Abstract
Background & aims
Prevalence and complications of oropharyngeal dysphagia (OD) and malnutrition (MN) in COVID-19 patients is unknown. Our aim was to assess the prevalence, risk factors and clinical outcomes of OD and MN in a general hospital during the first wave of the COVID-19 pandemic.
Methods
This was a prospective, observational study involving clinical assessment of OD (Volume-Viscosity Swallowing Test), and nutritional screening (NRS2002) and assessment (GLIM criteria) in COVID-19 patients hospitalized in general wards at the Consorci Sanitari del Maresme, Catalonia, Spain. The clinical characteristics and outcomes of patients were assessed at pre-admission, admission and discharge, and after 3 and 6-months follow-up.
Results
We included 205 consecutive patients (69.28 ± 17.52 years, Charlson 3.74 ± 2.62, mean hospital stay 16.8 ± 13.0 days). At admission, Barthel Index was 81.3 ± 30.3; BMI 28.5 ± 5.4 kg/m2; OD prevalence 51.7% (44.1% impaired safety of swallow); and 45.5% developed MN with a mean weight loss of 10.1 ± 5.0 kg during hospitalization. OD was an independent risk factor for MN during hospitalization (OR 3.96 [1.45–10.75]), and hospitalization was prolonged in patients with MN compared with those without (21.9 ± 14.8 vs 11.9 ± 8.9 days, respectively; p < 0.0001). OD was independently associated with comorbidities, neurological symptoms, and low functionality. At 6-month follow-up, prevalence of OD was still 23.3% and that of MN only 7.1%. Patients with OD at discharge showed reduced 6-month survival than those without OD at discharge (71.6% vs 92.9%, p < 0.001); in contrast, those with MN at discharge did not show 6-month survival differences compared to those without (85.4% vs 83.8%, p = 0.8).
Conclusions
Prevalence and burden of OD and MN in patients hospitalized in COVID-19 wards is very high. Our results suggest that optimizing the management of MN might shorten the hospitalization period but optimizing the management of OD will likely impact the nutritional status of COVID-19 patients and improve their clinical outcomes and survival after hospital discharge. ClinicalTrials.gov Identifier: NCT04346212.
Keywords: Swallowing disorders, Oropharyngeal dysphagia, COVID-19, Malnutrition, Nutritional risk, Fluid thickening
1. Introduction
Coronavirus disease 2019 (COVID-19) – caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – was first described in December 2019 [1] and the first case in Catalonia, Spain was reported on February 25th, 2020. A pandemic was declared in spring 2020 and the infection has since spread across the world, resulting in a high case rate, a high percentage of severely ill patients and high levels of mortality [2,3]. Manifestations of the disease continue to be documented by the WHO but the most common appear to be fever, cough, sore throat, breathing difficulties and fatigue [2,4]. While most cases are mild, the disease can prove life-threatening in older patients or in those with cardiac and respiratory disorders, which can evolve into pneumonia, acute respiratory distress syndrome and multiorgan failure [4].
COVID-19 is associated with a high risk of inadequate nutrition, which can impact significantly on critically ill patients; disease-related malnutrition (MN) is related to longer intensive care unit (ICU) stays, increased mortality, prolonged hospitalization, and increased morbidity following hospital discharge [5]. COVID-related MN arises due to increased nutritional requirements (hypercatabolism) and the presence of a severe inflammatory status. Furthermore, oropharyngeal dysphagia (OD) and other COVID symptoms such as cough, dyspnea, diarrhea, ageusia, and anosmia contribute to a hyporexic status, and can make food intake unsafe [6,7]. As such, MN worsens the already poor prognosis of COVID-19, especially in multimorbid older patients [6,8].
Oropharyngeal dysphagia (OD) is a particular concern in COVID-19 patients and is a common complication in post-ICU patients, particularly certain subpopulations, including those receiving intubation or mechanical ventilation, those with tracheostomies or nasogastric tubes (NGT), and those with acute respiratory infection, pneumonia and respiratory insufficiency [9,10]. OD is also a concern in patients admitted to COVID-19 wards during the viral response phase, the pulmonary phase and the hyperinflammation phase [11] and those discharged from acute hospitals to rehabilitation centers, nursing homes or other facilities, particularly older and more frail patients. All these scenarios are common with COVID-19. In addition, many complications related to COVID-19 (polyneuropathy, myopathy, and various neurological complications) [12] directly affect the swallowing network, making COVID-19 sufferers more susceptible to developing OD [7,13]. Invasion of peripheral nerves by SARS-CoV-2 causes anosmia and ageusia in a high proportion of COVID-19 patients [14,15]; as well as impaired pharyngeal sensory function, which could facilitate swallowing problems [7,13]. As a result of these nutritional concerns, various national bodies and research groups have published guidance on the management of dysphagia in COVID-19 patients [[16], [17], [18], [19], [20]].
Pathophysiology and prevalence of OD in COVID-19 patients and their relationship with nutritional complications and poor clinical outcome is as yet unclear and requires rapid elucidation to optimize the clinical management of patients. The aim of this observational study was to assess the prevalence, risk factors and clinical outcomes of OD and MN; to analyze the specific needs surrounding compensatory treatments; and to document 3- and 6-month clinical outcomes in patients with acute COVID-19 admitted to the general wards in a general hospital within Maresme, Spain. Maresme is located on the north coast of Catalonia and comprises 440,000 inhabitants. As we developed a specific protocol to assess OD and MN in patients with COVID-19 [21], we believe sharing collective clinical experiences may assist in clarifying the optimal treatment of COVID-19 patients on local and global levels.
2. Patients and methods
2.1. Study population
All SARS-CoV-2-positive patients admitted to the general wards of Consorci Sanitari del Maresme (CSdM, comprising the Hospital de Mataró; the Hospital de Sant Jaume i Santa Magdalena; and the medicalized Atenea Hotel) from 14th April to 30th July 2020 were prospectively enrolled in the study. Inclusion criteria were: SARS-CoV-2-infected patients (identified by reverse transcription polymerase chain reaction [RT-PCR]) with GeneXpert Dx (Cepheid, Sunnyvale, CA, USA) admitted to the CSdM for more than 48 h and patients able to be assessed for OD and nutritional status by their physician (fully awake in a stable respiratory situation and with an optimal PaO2/FiO2 ratio). The exclusion criterion was: uncontrolled risk of infection for healthcare professionals [21]. Patients who were admitted directly to the ICU from the Emergency Department of the hospital and then died were not included in the study due to the inability to perform protocol-mandated evaluations.
2.2. Study design
This was an observational, prospective study with COVID-19 patients admitted to the CSdM for more than 48 h. Demographic, clinical and nutritional characteristics of the study population, as well as severity of dysphagia symptoms, were collected for all participants. During the study, there were 4 evaluation time points: 1) within 24 h of hospitalization, pre-admission and admission data were collected; 2) at hospital discharge where all hospitalization data were collected; 3) 3 months post-discharge (data from discharge to 3 month follow up); and 4) 6 months post-discharge (from 3 month to 6 month follow up). Close clinical monitoring was conducted during admission and during the nutritional assessment. Study variables were collected by a multidisciplinary team and comprised three different assessments: a) clinical data (nurse and physician); b) swallowing assessment (nurses and speech language pathologist [SLP]); and c) nutritional assessment (study dietitian/nutritionist). To minimize the risk of cross-infection, all evaluations performed by study investigators were conducted telematically into hospital wards (by telephone call or videoconference with patients or their nurses/physicians) and by retrieving data from the patient's electronic medical record, according to a management protocol previously designed [21]. Telematic follow-up was conducted at 3 and 6 months post-discharge for the following variables: OD, MN, weight and percentage weight loss, adherence to fluid thickening and textural diet adaptation, and functional status (using Barthel Index).
The study protocol was approved by the Institutional Review Board (IRB) of the Hospital de Mataró (CEIm 34/20) and was conducted according to the principles and rules laid down in the Declaration of Helsinki and its subsequent amendments. Exemption of the informed consent form was granted by the Ethics Committee from Consorci Sanitari del Maresme and followed the Guidance on the Management of Clinical Trials during the COVID-19 pandemic (European Commission, version 4; 04/02/2021). ClinicalTrials.gov identifier: NCT04346212.
2.3. Clinical assessment
The clinical data collected included: patient origin at admission and destination at discharge (community, nursing home, intermediate care hospital); main clinical symptoms before and during admission; functionality using the Barthel Index (at pre-admission, admission and discharge) [22,23]; known comorbidities [24]; neurological symptoms at admission (seizures, encephalitis, delirium, headache, dyskinesia, stroke, parenthesis, ataxia, encephalitis, etc); ICU admission (including post-ICU neuromyopathy) and duration of stay; respiratory infections (including symptoms, severity and duration from the week prior to admission); need for oxygen therapy; duration of protonation; prescribed pharmacological treatment; hospitalization duration; diagnosed conditions at discharge (a] respiratory infection including SARS-CoV-2 pneumonia, bacterial respiratory co-infection [25], aspiration pneumonia [9,26], b] OD, and c] MN); and intrahospital mortality.
2.4. Swallowing assessment
Swallowing evaluation included several telematic explorations: 1) specific anamnesis of clinical signs and symptoms of OD and impaired chewing function consisting questions focused on the masticatory ability of patients with solids including omelette and puree, performed by two trained SLPs (VA, WN) with the help of the nursing staff; 2) the Eating Assessment Tool-10 (EAT-10) [27], which consists of 10 simple questions (a score ≥2 out of 40 indicates risk of dysphagia and the need for a more comprehensive evaluation) [27,28]; 3) the Volume-Viscosity Swallowing Test (V-VST) [29] – a validated clinical assessment tool used to explore safety and efficacy of swallow – which was simplified for COVID-19 patients with only one volume (intermediate; 10 mL) and the three usual viscosities (liquid, 250 mPa s and 800 mPa s prepared with 0, 2 and 5.5 g of Nutilis Clear [Nutricia N.V., Zoetermeer, The Netherlands], respectively, in 100 mL water). The V-VST was performed by COVID-19 ward nurses with telematic assistance from the SLP [21]. Recommendations on fluid adaptation given to patients, tolerance, and adherence levels were recorded during hospitalization and at 3 and 6 months post-discharge.
2.5. Nutritional assessment
Anthropometric measurements were collected and included weight, percentage weight loss, height, and body mass index (BMI). The Nutritional Risk Screening 2002 (NRS2002) score was also recorded at admission and discharge; this test is a validated nutritional screening tool for hospitalized adults, which has recently been recommended for COVID-19 patients [30]. If a patient's score is ≥ 3 points, they are deemed at risk of MN and a more comprehensive evaluation and management measures should be undertaken [31]. Prevalence of MN during admission was established according to the Global Leadership Initiative on Malnutrition (GLIM) criteria [32]. Information was also collected on the use of enteral or parenteral nutrition; nutritional intake at admission; nutritional recommendations during admission, at discharge and at 3 and 6 months follow-up (oral nutritional supplement [ONS], type of diet, and intake); patient's adherence to these recommendations during admission and follow-up; and blood analytical parameters (albumin, cholesterol, total proteins, total lymphocytes, ferritin and C-reactive protein [CRP]) at admission, discharge and follow-up when available. Reference intervals for blood analytical parameters were taken from the Reference Laboratory of Catalonia [33]: albumin (3.5–5.2 g/dL), cholesterol (120–200 mg/dL), total proteins (6–8.3 g/dL), lymphocytes (1 × 103–3x103/μL), ferritin (30–400 ng/mL) and CRP (<0.5 mg/dL). Supplementary information on NRS2002 and GLIM evaluations in Supplementary Material.
2.6. Triple adaptation of texture-modified foods during hospitalization
After the initial nutritional and dysphagia screening, patients received nutritional and textural adaptation of their food (normal, fork-mashable or pureed diets) according to their masticatory capacity, and fluid modification (liquid, 250 mPa s or 800 mPa s) according to their swallowing function [34,35]. Regarding caloric and proteic needs, patients with a normal nutritional status received the usual hospital diet (1750–2000 kcal +70–90 g proteins) and those with a positive nutritional risk screening (NRS2002 ≥ 3) at admission, received ONS according to their nutritional and rheological needs. The management algorithm used during the study for OD and MN is detailed in Supplementary Fig. 1.
2.7. Data management and statistical analysis
The main outcome measure was prevalence of OD and risk of MN among COVID-19 patients enrolled in the study. In addition, we aimed to assess whether patients with OD and those with MN have worse prognoses than those without these conditions. Qualitative data were presented as relative and absolute frequencies and analyzed by the Fisher's exact test or the Chi-square test. Continuous data were presented as mean standard deviation (SD) and compared using the t-test (intergroup comparisons) or paired t-test (intragroup comparisons). For those variables that did not follow a normal distribution, we used the nonparametric Mann–Whitney U test (intergroup comparisons), the Wilcoxon-paired test (intragroup comparisons) or the Kruskal–Wallis test for multiple comparisons with Dunn's multiple comparison test. To assess normality, we used the D'Agostino and Pearson omnibus normality test.
For the bivariate analysis we used the Chi-square test to assess the relationship of different categorical factors with OD (at discharge and at 3 and 6 months follow-up), MN (at discharge and at 3 and 6 months follow-up) and mortality (during admission). For the continuous factors we used Student t-test (normal distribution) and Mann–Whitney U test (non-normal distribution). Multivariate models were done with the factors significantly associated with (p < 0.05) and clinically relevant to the different outcomes. We used the Stepwise method to assess the independent factors. For the survival analysis we used the Kaplan–Meier method and Log Rank test to compare curves (OD versus no OD and MN versus no MN). Functionality and weight change during the study period was calculated and represented using data from patients that survived.
Results were interpreted according to the obtained p-value, the magnitude of the observed effect, and their clinical and biological plausibility. Statistical significance was accepted if p-values were <0.05. Statistical analysis was performed with GraphPad Prism 6 (San Diego, CA, USA).
3. Results
-
A)
Hospitalization
3.1. Demographics and clinical characteristics of the study population
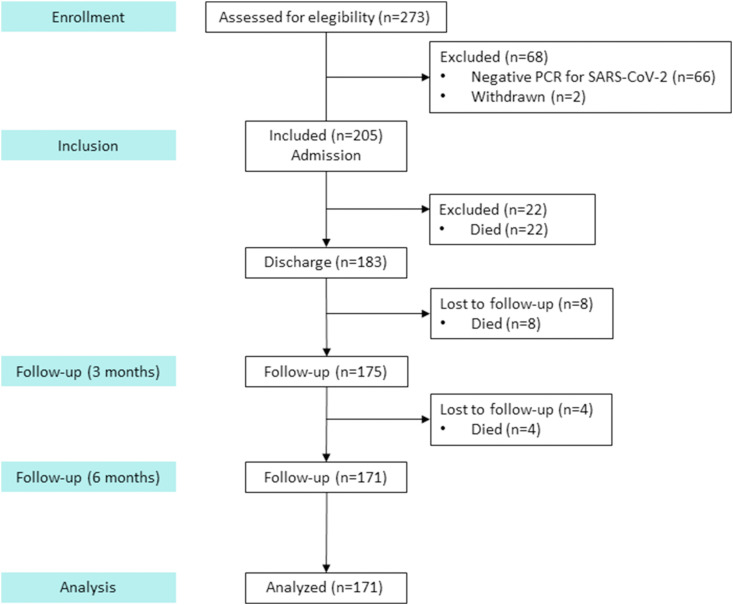
A total of 273 consecutive hospitalized patients were recruited into the study and 68 were excluded due to a negative SARS-CoV-2 RT-PCR test result (n = 66) or withdrawal of the subjects (n = 2) (Fig. 1 ). The mean age of participants was 69.3 ± 17.5 years; 52.2% were female. Patients presented with a considerably high number of comorbidities (mean Charlson score 3.7 ± 2.6) and mean Barthel Index on admission was 81.3 ± 30.3, indicating moderate dependence. Up to 66.3% (n = 136) of patients were admitted from the community, 30.2% (n = 62) from a nursing home, and 3.4% (n = 7) from an intermediate care hospital (Table 1 ).
Fig. 1.
Consort study flow chart for patients included in the study.
Table 1.
Demographics and clinical characteristics of the study population at admission.
| Pre -admission | Admission | Discharge | p-value | |
|---|---|---|---|---|
| Mean age (years), ±SD | 69.3 ± 17.5 | |||
| Sex (% female), n/N | 52.2 (107/205) | |||
| Patient origin/destination, % | ||||
| Community, n/N | 66.3 (136/205) | – | 63.2 (115/182) | 0.053 |
| Nursing home, n/N | 30.2 (62/205) | – | 27.5 (50/182) | |
| Intermediate care hospital, n/N | 3.4 (7/205) | – | 9.7 (17/183) | |
| Mean Charlson Score, ±SD | 3.74 ± 2.624 | |||
| Mean Barthel Index, ±SD | 90.4 ± 18.7 | 81.3 ± 30.3∗ | 80.7 ± 29.1∗ | <0.0001 |
| Slight dependence–independent (91–100), n/N | 56.6 (112/198) | 51.1 (92/180) | 56.2 (86/153) | 0.0438 |
| Moderate dependence (61–90), n/N | 23.7 (47/198) | 15.0 (27/180) | 17.6 (27/153) | |
| Severe dependence (21–60), n/N | 15.2 (30/198) | 22.8 (41/180) | 19.0 (29/153) | |
| Total dependence (0–20), n/N | 4.5 (9/198) | 11.1 (20/180) | 7.2 (11/153) | |
| Clinical symptoms, % | ||||
| Fever, n/N | 70.1 (143/204) | 32.2 (66/204) | – | <0.0001 |
| Cough, n/N | 52.9 (108/204) | 37.6 (77/204) | – | 0.003 |
| Dyspnea, n/N | 42.2 (86/204) | 31.7 (65/204) | – | 0.040 |
| Diarrhea, n/N | 23.0 (47/204) | 35.6 (73/204) | – | 0.007 |
| Ageusia, n/N | 7.4 (15/204) | 4.4 (9/204) | – | 0.293 |
| Anosmia, n/N | 6.4 (13/204) | 3.4 (7/204) | – | 0.251 |
| Vomiting, n/N | 6.4 (13/204) | 16.6 (34/204) | – | 0.251 |
∗p < 0.0001 vs. pre-admission.
Mean duration of symptoms prior to admission was 7.1 ± 4.9 days and the main clinical symptoms during that period were fever, cough, dyspnea, diarrhea, ageusia, anosmia and vomiting (Table 1). During admission, 62.4% of patients had at least one neurological symptom: confusion (40.0%), headache (28.3%), delirium (15.1%), ageusia (4.4%), anosmia (3.4%), encephalitis (2.0%), paresthesia (1.5%), stroke (0.5%) and dyskinesia (0.5%).
Mean hospital stay was 16.8 ± 13.0 days (median 13 [IQR 7–22]). Up to 61.0% (n = 125) of patients developed interstitial pneumonia due to SARS-COV-2; respiratory failure was diagnosed in 34.6% (n = 71); 12.4% (n = 25) had bacterial respiratory co-infection, and 3.9% (n = 8) had aspiration pneumonia according to cultures taken from the respiratory tract or bacterial blood samples. Severity of disease, measured using the NEWS and SOFA scales, showed a low degree of illness (4.6 ± 2.3 and 2.5 ± 2.3; respectively). Up to 37.6% (n = 77) of patients required high-flow oxygen therapy and 62.4% (n = 128) required low-flow (nose cannulas and facial mask). Furthermore, 12.2% (n = 25) of patients were referred from the ICU where 88.0% (n = 22) and 12.0% (n = 3) were treated with mechanical ventilation and non-invasive positive pressure ventilation, respectively. The average ICU stay was 15.0 ± 9.8 days and post-ICU myopathy was reported in 84.0% (n = 21) of cases. The prone position, irrespective of whether the patient was admitted to the ICU, was used in 20.5% (n = 42) of patients, and up to 18.2% of study patients received tocilizumab during admission.
3.2. Swallowing and masticatory function
The prevalence of patients with a previous diagnosis of OD before admission was 9.8%. Overall prevalence of OD at admission was 51.7%, with 48.0% and 44.1% of patients showing clinical signs of impaired swallow efficacy and safety, respectively. At discharge, 44.8% of patients still presented with OD. According to these results, 35.3% of included patients had a new diagnosis of OD.
Up to 39.9% dysphagic patients required fluid thickening at 250 mPa s and only 4.4% at 800 mPa s to maintain a safe swallow, and 75.1% of patients were adherent to this therapy. At discharge, there was a significant improvement in the number of patients that could be safely hydrated with thin liquid (78.6% vs. 56.1% at admission; p < 0.0001). Up to 53.7% of patients had mastication impairments at admission and this prevalence was significantly reduced to 39.0% at discharge. The need for texture-modified diets as a result of masticatory impairment also significantly improved from 53.7% at admission to 39.0% at discharge (p = 0.004). Patient adherence to texture-modified diets (easy mastication or puree) during hospitalization was 60.0%; and adherence to normal texture diet was 64.0%.
3.3. Nutritional status
Mean BMI at admission was 28.5 ± 5.4 (Table 2 ), indicating an overweight population, and 88.7% of patients presented with a NRS2002 ≥ 3, indicating nutritional risk. During hospitalization, 45.5% of patients developed MN according to GLIM criteria. Mean usual weight at pre-admission was 79.5 ± 18.4 kg. Mean weight loss was 6.5 ± 5.8 kg between first symptoms and hospital discharge for all patients, and 10.1 ± 5.0 kg in patients who had MN (p < 0.0001) (Fig. 2 ), with a weight loss ≥10 kg in 47.8% of malnourished patients (Table 2). The mean percentage weight loss was 7.8% in the whole study population, and 8.4% and 12.4% in those with OD and MN, respectively.
Table 2.
Anthropometric data and nutritional evaluation.
| Admission | Discharge | p-value | |
|---|---|---|---|
| Mean weight (kg), ±SD | 77.0 ± 18.4 | 72.8 ± 16.6 | <0.0001 |
| Mean BMI (kg/m2), ±SD | 28.5 ± 5.4 | 26.6 ± 5.7 | 0.009 |
| Mean weight loss (kg), ±SDa | 2.3 ± 3.2 | 6.5 ± 5.8 | <0.0001 |
| Weight loss (kg), %a | |||
| 0 kg (n/N) | 38.7 (43/111) | 20.3 (24/118) | <0.0001 |
| 1–3 kg (n/N) | 37.8 (42/111) | 19.5 (23/118) | |
| 3–6 kg (n/N) | 11.7 (13/111) | 22.0 (26/118) | |
| 6–10 kg (n/N) | 7.2 (8/111) | 18.6 (22/118) | |
| >10 kg (n/N) | 4.5 (5/111) | 19.5 (23/118) | |
| Main symptoms, % | |||
| Anorexia (n/N) | 59.6 (62/104) | 32.0 (39/122) | <0.0001 |
| Vomiting/nausea (n/N) | 13.6 (14/103) | 15.6 (19/122) | |
| Diarrhea (n/N) | 21.2 (22/104) | 33.6 (41/122) | |
| Incomplete diet intake (n/N) | 69.1 (85/123) | 42.3 (52/123) | |
| Others (n/N) | 23.3 (27/99) | 28.3 (34/120) | |
| Oral nutritional supplementation, % | |||
| Not prescribed (n/N) | 21.8 (44/202) | 33.3 (60/180) | <0.0001 |
| Prescribed (n/N) | 50.5(102/202) | 8.9 (16/180) | |
| Unknown (n/N) | 27.7 (56/202) | 57.8 (104/180) | |
Weight loss values are calculated from the appearance of the first COVID-19 symptoms to the timepoints described in the table. BMI: body mass index.
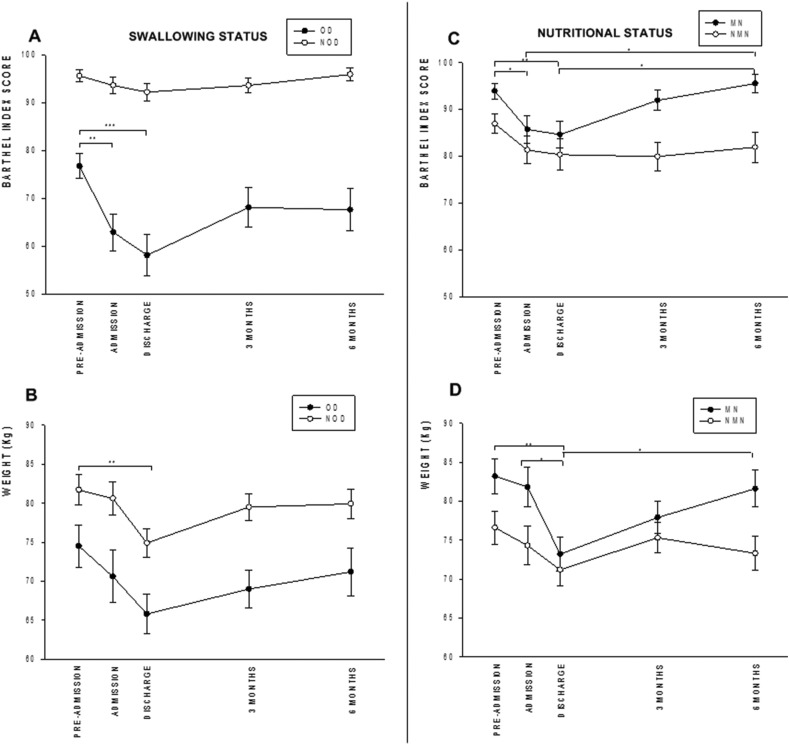
Fig. 2.
Change in functional status (Barthel Index) and weight over the 6-month study period according to swallowing and nutritional status.
At admission, up to 50.5% (n = 102) of patients were receiving ONS, 21.8% were not, and in 27.7% the ONS supplementation was not properly registered (and was therefore unknown) (Table 2). In addition, 14.9% (n = 29) required NGTs during hospitalization, most of them (86.2%) following mechanical ventilation in the ICU. The percentage of patients who consumed 100% of daily dietary requirements during pre-admission was only 31.7% (n = 38), and this significantly increased to 74.8% (n = 104) by discharge (p < 0.0001).
3.4. Biochemical analysis
Mean albumin concentration remained stable throughout hospitalization (3.2 ± 0.5 mg/dL at admission vs. 3.3 ± 0.7 at discharge; p = 0.676). Up to 60.1% of patients had albumin values below the lower limit of reference values (LLRV) at admission and 52.2% at discharge (p = 1.000). Mean cholesterol concentration was also below LLRV at admission and improved at discharge (131.2 ± 48.3 mg/dL vs. 189.5 ± 46.6 mg/dL, respectively; p = 0.0005). Up to 35.7% of patients had cholesterol values below the LLRV at admission while only 15.8% of patients had the same at discharge (p = 0.074). Mean total lymphocyte concentration increased during hospitalization (from 1.2 ± 0.6 × 103/μL at admission to 1.8 ± 1.6 × 103/μL at discharge; p < 0.0001); 41.4% of patients had total lymphocyte levels below the LLRV at admission and only 19.5% had the same at discharge (p < 0.0001). Mean total protein concentration remained stable throughout hospitalization (6.4 ± 0.7 g/dL admission vs. 6.2 ± 0.7 g/dL discharge; p = 0.095), however only 14.7% of patients had total protein levels below the LLRV at admission and this significantly worsened to 42.1% at discharge (p < 0.0001).
Inflammatory parameters such as ferritin and CRP were high at admission and improved at discharge (mean ferritin: 1024.0 ± 1420.0 ng/mL vs 912.5 ± 1217.0 ng/mL; p = 0.413; and mean CRP: 11.1 ± 10.1 mg/dL vs. 2.8 ± 4.9 mg/dL; p < 0.0001). A total of 62.1% of patients had ferritin levels above the upper limit of the reference values (ULRV) and 66% had the same at discharge (p = 0.484); 95.1% of patients had CRP values above the ULRV at admission and 61.5% at discharge (p < 0.0001).
3.5. Risk factors associated with OD, MN and mortality: bivariate, multivariate and survival analysis
3.5.1. Oropharyngeal dysphagia
Patients with OD were older (p = 0.0001), had a higher mean number of comorbidities (p < 0.0001), and had worse mean functional capacity at pre-admission (p < 0.0001) and during admission (p < 0.0001) than those not presenting with OD (Supplementary Table 1). The mean Barthel Index was lower in patients with OD and was severely impaired from pre-admission to admission (76.7 ± 19.9 vs. 62.9 ± 27.6; p < 0.01) and discharge (58.1 ± 30.0; p < 0.001) (Fig. 2A). OD patients were admitted from nursing homes or intermediate care centers (p < 0.0001). Patients with OD more frequently had neurological symptoms than those without OD (79.5% vs. 51.3%; p < 0.0001), with the most prevalent ones being confusion (68.9% vs. 18.0%; p < 0.0001) and delirium (28.9% vs. 4.5%; p < 0.0001). Patients with OD had a mean lower weight on pre-admission (p = 0.0026) and at discharge (p < 0.0001) and experienced higher mean weight loss from pre-admission to admission (p = 0.0043) than those without OD (Fig. 2B). The mean length of hospital stay among patients with OD (17.3 ± 13.1 days) was similar to those without OD (15.8 ± 11.8 days; p = 0.430). Analytical parameters showed lower mean albumin (p = 0.002) and total protein levels (p = 0.025) for OD patients. Patients with OD required more ONS (p < 0.0001) and texture-modified diets (p < 0.0001) than those without. Finally, OD patients were more frequently institutionalized at discharge than those without OD (p < 0.0001) (Supplementary Table 1).
Standard error of the mean is represented for each study point in both study variables. Note that the sharp decrease in Barthel Index during the acute phase in OD patients is not recovered during follow up; in contrast, patients with MN present a more moderate reduction in Barthel Index that is fully recovered. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. OD: oropharyngeal dysphagia; NOD: no oropharyngeal dysphagia; MN: malnourished; NMN: not malnourished. A: Barthel Index OD/NOD patients; B: Weight OD/NOD patients; C: Barthel index MN/NMN patients; D: Weight MN/NMN patients.
3.5.2. Malnutrition
Patients with MN were older (p = 0.008), had higher severity of disease (SOFA scale) (p < 0.0001), and more frequently came from community settings (p < 0.0001) than those without MN. Patients with MN also had a significantly longer length of hospital stay (mean 21.9 ± 14.8 days vs. 11.9 ± 8.9 among those without MN [p < 0.0001]). In patients with MN, mean functional capacity was impaired from pre-admission to admission (93.9 ± 14.0 vs. 85.7 ± 23.7; p < 0.05) and remained stable at discharge (84.6 ± 23.5; p < 0.01 vs. pre-admission) (Fig. 2C). Patients with MN had decreased food intake at pre-admission (p = 0.09) and during hospitalization (p = 0.04) compared with patients without MN. Their weight decreased from pre-admission to discharge (83.2 ± 18.7 kg vs. 73.2 ± 17.4 kg, respectively; p < 0.01) with a percentage mean weight loss of 12.2% (p < 0.001). In addition, they had a significant percentage of weight loss between admission and discharge (8.7%; p < 0.05) (Fig. 2D). Analytical parameters showed increased ferritin (p = 0.008) and CRP levels (p = 0.037), suggesting higher inflammatory response levels among patients with MN. Patients with MN required higher levels ONS (p < 0.0001) and were more frequently treated with tocilizumab than those without MN (34.9% vs. 5.0%, respectively; p < 0.0001). Finally, MN patients were more frequently discharged to community settings (p = 0.001) (Supplementary Table 2).
3.5.3. Mortality
Patients who died during hospitalization were older than those who did not die (81.3 ± 11.7 vs 67.8 ± 17.6 years, respectively; p < 0.0001), they had a worse functional status according to mean Barthel Index both at pre-admission (52.3 ± 38.2 vs. 86.3 ± 21.1; p < 0.0001) and during admission (27.5 ± 31.0 vs. 79.9 ± 28.6, p < 0.0001); they had a higher mean number of comorbidities (Charlson) (6.9 ± 2.5 vs. 3.4 ± 2.4; p < 0.0001); and were more institutionalized before admission (59.1% vs. 30.6%; p = 0.007). In addition, patients who eventually died presented with more impairments in swallow function requiring more thickeners (p < 0.0001) and showed a higher prevalence of previous OD (20.7% vs. 8.2%; p = 0.047) than those who survived. Regarding nutritional status, patients who died had a higher risk of MN at admission (95.2% vs. 73.1%; p = 0.029), reduced mean weight pre-admission (66.8 ± 12.2 vs. 80.1 ± 18.4 kg; p = 0.034), higher needs for texture-modified diets (p < 0.0001) and decreased number of lymphocytes on admission (1.0 ± 0.4 vs. 1.3 ± 0.7 × 103/μL; p = 0.036) than those who survived. Of those who died during hospitalization, 77.3% (n = 17) had OD, 50.0% (n = 11) had MN and 36.4% (n = 8) had both conditions.
3.6. Multivariate analysis: independent risk factors associated with OD, MN, and mortality
3.6.1. Oropharyngeal dysphagia
A multivariate logistic regression analysis showed that presence of delirium, comorbidities (Charlson), and low functional capacity pre-admission (Barthel) were independently associated with OD (Table 3 ). Delirium and a high number of comorbidities (Charlson) were risk factors for its development; and a higher Barthel pre-admission score was a protective factor for OD.
Table 3.
Multivariate logistic regression analysis of risk factors associated with oropharyngeal dysphagia at discharge, malnutrition and mortality.
| p-value | OR (95% CI) | |
|---|---|---|
| Oropharyngeal dysphagia at discharge | ||
| Delirium | 0.013 | 10.97 (1.64–73.31) |
| Charlson | 0.040 | 1.49 (1.02–2.18) |
| Barthel pre-admission | 0.008 | 0.92 (0.87–0.98) |
| Malnutrition | ||
| Dysphagia on admission | 0.007 | 3.96 (1.45–10.76) |
| Tocilizumab | <0.001 | 9.53 (2.79–32.59) |
| Patient origin (nursing home) | 0.003 | 0.16 (0.0–0.53) |
| Mortality | ||
| Charlson | 0.028 | 2.24 (1.09–4.58) |
| Barthel pre-admission | 0.004 | 0.91 (0.86–0.97) |
OR: odds ratio; CI: confidence interval.
3.6.2. Malnutrition
Presence of OD at admission, treatment with tocilizumab and admission from nursing home were independently associated with MN during hospitalization (Table 3). OD upon admission and tocilizumab treatment were risk factors for MN. In contrast, hospital admission from nursing homes was a protective factor for MN.
3.6.3. Mortality
Presence of comorbidities (Charlson) and functional capacity pre-admission (Barthel) were independently associated with intrahospital mortality (Table 3). A high number of comorbidities (Charlson) was a risk factor for mortality; and a high Barthel pre-admission score was a protective factor.
-
B)
Follow-up at 3 and 6 months
3.7. Oropharyngeal dysphagia
Prevalence of OD was significantly reduced from discharge to 3 months follow-up (44.8% vs. 24.0%; p < 0.0001) and remained stable up to 6 months follow-up (23.3%). Consequently, the percentage of patients that required fluid adaptation was reduced from 21.4% at discharge to 13.8% at 3 months (p = 0.069) and to 11.1% at 6 months follow-up (p = 0.031 vs. admission; p = 0.745 vs. 3 months). Masticatory function also improved from discharge to 3 months follow-up (with dietary adaptations in 39.0% vs. 19.8% patients, respectively; p = 0.037) and this remained stable at 6 months follow-up (20.4%).
In patients with OD at discharge we found that the mean Barthel Index score improved from hospital discharge to 3 months to 68.1 ± 31.3 and remained stable at 6 months, but pre-admission values were not recovered (Fig. 2A). Weight increased at 6 months follow-up (71.2 ± 19.7 kg) in OD patients without recovering to pre-admission values (Fig. 2B).
3.8. Malnutrition
Prevalence of MN was significantly reduced from discharge to 3 months follow-up (45.5% vs. 6.5%; p < 0.0001) and remained stable after 6 months follow-up (7.1%). At 3 months follow-up, the percentage of patients that were able to eat 100% of the diet increased to 89.1% (n = 139) (p = 0.003) and remained stable after 6 months (84.5%) (p = 0.546 vs. 3 months; p = 0.024 vs discharge). Regarding ONS supplementation and adherence, on hospital discharge, only 8.7% of patients were prescribed with ONS, 3.4% at 3 months, bringing treatment compliance very high (86.7%–66.7%). Mean BMI increased from 26.6 ± 5.7 at discharge to 27.9 ± 5.1 at 3 months to (p = 0.066) and to 28.3 ± 5.4 at 6 months follow-up. From discharge to 6 months follow-up, mean weight significantly increased to (81.6 ± 18.2 kg; p < 0.05) in MN patients, without recovering the pre-admission values (Fig. 2D). Regarding functional capacity, we found that between discharge and 6 months follow-up, mean Barthel Index of MN patients was recovered to pre-admission values (93.5 ± 15.2; p < 0.05 vs. discharge) (Fig. 2C).
3.9. Clinical outcomes and 6-month mortality
During the first three months after discharge, 14.9% patients were readmitted for any disease; 3.3% were diagnosed with a respiratory infection; and 17.7% visited the Emergency Room Service for any reason. From 3 to 6 months follow-up: 9.9% additional patients were readmitted for any disease; 1.8% were diagnosed with a respiratory infection; and 10.5% visited the Emergency Room Service for any reason. There were no statistically significant differences between these clinical outcomes among patients with or without OD and MN, neither at 3 or 6-months follow-up.
3.10. Survival analysis
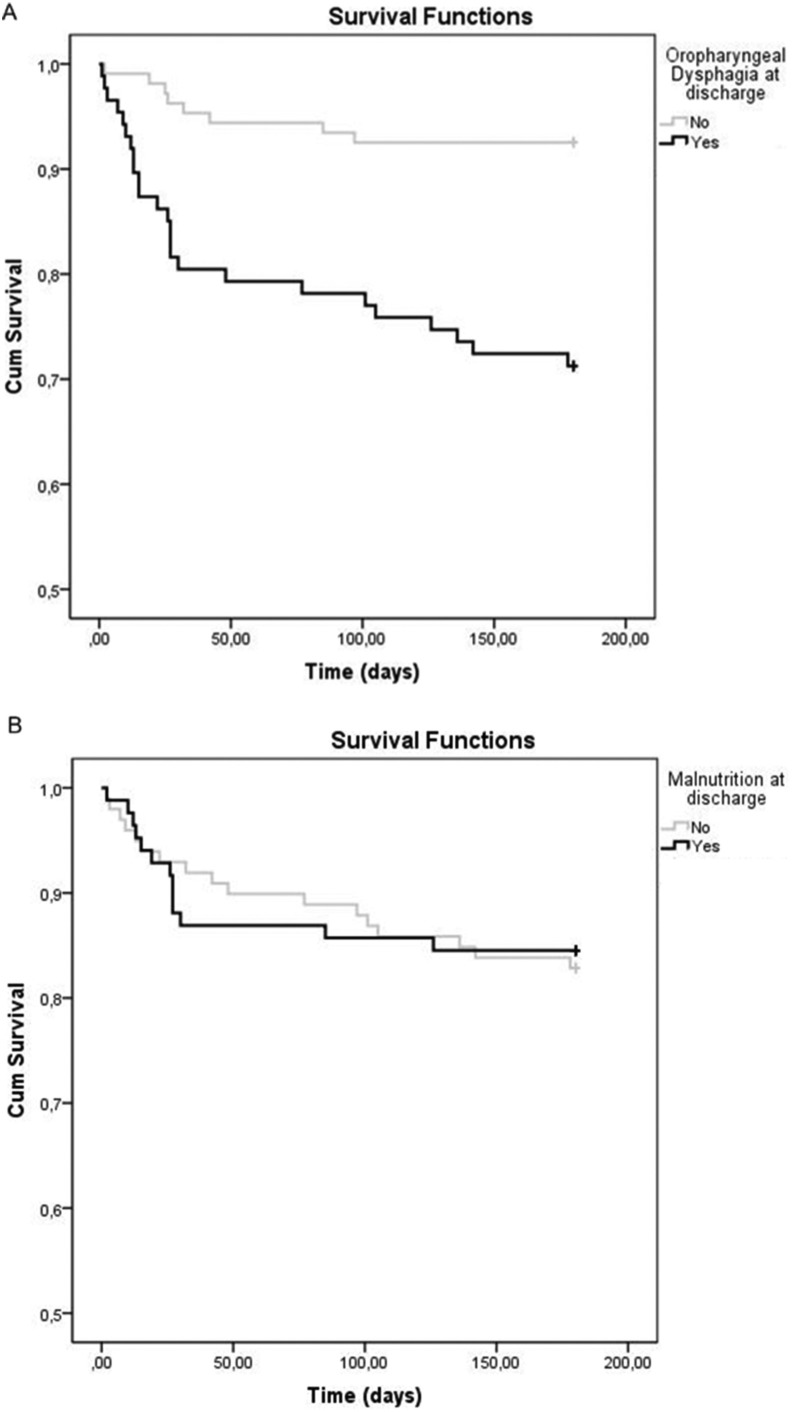
Mortality over the whole study period was 16.6% (n = 34): 10.7% (n = 22) during hospitalization; 4.4% (n = 8) at 3 months; and 2.3% (n = 4) at 6 months. Survival analysis showed a significant 6-month higher mortality for those patients with OD at discharge versus those without OD (28.4% vs. 7.1%; p < 0.001) (Fig. 3 A). Main causes of mortality during follow up were bronchoaspiration and sepsis (Supplementary table 3). In contrast, there were no significant differences in mortality between MN and no MN patients at the end of the study period (14.6% vs. 16.2%; p = 0.800) (Fig. 3B).
Fig. 3.
Survival curves of patients with and without OD (A) and MN (B) at hospital discharge A comparison p < 0.001; B comparison p = 0.800.
4. Discussion
This study evaluated 205 COVID-19 patients admitted consecutively to our general hospital during the first wave of the 2020 pandemic. Patients had a mean age of 69.28 years, were generally overweight, had a high prevalence of comorbidities and moderate functional impairment prior to admission. The majority were hospitalized from community settings after approximately one week of symptoms, presented with a high prevalence of OD at both admission and discharge, had a considerable prevalence of MN during hospitalization, and had notable weight loss before and during hospitalization. MN was associated with patient admission from the community, OD on admission, low visceral protein markers, hyperinflammation, treatment with tocilizumab, and weight loss >10 kg. OD was independently associated with comorbidities, neurological symptoms, and low functionality. We found that patients who died were older (81.3 ± 11.7) and had a higher prevalence of OD than survivors; comorbidities and low Barthel Index score pre-admission were found to be independent risk factors for intrahospital mortality. MN resolved in most patients during the 6-month follow-up period whereas OD persisted in up to 23.0% of patients, and 6-month survival was significantly decreased in patients with OD. Our results suggest that optimizing the management of MN might help shorten hospitalization duration, and that early diagnosis and effective treatment of OD might impact the nutritional status of COVID-19 patients during hospitalization, and improve their clinical outcomes and survival after discharge.
The patient demographic enrolled in this study is representative of the 656 patients admitted to our institution during the first wave of the COVID-19 pandemic in the Maresme area. Mean age and functionality of our study participants was similar to that reported in several Italian studies conducted [[36], [37], [38]]. Mean duration of symptoms prior to admission was 7.1 ± 4.9 days and consisted mainly of fever, cough, dyspnea, diarrhea and vomiting. Some studies observed similar symptoms prior to admission [39,40] but reported a higher symptom duration (11.3 ± 6.2 days) [41]. Length of hospital stay in our study (median 13 days [IQR 7–22]) was similar to that reported in Chinese studies [42], and our intrahospital mortality (10.7%) was lower than that reported by studies in similar Spanish populations (19.8% and 18.0%) [43,44]. Patients included from the ICU constituted 12.2% of our study population, similar to that reported by Bedock D et al. [45]. Furthermore, 61.0% of our patients had interstitial pneumonia, similar to other studies, which reported 54.2% and 82.8% [46,47]. Our patient series is fully representative of what happened during the first wave of COVID-19 pandemics in southern Europe, mainly in Spain and Italy. Pathophysiology of OD in SARS-CoV-2 patients is presumed to be related to the interaction of the virus with angiotensin converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), proteins that are present in relevant anatomic regions for swallowing function, such as oral, pharyngeal and nasal human mucosa [48,49]. SARS-CoV-2 causes oropharyngeal sensory dysfunction, probably related to glossopharyngeal and vagal sensory neuropathy, a major pathophysiological factor for OD [7,50]. Our results showed a low pre-admission prevalence of anosmia and ageusia (7.4% and 6.4%, respectively); however, other studies reported these symptoms in >40% of COVID-19 patients [[51], [52], [53], [54]], probably due to increased awareness and more sensitive methodology. In addition, stroke or encephalitis, can affect different parts of the neural swallowing network, making COVID-19 patients more prone to OD [13]. We have previously described that pharyngeal sensory alterations are key factors in the pathophysiology of OD in stroke patients and elderly people [[55], [56], [57]]. In this study, we found a high prevalence of OD (51.7%), which was higher than that reported by Dawson et al. in a similar aged cohort (28.9%) [58]. We suggest a different pathophysiology than post-extubation OD, that is a well-recognized complication in COVID-19 post-ICU patients [8], but a minor cause in our series (10.2% of OD patients). We have described in previous articles that the pathophysiology post COVID-19 OD could be associated with impaired pharyngeal sensory function [7,13]. The method we used for assessment of OD (V-VST) has robust psychometrics [28,29], was developed for our group, and has been adapted to virtual evaluation and telemedicine [59]. We also found that neurological symptoms, higher comorbidities and impaired functional status were independent risk factors for OD. These results are consistent with our previous publications on the pathophysiology of OD in older patients [[60], [61], [62]]. Our study also shows the main complications of OD in this COVID-19 population. We previously found that in elderly hospitalized patients, impaired safety of swallow causes respiratory infection and aspiration pneumonia with increased hospital readmissions [[63], [64], [65]], while impaired efficacy of swallow is associated with MN in the acute and chronic setting; while OD has been reported to be an independent risk factor for MN, and increasing mortality [62,66]. In this study, OD was an independent risk factor for MN, and was associated with greater weight loss from pre-admission to admission, institutionalization after discharge and higher mortality during follow-up. In addition, we found a high percentage of bacterial coinfection and aspiration pneumonia among the whole population (16.3%), further supporting the relevance of OD in bacterial respiratory co-infections in COVID-19 [50].
Pathophysiology of MN in SARS-CoV-2 infection has been recently reviewed in an ESPEN practical guidance document [8]. Our study identified that the majority of these well-known pathophysiological elements were associated with disease-related MN in COVID-19 patients, and included older age; anorexia and reduced food intake; respiratory failure; fever during the viral response phase; and catabolic changes due the host inflammatory response phase (increased ferritin and CRP levels, tocilizumab treatment, and higher disease severity). In addition, we observed a considerable reduction in muscle mass and visceral protein markers (albumin, total lymphocytes and cholesterol) at admission, as reported in other studies [41,67]. At discharge, we found significant improvements in levels of CRP, ferritin and T lymphocytes [41,67] and in some nutritional biomarkers that could be related to the favorable progression of the disease. Our contribution here is the identification of OD at admission as an independent factor for MN during hospitalization of COVID-19 patients. Our data showed that 88.7% of patients were at nutritional risk upon admission, according to NRS2002 score. Several studies, some of them using the same tool, have also identified a high percentage of patients at nutritional risk among COVID-19 sufferers (84.7–92.0%) [41,68]. In addition, 45.5% of our patients were malnourished at discharge, according to GLIM criteria, and this prevalence is similar to that reported in other studies with COVID-19 hospitalized patients (42.1%, 38.9% and 52.7%) [39,45,69]. We should recognize that our response to this nutritional emergency caused by the first wave of SARS-CoV-2 infection was still inadequate and late and this caused our clinical results to be suboptimal. Although mandated in our study protocol (Supplementary Fig. 1), only 50.5% of patients received ONS during hospitalization, and decreased food intake was also evident. In patients with MN, this prompted severe weight loss (12.4% of total weight from admission to discharge) and a hospitalization duration two times higher than those without MN.
Finally, we found that older age, worse functional status, higher number of comorbidities, OD at pre-admission, and nutritional risk on admission were associated with intrahospital mortality. In addition, comorbidities and functional capacity at pre-admission were revealed to have independent association with mortality. Other studies found male gender, severe obesity [70,71], increased age, low Barthel Index, longer disease duration, no pharmacological treatment and lymphocytopenia to be independent mortality risk factors in COVID-19 patients [40,70]. At 6-months follow-up, prevalence of OD was 23.3% while that of MN was only 7.1%. Prevalence of hospital readmissions and admission to the ER was high in this study in both OD and MN patients. Survival curves showed that OD was significantly associated with increased 6-month mortality, as previously reported in elderly people [60,62], while MN was not, probably due to the faster recovery of this condition as well as weight over the follow-up period. In addition, we have also found that some of these mortality causes (mainly bronchoaspiration and sepsis) could be attributed to OD.
This study presents some limitations. First the use of telematic screening, assessment and follow-up of COVID-19 patients limited the ability to perform a full evaluation in some cases and this could affect the prevalence described in the study. However, the reduction in infection risk for investigators justifies our methodology [21]. Second, it is possible that the mortality rate was underestimated, because the most severe patients who were admitted directly to the ICU and died were not included in the study. Third, neurological symptoms such as ageusia and anosmia were most likely underreported as monitoring for such symptoms was not usual clinical practice at the time and would have been hampered by the high workload during the pandemic. Fourth, only a limited number of clinical outcomes were collected due to the complexity of the study (hospital readmissions, respiratory infections, visits to the Emergency Room Service and mortality). For future studies it would be interesting to gather more data on the quality of life of patients, frailty and mobility. Additionally, we should recognize that although we used GLIM criteria for MN and these criteria have been recommended in COVID-19 patients, the definition of each phenotypic and etiologic component of GLIM system in our study was established according the specific clinical situation of our COVID-19 patients and the characteristics of our clinical management in each situation. Finally, the cohort presented here corresponds to the 1st wave of the Covid-19 pandemic in Catalonia and we should recognize that we were overwhelmed by the impact of the virus and that our initial nutritional management on discharge was quite poor, with low prevalence of patients prescribed with ONS. This has been amended during the 2nd and 3rd waves of the pandemic and the nutritional situation during follow up improved.
5. Conclusion
We conclude that prevalence and burden of OD and MN in patients hospitalized in COVID-19 wards is very high. Our results suggest that optimizing the management of MN might shorten the hospitalization period and that optimizing the management of OD will likely impact the nutritional status of COVID-19 patients and improve their clinical outcomes and survival after hospital discharge. Dysphagia and malnutrition is of importance in COVID-19 patients and simultaneous management of the two conditions must be proactive, aggressive and start upon admission to the ER.
Funding statement
Financial support received from: 1) NutriCOVer Program by Danone Trading Medical BV; 2) Strategic Action Grant in Oropharyngeal Dysphagia, Centro de Investigación Biomédica en Red en el Área de Enfermedades Hepáticas y Digestivas (CIBERehd), Insitituto de Salud Carlos III; 3) Strategic Plan for Research and Innovation in Health (PERIS), Generalitat de Catalunya (intensification grant of AM; SLT008/18/00162); 4) The Territorial Competitiveness Specialization Project (PECT) of Mataró-Maresme (PRE/161/2019) financed by the Government of Catalunya-Generalitat de Catalunya within the framework of the European Regional Developments Funds of Catalonia Operational Programme 2014-2020.
Author contribution
Alberto Martin–Martinez: Conceptualization, Methodology, Formal analysis, Writing - Original Draft, Project administration; Omar Ortega: Conceptualization, Methodology, Formal analysis, Writing - Original Draft, Writing - Review & Editing; Paula Viñas: Investigation, Writing - Original Draft; Viridiana Arreola: Investigation; Weslania Nascimento: Investigation; Alicia Costa: Investigation, Writing - Original Draft; Stephanie A. Riera: Investigation; Claudia Alarcon: Investigation; Pere Clavé: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Funding acquisition.
Conflict of interest
No competing interests declared. Nutricia-Danone Grant Support: Professor P. Clavé reports grants and contracts from Nutricia Advanced Medical Nutrition outside the submitted work.
Acknowledgements
Editorial support was provided by mXm Communications (Tina Morley) and funded by Danone Trading Medical BV. The authors would like to thank the dietitians, nurses, assistant nurses, healthcare workers, administrative staff and physicians from the COVID-19 Wards of Consorci sanitari del Maresme. We also want to acknowledge Elisabeth Palomeras and Mateu Serra-Prat from the Research Unit of CSdM for the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2021.06.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . January 5 2020. Pneumonia of unknown cause — China: disease outbreak news.https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Carretero Gómez J., Mafé Nogueroles M.C., Garrachón Vallo F., Escudero Álvarez E., Maciá Botejara E.M.G.J. La inflamación, la desnutrición y la infección por SARS-CoV-2: una combinación nefasta. Rev Clin Esp. 2020;220:511–517. doi: 10.1016/j.rce.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergara J., Lirani-Silva C., Brodsky M.B., Miles A., Clavé P., Nascimento W., et al. Potential influence of olfactory, gustatory, and pharyngolaryngeal sensory dysfunctions on swallowing physiology in COVID-19. Otolaryngol - Head Neck Surg (United States) 2020;164(6):1134–1135. doi: 10.1177/0194599820972680. [DOI] [PubMed] [Google Scholar]
- 8.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marik P.E., Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–336. doi: 10.1378/chest.124.1.328. [DOI] [PubMed] [Google Scholar]
- 10.Zuercher P., Moret C.S., Dziewas R., Schefold J.C. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23:1–11. doi: 10.1186/s13054-019-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dziewas R., Warnecke T., Zürcher P., Schefold J.C. Dysphagia in COVID-19 – multilevel damage to the swallowing network? Eur J Neurol. 2020;27:e46–e47. doi: 10.1111/ene.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130:1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moro E., Priori A., Beghi E., Helbok R., Campiglio L., Bassetti C.L., et al. The international European Academy of Neurology survey on neurological symptoms in patients with COVID-19 infection. Eur J Neurol. 2020;27:1727–1737. doi: 10.1111/ene.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ku P.K.M., Holsinger F.C., Chan J.Y.K., Yeung Z.W.C., Chan B.Y.T., Tong M.C.F., et al. Management of dysphagia in the patient with head and neck cancer during COVID-19 pandemic: practical strategy. Head Neck. 2020;42:1491–1496. doi: 10.1002/hed.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C., Viana A., Wang Y., Wei H quan, Yan A hui, Capasso R. Otolaryngology during COVID-19: preventive care and precautionary measures. Am J Otolaryngol - Head Neck Med Surg. 2020;41:102508. doi: 10.1016/j.amjoto.2020.102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattei A., Amy de la Bretèque B., Crestani S., Crevier-Buchman L., Galant C., Hans S., et al. Guidelines of clinical practice for the management of swallowing disorders and recent dysphonia in the context of the COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137:173–175. doi: 10.1016/j.anorl.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu D., Wang H., Yu R., Yang H., Zhao Y. Integrated infection control strategy to minimize nosocomial infection of coronavirus disease 2019 among ENT healthcare workers. J Hosp Infect. 2020;104:454–455. doi: 10.1016/j.jhin.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindler A., Baijens L.W.J., Clave P., Degen B., Duchac S., Dziewas R., et al. ESSD commentary on dysphagia management during COVID pandemia. Dysphagia. 2020:17–20. doi: 10.1007/s00455-020-10194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clavé P., Arreola V., Martín A., Costa A., Nascimento W., Carrión S., et al. 2020. Basic procedures to assess and treat oropharyngeal dysphagia in patients with COVID-19 infection.https://www.furega.com/covid-19/covid-eng.pdf [Google Scholar]
- 22.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:56–61. [PubMed] [Google Scholar]
- 23.Shah S., Vanclay F.C.B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 24.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almirall J., Cabré M., Clavé P. Complications of oropharyngeal dysphagia: aspiration pneumonia. Nestle Nutr Inst Workshop Ser. 2012;72:67–76. doi: 10.1159/000339989. [DOI] [PubMed] [Google Scholar]
- 27.Belafsky P.C., Mouadeb D.A., Rees C.J., Pryor J.C., Postma G.N., Allen J., et al. Validity and reliability of the eating assessment tool (EAT-10) Ann Otol Rhinol Laryngol. 2008;117:919–924. doi: 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 28.Rofes L., Arreola V., Mukherjee R., Clavé P. Sensitivity and specificity of the eating assessment tool and the volume-viscosity swallow test for clinical evaluation of oropharyngeal dysphagia. Neuro Gastroenterol Motil. 2014;26:1256–1265. doi: 10.1111/nmo.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clavé P., Arreola V., Romea M., Medina L., Palomera E., Serra-Prat M. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin Nutr. 2008;27:806–815. doi: 10.1016/j.clnu.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Med J Chin Peoples Lib Army. 2020;45:1–20. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondrup J., Ramussen H.H., Hamberg O., Stanga Z., Camilo M., Richardson R., et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 32.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Laboratori de Referència de Catalunya Analytical reference values of the Laboratori de Referència de Catalunya. http://central.lrc.cat/cgi-bin/nph-mgwcgi.exe?App=CATALOGO n.d.
- 34.Costa A., Carrión S., Puig-Pey M., Juárez F., Clavé P. Triple adaptation of the mediterranean diet: design of a meal plan for older people with oropharyngeal dysphagia based on home cooking. Nutrients. 2019;11:1–17. doi: 10.3390/nu11020425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolivar-Prados M., Rofes L., Arreola V., Guida S., Nascimento W.V., Martin A., et al. Effect of a gum-based thickener on the safety of swallowing in patients with poststroke oropharyngeal dysphagia. Neuro Gastroenterol Motil. 2019;31:1–11. doi: 10.1111/nmo.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sale P., Madio C., Pignataro A.F.G. Nutritional aspects in patients with COVID-19 admitted for rehabilitation. J Orthop Rheum. 2020;4:71–75. [Google Scholar]
- 37.Pironi L., Sasdelli A.S., Ravaioli F., Baracco B., Battaiola C., Bocedi G., et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2020;40(3):1330–1337. doi: 10.1016/j.clnu.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Formisano E., Di Maio P., Ivaldi C., Sferrazzo E., Arieta L., Bongiovanni S., et al. Nutritional therapy for patients with coronavirus disease 2019 (COVID-19): practical protocol from a single center highly affected by an outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nutrition. 2021;82:111048. doi: 10.1016/j.nut.2020.111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allard L., Ouedraogo E., Molleville J., Bihan H., Giroux-Leprieur B., Sutton A., et al. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020;12:1–14. doi: 10.3390/nu12123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Linli Z., Lei Y., Yang Y., Liu Z., Xia Y., et al. Risk factors for mortality in critically ill patients with COVID-19 in Huanggang, China: a single-center multivariate pattern analysis. J Med Virol. 2020;93:2046–2055. doi: 10.1002/jmv.26572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X., Li Y., Ge Y., Shi Y., Lv P., Zhang J., et al. Evaluation of nutrition risk and its association with mortality risk in severely and critically ill COVID-19 patients. J Parenter Enteral Nutr. 2021;45:32–42. doi: 10.1002/jpen.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rees E.M., Nightingale E.S., Jafari Y., Waterlow N.R., Clifford S., Pearson C.A.B., et al. COVID-19 length of hospital stay: a systematic review and data synthesis. MedRxiv. 2020;18:270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laosa O., Pedraza L., Álvarez-bustos A. Rapid assessment at hospital admission of mortality risk from COVID-19: the role of functional status. J Am Med Dir Assoc. 2020;21:1798–1802.e2. doi: 10.1016/j.jamda.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martos Pérez F., Luque del Pino J., Jiménez García N., Mora Ruiz E., Asencio Méndez C., García Jiménez J.M., et al. Comorbidity and prognostic factors on admission in a COVID-19 cohort of a general hospital. Rev Clin Esp. 2020:1–7. doi: 10.1016/j.rceng.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung H.K., Kim J.Y., Lee M.S., Lee J.Y., Park J.S., Hyun M., et al. Characteristics of COVID-19 patients who progress to pneumonia on follow-up chest radiograph: 236 patients from a single isolated cohort in Daegu, South Korea. Korean J Radiol. 2020;21:1265–1272. doi: 10.3348/kjr.2020.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossi R., Coppi F., Talarico M., Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID-19 era. Eur J Intern Med. 2020;77:158–160. doi: 10.1016/j.ejim.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehraeen E., Behnezhad F., Salehi M.A., Noori T., Harandi H., SeyedAlinaghi S.A. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): a review of current evidence. Eur Arch Oto-Rhino-Laryngol. 2021;278:307–312. doi: 10.1007/s00405-020-06120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lechien J.R., Radulesco T., Calvo-Henriquez C., Chiesa-Estomba C.M., Hans S., Barillari M.R., et al. ACE2 & TMPRSS2 Expressions in head and neck tissues: a systematic review. Head Neck Pathol. 2020;15(1):225–235. doi: 10.1007/s12105-020-01212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aoyagi Y., Ohashi M., Funahashi R., Otaka Y., Saitoh E. Oropharyngeal dysphagia and aspiration pneumonia following coronavirus disease 2019: a case report. Dysphagia. 2020;35:545–548. doi: 10.1007/s00455-020-10140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu C., Cui C., Hautefort C., Haehner A., Zhao J., Yao Q., et al. Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg. 2020;163:714–721. doi: 10.1177/0194599820934376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speth M.M., Singer-Cornelius T., Oberle M., Gengler I., Brockmeier S.J., Sedaghat A.R. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol - Head Neck Surg (United States) 2020;163:114–120. doi: 10.1177/0194599820929185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paderno A., Mattavelli D., Rampinelli V., Grammatica A., Raffetti E., Tomasoni M., et al. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol - Head Neck Surg (United States) 2020;163:1144–1149. doi: 10.1177/0194599820939538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beltrán-Corbellini, Chico-García J.L., Martínez-Poles J., Rodríguez-Jorge F., Natera-Villalba E., Gómez-Corral J., et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case–control study. Eur J Neurol. 2020;27:1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rofes L., Ortega O., Vilardell N., Mundet L., Clavé P. Spatiotemporal characteristics of the pharyngeal event-related potential in healthy subjects and older patients with oropharyngeal dysfunction. Neuro Gastroenterol Motil. 2016;29:1–11. doi: 10.1111/nmo.12916. [DOI] [PubMed] [Google Scholar]
- 56.Cabib C., Ortega O., Vilardell N., Mundet L., Clavé P., Rofes L. Chronic post-stroke oropharyngeal dysphagia is associated with impaired cortical activation to pharyngeal sensory inputs. Eur J Neurol. 2017;24:1355–1362. doi: 10.1111/ene.13392. [DOI] [PubMed] [Google Scholar]
- 57.Cabib C., Nascimento W., Rofes L., Arreola V., Tomsen N., Mundet L., et al. Neurophysiological and biomechanical evaluation of the mechanisms which impair safety of swallow in chronic post-stroke patients. Transl Stroke Res. 2020;11:16–28. doi: 10.1007/s12975-019-00701-2. [DOI] [PubMed] [Google Scholar]
- 58.Dawson C., Capewell R., Ellis S., Matthews S., Adamson S., Wood M., et al. Dysphagia presentation and management following coronavirus disease 2019: an acute care tertiary centre experience. J Laryngol Otol. 2020;134:981–986. doi: 10.1017/S0022215120002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soldatova L., Williams C., Postma G.N., Falk G.W., Mirza N. Virtual Dysphagia Evaluation: practical guidelines for dysphagia management in the context of the COVID-19 pandemic. Otolaryngol - Head Neck Surg (United States) 2020;163:455–458. doi: 10.1177/0194599820931791. [DOI] [PubMed] [Google Scholar]
- 60.Cabre M., Serra-Prat M., Palomera E., Almirall J., Pallares R., Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39:39–45. doi: 10.1093/ageing/afp100. [DOI] [PubMed] [Google Scholar]
- 61.Serra-Prat M., Hinojosa G., Lõpez D., Juan M., Fabré E., Voss D.S., et al. Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J Am Geriatr Soc. 2011;59:186–187. doi: 10.1111/j.1532-5415.2010.03227.x. [DOI] [PubMed] [Google Scholar]
- 62.Carrión S., Cabré M., Monteis R., Roca M., Palomera E., Serra-Prat M., et al. Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clin Nutr. 2015;34:436–442. doi: 10.1016/j.clnu.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Almirall J., Rofes L., Serra-Prat M., Icart R., Palomera E., Arreola V., et al. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J. 2013;41:923–926. doi: 10.1183/09031936.00019012. [DOI] [PubMed] [Google Scholar]
- 64.Cabré M., Serra-Prat M., Force L., Almirall J., Palomera E., Clavé P. Oropharyngeal dysphagia is a risk factor for readmission for pneumonia in the very elderly persons: observational prospective study. J Gerontol - Ser A Biol Sci Med Sci. 2014;69 A:330–337. doi: 10.1093/gerona/glt099. [DOI] [PubMed] [Google Scholar]
- 65.Martín A., Ortega O., Roca M., Arús M., Clavé Civit P. Effect of a minimal-massive intervention in hospitalized older patients with oropharyngeal dysphagia: a proof of concept study. J Nutr Health Aging. 2018;22:739–747. doi: 10.1007/s12603-018-1043-3. [DOI] [PubMed] [Google Scholar]
- 66.Carrión S., Costa A., Ortega O., Verin E., Clavé P., Laviano A. In: Dysphagia. Medical radiology. Ekberg O., editor. Springer; Cham: 2019. Complications of oropharyngeal dysphagia: malnutrition and aspiration pneumonia. [Google Scholar]
- 67.Jin X.H., Zhou H.L., Chen L.L., Wang G.F., Han Q.Y., Zhang J.G., et al. Peripheral immunological features of COVID-19 patients in Taizhou, China: a retrospective study. Clin Immunol. 2021;222:108642. doi: 10.1016/j.clim.2020.108642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu G., Zhang S., Mao Z., Wang W., Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74:876–883. doi: 10.1038/s41430-020-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74:871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heras E., Garibaldi P., Boix M., Valero O., Castillo J., Curbelo Y., et al. COVID-19 mortality risk factors in older people in a long-term care center. Eur Geriatr Med. 2020;27:1–7. doi: 10.1007/s41999-020-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., et al. Severe obesity is associated with higher in-hospital mortality in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.