zhang L. (2017) Blocking of stromal interaction molecule 1 expression influence cell proliferation and promote cell apoptosis in vitro and inhibit tumor growth in vivo in head and neck squamous cell carcinoma. PLoS One 12 (5), e0177484. 10.1371/journal.pone.0177484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiris A.; Karamouzis M. V; Raben D.; Ferris R. L (2008) Head and neck cancer. Lancet 371, 1695–1709. 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels J. P.; René Leemans C.; Golusinski W.; Grau C.; Licitra L.; Gregoire V. (2020) Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 31 (11), 1462–1475. 10.1016/j.annonc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- Mapanao A. K.; Santi M.; Voliani V. (2021) Combined chemo-photothermal treatment of three-dimensional head and neck squamous cell carcinomas by gold nano-architectures. J. Colloid Interface Sci. 582, 1003–1011. 10.1016/j.jcis.2020.08.059. [DOI] [PubMed] [Google Scholar]
- Lango M. N. (2009) Multimodal Treatment for Head and Neck Cancer. Surg. Clin. North Am. 89 (1), 43–52. 10.1016/j.suc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Cognetti D. M.; Weber R. S.; Lai S. Y. (2008) Head and neck Cancer an evolving treatment paradigm. Cancer 113 (7), 1911–1932. 10.1002/cncr.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E.; Mazul A. L.; Farquhar D.; Brennan P.; Anantharaman D.; Abedi-Ardekani B.; Weissler M. C.; Hayes D. N.; Olshan A. F.; Zevallos J. P. (2019) Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope 129, 2506–2513. 10.1002/lary.27807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi M.; Mapanao A. K.; Biancalana L.; Marchetti F.; Voliani V. (2021) Ruthenium arene complexes in the treatment of 3D models of head and neck squamous cell carcinomas. Eur. J. Med. Chem. 212, 113143. 10.1016/j.ejmech.2020.113143. [DOI] [PubMed] [Google Scholar]
- Santi M.; Mapanao A. K.; Cassano D.; Vlamidis Y.; Cappello V.; Voliani V. (2020) Endogenously-activated ultrasmall-in-nano therapeutics: Assessment on 3d head and neck squamous cell carcinomas. Cancers 12 (5), 1063. 10.3390/cancers12051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R. I.; Shin D. M. (2008) Recent advances in head and neck cancer reconstruction. N. Engl. J. Med. 359, 1143–1154. 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- Mapanao A. K.; Giannone G.; Summa M.; Ermini M. L.; Zamborlin A.; Santi M.; Cassano D.; Bertorelli R.; Voliani V. (2020) Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures. Nanoscale Adv. 2, 3815–3820. 10.1039/D0NA00521E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano D.; Mapanao A.-K.; Summa M.; Vlamidis Y.; Giannone G.; Santi M.; Guzzolino E.; Pitto L.; Poliseno L.; Bertorelli R.; Voliani V. (2019) Biosafety and biokinetics of noble metals: the impact of their chemical nature. ACS Appl. Bio Mater. 2 (10), 4464–4470. 10.1021/acsabm.9b00630. [DOI] [PubMed] [Google Scholar]
- Cassano D.; Summa M.; Pocoví-Martínez S.; Mapanao A.-K.; Catelani T.; Bertorelli R.; Voliani V. (2019) Biodegradable ultrasmall-in-nano gold architectures: mid-period in vivo biodistribution and excretion assessment. Part. Part. Syst. Charact. 36 (2), 1800464. 10.1002/ppsc.201800464. [DOI] [Google Scholar]
- European Parliament . Directive 2010/63/EU - On the protection of animals used for scientific purposes. Off. J. Eur. Communities: Legis. 2010, 276, 33–79. [Google Scholar]
- Mapanao A. K.; Voliani V. (2020) Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today. 19, 100552. 10.1016/j.apmt.2019.100552. [DOI] [Google Scholar]
- Ribatti D. (2016) The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 141, 70–77. 10.1016/j.mod.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Santi M.; Mapanao A. K.; Cappello V.; Voliani V. (2020) Production of 3D tumor models of head and neck squamous cell carcinomas for nanotheranostics assessment. ACS Biomater. Sci. Eng. 6 (9), 4862–4869. 10.1021/acsbiomaterials.0c00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapanao A. K.; Che P. P.; Sarogni P.; Sminia P.; Giovannetti E.; Voliani V. (2021) Tumor grafted - chick chorioallantoic membrane as an alternative model for biological cancer research and conventional/nanomaterial-based theranostics evaluation. Expert Opin. Drug Metab. Toxicol. 00 (00), 1–22. 10.1080/17425255.2021.1879047. [DOI] [PubMed] [Google Scholar]
- Kunz P.; Schenker A.; Sähr H.; Lehner B.; Fellenberg J. (2019) Optimization of the chicken chorioallantoic membrane assay as reliable in vivo model for the analysis of osteosarcoma. PLoS One 14 (4), e0215312. 10.1371/journal.pone.0215312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M.; Javerzat S.; Gilges D.; et al. (2005) Accessing key steps of human tumor progression in vivo by using an avian embryo model. Proc. Natl. Acad. Sci. U. S. A. 102 (5), 1643–1648. 10.1073/pnas.0408622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovithi M.; Avan A.; Funel N.; Leon L. G.; Gomez V. E.; Wurdinger T.; Griffioen A. W.; Verheul H. M. W.; Giovannetti E. (2017) Development of bioluminescent chick chorioallantoic membrane (CAM) models for primary pancreatic cancer cells: A platform for drug testing. Sci. Rep. 7, 44686. 10.1038/srep44686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecilia Subauste M.; Kupriyanova T. A.; Conn E. M.; Ardi V. C.; Quigley J. P.; Deryugina E. I. (2009) Evaluation of metastatic and angiogenic potentials of human colon carcinoma cells in chick embryo model systems. Clin. Exp. Metastasis 26 (8), 1033–1047. 10.1007/s10585-009-9293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P.; Casar B. (2016) The Chick Embryo Chorioallantoic Membrane as an in vivo Model to Study Metastasis. Bio-Protocol. 6 (20), 1–11. 10.21769/BioProtoc.1962.27642615 [DOI] [Google Scholar]
- Sys G. M. L.; Lapeire L.; Stevens N.; et al. (2013) The in ovo CAM-assay as a xenograft model for sarcoma. J. Visualized Exp. (77), 1–7. 10.3791/50522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleibeuker E. A.; Schulkens I. A. E.; Castricum K. C. M.; Griffioen A. W.; Thijssen V. L. J. L. (2015) Examination of the Role of Galectins During In Vivo Angiogenesis Using the Chick Chorioallantoic Membrane Assay. Methods Mol. Biol. 1207, 305–315. 10.1007/978-1-4939-1396-1_20. [DOI] [PubMed] [Google Scholar]
- Kunz et al. demonstrated that the application of 70% ethanol or other disinfectants on the shell considerably reduces the viability of the embryo by approximately 30%, thereby affecting the overall execution of the experiment.17
- The tilting racks are usually provided upon the purchase of the incubator. They have a metal pin that fits into a small hole in the back of the incubator, hence allowing the automatic movement. If no tilting racks are available, then the trays can be manually moved through 180° at least 2 or 3 times per day.
- Once all the eggs are inside and incubation starts, it is recommend to avoid frequent opening of the incubator in order to keep the temperature and humidity constantly on the set parameter and not interfere with the growth of the embryo. The percentage of the unfertilized eggs may interfere with the final outcome of the experiment, and for this reason, it is strongly recommended to start with a large number of eggs to obtain consistent result, e.g., 10% more than expected eggs for the experiment. It should also take in consideration that the fertilization rate of the eggs can be seasonal depending on the supplier.
- Placing a bright light source on top/behind the egg (“candling”) allows you to see through the shell. The air chamber should be located in the blunt end of the egg. The incubated eggs can be also candled to check for fertilization. While the fertilization of white eggs can be checked at the third day of incubation, the brown-shelled eggs may require a few more days to check this parameter (Figure 2B). The presence of vessels is an indication of fertilized egg.
- The small window should be carefully opened to avoid shell debris falling onto the membrane. If this happens, then try to carefully remove the debris with sterile tweezers, without damaging the membrane, to prevent infections or microorganism contamination.
- SCC-25 cells are maintained in a complete growth medium composed of a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium supplemented with 10% of fetal bovine serum (FBS), 4 mM l-glutamine, 1mM sodium pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin. The medium is also supplemented with 400 ng/mL hydrocortisone. It should be noted that SCC-25 is a very adherent cell line. Thus, a 5 min incubation in trypsin may not be sufficient to completely detach the cells from the culture dish. After the medium is removed, it is recommended to first wash the cells with PBS and, then, with a small amount of trypsin. After discarding, add trypsin again and incubate the cells for 5–10 min to harvest a high and consistent number of cells. Knowing the doubling time of your cells may be useful for setting up the experimental design. For SCC-25, the doubling time was observed to be approximately 45 hours. It is also suggested to use 150 mm plates to promote an extensive cell growth and to lower the frequency of splitting the cells.
- The final cell number per egg was empirically determined. It has been observed that 2 × 106 cells for SCC-25 is the minimum amount needed to obtain solid tumor formation by EDD 10 (i.e., 4 days post-grafting and assigned EDD in which treatments are initially administered).
- Matrigel is a protein mixture that resembles the extracellular environment required to have a conducive substrate for cell growth. Moreover, it is a dense mixture and keeps the cells together once deposited on the membrane. The Matrigel solution is stored at −20°C. It needs to be thawed overnight at 4°C before the grafting experiment. It is recommended to prepare Matrigel aliquots to keep the stocks in proper storage temperature and avoid repeated freeze–thaw cycles. While working with Matrigel, it should be kept constantly on ice to prevent polymerization while preparing the working solution (12.5μL Matrigel + 12.5μL medium without FBS per egg) or dispensing the cells on the membrane. Importantly, the tumor grafting efficiency at EDD 10 is only 20% if the SCC-25 cells deposited on the CAM are suspended in the medium alone.
- The veins and arteries of the CAM are easily distinguished from each other by the different blood color and vessel motility.25 The arteries are darker, and their size is larger compared to the veins. Also, the movement of the arteries is more active, and their walls are thicker, making them difficult to rupture during cell grafting.
- It usually takes 4 days post-grafting for the SCC-25 cells to grow and form a solid visible tumor on the CAM. This timeframe may change for other cell lines. It is recommended to have at least 8–9 eggs per treatment condition in order to have consistent statistics as well as to employ at least 3 eggs as a control group without any treatment.
- The treatment solution must be freshly prepared just before its application. It has been observed that 30 μL of solution is enough to adequately cover the tumor mass. Do not touch the tumor with the tip while applying the treatment in order to avoid tissue damage.
- The tumor measurements from EDD10 to EDD14 are taken from a superficial angle. Indeed, their volume is calculated by considering the width and length of the visible tumor estimated from the top of the CAM. It should be considered that some neoplasms may grow below or above the membrane. Image acquisition can be time consuming, especially when handling a lot of samples. It is recommended to always keep the same magnification for all the collected images. Any magnification changes require a software recalibration. The light and embryo movements may interfere with the acquisition of good quality images. It is also important to adjust the light and polarization of the microscope to remove as much light reflection as possible on the membrane in order to collect high-quality images. Eggs should be taken out from the incubator in small batches during visual analysis to prevent the frequent opening and closing of the incubator, which can affect the settings.
- Considering that the appearance of the membranes and the tumors can change after 2 hours at 4 °C due to the wrinkling of the CAM, the visualization of the tumor can be difficult. In this regard, the images from previous EDDs may be helpful to locate the position of the tumor. While harvesting the tumor, cut as little of the membrane as possible since it might interfere with the weight of the tumor and other downstream assays such as RNA extraction and analysis of gene expression profile, protein extraction and detection of tumor marker, and qualitative information about the structure of the tissue.
- Livak K. J.; Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25 (4), 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nam K. H.; Kim J.; Ra G.; Lee C. H.; Paeng D. G. (2015) Feasibility study of Ex Ovo chick chorioallantoic artery model for investigating pulsatile variation of arterial geometry. PLoS One 10 (12), e0145969. 10.1371/journal.pone.0145969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina E. (2016) Chorioallantoic Membrane Microtumor Model to Study the Mechanisms of Tumor Angiogenesis, Vascular Permeability, and Tumor Cell Intravasation. Methods Mol. Biol. 1430, 283–298. 10.1007/978-1-4939-3628-1. [DOI] [PubMed] [Google Scholar]
- Handy B. (2009) The clinical utility of tumor markers. Lab. Med. 40 (2), 99–103. 10.1309/LMTRKSKYW4GI6SBJ. [DOI] [Google Scholar]
- Shan L.; Hao Y.; Wang S.; Korotcov A.; Zhang R.; Wang T.; Califano J.; Gu X.; Sridhar R.; Bhujwalla Z. M.; Wang P. C. (2008) Visualizing head and neck tumors in vivo using near-infrared fluorescent transferrin conjugate. Mol. Imaging 7 (1), 42–49. 10.2310/7290.2008.0006. [DOI] [PubMed] [Google Scholar]
- Högemann-Savellano D.; Bos E.; Blondet C.; Sato F.; Abe T.; Josephson L.; Weissleder R.; Gaudet J.; Sgroi D.; Peters P. J.; Basilion J. P. (2003) The Transferrin Receptor: A Potential Molecular Imaging Marker for Human Cancer. Neoplasia 5 (6), 495–506. 10.1016/S1476-5586(03)80034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman A. T.; Wolfe W. (2014) Tissue Processing and Hematoxylin and Eosin Staining. Methods Mol. Biol. 1180, 283–291. 10.1007/978-1-4939-1050-2. [DOI] [PubMed] [Google Scholar]
- Neufeld G.; Kessler O. (2006) Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer Metastasis Rev. 25 (3), 373–385. 10.1007/s10555-006-9011-5. [DOI] [PubMed] [Google Scholar]
- McDermott J. D.; Bowles D. W. (2019) Epidemiology of Head and Neck Squamous Cell Carcinomas: Impact on Staging and Prevention Strategies. Curr. Treat Options Oncol. 20 (5), 1–13. 10.1007/s11864-019-0650-5. [DOI] [PubMed] [Google Scholar]
- DeBord L. C.; Pathak R. R.; Villaneuva M.; Liu H.-C.; Harrington D. A.; Yu W.; Lewis M. T.; Sikora A. G. (2018) The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 8 (8), 1642–1660. [PMC free article] [PubMed] [Google Scholar]
- Vlamidis Y.; Voliani V. (2018) Bringing again noble metal nanoparticles to the forefront of cancer therapy. Front. Bioeng. Biotechnol. 6, 143. 10.3389/fbioe.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]