Abstract
Hospital discharge planning can be complex and hospital space is often limited. Patients, including those with COVID-19, can have prolonged symptoms after discharge and often require ongoing monitoring. Furthermore, prolonging hospital stays primarily for monitoring can expose patients to iatrogenic and infectious risks. The patient's overall condition and their home support system factor into the decisions of when and where to discharge patients. Innovations in remote patient monitoring (RPM) now allow for more options in the discharge process. This case report presents a patient with severe COVID-19 pneumonia where RPM was used at discharge to improve home monitoring and clinical follow-up. Additional experience with RPM is necessary to refine its role in post-acute care monitoring.
Keywords: COVID-19, Remote patient monitoring, Post-acute care management, Respiratory monitoring, Wearable sensors
Highlights
-
•
A COVID-19 infected patient was followed post-discharged using a wearable cardiorespiratory monitor.
-
•
RPM informed discharge planning in light of the feasibility of ongoing monitoring.
-
•
Physiological changes on RPM mirrored the patient's clinical and radiographic improvements.
1. Introduction
The recovery time from COVID-19 can be prolonged with most post-hospitalized patients reporting persistent symptoms at 2 months [1]. The risk of readmission after COVID-19 hospitalization is substantial with studies showing rates of 60-day readmission as high as 20% [2]. Prolonging hospitalization increases iatrogenic and infectious risks to the patient as well as increases financial costs to the healthcare system [3,4]. For these reasons as well as the limitations of hospital capacity, optimizing hospital discharge plans and follow-up is imperative.
When patients reach a level of stability in the hospital, medical teams must decide when it is appropriate to discharge a patient home. This can be challenging when patients have not returned to their baseline and when it is uncertain if the home support system can monitor the patient appropriately. Here we present a case of post-acute care monitoring using digital, non-invasive RPM at home in a patient following admission for respiratory failure as a result of severe COVID-19 pneumonia. By using a respiration-focused RPM in conjunction with a proactive program which responds to physiologic alerts, we hope to improve patient and physician comfort at the time of discharge while providing optimal follow-up care.
2. Case
The patient is a 75-year-old male whose only prior significant medical history included hyperlipidemia. He was diagnosed with COVID-19 in the setting of fatigue, fever and dry cough. Over the next 3 weeks, his symptoms seemed to initially improve slightly but he then developed worsening shortness of breath, cough, and recurrent fever. On presentation to the emergency room he was hypoxic to levels as low as 83% with ambulation while on room air. He had significant rales on exam with a CT scan demonstrating patchy bilateral ground glass opacities consistent with viral pneumonia (Fig. 1, left panel). The patient was subsequently admitted to the hospital and started on systemic steroids with dexamethasone. After five days on therapy, his ambulatory oxygen saturations improved to 93% while on room air, though rales persisted on exam. The Pulmonary team felt he was stable for discharge but wanted to ensure optimal home monitoring given his persistent symptoms and risk for decompensation based on his age. He was discharged with a 48-h telemedicine follow up visit and a plan to initiate a remote cardiorespiratory monitoring program. The program included a garment-adhered wearable sensor that includes photoplethysmography, tri-axis accelerometers, and a dedicate sensor of respiratory effort. The resulting metrics used were respiratory rate, pulse rate, and steps [5]. The program included a 7-day-per-week clinical monitoring service in which respiratory therapists proactively engage the patient by telephone in the event of significant changes in physiologic variables or device adherence.
Fig. 1.
Axial CT image 3 (left) and 6 (right) weeks after initial COVID-19 diagnosis.
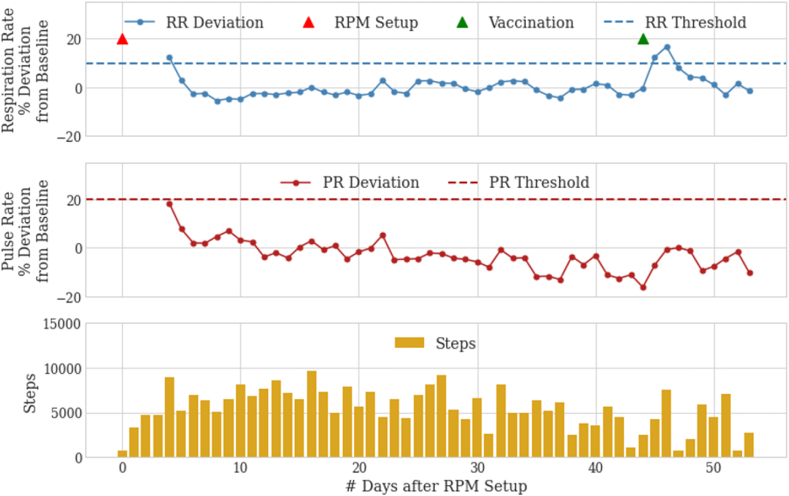
After discharge, the patient reported slow but continued improvement in his shortness of breath with increasing exercise capacity. However, the patient did report feeling tachycardic at times. At telemedicine and in-person clinic visits, the patient's progress was discussed and visualized using the patient's RPM. The patient was reassured that his cardiac and respiratory parameters remained stable. In fact, steady declines in his respiratory rate and pulse rate deviations were noted post-discharge (Fig. 2). Three weeks after discharge, his ambulatory oxygenation improved to 94% or higher while on room air. Follow-up imaging 6 weeks after the initial diagnosis showed significant improvement but not complete resolution of the parenchymal changes (Fig. 1, right). Except for a notable 2-day increase in respiratory rate corresponding to symptoms after COVID vaccination, the patient showed continued stability in respiratory rate and heart rate two months after hospital discharge (Fig. 2).
Fig. 2.
COVID-19 pneumonia post-discharge physiological data as measured by the RPM device. Decreasing respiratory and pulse rates are shown in the days immediately after device setup. Each data point on the respiratory and pulse rate deviation rows refers to the deviation of that day's median rate with the patient's all-time median baseline value. RPM setup is followed by a period of physiological stability then and a 2-day temporary increase in respiratory rate alone following COVID-19 vaccination.
3. Discussion
The current U.S. model for hospital discharge generally includes scheduled healthcare provider appointments and the encouragement of patients to reach out to their provider when they have new or worsening symptoms. When feasible and appropriate, home health services may also be ordered. The existing approach suffers from several limitations. Patients may become ill before their scheduled appointment. They may not recognize their symptoms as significant enough to warrant reaching out to their doctor sooner [6]. Additionally, patients may avoid seeking care for subjective or logistical reasons such as not wanting to burden themselves or the practice [6]. Concerns of increasing exposure to COVID-19 have also been shown to delay care, including for emergent medical issues [7]. Consequently, healthcare providers may be resistant to discharge patients due to concerns about inadequate follow-up and monitoring.
The concept of virtual wards has been promoted recently to maintain the quality of medical care while increasing hospital capacity [8]. This solution typically involves self-monitoring at home, supplemented by frequent telephone contact. The incorporation of remote monitoring technology has been suggested as a way to further improve quality and lessen resource utilization [9]. Current technology now allows for enhanced monitoring and a more targeted approach to post-hospital care. Some medical-grade RPM devices allow for 24-h surveillance of a patient's respiratory rate, heart rate and activity level similar to, and in some cases more than, what they receive in the hospital setting [5]. While RPM has often been focused on behavioral interventions and the management of chronic disease [10], opportunities for its application exist in post-acute respiratory monitoring.
This case demonstrates a promising use for RPM in a patient recovering from COVID-19. The increased surveillance provided by RPM gave the clinician additional confidence that upon discharge the patient would be monitored and in the event of significant physiologic changes, the patient would be proactively engaged. Respiratory rate elevation alone has been suggested as an important triage tool for COVID-19 and the remote monitor used removes the problems of manually counted rates [11]. The respiratory rate increase noted after COVID-19 vaccine points to the sensitivity of RPM to identify physiologic stressors but also highlights the need for a subsequent clinical assessment to determine significance and to limit false positives. Additionally, the RPM data confirmed the patient-reported improvements were consistent with objective data. Given the increasingly recognized long-term sequelae of COVID-19 [12], RPM also provides opportunity for longitudinal assessment of patient progress and functional status through the reporting of daily step counts and physiologic changes.
4. Conclusion
The potential for RPM to allow patients to be discharged sooner and for healthcare services to be concentrated on those with the most need is significant and not isolated to cases of COVID-19. Hospital bed shortages have been almost universally reported around the world as a result of the COVID-19 pandemic [[13], [14], [15]]. Virtual wards have been promoted in the United Kingdom and has shown significant cost savings [16]. Earlier discharge has the potential to reduce iatrogenic complications such as hospital acquired infections and deep vein thrombosis [3,4]. The use of RPM technology has the ability to improve the capacity, efficiency, and quality of complex home-based care. This case demonstrates the utility of RPM in enhancing clinician confidence in the discharge plan for a patient with COVID-19 as well as in aiding the communication of patient progress. Research focusing on the optimal utilization and protocols for RPM use in post-acute care for COVID-19 and other disease states is necessary.
Author contributions
Michael Polsky: Conceptualization, Methodology, Original draft preparation.
Neema Moraveji: Data curation, Visualization, Reviewing and Editing.
Declaration of competing interest
Dr. Polsky is a paid consultant of Spire Health. Dr. Moraveji is an employee of Spire Health.
Contributor Information
Michael B. Polsky, Email: mpolsky@paraccess.com.
Neema Moraveji, Email: neema@spirehealth.com.
References
- 1.Mandal S., Barnett J., Brill S.E. 10 November 2020. ‘Long-COVID’: a Cross-Sectional Study of Persisting Symptoms, Biomarker and Imaging Abnormalities Following Hospitalisation for COVID-19. Thorax Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly J.P., Wang X.Q., Iwashyna T.J., Prescott H.C. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. J. Am. Med. Assoc. 2021;325(3):304–306. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosman M., Rachminov O., Segal O., Segal G. Prolonged patients' In-Hospital Waiting Period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv. Res. 2015;15:246. doi: 10.1186/s12913-015-0929-6. Published 2015 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin A., Neuman W.R., Lingohr-Smith M., Menges B., Lin J. Influence of the duration of hospital length of stay on frequency of prophylaxis and risk for venous thromboembolism among patients hospitalized for acute medical illnesses in the USA. Drugs Context. 2019;8:212568. doi: 10.7573/dic.212568. Published 2019 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moraveji Neema. 2019 IEEE International Symposium on Medical Measurements and Applications. IEEE; 2019. Long-term, ambulatory respiratory monitoring of COPD patients using garment-adhered sensors. [Google Scholar]
- 6.Raza K. SP0158 why do patients delay in seeking help. Ann. Rheum. Dis. 2014;73(Suppl 2) 42-42. [Google Scholar]
- 7.Czeisler M.É., Marynak K., Clarke K.E. Delay or avoidance of medical care because of COVID-19–related concerns — United States, june 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virtual Wards: Bringing the hospital home. Healthcare at Home. URL: https://hah.co.uk/wp-content/uploads/2017/07/Virtual-Hospital-Report_AW_Final2.pdf [accessed 2021-01-07].
- 9.Shah S.S., Gvozdanovic A., Knight M., Gagnon J. Mobile app-based remote patient monitoring in acute medical conditions: prospective feasibility study exploring digital health solutions on clinical workload during the COVID crisis. JMIR Form Res. 2021;5(1) doi: 10.2196/23190. Published 2021 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vegesna A., Tran M., Angelaccio M., Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed. J. e Health. 2017 Jan;23(1):3–17. doi: 10.1089/tmj.2016.0051. Epub 2016 Apr 26. PMID: 27116181; PMCID: PMC5240011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massaroni C., Nicolò A., Schena E., Sacchetti M. Remote respiratory monitoring in the time of COVID-19. Front. Physiol. 2020;11:635. doi: 10.3389/fphys.2020.00635. Published 2020 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020 Nov 1;26 doi: 10.12659/MSM.928996. PMID: 33177481; PMCID: PMC7643287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baughman A.W., Hirschberg R.E., Lucas L.J. Pandemic care through collaboration: lessons from a COVID-19 field hospital. J. Am. Med. Dir. Assoc. 2020;21(11):1563–1567. doi: 10.1016/j.jamda.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Requia W.J., Kondo E.K., Adams M.D., Gold D.R., Struchiner C.J. Risk of the Brazilian health care system over 5572 municipalities to exceed health care capacity due to the 2019 novel coronavirus (COVID-19) Sci. Total Environ. 2020 Aug 15:730. doi: 10.1016/j.scitotenv.2020.139144. 139144, Epub 2020 May 1. PMID: 32380368; PMCID: PMC7252142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carenzo L., Costantini E., Greco M., Barra F.L., Rendiniello V., Mainetti M., Bui R., Zanella A., Grasselli G., Lagioia M., Protti A., Cecconi M. Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy. Anaesthesia. 2020 Jul;75(7):928–934. doi: 10.1111/anae.15072. Epub 2020 Apr 22. Erratum in: Anaesthesia. 2020 Nov;75(11):1540. PMID: 32246838. [DOI] [PubMed] [Google Scholar]
- 16.Colligan J. Royal College of Nursing; 2015. The Virtual Ward, Managing the Care of Patients with Chronic Conditions in the Community - an Economic Assessment of the South Eastern Trust Virtual Ward Internet.https://www.rcn.org.uk/professional-development/research-and-innovation/innovation-in-nursing/case-studies-demonstrating-the-value-of-nursing/janice-colligan accessed 2021-01-07. [Google Scholar]